Abstract
The increasing threat of antimicrobial resistance (AMR), along with the limited availability of new lead compounds in the drug development pipeline, highlights the urgent need to discover antimicrobial agents with innovative mechanisms of action. In this regard, metal complexes offer a unique opportunity to access mechanisms distinct from those of conventional antibiotics. Although iron (Fe) is an essential element for all forms of life, including pathogenic bacteria, it also poses a serious risk of cytotoxicity due to its redox activity, which can trigger the production of reactive oxygen species (ROS) via the Fenton reaction. This review highlights recent advances in the development of iron-based antimicrobial agents that harness the toxicity resulting from dysregulated iron uptake, thereby inducing bacterial cell death through oxidative stress. These findings may guide the development of effective treatments for pathogenic infections and offer new perspectives on leveraging redox chemistry of iron to combat the growing threat of global bacterial resistance.
1. Introduction
Antimicrobial resistance (AMR) poses a significant challenge to global health, which is further heightened by the declining number of promising antimicrobial agents in the pharmaceutical pipeline [1,2,3,4]. AMR has increased dramatically over the past few years, becoming a serious worldwide issue in the healthcare system [2]. This situation has led to higher rates of morbidity and mortality, along with increased healthcare costs associated with bacterial infections [5]. Annually, over 2 million infections and approximately 23,000 deaths in the USA are caused by antibiotic-resistant Staphylococcus aureus, Pseudomonas aeruginosa [6], Enterococcus faecium, Acinetobacter baumannii, and Enterobacter (ESKAPE) pathogens [7,8,9]. Methicillin-resistant Staphylococcus aureus (MRSA) infections have become a public health crisis [10,11], leading to untreatable cases of bacteremia, pneumonia, and skin and soft tissue infections along with significant clinical complications [7]. More than 30% of clinical isolates of Pseudomonas aeruginosa sourced from patients in intensive care units (ICUs) or nursing homes are now resistant to three or more antibiotic drugs [12]. Although current antibiotic classes have distinct modes of action, certain bacteria, such as Staphylococcus aureus, Pseudomonas aeruginosa, and Mycobacterium tuberculosis, are resistant to numerous classes of antibiotics, decreasing their ability for effective medication [13,14,15]. There is increasing interest in the application of metal-based antimicrobial compounds for the treatment of bacterial infections, owing to their distinct mechanisms of action compared to conventional antibiotics, which may enable them to circumvent existing resistance pathways and provide effective treatment for persistent infections [1,16,17,18].
Mechanisms of AMR in Bacteria
Bacteria can rapidly evolve into novel mechanisms to resist antibiotics and reduce the efficiency of existing conventional antibiotics [19]. Several mechanisms of adaptation enable bacteria to mediate AMR in their efforts to survive against antibiotic treatments [20,21,22,23]. Notably, these adaptations include strategies that lower the concentration of antibiotics within bacterial cells and prevent their activity. This can be achieved either by decreasing cellular permeability to limit the uptake of antibiotics into the cells or by enhancing the expression of multidrug efflux pumps, which actively transport antibiotics out of the cells [22,24]. (i) reduced uptake—regulating the activity of membrane transporters responsible for the uptake of essential metals, thereby limiting the influx of antibiotics, (ii) efflux—bacteria can increase the expression of membrane transporter proteins that transport specific toxic metals and antibiotics out of the bacterial cell, (iii) extracellular sequestration an upregulating extracellular polymer expression that bind to antibiotics; (iv) chemical modification—conversion of antibiotics into less toxic or inactive forms inside the cells [25]; (v) degrade by bacterial enzymes—bacteria can degrade or modify target site through bacterial enzymes, thereby inhibiting their antibacterial activity (Figure 1) [26,27,28].
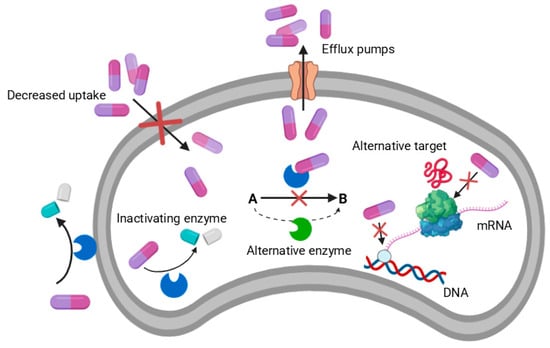
Figure 1.
Overview of major mechanisms used by microorganisms to resist antibiotic action.
Many antibiotics currently used in clinical settings are natural products derived from microorganisms [29]. The challenge of combating drug resistance by developing new antibiotics is greatly exacerbated by the fact that, in the last three decades, only one new antibiotic with a novel mode of action “linezolid” has been developed for clinical use [30]. There has been a significant decrease in the number of new antimicrobials across the world for clinical treatments while only a few new antibiotics are currently in advanced development [3]. Therefore, this critical situation necessitates a paradigm shift in the development of next-generation antibiotics with distinct modes of action in contrast to conventional antibiotics to effectively tackle bacterial infections caused by multidrug-resistant organisms [23,31,32,33]. Recently, metal-based compounds have attracted significant attention for their potential in treating bacterial infections, offering a distinct mode of action compared to conventional antibiotics that may lead to new strategies for treating persistent microbial infections [1,34,35]. A recent analysis of 906 metal-containing compounds screened by the Community for Open Antimicrobial Drug Discovery (CO-ADD) identified several metals, including Mn, Co, Zn, Ru, Ag, Eu, Ir, and Pt, whose complexes demonstrated activity and non-toxicity against at least one pathogenic organism, thus highlighting their promise as lead compounds in antimicrobial development [1,36]. However, the development of iron (Fe)-based antimicrobial agents remained paradoxical and largely unexplored, due to their double-edged role in biological systems [37,38]. Iron is generally recognized as a bacterial growth promoter rather than a bactericidal agent [39]. As a result, almost no attempt has been made by the worldwide research community to develop iron-based compounds as antimicrobial agents.
This review summarizes recent developments in this growing research field, focusing on advances in the development of iron-based antimicrobial agents. Particular focus is given to the utilization of non-siderophore chelating ligands capable of transporting Fe(III) across bacterial cell membranes, circumventing the regulation of bacterial iron homeostasis and subsequently triggering the Fenton catalytic production of reactive oxygen species (ROS), thereby inducing bacterial cell death through oxidative stress. This innovative approach offers an alternative mode of action, different from conventional antibiotics, positioning iron complexes as a novel class of antimicrobial agents with significant potential in the fight against drug-resistant pathogens.
2. Materials and Methods
A comprehensive literature search was conducted using PubMed and Google Scholar to identify relevant studies on iron-based antimicrobial agents and their mechanisms of action against antibiotic-resistant bacteria. Keywords used included: antimicrobial resistance, iron-based antimicrobials, Ferroptosis, Fe-based compounds, Fenton reaction, and bacterial iron metabolism. All references were included using EndNote X8 citation software.
3. Discussion
3.1. Iron in Bacteria
Iron is a transitional metal and an essential micronutrient necessary for the pathogenic microorganism survival, growth, and propagation of bacteria [40,41,42]. It plays vital roles in DNA replication, transcription, repair, biosynthesis of cofactors, ATP synthesis, gene regulation, cellular respiration, nucleotide biosynthesis, and so on [43,44,45]. On the other hand, iron is known to be a double-edged sword in biology, as excess levels in the intracellular free iron store can be toxic to the host cells. Such cytotoxicity stems from the ability of Fe(II) to catalyze the Fenton reaction that generates reactive oxygen species (ROS). Due to the redox activity of iron [Fe(II) /Fe(III)] and the poor solubility of its predominant ferric form, free iron concentrations in mammalian hosts are extremely low, approximately 10−18 M in the blood. This iron content is substantially lower than the amount required for microbiological growth and replication (10−6 M) [43,46,47]. Bacteria must sequester iron from the host cells and transport it inside the bacterial cell to ensure their survival. To deal with these difficulties, nature has evolved sophisticated biochemical systems to execute and regulate all aspects of iron metabolism in bacteria [48].
3.2. Iron Metabolism in Bacteria
Bacteria have developed sophisticated mechanisms to hijack iron from host proteins, while in response, humans have evolved proteins that specifically target and neutralize bacterial iron acquisition strategies [49]. Pathogens use three major mechanisms to scavenge and transport iron based on the iron resource: (i) transferrin/lactoferrin receptor-assisted uptake mechanism; (ii) heme-acquisition mechanism; and (iii) siderophore-assisted uptake mechanism (Figure 2). Siderophore-mediated iron acquisition is the most widespread and successful, allowing bacteria to scavenge iron from any source in conditions of iron deficiency [49,50]. Siderophores are small molecules (generally <1 kDa) with a high affinity for ferric; Fe(III) ions [49,50]. Siderophores are categorized into four primary classes based on the functional groups and chemical properties of the moieties that donate oxygen ligands for Fe(III) coordination: (i) catecholates, (ii) phenolates, (iii) hydroxamates, and (iv) (α-hydroxy) carboxylates [51]. Bacteria synthesize siderophores during iron deficiency when their intracellular iron content falls below the essential threshold of around 10−6 M for microbial growth [49,52]. Structurally, siderophores contains lipophilic chelating agents compose of hard-Lewis base O or N donor atoms that have a high affinity for Fe(III) to form thermodynamically stable and kinetically nonlabile soluble Fe(III)-siderophore complexes [46]. Also, it effectively sequesters Fe(III) within a tight octahedral coordination environment to prevent ligand displacement, trans-metalation, and catalytic reactions [53]. Then the uptake of a specific Fe(III)-siderophore complex is a receptor-mediated process that is tightly regulated to ensure no excessive uptake would occur to increase the iron level in the intracellular free iron store [54]. Once the Fe(III)-siderophore complex is transported across the cell membrane, the Fe(III) center is reduced to Fe(II) by the bacterial ferric reductase or other intracellular antioxidants [54]. This reduction is essential for iron release, as siderophores exhibit relatively low affinity for Fe(II), a soft Lewis acid.
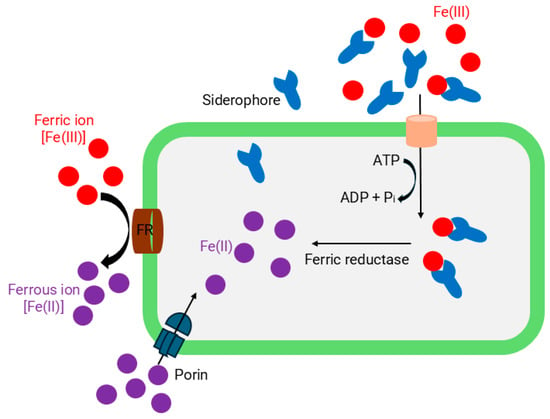
Figure 2.
Mechanisms of bacterial iron acquisition and transport.
3.3. Redox-Active Iron Complexes as ROS-Inducing Anticancer Agents
Many iron complexes have been investigated for biological applications such as anticancer activity [55], neurodegenerative diseases, and iron deficiency anemia [56]. The limitations associated with platinum-based anticancer drugs such as toxicity, resistance, and high cost have prompted the exploration of more effective and safer non-platinum anticancer drugs. In this regard, iron complexes represent a promising class of metallodrugs, as evidenced by more than 214 research articles reporting their cytotoxicity against various cancer cell lines [56,57]. Cancer cells are known to experience elevated oxidative stress due to factors like oncogene activation, higher metabolic activity, and mitochondrial dysfunction [58]. Excessive ROS production can suppress tumor growth by activating cell cycle inhibition, triggering cell death pathways. Such pathways are often referred to as ferroptosis, is a distinct form of regulated cell death that is iron-dependent and characterized by the accumulation of lipid peroxides, which leads to oxidative damage and ultimately cell death [39,59,60]. Many chemotherapeutic agents leverage this vulnerability by further increasing intracellular ROS to cytotoxic levels. To this end, iron metal has emerged as a powerful redox-active element capable of enhancing oxidative stress within cancer cells. Iron has a unique ability to undergo redox reaction between ferrous [Fe(II)] and ferric [Fe(III)] oxidation states allows it to participate in Fenton-type reaction, generating highly reactive ROS like hydroxyl radicals (•OH) [60,61]. These radicals contribute to oxidative damage of DNA, lipids, and proteins, ultimately disrupting cellular homeostasis [47,51,62,63,64]. This redox versatility underpins iron’s involvement in the generation of reactive oxygen species (ROS), which is a key mechanism in the anticancer activity of many iron complexes. Indeed, the first reports on anticancer activity of iron-based compounds such as ferrocenium picrate and ferrocenium trichloroacetate salts linked their cytotoxicity to ROS-induced oxidative damage to cellular DNA [55]. Several iron complexes have been reported to exert their anticancer activity through the generation of ROS [65,66,67,68,69,70]. For instance, Oliveira et al. reported the anticancer activities of 2-ferrocenyl-1, 1-diphenylbut-1-ene against multiple cancer cell lines, primarily occurs through apoptosis [71]. Similarly, James et al. reported ferrocenyl nucleoside analogs capable of inducing apoptosis in BJAB cells and lymphoblasts from pediatric acute lymphoblastic leukemia patients [57]. Vanco et al. further expanded this field by reporting the broad-spectrum cytotoxicity of iron (II/III) salophen complexes bearing monodentate azole-derived ligands [72]. These complexes showed potent activity against six human cancer cell lines and several folds more active than cisplatin. A notable example of a redox-active iron compound is the ferrocene-tamoxifen hybrid, which has shown potent antiproliferative effects attributed to its capacity of generating ROS [73]. Similarly, iron (II) complexes with pentadentate pyridyl ligands have demonstrated strong cytotoxicity and apoptosis induction in various cancer cell lines [74]. Shao et al. reported that a ferrous triapine complex can inactivate human ribonucleotide reductase by ROS-mediated mechanisms, thereby inhibiting DNA synthesis and repairing rapidly proliferating cells [70].
However, most of these complexes have been discovered serendipitously rather than through rational design [75]. Therefore, the limited cellular penetration and suboptimal safety of iron-based anticancer agents remain significant challenges [76]. To effectively harness the anticancer activity of iron while addressing these two major limitations, a biomimetic approach using highly lipophilic chelating agents that favor the formation of Fe(III) complexes over their Fe(II) counterparts has recently emerged as a critical strategy. This approach enhances the ability of Fe(III) complex to penetrate cell membranes while preventing the premature release of iron into the bloodstream, thereby minimizing systemic cytotoxicity.
We recently reported that a highly lipophilic tropolone derivative referred to as hinokitiol (2-hydroxy-4-isopropyl-2, 4, 6-cycloheptatrien-1-one or β-thujaplicin) exhibits a remarkable ability to transport iron across cancer cells in the form of Fe(hinok)3, thus effectively bypassing the regulation of cellular iron homeostasis, which in turn should trigger the production of ROS in cancer cells to cause cell death [77]. As a result, Fe(hinok)3 demonstrates potent broad-spectrum anticancer activity against five different cell lines including cisplatin-resistant ovarian cancer cells. These findings further revealed that the cytotoxic activity of Fe(hinok)3 is associated with iron-triggered ROS-mediated damage to both mitochondria and the cell membrane [77]. Supporting this mechanism, Hongting Zhao et al. reported that the Fe(hinok)3 induces ferroptosis by enhancing iron-dependent lipid peroxidation in both in vitro and in vivo models of triple-negative breast cancer (TNBC) [78].
Similarly, we also reported the lipophilic chlorquinaldol (L; 5, 7-dichloro-8-hydroxy-2-methylquinoline) strongly favors the formation of a highly stable binuclear Fe(III) complex, [(L2Fe)2(μ-O)], which mimics the function of the Fe(III)-transferrin complex by strongly binding Fe(III) and enabling the facile release of Fe(II) upon reduction [79]. Notably, the cellular uptake of this complex is facilitated by the high lipophilicity of chlorquinaldol. Once transported across the cell membrane, Fe(III) can be reduced by ferric reductase or other intracellular antioxidants to Fe(II), triggering the Fenton reaction and initiating ROS-mediated cytotoxicity. Consequently, this transferrin-inspired iron-delivery strategy significantly lowers the cytotoxicity of this Fe-complex in normal human embryonic kidney (HEK 293) cells and reduces hemolytic activity in human red blood cells (hRBCs), thereby conferring tumor-specific anticancer activity [79].
The above literature raises the possibility of harnessing iron by triggering ROS signaling pathways to develop iron-based metallodrugs for cancers. The deleterious cellular effects of ROS on crucial cellular components include lipid peroxidation of membranes, oxidation of proteins, cleavage of DNA and even activation of apoptotic cell death pathways referred to as ferroptosis [59]. While ferroptosis has primarily been explored in the context of cancer therapy, its underlying mechanism iron-dependent lipid peroxidation and oxidative damage offers intriguing potential in the development of novel antimicrobial agents. Similarly to cancer cells, many pathogenic microorganisms are sensitive to disruptions in redox homeostasis. Leveraging the redox-active nature of iron to induce oxidative stress in microbes presents a promising strategy to combat infections, particularly those caused by multidrug-resistant (MDR) pathogens.
Recent studies have suggested that ferroptosis-like mechanisms can be harnessed to target bacteria by overwhelming their antioxidant defenses and promoting lethal lipid peroxidation [80]. In fact, certain Gram-positive bacteria are particularly vulnerable to oxidative membrane damage due to their outer membrane composition. By designing iron-based compounds capable of triggering such oxidative stress selectively in microbes either through Fenton chemistry or by interfering with microbial iron metabolism researchers can potentially exploit a ferroptosis-inspired mode of action. Moreover, antimicrobial resistance to traditional antibiotics often involves mechanisms that evade specific molecular targets; however, ferroptosis-like death bypasses these pathways by attacking fundamental cellular structures like membranes via reactive oxygen species [81]. This mode of action is less likely to elicit cross-resistance, making ferroptosis-inducing agent’s attractive candidates in the fight against antibiotic resistance.
However, since iron is generally regarded as a bacterial growth promoter rather than a bactericidal agent [40,82], there has been little effort by the global research community to develop iron-based compounds as antimicrobial agents. As a result, rational design strategies for iron complexes targeting microbial pathogens have remained largely unexplored. Despite this long-standing assumption, some studies have shown the potential of iron in antimicrobial therapy. One notable advancement was the incorporation of ferrocene into β-lactam antibiotics, such as penicillin and cephalosporins, marked a significant milestone in iron-based antimicrobial development. In 1975, Edwards first demonstrated the synthesis of ferrocene-modified β-lactam antibiotics [83]. This was followed by the development of additional ferrocenylamide derivatives, thereby expanding the library of ferrocene-containing antibiotics [84]. These iron compounds exhibited promising antibiotic activity, particularly against Staphylococcus aureus bacteria [85], indicating that ferrocene incorporation not only enhanced the structural diversity of β-lactam antibiotics but also opened new avenues for combating resistant bacterial strains. Iron compounds have also been considered as a potential candidate for combined treatment with antibiotics [86]. For instance, the complexation of amoxicillin with Fe(III) led to the formation of AMX-Fe complex, which demonstrated a bactericidal effect against Escherichia coli, reducing the bacterial count (log CFU/mL) by more than 3 logs after 24 h and outperforming amoxicillin alone [87]. This suggests that iron antibiotic complexes may offer additive or synergistic benefits that improve efficacy and help overcome drug resistance [86].
3.4. Design and Development of Novel Iron-Based Antimicrobial Agents
Although ferroptosis has not been definitively observed in bacteria as it is in mammalian cells, ferroptosis-like mechanisms may be mimicked in bacterial cells through iron-induced oxidative stress [88]. Recently, research has focused on the rational design of iron-based antimicrobial agents by mimicking ferroptosis cell damage in drug-resistant pathogenic bacteria as a novel antimicrobial strategy [42]. However, iron uptake in bacteria is tightly regulated by siderophores and heme-uptake systems in order to maintain homeostasis, which severely limits iron trafficking into bacterial cells via other pathways [89]. Therefore, penetration of iron across bacterial membrane poses significant challenge for leveraging iron toxicity for the development of iron-based antimicrobial agents. Iron-based metal complexes with high lipophilicity have shown to facilitate transmembrane iron transport in the absence of iron-transporter proteins [90]. A prominent example is the iron maltol complex, originally developed for the treatment of iron deficiency anemia (IDA) between 1979 and 1982 [91]. Its efficacy stems from its high lipophilicity, which enables effective iron transport across lipid-rich membranes, such as those of red blood cells and intestinal epithelium [59]. Beyond membrane permeability, the iron-maltol complex exhibits high chemical stability and favorable protein binding characteristics, further supporting its potential for therapeutic applications [91,92,93]. These properties make it a promising candidate for developing iron-based antimicrobials.
Building on this foundation, researchers have begun to exploit bacterial iron uptake pathways as a vulnerability for the design of novel iron-based antimicrobial agents. Neutral octahedral Fe(III)-complexes formed with various lipophilic bidentate chelating ligands containing the hard Lewis donor atoms of O and/or N have demonstrated potential to disrupt bacterial iron regulation. These lipophilic, non-siderophore iron complexes can penetrate bacterial membranes via passive diffusion, bypassing receptor-mediated uptake mechanisms. Once inside the cell, Fe(III) is reduced to Fe(II) by ferric reductase or intracellular antioxidants, elevating the intracellular free iron pool. In biological systems, iron is required to catalyze the production of hydroxyl free radicals (•HO), hence the reduction of iron (III) to iron (II) catalyzes the electron transfer chain [Equation (1)] followed by the Fenton reaction [Equation (2)] to produce hydroxyl free radicals, resulting in the Haber-Weiss reaction [Equation (3)].
Fe(III) + O2•− → Fe(III) + O2
Fe(II) + H2O2 → Fe(III) + HO− + HO• Fenton reaction
The net reaction is as follows:
O2•− + H2O2 → O2 + HO− + HO• Haber Weiss reaction
Reactive oxygen species (ROS), such as hydroxyl radicals (·OH) generated via the Fenton reaction, as well as superoxide anions (O2−) and hydrogen peroxide (H2O2) produced during iron redox cycling in aerobic environments, can cause extensive damage on lipids, proteins, DNA, and other essential cellular components [87,94,95]. Iron (Fe) is not known to upregulate the production of nitric oxide (NO) free radicals which are primarily produced by nitric oxide synthases (NOS). While the Fenton reaction itself does not directly generate reactive nitrogen species (RNS), it can indirectly contribute to their formation through its interplay with cellular nitric oxide (NO·). The reaction between NO· and O2− leads to the formation of peroxynitrite (ONOO−), a highly reactive RNS that further contributes oxidative and nitrative stress within bacterial cells, ultimately leading to iron-mediated cell death.
The rationale behind this approach lies in the formation of Fe(III) complexes with D3 symmetry, which effectively reduces molecular polarity and conceals the ionic nature of the metal center within an octahedral geometry. This structural arrangement imparts a “greaseball-like” characteristic to the complexes, enhancing their hydrophobicity and facilitating them across bacterial cell membranes. This dual mechanism: membrane penetration and redox-induced cytotoxicity represents a novel and promising strategy for targeting bacterial iron homeostasis and inducing oxidative stress as an antimicrobial approach.
3.5. Recent Advances of Iron-Based Antimicrobial Agents
Recent studies have highlighted the therapeutic potential of the Fe(hinok)3, where hinok is the naturally occurring metal chelator hinokitiol, with high lipophilicity and the nonpolar nature (Figure 3a). Fe(hinok)3 is able to effectively penetrate the bacterial cell membrane of both against both methicillin-sensitive S. aureus (MSSA, ATCC 6538) and methicillin-resistant S. aureus (MRSA, ATCC BAA-44) strains. It bypasses the regulation of siderophore-mediated bacterial iron homeostasis to deliver iron into these cells, thereby triggering the production of ROS and causing bacterial cell death. The in vitro evaluation of antimicrobial efficacy results showed that the iron complex was significantly more active against the MSSA strain, with a MIC value of 1.65 μg/mL than the non-coordinated hinokitiol, which had a MIC value of 9.00 μg/mL. A similar trend was observed with MRSA, where Fe(hinok)3 achieved an MIC of 3.50 μg/mL, again outperforming the free ligand (Figure 3b,c) [96]. The in vivo evaluation of antimicrobial efficacy against S. aureus in the murine model of skin wound infection confirms that the treatment with a single dose of the Fe(hinok)3 can decrease the bacterial burden by 83% compared to untreated vehicle control (Figure 3d). Interestingly, the development of resistance in S. aureus toward Fe(hinok)3 is considerably hampered in comparison to ciprofloxacin (Figure 3e). Additionally, results demonstrated that the potent antimicrobial activity of Fe(hinok)3 primarily stems from reactive oxygen species (ROS) production through the Fenton reaction, driven by the Fe(II)/Fe(III) redox cycle (Figure 3f,g). Upon cellular entry, Fe(III) in the Fe(hinok)3 complex is reduced to Fe(II), which subsequently triggers the Fenton reaction, generating ROS that cause oxidative damage to cellular components. These findings suggest that intracellular ROS generation, leading to oxidative stress, is the primary mechanism of bacterial cell death induced by Fe(hinok)3. Fe(hinok)3 complex has demonstrated promising activity against MRSA biofilms, effectively inhibiting biofilm formation which was confirmed by confocal microscopy and quantitative CFU analysis (Figure 3h). Results showed that the activity of anti-biofilm is indeed dose-dependent, affording 18% inhibition at the concentration of 2.00 µg/mL, 90% inhibition at the concentration of 3.00 µg/mL, and 99% inhibition at the concentration of 4.00 µg/mL showing potentially enhancing susceptibility to conventional antibiotics [96]. Fe(hinok)3, complex shown to bypass the conventional resistance pathways commonly seen with traditional antibiotics. Lipophilic Fe(hinok)3 has demonstrated a significantly lower tendency to develop drug resistance compared to ciprofloxacin in S. aureus. These findings suggest that the molecular targets of Fe(hinok)3 are likely distinct and non-overlapping with those of conventional antibiotics, highlighting its potential as a novel therapeutic agent in the fight against antibiotic-resistant infections. Notably, the antimicrobial efficacy of Fe(hinok)3 can be further enhanced when used in combination with conventional antibiotics. A fractional inhibitory concentration index (FICI) of 0.5 was reported for the combination of Fe(hinok)3 and ofloxacin, indicating a borderline synergistic interaction (FICI ≤ 0.5). Additionally, combinations with ciprofloxacin and vancomycin yielded additive effects, with FICI values of 0.75 (within the range of 0.5 < FICI < 4) (Table 1). These findings suggest that Fe(hinok)3 has the potential to be used as a combination therapy to enhance the efficacy of existing antibiotics, particularly in combating resistant strains such as MRSA.
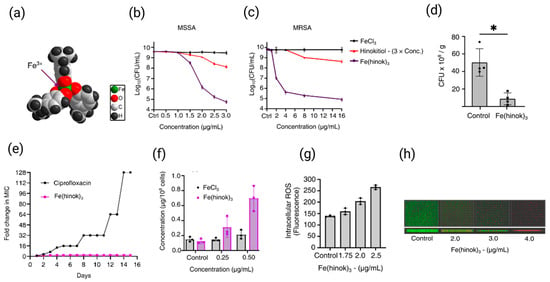
Figure 3.
Molecular structure of Fe(hinok)3 depicted by the space-filling model (a), the growth-inhibitory effect of Fe(hinok)3 against MSSA (b) and MRSA (c), cellular uptake of Fe(hinok)3 in MSSA bacteria as determined by the Fe concentration in the cell lysate (d), development of resistance towards Fe(hinok)3 in comparison with that towards ciprofloxacin in MSSA (e), normalized ROS yield after the bacterial cells were treated with varying concentrations of Fe(hinok)3 in MSSA (f), representative fluorescence images of the top-view and sideview of MRSA biofilms treated with Fe(hinok)3 (g), and results of wound infections induced by MSSA in a murine model treated with Fe(hinok)3 or vehicle control (h) (mean ± s.d, n = 3 replicates; * p < 0.05). Reproduced and adapted from N. Abeydeera et al., Ref. [96], with permission from the Royal Society of Chemistry.
Similarly, Fe(8-hq)3, a 1:3 complex formed between Fe(III) and 8-hydroxyquinoline (8-hq) (Figure 4a), demonstrates potent antimicrobial activity against MRSA by facilitating iron transport into bacterial cells and disrupting intracellular iron homeostasis [97]. In vitro antimicrobial studies revealed that Fe(8-hq)3 exhibited significantly greater activity against both MSSA and MRSA strains, with a MIC of 4.0 μM, compared to the 8-hydroxyquinoline, which showed MIC values ranging from 16 to 32 μM. These results are particularly noteworthy when compared to 8-hydroxyquinoline alone, indicating that the coordination of 8-hq to Fe(III) significantly enhances bacterial inhibition. Fe(8-hq)3 can readily deliver Fe(III) into bacterial cells, exerting a dual antimicrobial effect: the 8-hq ligand chelates essential metal ions such as Mn2+, Zn2+, and Cu2+, disrupting intracellular metal homeostasis, while the iron metal center induces Fenton reaction that generates ROS which contributes to bactericidal activity (Figure 4b). Resistance development by SA toward Fe(8-hq)3 is considerably hampered as compared with ciprofloxacin and 8-hq (Figure 4c). Additionally, Fe(8-hq)3 can overcome the mupirocin resistance developed in S. aureus mutant and MRSA mutant bacteria (Figure 4d). Notably, Fe(8-hq)3 also exhibited a synergistic effect when combined with either ciprofloxacin or imipenem, with a FICI of 0.375, potentially offering a promising strategy for combating resistant infections (Table 1). Scanning electron microscopy (SEM) revealed that Fe(8-hq)3 induces significant morphological changes and membrane damage in bacterial cells effects not observed with 8-hq alone highlighting the unique contribution of the iron center (Figure 4e). This membrane disruption is likely a consequence of reactive oxygen species (ROS) generated via intracellular Fenton reactions, further contributing to the Fe(8-hq)3 bactericidal activity.
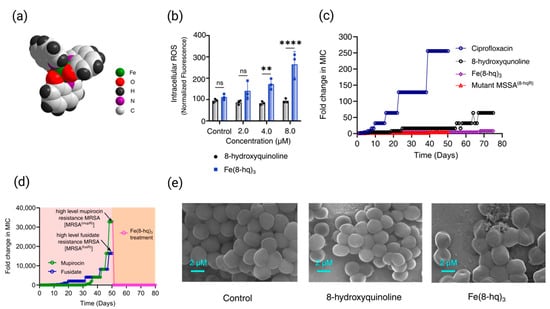
Figure 4.
X-ray structure of Fe(8-hq)3with the space-filling presentation (a), relative yields of intracellular ROS generation in MRSA bacterial cells treated with Fe(8-hq)3 in comparison with molar equivalents of 8-hq (b), drug resistance development of Fe(8-hq)3 vs. ciprofloxacin and 8-hq in MSSA and the overcome of drug resistance in mutant MSSA8-hqR (a strain resistant to 8-hq) bacteria by Fe(8-hq)3 (c), resistance development profile of mupirocin and fusidate in MRSA and the overcome of high-level mupirocin resistance mutant bacteria [MRSA(mupR)] by Fe(8-hq)3 (d), and SEM images of MRSA treated with 8-hq and Fe(8-hq)3 (e) (mean ± s.d, n = 3 replicates; ** p < 0.01, **** p < 0.0001 and ns = not significant). Reproduced with the permission from Ref. [97]. Adapted from N. Abeydeera et al. (2023) under the Creative Commons Attribution (CC BY) license.
This study also aims to develop novel topical antimicrobial agents for the treatment of skin and soft tissue infections (SSTIs) caused by mupirocin-resistant and fusidate-resistant MRSA strains. In vivo evaluation of a 2% Fe(8-hq)3 topical ointment demonstrated significant antimicrobial efficacy, highlighting the therapeutic potential of this non-antibiotic iron complex in treating drug-resistant SSTIs. Fe(8-hq)3 possesses several desirable attributes for clinical application, including potent bactericidal activity, a novel mechanism of action involving ROS generation, and a low propensity for cross-resistance with conventional antibiotics. These features of Fe(8-hq)3 have positioned it as a promising candidate for over-the-counter (OTC) use, potentially serving as an effective alternative to topical agents such as mupirocin and fusidate in the treatment of resistant S. aureus infections (Figure 5). However, it is important to note the limitations of murine infection models, as there are significant differences between murine and human immune systems. Future studies should be encouraged to utilize alternative, human-relevant platforms such as organ on-a-chip systems, ex vivo tissue models, or advanced in vitro approaches to mimic how infections and immune responses occur in the human body.
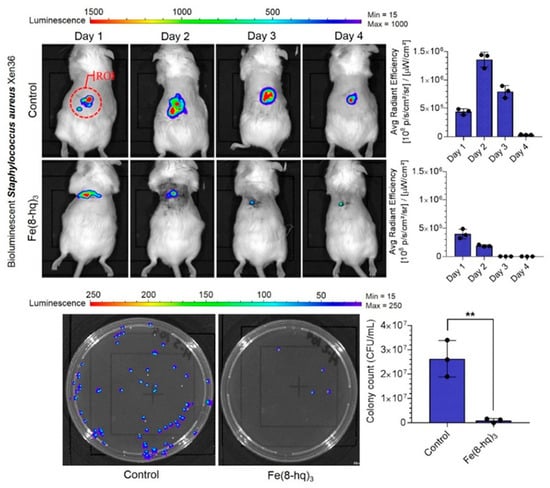
Figure 5.
Results of in vivo bioluminescent imaging studies of mice with ROI measurement, and representative images of CFU enumeration. Reproduced with the permission from Ref. [97]. Adapted from N. Abeydeera et al. (2023) under the Creative Commons Attribution (CC BY) license. ** p < 0.01.
In another study, Ana B. Polo et al. reported the antibacterial activity of cyclam-based iron compound [Fe(cyclam)ox]PF6; cyclam (1, 4, 8, 11-tetraazacyclotetradecane) and ox (oxalate) against pathogenic bacterial species, including multidrug-resistant clinical specimens [98]. MIC assays demonstrated that [Fe(cyclam)(ox)]PF6 exhibited broad-spectrum antibacterial activity against both Gram-positive and Gram-negative strains. The compound was effective against reference strains of Cutibacterium acnes (16.67 μg/mL), Staphylococcus aureus (23.61 μg/mL), Salmonella enterica serovar Typhi and Acinetobacter baumannii (33.33 μg/mL), and Pseudomonas aeruginosa (91.67 μg/mL). Notably, the compound also retained activity against clinical isolates, including S. aureus (17.54 μg/mL), A. baumannii (31.25 μg/mL), and P. aeruginosa (125.92 μg/mL). In addition, [Fe(cyclam)(ox)]PF6 exhibited low cytotoxicity toward fibroblast and keratinocyte cell lines and showed favorable pharmacokinetic properties based on in silico analysis.
Similarly, Matheus T. Branca et al. evaluated the antibacterial activity of another cyclam-based iron complex, [Fe(cyclam)(sal)]PF6, where sal denotes the salicylate ion, against multiple S. aureus strains, including S. aureus ATCC 25904, ATCC 33591, and a clinical isolate, S. aureus 05−0052 [99]. Their findings revealed that [Fe(cyclam)(sal)]PF6 exhibited potent activity against both reference and clinical strains, with a MIC value of 12.5 μg/mL. A noteworthy finding of this study was the potent anti-biofilm activity of [Fe(cyclam)sal]PF6, which effectively inhibited both the initial adhesion phase and disrupted preformed biofilms of S. aureus, a key pathogen in biofilm-associated infections. Biofilms form when bacterial cells adhere irreversibly to surfaces, grow into colonies, and secrete extracellular polymeric substances that inhibit the penetration of drugs, fostering an environment where persister cells exhibit enhanced antibiotic resistance up to 10 to 1000 times greater than that of planktonic cells [100,101,102,103]. These results highlight [Fe(cyclam)sal]PF6 as a promising dual-action anti-biofilm agent, particularly valuable in the context of antibiotic-resistant infections. The antibacterial mechanism of [Fe(cyclam)sal]PF6 is believed to involve disruption of the cell envelope, alteration of enzyme activities through ligand exchange, formation of ROS and ferroptosis was suggested as part of the mechanism of action.
Ludmila Kosaristanova et al. has reported the synergistic antibacterial action of the Fe16; [Fe(nphen)3](fu)·7H2O (where fu; fumarate and nphen; 5-nitro-1,10-phenanthroline) and ampicillin against S. aureus [104]. The minimum inhibitory concentration (MIC) of Fe16 alone was 125 μg/mL, and that of ampicillin was 2 μg/mL. Remarkably, when used in combination, the MIC values dropped to 31 μg/mL for Fe16 and 0.5 μg/mL for ampicillin, indicating a strong synergistic interaction that significantly enhanced antibacterial efficacy against S. aureus (Table 1). Morphological analysis by SEM revealed no substantial changes in S. aureus cells treated with Fe16 or ampicillin alone. However, cells exposed to the Fe16 + ampicillin combination at a sub-inhibitory concentration (0.25 μg/mL) for 24 h exhibited pronounced structural damage, including disruption of the cell wall and compromised membranes, highlighting a possible mechanism of synergy. These observations suggest that Fe16 may facilitate the penetration of ampicillin through the bacterial cell wall, thereby enhancing its bactericidal activity. Fe16 alone was also found to upregulate the expression of both efflux pumps proteins; mepA and norA. This is likely a consequence of reactive oxygen species (ROS) generated by the iron complex, which induces cellular stress and disrupts key cellular components. Based on these findings, it is proposed that the combination of Fe16 and ampicillin (AMP) imposes additional stress on S. aureus, thereby facilitating AMP penetration through the cell wall and simultaneously interfering with the function of efflux pumps and ABC transporters.

Table 1.
FIC indexes of Fe complexes in combination with different antibiotics.
Table 1.
FIC indexes of Fe complexes in combination with different antibiotics.
| MICs Against MRSA (ATCC BAA-44) | FIC Index | Ref. | |||
| Ciprofloxacin only | Ciprofloxacin with Fe(hinok)3 | Fe(hinok)3 only | Fe(hinok)3 with Ciprofloxacin | 0.75 | [96] |
| 16 µg/mL | 8 µg/mL | 4 µg/mL | 1 µg/mL | ||
| Ofloxacin only | Ofloxacin with Fe(hinok)3 | Fe(hinok)3 only | Fe(hinok)3 with Ofloxacin | 0.50 | [96] |
| 8 µg/mL | 2 µg/mL | 4 µg/mL | 1 µg/mL | ||
| Vancomycin only | Vancomycin with Fe(hinok)3 | Fe(hinok)3 only | Fe(hinok)3 with Vancomycin | 0.75 | [96] |
| 1 µg/mL | 0.5 µg/mL | 4 µg/mL | 1 µg/mL | ||
| MICs Against MRSA (ATCC BAA-44) | FIC Index | Ref. | |||
| Ciprofloxacin only | Ciprofloxacin with Fe(8-hq)3 | Fe(8-hq)3 only | Fe(8-hq)3 with Ciprofloxacin | 0.375 | [97] |
| 48.0 µM | 6.0 µM | 4.0 µM | 1.0 µM | ||
| Imipenem only | Imipenem with Fe(8-hq)3 | Fe(8-hq)3 only | Fe(8-hq)3 with Imipenem | 0.375 | [97] |
| 50.0 µM | 6.25 µM | 4.0 µM | 1.0 µM | ||
| MICs Against Staphylococcus aureus CCM 4223 | FIC Index | Ref. | |||
| Ampicillin only | Ampicillin with Fe16 | Fe16 only | Fe16 with Ampicillin | 0.498 | [104] |
| 2 µg/mL | 0.5 µg/mL | 125 µg/mL | 31 µg/mL | ||
Hrioua et al. reported that the metal-amoxicillin complexes have better antibacterial activity against Escherichia coli (E. coli) than the free ligand (amoxicillin) due to the AMX protection against oxidation after complexation [87]. The binding constant/association constant (K) of the AMX with Fe(III) were found to be 7.65 × 102 L mol−1. It was also noticed that Fe-AMX complex exhibited the highest antibacterial activity with MIC 25 ug/mL and bactericidal effect with greater than a 3-log CFU reduction after 24 h preexposure to Fe-AMX complex. In addition to its antibacterial effects, the Fe-AMX complex significantly inhibited biofilm formation by E. coli when compared to the control. After 48 h of incubation, biofilm biomass was reduced by 66.5% relative to the control and 45.16% relative to AMX alone, confirming the anti-biofilm potential of this Fe-AMX complex. These findings underscore the promise of iron based AMX complexes as next-generation antimicrobial and anti-biofilm agents. Square wave voltammetry (SWV) analysis of the Fe(III)-AMX complex revealed a sequential decrease in anodic peak current, along with the emergence of a new cathodic peak at approximately 0.5 V, which is attributed to the reduction of Fe(III) to Fe(II). This redox behavior suggests the potential involvement of Fenton-like reactions, wherein Fe(II) catalyzes the conversion of hydrogen peroxide into highly reactive hydroxyl radicals (•OH), contributing to reactive oxygen species (ROS) generation. The resulting oxidative stress may underline the antibacterial mechanism of the Fe(III)-AMX complex, leading to damage of bacterial membranes, proteins, and DNA. The enhanced antibacterial activity of Fe-AMX complex can be largely attributed to the chelation of metal ions with the AMX ligand. During chelation, the polarity of the metal ion is significantly reduced due to the partial sharing of its positive charge with the donor atoms of the ligand [105]. This process leads to increased π-electron delocalization across the chelate ring, which in turn enhances the lipophilicity of the resulting complex [106]. The increased lipophilicity facilitates the penetration of the complex into the lipid membranes of bacterial cells, thereby improving intracellular accumulation. Furthermore, these lipophilic metal complexes may interfere with essential metal-binding sites in bacterial enzymes, disrupting vital metabolic processes and contributing to their antimicrobial effect.
To further support the role of ROS-mediated iron-based antimicrobial mechanisms, Zhu et al. explored a novel approach to inactivate Listeria monocytogenes in its viable but non-culturable (VBNC) state [107]. Previous studies have shown that FeSO4 alone can induce VBNC formation in L. monocytogenes, allowing bacteria to retain virulence and resist antibiotics [108]. Zhu et al. investigated the application of blue light-assisted FeSO4 (Fe-BL) treatment, enhanced by three physical fields magnetic field, ultrasound, and blue light [107]. Among these, blue light was most effective in reversing the VBNC state and inducing bacterial death. Fe-BL treatment led to a substantial increase in cell membrane permeability, facilitating intracellular Fe2+ accumulation, triggering ROS bursts, and initiating lipid peroxidation hallmarks of Fenton reaction-driven ferroptosis-like cell death. The study highlights the potential of iron-based ROS generation, in combination with physical stimuli, to induce irreversible oxidative damage in persistent bacterial states. Additionally, Fe2+ loaded hyaluronic acid [81], and alginate hydrogels [109] have demonstrated significant potential in eliminating antibiotic-resistant bacterial infections while promoting wound healing in murine models. Furthermore, the incorporation of Fe3+/ethylenediaminetetraacetic acid (EDTA) complexes into hyaluronic acid [110], and alginate/hyaluronic acid [111] hydrogels has been employed to endow these biomaterials with sustained bactericidal activity, primarily through the controlled release of iron ions.
A recent study reported the development of an injectable hydrogel formed via the electrostatically triggered self-assembly of Fmoc-FFpY (fluorenylmethoxycarbonyl-diphenylalanine-phosphotyrosine) in the presence of Fe(III) ions [112]. The resulting β-sheet nanostructures, organized into twisted fibrillar helices, facilitated Fe(III)-mediated membrane disruption in both S. aureus and P. aeruginosa, promoting Fe(III) uptake and triggering reactive oxygen species (ROS) generation. Molecular dynamics simulations supported these findings, revealing that aggregated Fmoc-FFpY/Fe(III) complexes integrate into bacterial membranes and potentiate ROS production. This work highlights the potential of Fe(III) loaded peptide hydrogels as ferroptosis-inducing materials for combating bacterial infections.
The incorporation of ferroptosis-inspired chemistry into antimicrobial drug design thus represents an exciting interdisciplinary approach, bridging cancer biology and infectious disease therapeutics (Figure 6). Continued investigation into the molecular triggers and microbial responses to iron-induced oxidative stress may open up new avenues for the development of redox-active, metal-based antimicrobials.
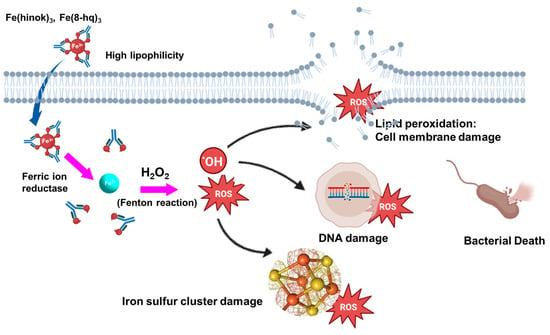
Figure 6.
Mechanism of action by lipophilic Fe (III) complexes against bacteria cells via the intracellular ROS production that in turn causes damage to nuclear DNA, iron-sulfur cluster, and cell membrane to trigger bacterial cell death.
4. Conclusions and Future Perspectives
The development of iron-based antimicrobial agents represents a promising frontier in combating antibiotic-resistant infections, particularly those caused by MRSA and other challenging pathogens. By exploiting iron’s essential yet potentially toxic nature, researchers have designed innovative strategies that disrupt microbial iron homeostasis, induce oxidative stress through Fenton reaction, and impair critical biological processes. Lipophilic small molecule iron chelators and iron-coordinating complexes, such as hinokitiol and 8-hydroxyquinoline and their derivatives, have demonstrated potent antimicrobial and anti-biofilm activities, offering new avenues for antimicrobial agents. Emerging strategies such as Fe(III)-loaded peptide hydrogels and light-assisted iron therapies further underscore the potential of ferroptosis-inducing platforms for localized and targeted antibacterial action. These approaches mimic key features of ferroptosis, including lipid peroxidation and ROS-mediated membrane damage, offering new avenues for treating persistent and non-culturable bacterial populations.
Despite these advances, challenges remain in optimizing the selectivity, stability, and toxicity profiles of iron-based agents for clinical application. Future research must focus on fine-tuning the balance between antimicrobial efficacy and host safety, understanding mechanisms of resistance, and exploring synergistic combinations with existing antibiotics. With continued innovation and interdisciplinary collaboration, iron-based therapeutics have the potential to expand our antimicrobial agents and address the urgent threat of multidrug-resistant infections. Investigations into the molecular underpinnings of bacterial ferroptosis-like mechanisms, combined with in vivo validation, will be critical for translating these discoveries into clinical applications. Altogether, harnessing iron-induced ferroptosis presents a novel and powerful strategy to overcome antimicrobial resistance and reinvigorate the development of next-generation antibacterial therapies.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Author would like to thanks to Department of Chemistry and Biochemistry at Kent State University.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMR | Antimicrobial Resistance |
| ICUs | Intensive Care Units |
| CO-ADD | Community for Open Antimicrobial Drug Discovery |
| ROS | Reactive Oxygen Species |
| MSSA | Methicillin-Sensitive Staphylococcus aureus |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| DNA | Deoxyribonucleic Acid |
| ATP | Adenosine Triphosphate |
| TNBC | Triple-Negative Breast Cancer |
| HEK 293 | Human Embryonic Kidney cells |
| hRBCs | human Red Blood Cells |
| MDR | Multidrug Resistant |
| IDA | Iron Deficiency Anemia |
| CFU | Colony Forming Unit |
| MIC | Minimum Inhibitory Concentration |
| FICI | Fractional Inhibitory Concentration Index |
| AMP | Ampicillin |
| SSTIs | Skin and Soft Infections |
| VBNC | Viable But Non-Culturable |
| SWV | Square Wave Voltammetry |
| EDTA | Ethylenediaminetetraacetic Acid |
References
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Rai, J.; Randhawa, G.K.; Kaur, M. Recent advances in antibacterial drugs. Int. J. Appl. Basic Med. Res. 2013, 3, 3–10. [Google Scholar]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Benin, B.M.; Yu, B.; Bunge, S.D.; Abeydeera, N.; Huang, S.D.; Kim, M.-H. Lipophilic Ga Complex with Broad-Spectrum Antimicrobial Activity and the Ability to Overcome Gallium Resistance in both Pseudomonas aeruginosa and Staphylococcus aureus. J. Med. Chem. 2021, 64, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2020.
- Pant, B.D.; Abeydeera, N.; Dubadi, R.; Kim, M.-H.; Huang, S.D. Broad-Spectrum Antimicrobial Activity of Ultrafine (BiO)2CO3 NPs Functionalized with PVP That Can Overcome the Resistance to Ciprofloxacin, AgNPs and Meropenem in Pseudomonas aeruginosa. Antibiotics 2023, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Mudarmah, K.; Abeydeera, N.; Chen, G.; Jogadi, W.; Krause, J.; Budzik, J.M.; Huang, S.D. Synthesis, Structural Characterization, and Antimicrobial Activity of Zn (cloxyquin) 2: Towards Harnessing Zinc Intoxication and Immune Response Restoration to Combat Staphylococcus aureus and Mycobacterium tuberculosis. Dalton Trans. 2025, 54, 9975–9983. [Google Scholar] [CrossRef]
- Dassanayake, T.M.; Dassanayake, A.C.; Abeydeera, N.; Pant, B.D.; Jaroniec, M.; Kim, M.-H.; Huang, S.D. An aluminum lining to the dark cloud of silver resistance: Harnessing the power of potent antimicrobial activity of γ-alumina nanoparticles. Biomater. Sci. 2021, 9, 7996–8006. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013; Centres for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2013.
- Hameed, H.A.; Islam, M.M.; Chhotaray, C.; Wang, C.; Liu, Y.; Tan, Y.; Li, X.; Tan, S.; Delorme, V.; Yew, W.W. Molecular targets related drug resistance mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis strains. Front. Cell. Infect. Microbiol. 2018, 8, 114. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler Jr, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Harkins, C.P.; Pichon, B.; Doumith, M.; Parkhill, J.; Westh, H.; Tomasz, A.; de Lencastre, H.; Bentley, S.D.; Kearns, A.M.; Holden, M.T. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Benin, B.M.; Abeydeera, N.; Kim, M.-H.; Huang, S.D. Bi2O3 nanoparticles exhibit potent broad-spectrum antimicrobial activity and the ability to overcome Ag-, ciprofloxacin- and meropenem-resistance in P. aeruginosa: The next silver bullet of metal antimicrobials? Biomater. Sci. 2022, 10, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Mudarmah, K.; Bagale, B.; Chen, G.; Krause, J.A.; Mighion, J.D.; Huang, S.D. Harnessing the dual antimicrobial mode of action with a lipophilic Mn (II) complex using the principle of the Irving–Williams Series to completely eradicate Staphylococcus aurous. Dalton Trans. 2023, 52, 12203–12207. [Google Scholar] [CrossRef]
- Alamri, H.; Chen, G.; Huang, S.D. Development of Biocompatible Ga2 (HPO4)3 Nanoparticles as an Antimicrobial Agent with Improved Ga Resistance Development Profile against Pseudomonas aeruginosa. Antibiotics 2023, 12, 1578. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef]
- Blair, J.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Lin, J.; Nishino, K.; Roberts, M.C.; Tolmasky, M.; Aminov, R.I.; Zhang, L. Mechanisms of antibiotic resistance. Front. Microbiol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: A review. J. Med. Chem. 2019, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Niu, G.; Li, W. Next-generation drug discovery to combat antimicrobial resistance. Trends Biochem. Sci. 2019, 44, 961–972. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping chemists discover new antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Evans, A.; Kavanagh, K.A. Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens. J. Med. Microbiol. 2021, 70, 001363. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9. [Google Scholar] [CrossRef]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; Van Lanen, S.G.; Boros, E. Theranostic gallium siderophore ciprofloxacin conjugate with broad spectrum antibiotic potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef]
- Skaar, E.P.; Humayun, M.; Bae, T.; DeBord, K.L.; Schneewind, O. Iron-source preference of Staphylococcus aureus infections. Science 2004, 305, 1626–1628. [Google Scholar] [CrossRef]
- Létoffé, S.; Heuck, G.; Delepelaire, P.; Lange, N.; Wandersman, C. Bacteria capture iron from heme by keeping tetrapyrrol skeleton intact. Proc. Natl. Acad. Sci. USA 2009, 106, 11719–11724. [Google Scholar] [CrossRef] [PubMed]
- Frederick, R.E.; Mayfield, J.A.; DuBois, J.L. Iron trafficking as an antimicrobial target. Biometals 2009, 22, 583. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, J.R.; Heinrichs, D.E. Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol. Rev. 2015, 39, 592–630. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Frangipani, E.; Bonchi, C.; Minandri, F.; Imperi, F.; Visca, P. Pyochelin potentiates the inhibitory activity of gallium on Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 5572–5575. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Ratledge, C.; Dover, L.G. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Petrik, M.; Zhai, C.; Haas, H.; Decristoforo, C. Siderophores for molecular imaging applications. Clin. Transl. Imaging 2017, 5, 15–27. [Google Scholar] [CrossRef]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in iron metabolism: From mechanism to therapy potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator–Siderophore: A review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- Weller, M.; Overton, T.; Rourke, J.; Armstrong, F.A. Inorganic Chemistry; Oxford University Press: Oxford, UK, 2018. [Google Scholar][Green Version]
- Wandersman, C.; Delepelaire, P. Bacterial iron sources: From siderophores to hemophores. Annu. Rev. Microbiol. 2004, 58, 611–647. [Google Scholar] [CrossRef] [PubMed]
- Schröder, I.; Johnson, E.; De Vries, S. Microbial ferric iron reductases. FEMS Microbiol. Rev. 2003, 27, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Köpf-Maier, P.; Köpf, H.; Neuse, E.W. Ferricenium complexes: A new type of water-soluble antitumor agent. J. Cancer Res. Clin. Oncol. 1984, 108, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Deferiprone and Iron–Maltol: Forty Years since Their Discovery and Insights into Their Drug Design, Development, Clinical Use and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4970. [Google Scholar] [CrossRef]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Hun, L.T. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Ratledge, C. Iron metabolism and infection. Food Nutr. Bull. 2007, 28, S515–S523. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Cornelis, P. Role of Iron in Bacterial Pathogenesis. Front. Cell. Infect. Microbiol. 2018, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Herchel, R.; Šindelář, Z.; Trávníček, Z.; Zbořil, R.; Vančo, J. Novel 1D chain Fe (III)-salen-like complexes involving anionic heterocyclic N-donor ligands. Synthesis, X-ray structure, magnetic, 57Fe Mössbauer, and biological activity studies. Dalton Trans. 2009, 44, 9870–9880. [Google Scholar] [CrossRef] [PubMed]
- Basu, U.; Khan, I.; Koley, D.; Saha, S.; Kondaiah, P.; Chakravarty, A.R. Nuclear targeting terpyridine iron (II) complexes for cellular imaging and remarkable photocytotoxicity. J. Inorg. Biochem. 2012, 116, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, E. Recent advances in multinuclear complexes as potential anticancer and DNA binding agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2014, 14, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Heldt, J.M.; Guille-Collignon, M.; Lemaître, F.; Jaouen, G.; Vessières, A.; Amatore, C. Quantitative analyses of ROS and RNS production in breast cancer cell lines incubated with ferrocifens. ChemMedChem 2014, 9, 1286–1293. [Google Scholar] [CrossRef]
- Franke, J.C.; Plötz, M.; Prokop, A.; Geilen, C.C.; Schmalz, H.-G.; Eberle, J. New caspase-independent but ROS-dependent apoptosis pathways are targeted in melanoma cells by an iron-containing cytosine analogue. Biochem. Pharmacol. 2010, 79, 575–586. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, B.; Di Bilio, A.J.; Zhu, L.; Wang, T.; Qi, C.; Shih, J.; Yen, Y. A Ferrous-Triapine complex mediates formation of reactive oxygen species that inactivate human ribonucleotide reductase. Mol. Cancer Ther. 2006, 5, 586–592. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.; Da Silva, E.G.; Rocha, D.D.; Hillard, E.A.; Pigeon, P.; Jaouen, G.; Rodrigues, F.A.; De Abreu, F.C.; da Rocha Ferreira, F.; Goulart, M.O. Molecular Mechanism of Action of 2-Ferrocenyl-1, 1-diphenylbut-1-ene on HL-60 Leukemia Cells. ChemMedChem 2014, 9, 2580–2586. [Google Scholar] [CrossRef]
- Vančo, J.; Šindelář, Z.; Dvořák, Z.; Trávníček, Z. Iron-salophen complexes involving azole-derived ligands: A new group of compounds with high-level and broad-spectrum in vitro antitumor activity. J. Inorg. Biochem. 2015, 142, 92–100. [Google Scholar] [CrossRef]
- Ahmad, I.; Nelson, D.J.; Hussain, M.I.; Nasar, N.A. Potential of covalently linked tamoxifen hybrids for cancer treatment: Recent update. RSC Med. Chem. 2024, 15, 1877–1898. [Google Scholar] [CrossRef]
- Kwong, W.L.; Lok, C.N.; Tse, C.W.; Wong, E.L.M.; Che, C.M. Anti-Cancer Iron (II) Complexes of Pentadentate N-Donor Ligands: Cytotoxicity, Transcriptomics Analyses, and Mechanisms of Action. Chem. A Eur. J. 2015, 21, 3062–3072. [Google Scholar] [CrossRef]
- Hargrave-Thomas, E.; Yu, B.; Reynisson, J. Serendipity in anticancer drug discovery. World J. Clin. Oncol. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Mao, X.; Wu, S.; Huang, D.; Li, C. Complications and comorbidities associated with antineoplastic chemotherapy: Rethinking drug design and delivery for anticancer therapy. Acta Pharm. Sin. B 2024, 14, 2901–2926. [Google Scholar] [CrossRef]
- Abeydeera, N.; Stilgenbauer, M.; Pant, B.D.; Mudarmah, K.; Dassanayake, T.M.; Zheng, Y.-R.; Huang, S.D. Lipophilic Fe (III)-Complex with Potent Broad-Spectrum Anticancer Activity and Ability to Overcome Pt Resistance in A2780cis Cancer Cells. Molecules 2023, 28, 4917. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, M.; Zhang, J.; Sun, Z.; Zhang, W.; Dong, W.; Cheng, C.; Yao, Y.; Li, K. Hinokitiol-iron complex is a ferroptosis inducer to inhibit triple-negative breast tumor growth. Cell Biosci. 2023, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Mudarmah, K.; Pant, B.D.; Krause, J.A.; Zheng, Y.-R.; Huang, S.D. Transferrin-inspired iron delivery across the cell membrane using [(L2 Fe)2 (μ-O)](L= chlorquinaldol) to harness anticancer activity of ferroptosis. Dalton Trans. 2024, 53, 3206–3214. [Google Scholar] [CrossRef]
- Guo, R.; Fang, X.; Shang, K.; Wen, J.; Ding, K. Induction of ferroptosis: A new strategy for the control of bacterial infections. Microbiol. Res. 2024, 284, 127728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Zhou, W.; Lee, J.; Liu, Z.; An, Z.; Xu, D.; Mo, H.; Hu, L.; Zhou, X. Ferrous sulfate-loaded hydrogel cures Staphylococcus aureus infection via facilitating a ferroptosis-like bacterial cell death in a mouse keratitis model. Biomaterials 2022, 290, 121842. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H. Iron and microbial infection. Nat. Rev. Microbiol. 2004, 2, 946–953. [Google Scholar] [CrossRef]
- Edwards, E.I.; Epton, R.; Marr, G. Organometallic derivatives of penicillins and cephalosporins a new class of semi-synthetic antibiotics. J. Organomet. Chem. 1975, 85, C23–C25. [Google Scholar] [CrossRef]
- Edwards, E.I.; Epton, R.; Marr, G. A new class of semi-synthetic antibiotics: Ferrocenyl-penicillins and-cephalosporins. J. Organomet. Chem. 1976, 107, 351–357. [Google Scholar] [CrossRef]
- Edwards, E.; Epton, R.; Marr, G. 1, 1′-Ferrocenyldiacetic Acid Anhydride and its Use in the preparation of heteroannularly substituted ferrocenyl-penicillins and-cephalosporins. J. Organomet. Chem. 1976, 122, C49–C53. [Google Scholar] [CrossRef]
- Santos, J.V.d.O.; Porto, A.L.F.; Cavalcanti, I.M.F. Potential application of combined therapy with lectins as a therapeutic strategy for the treatment of bacterial infections. Antibiotics 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Hrioua, A.; Loudiki, A.; Farahi, A.; Laghrib, F.; Bakasse, M.; Lahrich, S.; Saqrane, S.; El Mhammedi, M. Complexation of amoxicillin by transition metals: Physico-chemical and antibacterial activity evaluation. Bioelectrochemistry 2021, 142, 107936. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, H.; Fan, S.; Zheng, B.; Wu, J.; Zhang, J.; Pi, J.; Xu, J.-F. Ferroptosis: A mixed blessing for infectious diseases. Front. Pharmacol. 2022, 13, 992734. [Google Scholar] [CrossRef]
- Parrello, D.; Zegeye, A.; Mustin, C.; Billard, P. Siderophore-mediated iron dissolution from nontronites is controlled by mineral cristallochemistry. Front. Microbiol. 2016, 7, 423. [Google Scholar] [CrossRef]
- Grillo, A.S.; SantaMaria, A.M.; Kafina, M.D.; Cioffi, A.G.; Huston, N.C.; Han, M.; Seo, Y.A.; Yien, Y.Y.; Nardone, C.; Menon, A.V. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 2017, 356, 608–616. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Design of Orally Active Iron Chelators for the Treatment of Thalassaemia. Ph.D. Thesis, University of Essex, Colchester, UK, 1982; pp. 1–243. [Google Scholar]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators intended for clinical use in iron overload, other diseases of iron imbalance and free radical pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, W.; Huang, H.; Tang, Y.; Wang, W.; Meng, F.; Wang, H.; Zheng, Y. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New antimicrobial strategies based on metal complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Abeydeera, N.; Yu, B.; Pant, B.D.; Kim, M.-H.; Huang, S.D. Harnessing the toxicity of dysregulated iron uptake for killing Staphylococcus aureus: Reality or mirage? Biomater. Sci. 2022, 10, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Benin, B.M.; Mudarmah, K.; Pant, B.D.; Chen, G.; Shin, W.S.; Kim, M.-H.; Huang, S.D. Harnessing the Dual Antimicrobial Mechanism of Action with Fe (8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections. Antibiotics 2023, 12, 886. [Google Scholar] [CrossRef]
- Polo, A.B.; Lemos, A.S.; Martins da Mata, C.P.; Oliveira, V.S.; Pontes, A.C.; Pontes, D.L.; Tavares, G.D.; Fabri, R.L.; M Apolônio, A.C. In vitro activity of the novel Fe-cyclam complex against clinical multidrug-resistant bacterial isolates from Brazil. Future Microbiol. 2023, 18, 897–909. [Google Scholar] [CrossRef]
- Branca, M.T.; Silva, T.P.; Lemos, A.S.; Campos, L.M.; Souza, T.F.; Palazzi, C.; Oliveira, V.S.; Coimbra, E.S.; Silva, F.O.; FB Pontes, A.C. The Fe-Cyclam-Derived Compound [Fe (cyclam) sal] PF6 Restrains Drug-Resistant Staphylococcus aureus Proliferation and Biofilm Formation. ACS Omega 2025, 10, 11386–11396. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef]
- Kosaristanova, L.; Rihacek, M.; Sucha, F.; Milosavljevic, V.; Svec, P.; Dorazilova, J.; Vojtova, L.; Antal, P.; Kopel, P.; Patocka, Z. Synergistic antibacterial action of the iron complex and ampicillin against Staphylococcus aureus. BMC Microbiol. 2023, 23, 288. [Google Scholar] [CrossRef]
- Mishra, P. Biocoordination, Computational Modeling and Antibacterial Sensitivities of Cobalt (II), Nickel (II), Copper (II) and Bismuth (V) with Gentamicin and Amoxicillin Antibiotics mixed Ligands. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 145–156. [Google Scholar]
- Singh, H.L.; Singh, J.; Mukherjee, A. Synthesis, spectral, and in vitro antibacterial studies of organosilicon (IV) complexes with schiff bases derived from amino acids. Bioinorg. Chem. Appl. 2013, 2013, 425832. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Peng, S.; Zhang, J.; Li, H.; Mo, H.; Hu, L. Physical fields reverse FeSO4-induced VBNC state in Listeria monocytogenes and facilitate ferroptosis. Food Microbiol. 2025, 131, 104796. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Zhang, N.; Zhang, H.; Li, H.; Qi, Y.; Mo, H.; Hu, L. Direct ferrous sulfate exposure facilitates the VBNC state formation rather than ferroptosis in Listeria monocytogenes. Microbiol. Res. 2023, 269, 127304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; An, Z.; Richel, A.; Huang, M.; Gou, X.; Xu, D.; Zhang, M.; Mo, H.; Hu, L.; Zhou, X. Ferrous sulfate remodels the properties of sodium alginate-based hydrogel and facilitates the healing of wound infection caused by MRSA. Carbohydr. Polym. 2024, 346, 122554. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of self-healing hydrogels with on-demand antimicrobial activity and sustained biomolecule release for infected skin regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef] [PubMed]
- Shuai, F.; Zhang, Y.; Yin, Y.; Zhao, H.; Han, X. Fabrication of an injectable iron (III) crosslinked alginate-hyaluronic acid hydrogel with shear-thinning and antimicrobial activities. Carbohydr. Polym. 2021, 260, 117777. [Google Scholar] [CrossRef]
- Loth, C.; Barbault, F.; Guégan, C.; Lemaire, F.; Contal, C.; Carvalho, A.; Hellé, S.; Champion, M.; Kerdjoudj, H.; Chan-Seng, D. Experimental and Computational Study of Injectable Iron (III)/Ultrashort Peptide Hydrogels: A Candidate for Ferroptosis-Induced Treatment of Bacterial Infections. Small Sci. 2025, 5, 2400618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).