Recent Development of Exploring Ferroptosis-Inspired Effect of Iron as a Feasible Strategy for Combating Multidrug Resistant Bacterial Infections
Abstract
1. Introduction
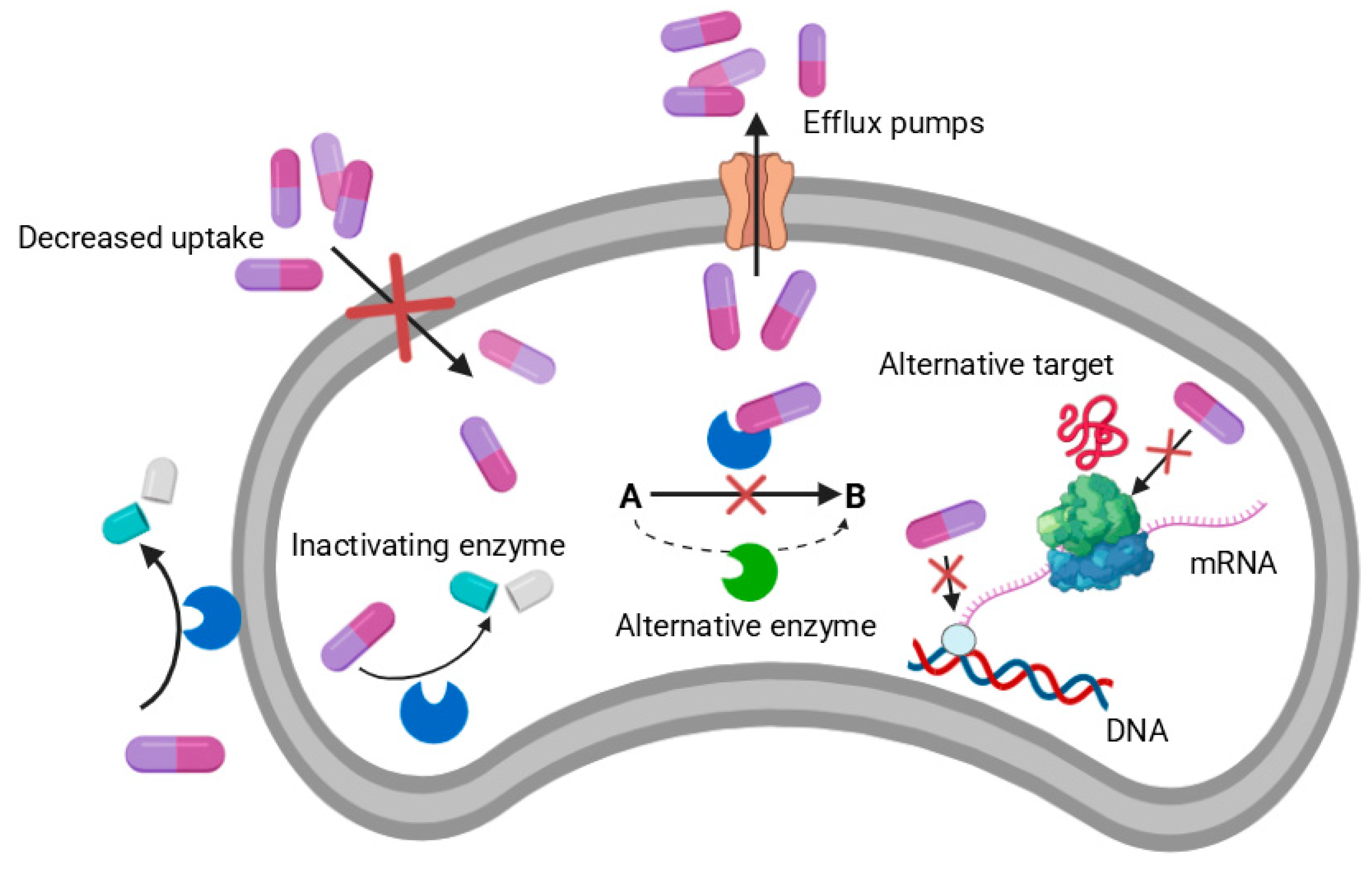
Mechanisms of AMR in Bacteria
2. Materials and Methods
3. Discussion
3.1. Iron in Bacteria
3.2. Iron Metabolism in Bacteria
3.3. Redox-Active Iron Complexes as ROS-Inducing Anticancer Agents
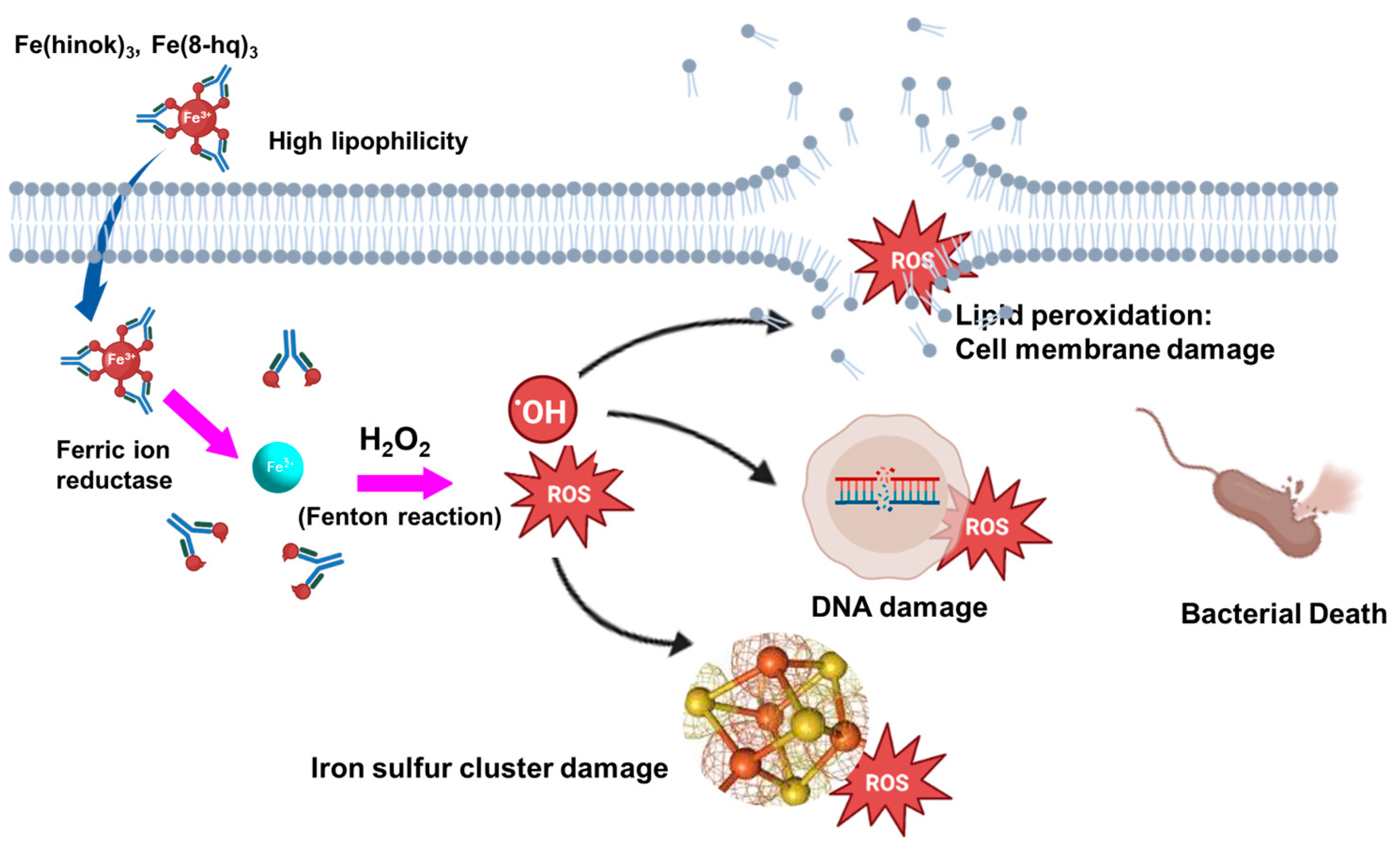
3.4. Design and Development of Novel Iron-Based Antimicrobial Agents
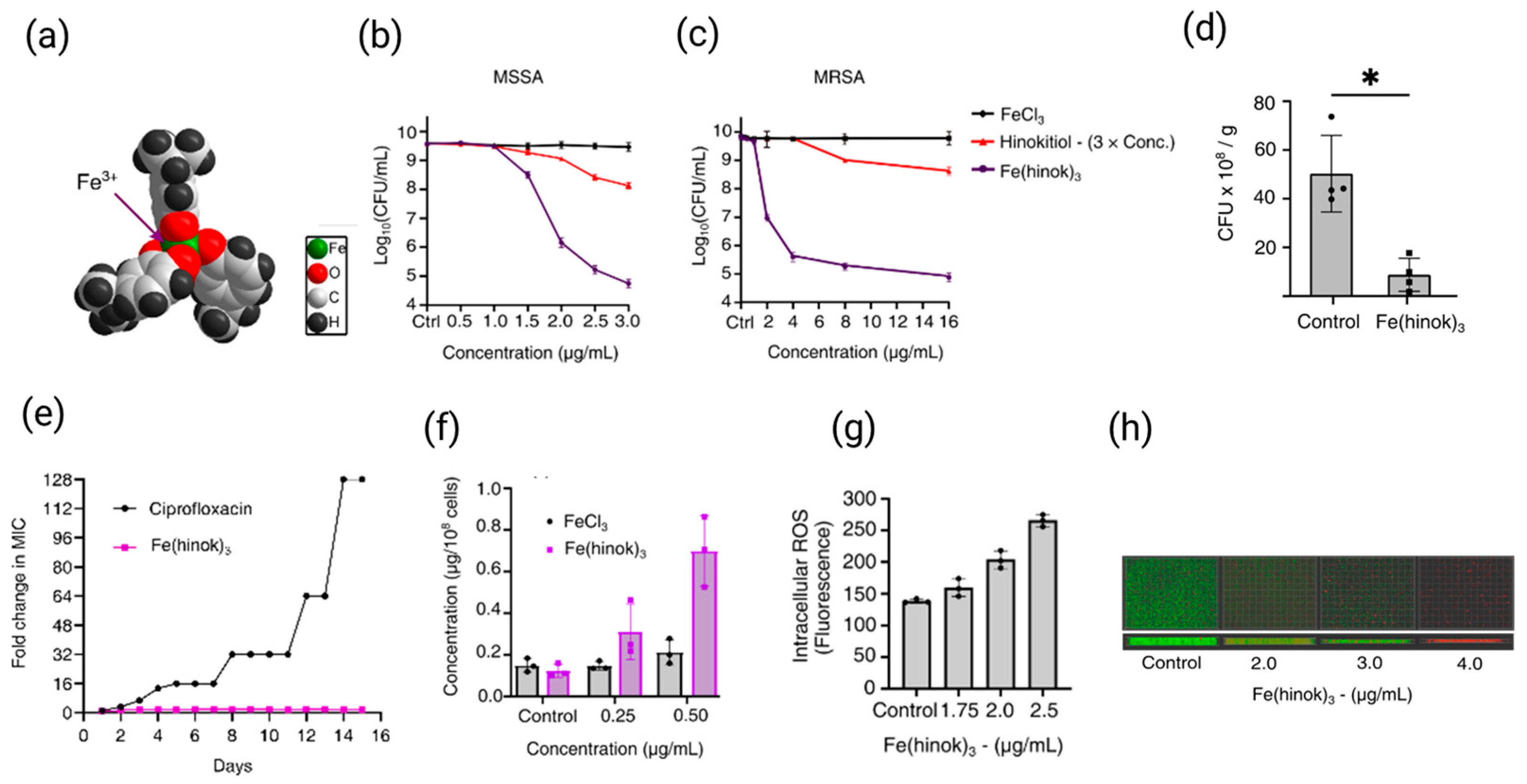
3.5. Recent Advances of Iron-Based Antimicrobial Agents
| MICs Against MRSA (ATCC BAA-44) | FIC Index | Ref. | |||
| Ciprofloxacin only | Ciprofloxacin with Fe(hinok)3 | Fe(hinok)3 only | Fe(hinok)3 with Ciprofloxacin | 0.75 | [96] |
| 16 µg/mL | 8 µg/mL | 4 µg/mL | 1 µg/mL | ||
| Ofloxacin only | Ofloxacin with Fe(hinok)3 | Fe(hinok)3 only | Fe(hinok)3 with Ofloxacin | 0.50 | [96] |
| 8 µg/mL | 2 µg/mL | 4 µg/mL | 1 µg/mL | ||
| Vancomycin only | Vancomycin with Fe(hinok)3 | Fe(hinok)3 only | Fe(hinok)3 with Vancomycin | 0.75 | [96] |
| 1 µg/mL | 0.5 µg/mL | 4 µg/mL | 1 µg/mL | ||
| MICs Against MRSA (ATCC BAA-44) | FIC Index | Ref. | |||
| Ciprofloxacin only | Ciprofloxacin with Fe(8-hq)3 | Fe(8-hq)3 only | Fe(8-hq)3 with Ciprofloxacin | 0.375 | [97] |
| 48.0 µM | 6.0 µM | 4.0 µM | 1.0 µM | ||
| Imipenem only | Imipenem with Fe(8-hq)3 | Fe(8-hq)3 only | Fe(8-hq)3 with Imipenem | 0.375 | [97] |
| 50.0 µM | 6.25 µM | 4.0 µM | 1.0 µM | ||
| MICs Against Staphylococcus aureus CCM 4223 | FIC Index | Ref. | |||
| Ampicillin only | Ampicillin with Fe16 | Fe16 only | Fe16 with Ampicillin | 0.498 | [104] |
| 2 µg/mL | 0.5 µg/mL | 125 µg/mL | 31 µg/mL | ||
4. Conclusions and Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| ICUs | Intensive Care Units |
| CO-ADD | Community for Open Antimicrobial Drug Discovery |
| ROS | Reactive Oxygen Species |
| MSSA | Methicillin-Sensitive Staphylococcus aureus |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| DNA | Deoxyribonucleic Acid |
| ATP | Adenosine Triphosphate |
| TNBC | Triple-Negative Breast Cancer |
| HEK 293 | Human Embryonic Kidney cells |
| hRBCs | human Red Blood Cells |
| MDR | Multidrug Resistant |
| IDA | Iron Deficiency Anemia |
| CFU | Colony Forming Unit |
| MIC | Minimum Inhibitory Concentration |
| FICI | Fractional Inhibitory Concentration Index |
| AMP | Ampicillin |
| SSTIs | Skin and Soft Infections |
| VBNC | Viable But Non-Culturable |
| SWV | Square Wave Voltammetry |
| EDTA | Ethylenediaminetetraacetic Acid |
References
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Rai, J.; Randhawa, G.K.; Kaur, M. Recent advances in antibacterial drugs. Int. J. Appl. Basic Med. Res. 2013, 3, 3–10. [Google Scholar]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Benin, B.M.; Yu, B.; Bunge, S.D.; Abeydeera, N.; Huang, S.D.; Kim, M.-H. Lipophilic Ga Complex with Broad-Spectrum Antimicrobial Activity and the Ability to Overcome Gallium Resistance in both Pseudomonas aeruginosa and Staphylococcus aureus. J. Med. Chem. 2021, 64, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2020.
- Pant, B.D.; Abeydeera, N.; Dubadi, R.; Kim, M.-H.; Huang, S.D. Broad-Spectrum Antimicrobial Activity of Ultrafine (BiO)2CO3 NPs Functionalized with PVP That Can Overcome the Resistance to Ciprofloxacin, AgNPs and Meropenem in Pseudomonas aeruginosa. Antibiotics 2023, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Mudarmah, K.; Abeydeera, N.; Chen, G.; Jogadi, W.; Krause, J.; Budzik, J.M.; Huang, S.D. Synthesis, Structural Characterization, and Antimicrobial Activity of Zn (cloxyquin) 2: Towards Harnessing Zinc Intoxication and Immune Response Restoration to Combat Staphylococcus aureus and Mycobacterium tuberculosis. Dalton Trans. 2025, 54, 9975–9983. [Google Scholar] [CrossRef]
- Dassanayake, T.M.; Dassanayake, A.C.; Abeydeera, N.; Pant, B.D.; Jaroniec, M.; Kim, M.-H.; Huang, S.D. An aluminum lining to the dark cloud of silver resistance: Harnessing the power of potent antimicrobial activity of γ-alumina nanoparticles. Biomater. Sci. 2021, 9, 7996–8006. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013; Centres for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2013.
- Hameed, H.A.; Islam, M.M.; Chhotaray, C.; Wang, C.; Liu, Y.; Tan, Y.; Li, X.; Tan, S.; Delorme, V.; Yew, W.W. Molecular targets related drug resistance mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis strains. Front. Cell. Infect. Microbiol. 2018, 8, 114. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler Jr, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Harkins, C.P.; Pichon, B.; Doumith, M.; Parkhill, J.; Westh, H.; Tomasz, A.; de Lencastre, H.; Bentley, S.D.; Kearns, A.M.; Holden, M.T. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Benin, B.M.; Abeydeera, N.; Kim, M.-H.; Huang, S.D. Bi2O3 nanoparticles exhibit potent broad-spectrum antimicrobial activity and the ability to overcome Ag-, ciprofloxacin- and meropenem-resistance in P. aeruginosa: The next silver bullet of metal antimicrobials? Biomater. Sci. 2022, 10, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Mudarmah, K.; Bagale, B.; Chen, G.; Krause, J.A.; Mighion, J.D.; Huang, S.D. Harnessing the dual antimicrobial mode of action with a lipophilic Mn (II) complex using the principle of the Irving–Williams Series to completely eradicate Staphylococcus aurous. Dalton Trans. 2023, 52, 12203–12207. [Google Scholar] [CrossRef]
- Alamri, H.; Chen, G.; Huang, S.D. Development of Biocompatible Ga2 (HPO4)3 Nanoparticles as an Antimicrobial Agent with Improved Ga Resistance Development Profile against Pseudomonas aeruginosa. Antibiotics 2023, 12, 1578. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef]
- Blair, J.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Lin, J.; Nishino, K.; Roberts, M.C.; Tolmasky, M.; Aminov, R.I.; Zhang, L. Mechanisms of antibiotic resistance. Front. Microbiol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: A review. J. Med. Chem. 2019, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Niu, G.; Li, W. Next-generation drug discovery to combat antimicrobial resistance. Trends Biochem. Sci. 2019, 44, 961–972. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping chemists discover new antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Evans, A.; Kavanagh, K.A. Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens. J. Med. Microbiol. 2021, 70, 001363. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9. [Google Scholar] [CrossRef]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; Van Lanen, S.G.; Boros, E. Theranostic gallium siderophore ciprofloxacin conjugate with broad spectrum antibiotic potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef]
- Skaar, E.P.; Humayun, M.; Bae, T.; DeBord, K.L.; Schneewind, O. Iron-source preference of Staphylococcus aureus infections. Science 2004, 305, 1626–1628. [Google Scholar] [CrossRef]
- Létoffé, S.; Heuck, G.; Delepelaire, P.; Lange, N.; Wandersman, C. Bacteria capture iron from heme by keeping tetrapyrrol skeleton intact. Proc. Natl. Acad. Sci. USA 2009, 106, 11719–11724. [Google Scholar] [CrossRef] [PubMed]
- Frederick, R.E.; Mayfield, J.A.; DuBois, J.L. Iron trafficking as an antimicrobial target. Biometals 2009, 22, 583. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, J.R.; Heinrichs, D.E. Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol. Rev. 2015, 39, 592–630. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Frangipani, E.; Bonchi, C.; Minandri, F.; Imperi, F.; Visca, P. Pyochelin potentiates the inhibitory activity of gallium on Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 5572–5575. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Ratledge, C.; Dover, L.G. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Petrik, M.; Zhai, C.; Haas, H.; Decristoforo, C. Siderophores for molecular imaging applications. Clin. Transl. Imaging 2017, 5, 15–27. [Google Scholar] [CrossRef]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in iron metabolism: From mechanism to therapy potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator–Siderophore: A review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- Weller, M.; Overton, T.; Rourke, J.; Armstrong, F.A. Inorganic Chemistry; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Wandersman, C.; Delepelaire, P. Bacterial iron sources: From siderophores to hemophores. Annu. Rev. Microbiol. 2004, 58, 611–647. [Google Scholar] [CrossRef] [PubMed]
- Schröder, I.; Johnson, E.; De Vries, S. Microbial ferric iron reductases. FEMS Microbiol. Rev. 2003, 27, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Köpf-Maier, P.; Köpf, H.; Neuse, E.W. Ferricenium complexes: A new type of water-soluble antitumor agent. J. Cancer Res. Clin. Oncol. 1984, 108, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Deferiprone and Iron–Maltol: Forty Years since Their Discovery and Insights into Their Drug Design, Development, Clinical Use and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4970. [Google Scholar] [CrossRef]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Hun, L.T. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Ratledge, C. Iron metabolism and infection. Food Nutr. Bull. 2007, 28, S515–S523. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Cornelis, P. Role of Iron in Bacterial Pathogenesis. Front. Cell. Infect. Microbiol. 2018, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Herchel, R.; Šindelář, Z.; Trávníček, Z.; Zbořil, R.; Vančo, J. Novel 1D chain Fe (III)-salen-like complexes involving anionic heterocyclic N-donor ligands. Synthesis, X-ray structure, magnetic, 57Fe Mössbauer, and biological activity studies. Dalton Trans. 2009, 44, 9870–9880. [Google Scholar] [CrossRef] [PubMed]
- Basu, U.; Khan, I.; Koley, D.; Saha, S.; Kondaiah, P.; Chakravarty, A.R. Nuclear targeting terpyridine iron (II) complexes for cellular imaging and remarkable photocytotoxicity. J. Inorg. Biochem. 2012, 116, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, E. Recent advances in multinuclear complexes as potential anticancer and DNA binding agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2014, 14, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Heldt, J.M.; Guille-Collignon, M.; Lemaître, F.; Jaouen, G.; Vessières, A.; Amatore, C. Quantitative analyses of ROS and RNS production in breast cancer cell lines incubated with ferrocifens. ChemMedChem 2014, 9, 1286–1293. [Google Scholar] [CrossRef]
- Franke, J.C.; Plötz, M.; Prokop, A.; Geilen, C.C.; Schmalz, H.-G.; Eberle, J. New caspase-independent but ROS-dependent apoptosis pathways are targeted in melanoma cells by an iron-containing cytosine analogue. Biochem. Pharmacol. 2010, 79, 575–586. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, B.; Di Bilio, A.J.; Zhu, L.; Wang, T.; Qi, C.; Shih, J.; Yen, Y. A Ferrous-Triapine complex mediates formation of reactive oxygen species that inactivate human ribonucleotide reductase. Mol. Cancer Ther. 2006, 5, 586–592. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.; Da Silva, E.G.; Rocha, D.D.; Hillard, E.A.; Pigeon, P.; Jaouen, G.; Rodrigues, F.A.; De Abreu, F.C.; da Rocha Ferreira, F.; Goulart, M.O. Molecular Mechanism of Action of 2-Ferrocenyl-1, 1-diphenylbut-1-ene on HL-60 Leukemia Cells. ChemMedChem 2014, 9, 2580–2586. [Google Scholar] [CrossRef]
- Vančo, J.; Šindelář, Z.; Dvořák, Z.; Trávníček, Z. Iron-salophen complexes involving azole-derived ligands: A new group of compounds with high-level and broad-spectrum in vitro antitumor activity. J. Inorg. Biochem. 2015, 142, 92–100. [Google Scholar] [CrossRef]
- Ahmad, I.; Nelson, D.J.; Hussain, M.I.; Nasar, N.A. Potential of covalently linked tamoxifen hybrids for cancer treatment: Recent update. RSC Med. Chem. 2024, 15, 1877–1898. [Google Scholar] [CrossRef]
- Kwong, W.L.; Lok, C.N.; Tse, C.W.; Wong, E.L.M.; Che, C.M. Anti-Cancer Iron (II) Complexes of Pentadentate N-Donor Ligands: Cytotoxicity, Transcriptomics Analyses, and Mechanisms of Action. Chem. A Eur. J. 2015, 21, 3062–3072. [Google Scholar] [CrossRef]
- Hargrave-Thomas, E.; Yu, B.; Reynisson, J. Serendipity in anticancer drug discovery. World J. Clin. Oncol. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Mao, X.; Wu, S.; Huang, D.; Li, C. Complications and comorbidities associated with antineoplastic chemotherapy: Rethinking drug design and delivery for anticancer therapy. Acta Pharm. Sin. B 2024, 14, 2901–2926. [Google Scholar] [CrossRef]
- Abeydeera, N.; Stilgenbauer, M.; Pant, B.D.; Mudarmah, K.; Dassanayake, T.M.; Zheng, Y.-R.; Huang, S.D. Lipophilic Fe (III)-Complex with Potent Broad-Spectrum Anticancer Activity and Ability to Overcome Pt Resistance in A2780cis Cancer Cells. Molecules 2023, 28, 4917. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, M.; Zhang, J.; Sun, Z.; Zhang, W.; Dong, W.; Cheng, C.; Yao, Y.; Li, K. Hinokitiol-iron complex is a ferroptosis inducer to inhibit triple-negative breast tumor growth. Cell Biosci. 2023, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Mudarmah, K.; Pant, B.D.; Krause, J.A.; Zheng, Y.-R.; Huang, S.D. Transferrin-inspired iron delivery across the cell membrane using [(L2 Fe)2 (μ-O)](L= chlorquinaldol) to harness anticancer activity of ferroptosis. Dalton Trans. 2024, 53, 3206–3214. [Google Scholar] [CrossRef]
- Guo, R.; Fang, X.; Shang, K.; Wen, J.; Ding, K. Induction of ferroptosis: A new strategy for the control of bacterial infections. Microbiol. Res. 2024, 284, 127728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Zhou, W.; Lee, J.; Liu, Z.; An, Z.; Xu, D.; Mo, H.; Hu, L.; Zhou, X. Ferrous sulfate-loaded hydrogel cures Staphylococcus aureus infection via facilitating a ferroptosis-like bacterial cell death in a mouse keratitis model. Biomaterials 2022, 290, 121842. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H. Iron and microbial infection. Nat. Rev. Microbiol. 2004, 2, 946–953. [Google Scholar] [CrossRef]
- Edwards, E.I.; Epton, R.; Marr, G. Organometallic derivatives of penicillins and cephalosporins a new class of semi-synthetic antibiotics. J. Organomet. Chem. 1975, 85, C23–C25. [Google Scholar] [CrossRef]
- Edwards, E.I.; Epton, R.; Marr, G. A new class of semi-synthetic antibiotics: Ferrocenyl-penicillins and-cephalosporins. J. Organomet. Chem. 1976, 107, 351–357. [Google Scholar] [CrossRef]
- Edwards, E.; Epton, R.; Marr, G. 1, 1′-Ferrocenyldiacetic Acid Anhydride and its Use in the preparation of heteroannularly substituted ferrocenyl-penicillins and-cephalosporins. J. Organomet. Chem. 1976, 122, C49–C53. [Google Scholar] [CrossRef]
- Santos, J.V.d.O.; Porto, A.L.F.; Cavalcanti, I.M.F. Potential application of combined therapy with lectins as a therapeutic strategy for the treatment of bacterial infections. Antibiotics 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Hrioua, A.; Loudiki, A.; Farahi, A.; Laghrib, F.; Bakasse, M.; Lahrich, S.; Saqrane, S.; El Mhammedi, M. Complexation of amoxicillin by transition metals: Physico-chemical and antibacterial activity evaluation. Bioelectrochemistry 2021, 142, 107936. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, H.; Fan, S.; Zheng, B.; Wu, J.; Zhang, J.; Pi, J.; Xu, J.-F. Ferroptosis: A mixed blessing for infectious diseases. Front. Pharmacol. 2022, 13, 992734. [Google Scholar] [CrossRef]
- Parrello, D.; Zegeye, A.; Mustin, C.; Billard, P. Siderophore-mediated iron dissolution from nontronites is controlled by mineral cristallochemistry. Front. Microbiol. 2016, 7, 423. [Google Scholar] [CrossRef]
- Grillo, A.S.; SantaMaria, A.M.; Kafina, M.D.; Cioffi, A.G.; Huston, N.C.; Han, M.; Seo, Y.A.; Yien, Y.Y.; Nardone, C.; Menon, A.V. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 2017, 356, 608–616. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Design of Orally Active Iron Chelators for the Treatment of Thalassaemia. Ph.D. Thesis, University of Essex, Colchester, UK, 1982; pp. 1–243. [Google Scholar]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators intended for clinical use in iron overload, other diseases of iron imbalance and free radical pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, W.; Huang, H.; Tang, Y.; Wang, W.; Meng, F.; Wang, H.; Zheng, Y. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New antimicrobial strategies based on metal complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Abeydeera, N.; Yu, B.; Pant, B.D.; Kim, M.-H.; Huang, S.D. Harnessing the toxicity of dysregulated iron uptake for killing Staphylococcus aureus: Reality or mirage? Biomater. Sci. 2022, 10, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Benin, B.M.; Mudarmah, K.; Pant, B.D.; Chen, G.; Shin, W.S.; Kim, M.-H.; Huang, S.D. Harnessing the Dual Antimicrobial Mechanism of Action with Fe (8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections. Antibiotics 2023, 12, 886. [Google Scholar] [CrossRef]
- Polo, A.B.; Lemos, A.S.; Martins da Mata, C.P.; Oliveira, V.S.; Pontes, A.C.; Pontes, D.L.; Tavares, G.D.; Fabri, R.L.; M Apolônio, A.C. In vitro activity of the novel Fe-cyclam complex against clinical multidrug-resistant bacterial isolates from Brazil. Future Microbiol. 2023, 18, 897–909. [Google Scholar] [CrossRef]
- Branca, M.T.; Silva, T.P.; Lemos, A.S.; Campos, L.M.; Souza, T.F.; Palazzi, C.; Oliveira, V.S.; Coimbra, E.S.; Silva, F.O.; FB Pontes, A.C. The Fe-Cyclam-Derived Compound [Fe (cyclam) sal] PF6 Restrains Drug-Resistant Staphylococcus aureus Proliferation and Biofilm Formation. ACS Omega 2025, 10, 11386–11396. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef]
- Kosaristanova, L.; Rihacek, M.; Sucha, F.; Milosavljevic, V.; Svec, P.; Dorazilova, J.; Vojtova, L.; Antal, P.; Kopel, P.; Patocka, Z. Synergistic antibacterial action of the iron complex and ampicillin against Staphylococcus aureus. BMC Microbiol. 2023, 23, 288. [Google Scholar] [CrossRef]
- Mishra, P. Biocoordination, Computational Modeling and Antibacterial Sensitivities of Cobalt (II), Nickel (II), Copper (II) and Bismuth (V) with Gentamicin and Amoxicillin Antibiotics mixed Ligands. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 145–156. [Google Scholar]
- Singh, H.L.; Singh, J.; Mukherjee, A. Synthesis, spectral, and in vitro antibacterial studies of organosilicon (IV) complexes with schiff bases derived from amino acids. Bioinorg. Chem. Appl. 2013, 2013, 425832. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Peng, S.; Zhang, J.; Li, H.; Mo, H.; Hu, L. Physical fields reverse FeSO4-induced VBNC state in Listeria monocytogenes and facilitate ferroptosis. Food Microbiol. 2025, 131, 104796. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Zhang, N.; Zhang, H.; Li, H.; Qi, Y.; Mo, H.; Hu, L. Direct ferrous sulfate exposure facilitates the VBNC state formation rather than ferroptosis in Listeria monocytogenes. Microbiol. Res. 2023, 269, 127304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; An, Z.; Richel, A.; Huang, M.; Gou, X.; Xu, D.; Zhang, M.; Mo, H.; Hu, L.; Zhou, X. Ferrous sulfate remodels the properties of sodium alginate-based hydrogel and facilitates the healing of wound infection caused by MRSA. Carbohydr. Polym. 2024, 346, 122554. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of self-healing hydrogels with on-demand antimicrobial activity and sustained biomolecule release for infected skin regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef] [PubMed]
- Shuai, F.; Zhang, Y.; Yin, Y.; Zhao, H.; Han, X. Fabrication of an injectable iron (III) crosslinked alginate-hyaluronic acid hydrogel with shear-thinning and antimicrobial activities. Carbohydr. Polym. 2021, 260, 117777. [Google Scholar] [CrossRef]
- Loth, C.; Barbault, F.; Guégan, C.; Lemaire, F.; Contal, C.; Carvalho, A.; Hellé, S.; Champion, M.; Kerdjoudj, H.; Chan-Seng, D. Experimental and Computational Study of Injectable Iron (III)/Ultrashort Peptide Hydrogels: A Candidate for Ferroptosis-Induced Treatment of Bacterial Infections. Small Sci. 2025, 5, 2400618. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeydeera, N. Recent Development of Exploring Ferroptosis-Inspired Effect of Iron as a Feasible Strategy for Combating Multidrug Resistant Bacterial Infections. Appl. Microbiol. 2025, 5, 73. https://doi.org/10.3390/applmicrobiol5030073
Abeydeera N. Recent Development of Exploring Ferroptosis-Inspired Effect of Iron as a Feasible Strategy for Combating Multidrug Resistant Bacterial Infections. Applied Microbiology. 2025; 5(3):73. https://doi.org/10.3390/applmicrobiol5030073
Chicago/Turabian StyleAbeydeera, Nalin. 2025. "Recent Development of Exploring Ferroptosis-Inspired Effect of Iron as a Feasible Strategy for Combating Multidrug Resistant Bacterial Infections" Applied Microbiology 5, no. 3: 73. https://doi.org/10.3390/applmicrobiol5030073
APA StyleAbeydeera, N. (2025). Recent Development of Exploring Ferroptosis-Inspired Effect of Iron as a Feasible Strategy for Combating Multidrug Resistant Bacterial Infections. Applied Microbiology, 5(3), 73. https://doi.org/10.3390/applmicrobiol5030073





