Purification and Characterization of the Recombinant Chitinase ChiBlUV02 of Bacillus licheniformis UV01 with a Choleoptericidal Effect on Hive Beetle (Aethina tumida)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactivation and Growth of Bacillus licheniformis UV01
2.2. Chitinase Gene Amplification
2.3. Purification and Quantification of the PCR Products
2.4. Cloning of the Chitinase Gene Chibluv01
2.5. Recombinant Protein Expression
2.6. Preparation of Colloidal Chitin
2.7. Quantification of the Protein Concentration
2.8. Enzymatic Assay
2.9. Purification via Affinity Chromatography
2.10. Determination of the Optimal Temperature and pH for Chitinolytic Activity
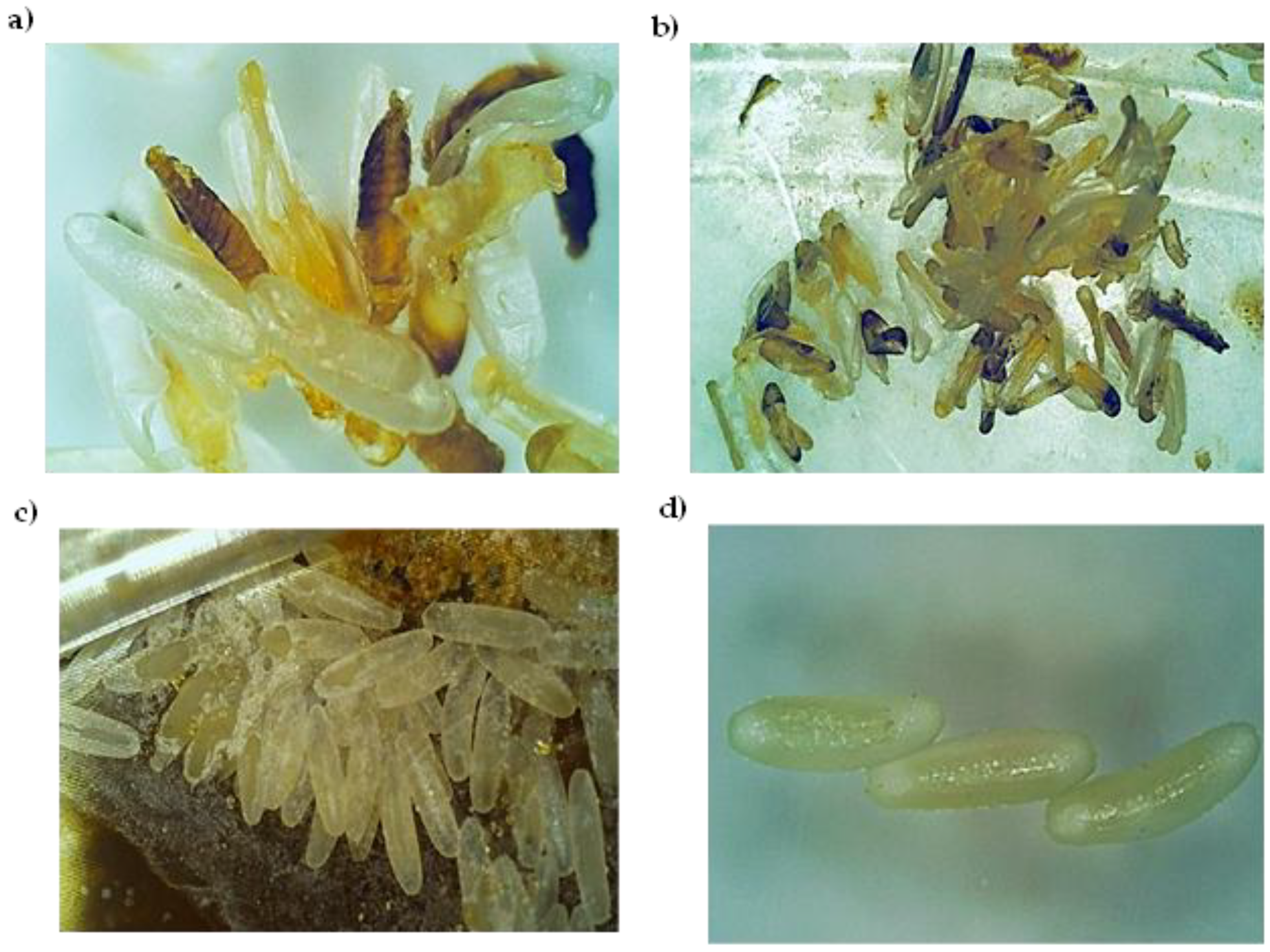
2.11. Adaptation of Aethina tumida
2.12. Choleoptericidal Activity on Aethina tumida
2.13. Statistical Analysis
3. Results
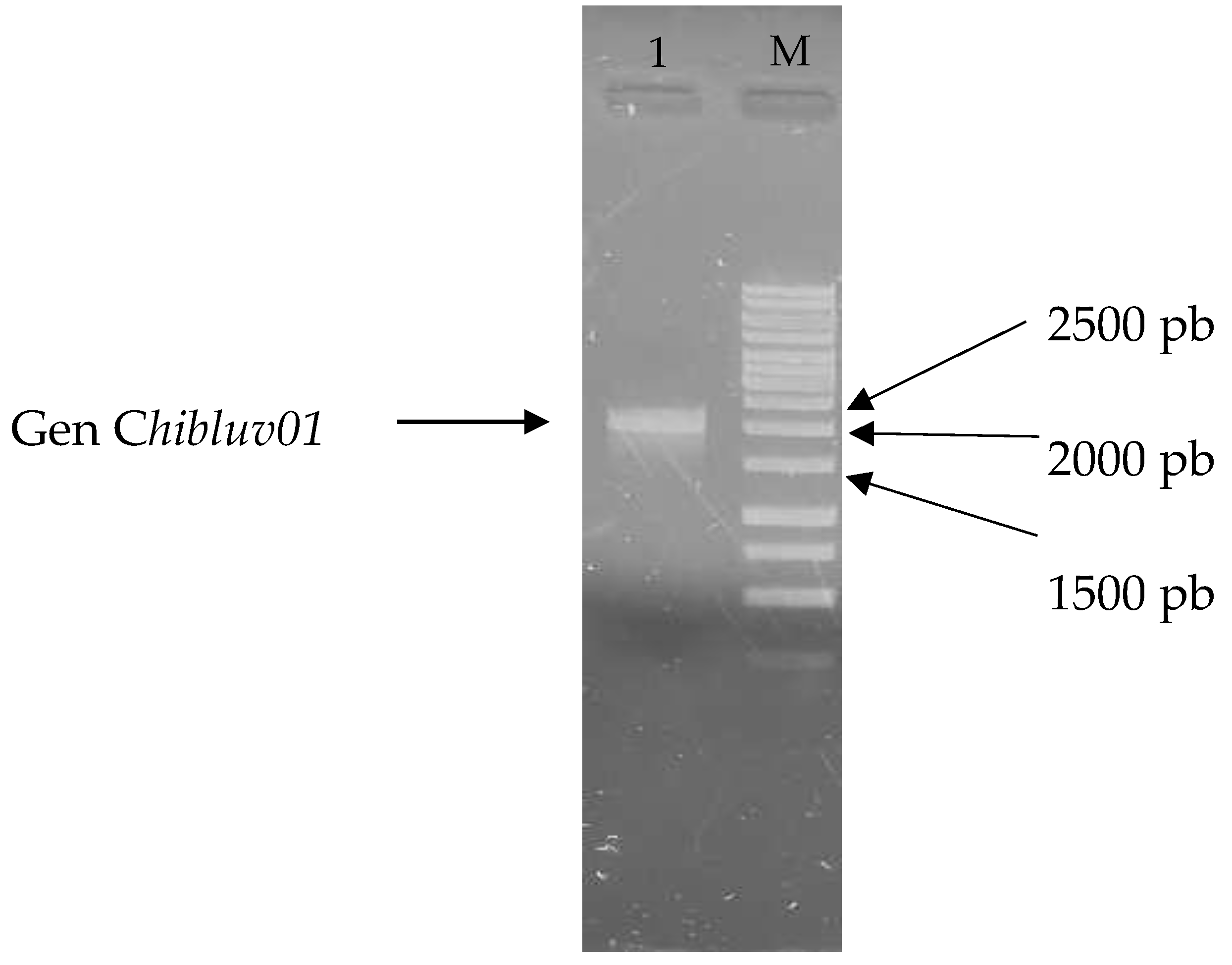
3.1. Recovery of the Gene chiBLUV01
3.2. Identification of the Transformed Cells
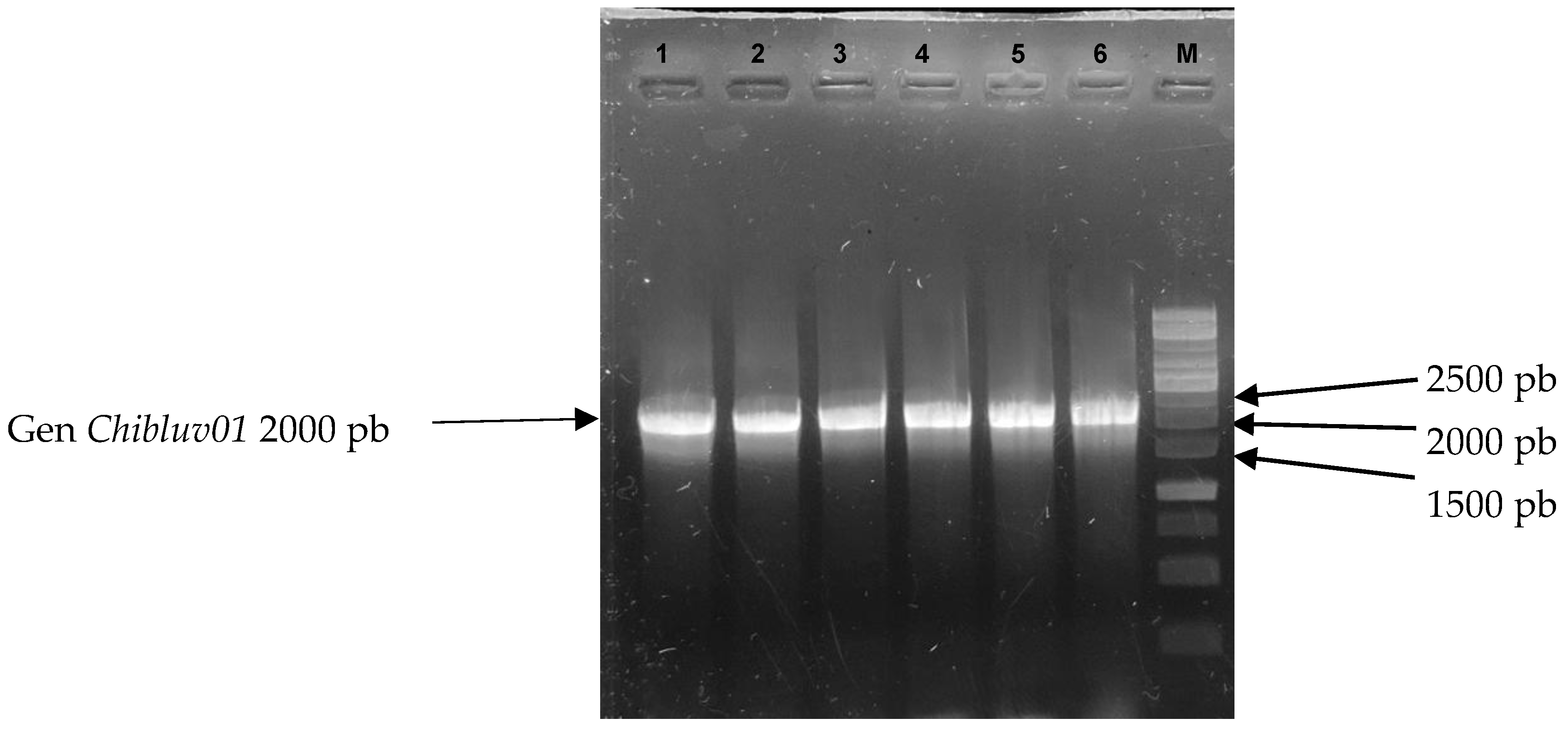
3.3. Induction of Chibluv01 Gene Expression
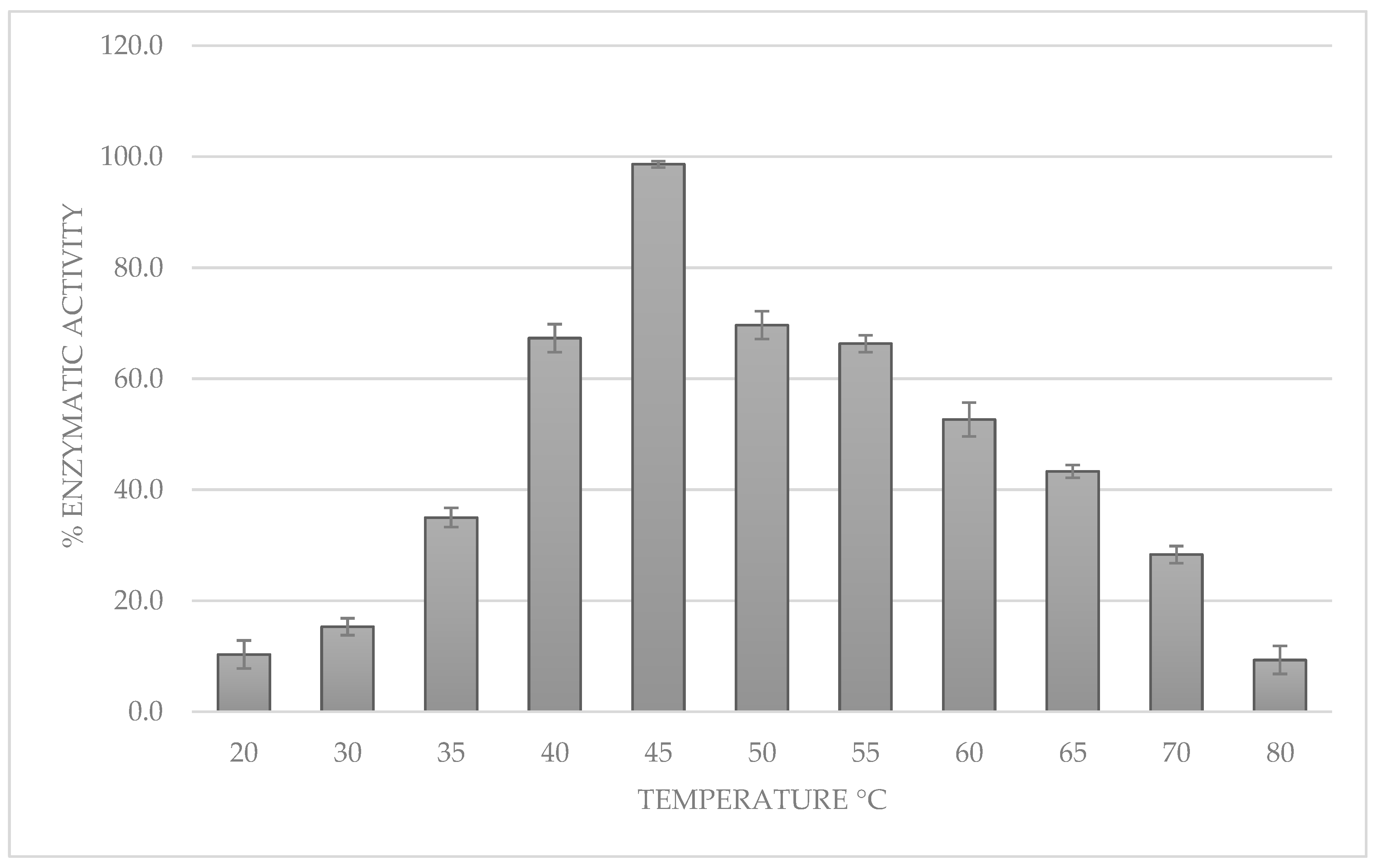
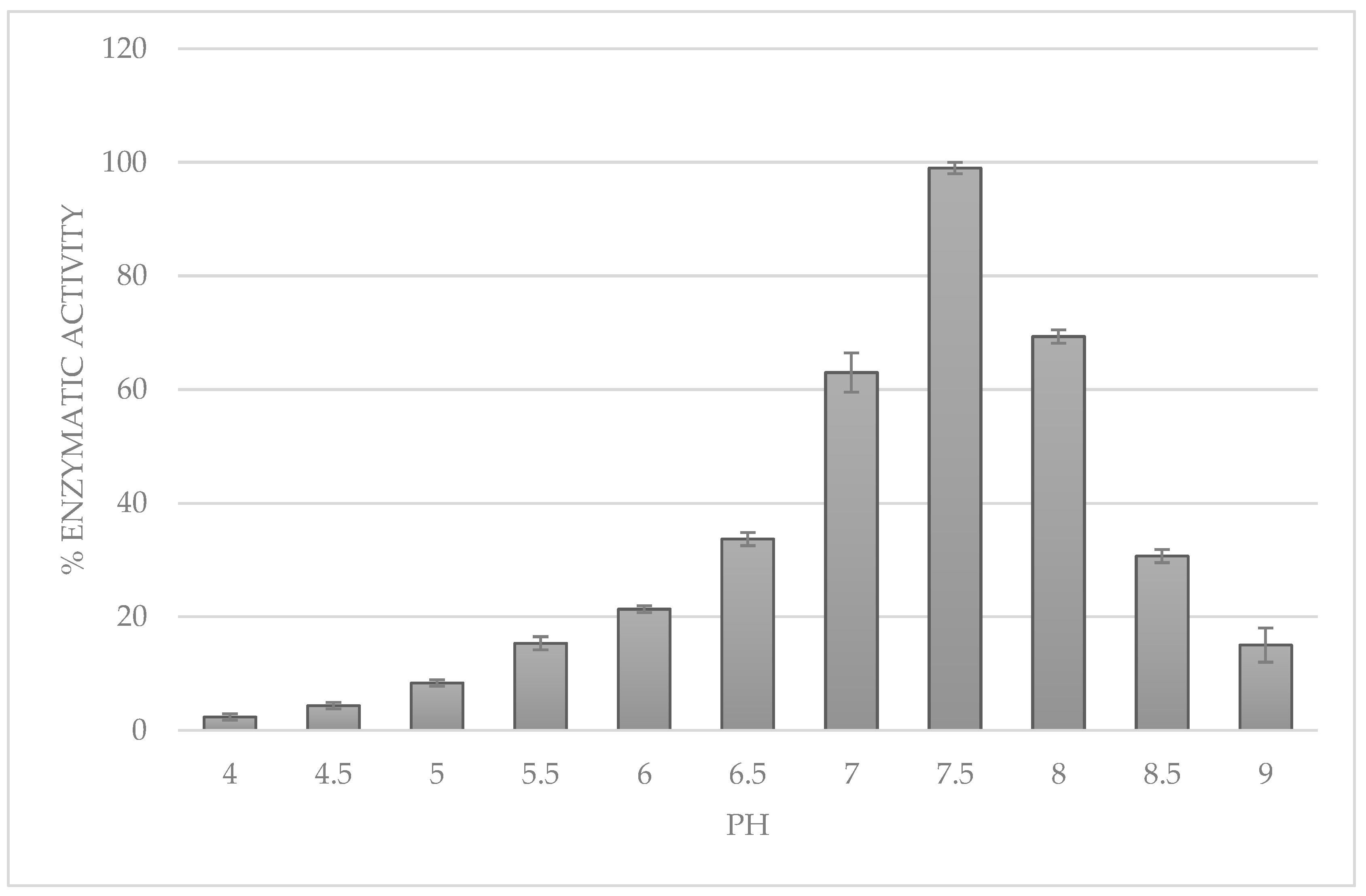
3.4. Determination of the Optimal pH and Temperature
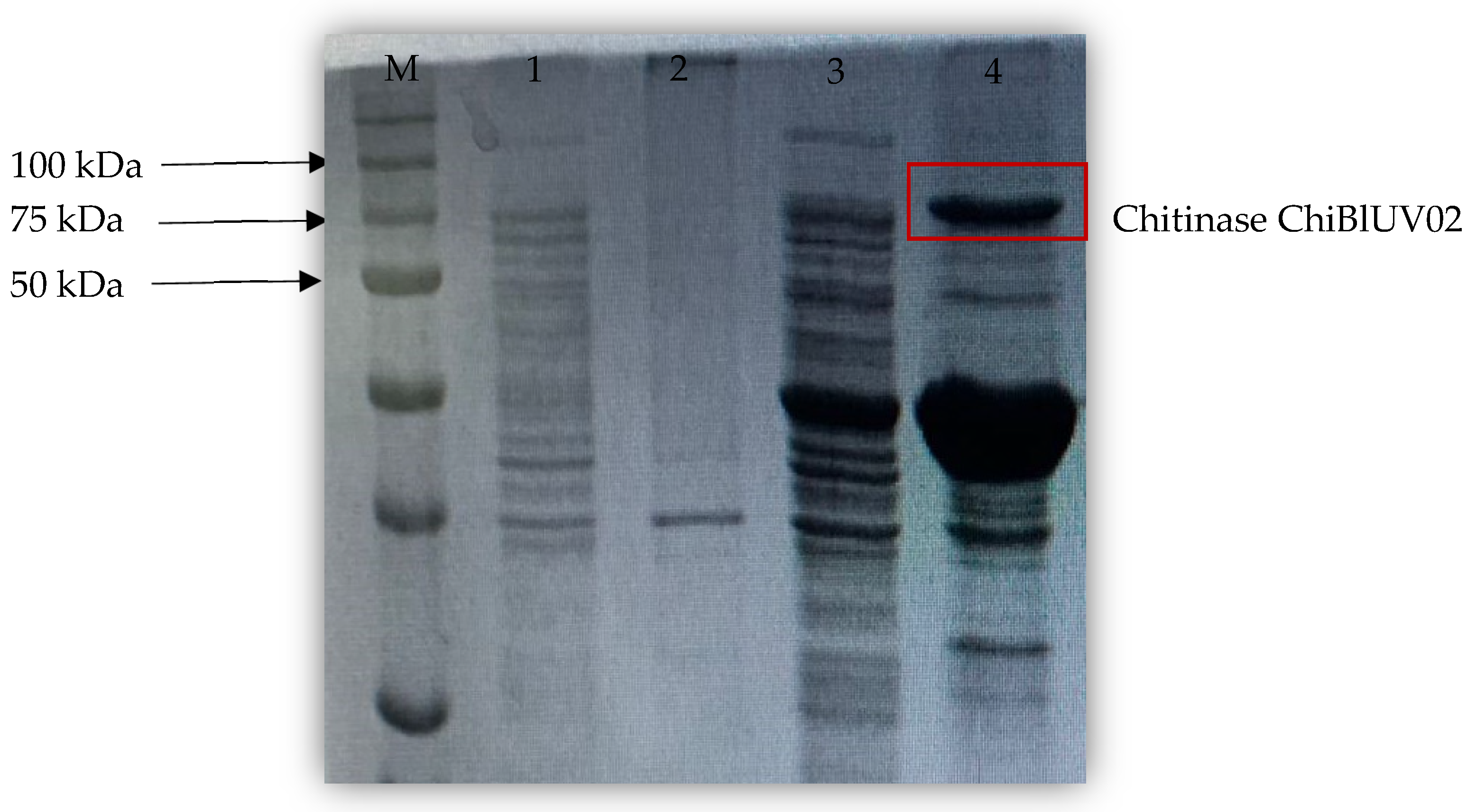
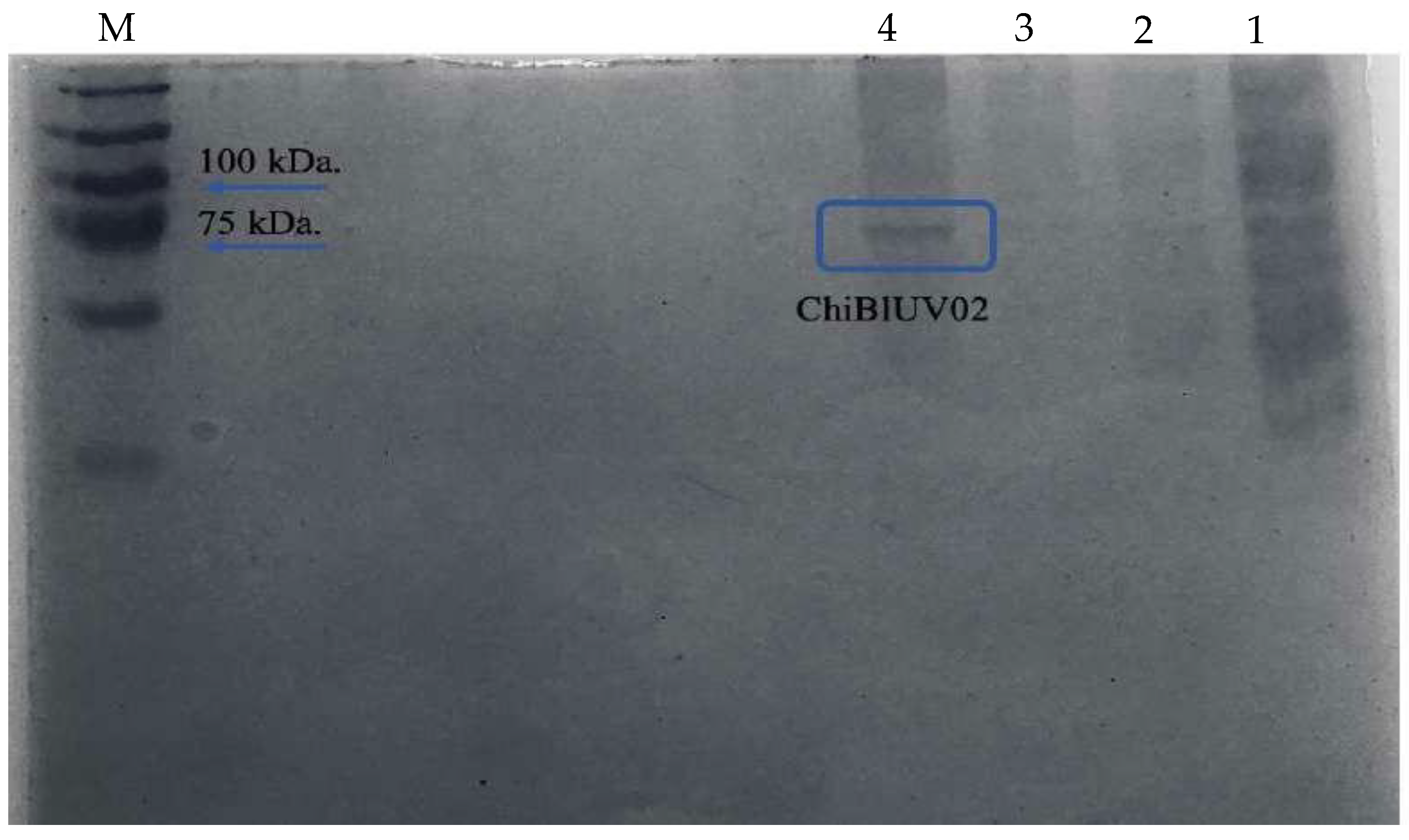
3.5. ChiBlUV02 Chitinase Purification
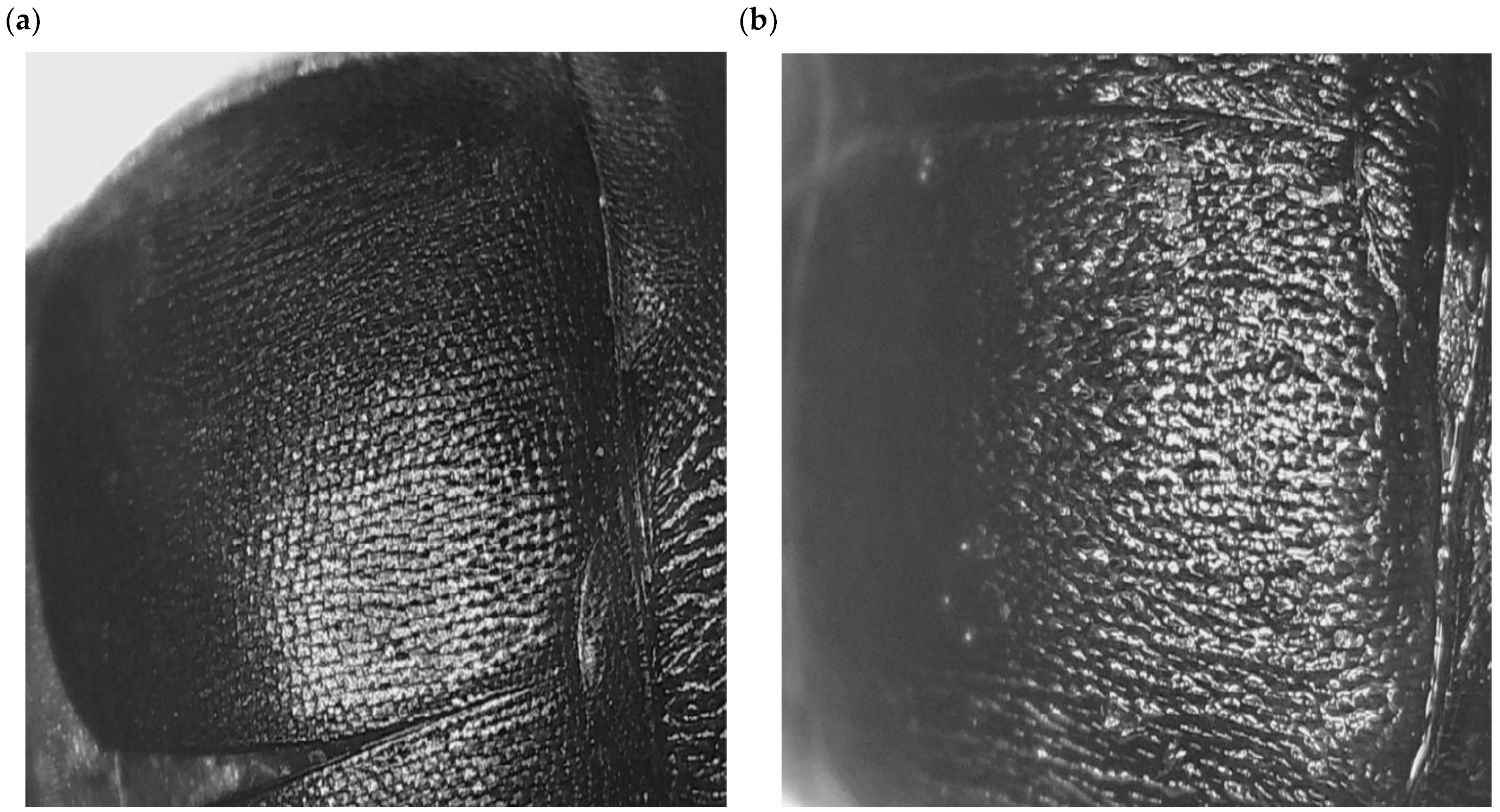
3.6. Choleoptericidal Activity on the Larvae and Adults of Aethina tumida
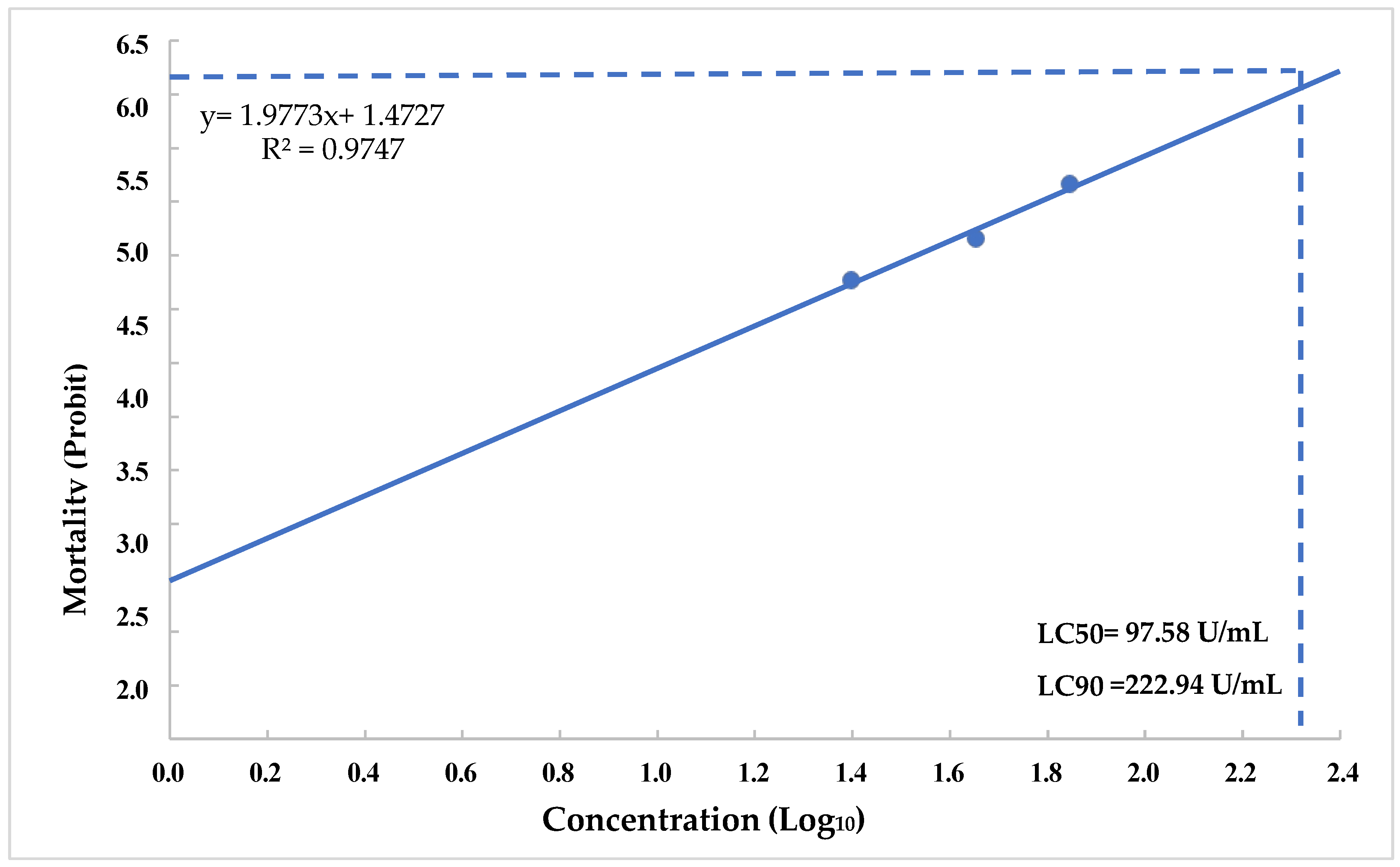
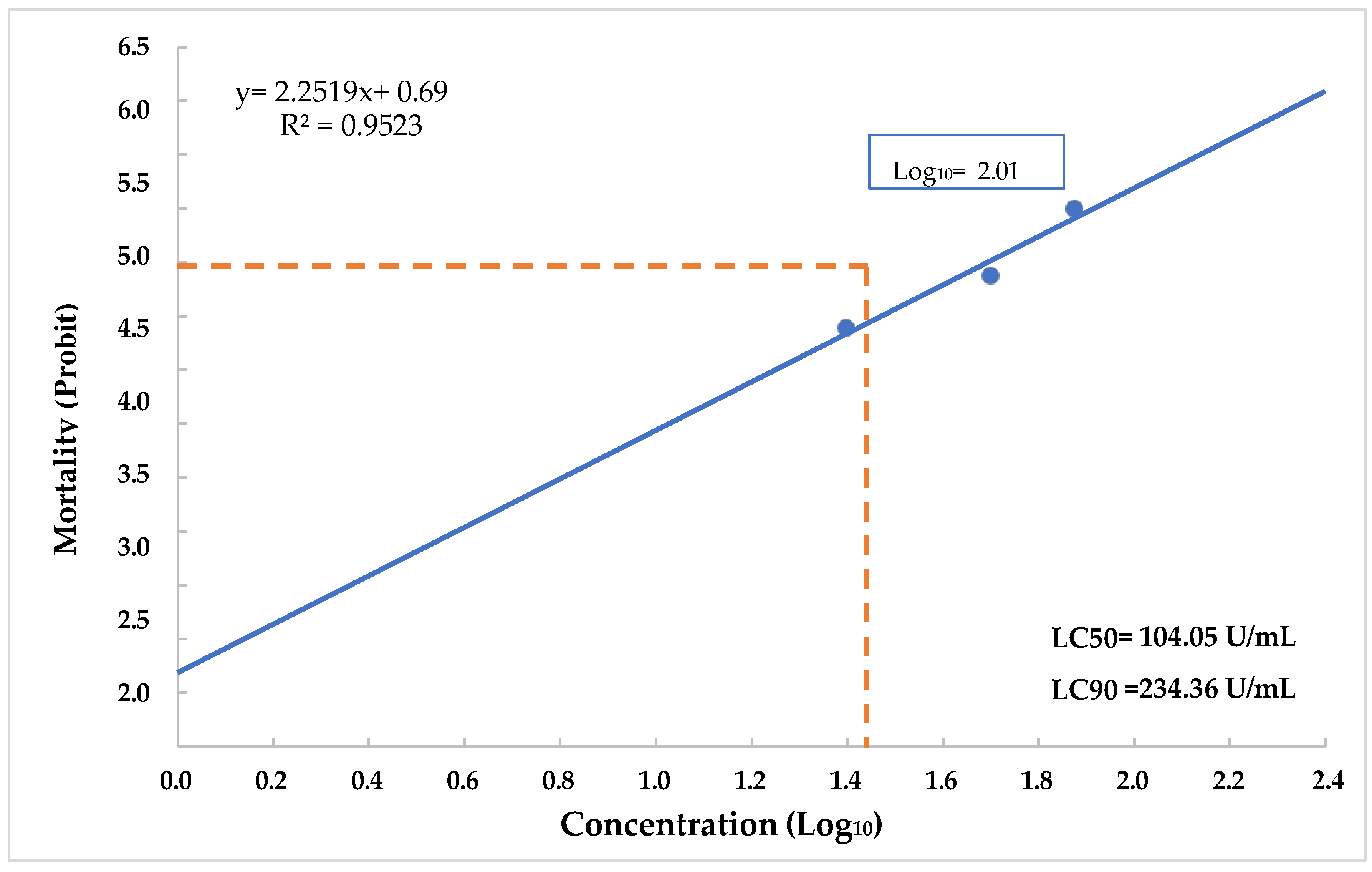
3.7. Determination of LC50 and LC90
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langner, T.; Göhre, V. Fungal chitinases: Function, regulation, and potential roles in plant/pathogen interactions. Curr. Genet. 2016, 62, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Salazar, U.; Inca, A.; Falcón, G.; Carbonero, P.; Rodríguez, B.; del Campo, J.; Parrado, J.; Bautista, J. Production of Chitinases from By-Products of the Food: Industry Application to Edible Mushrooms and Crustaceans. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nagpure, A.; Gupta, R.K. Bacterial chitinases: Properties and potential. Crit. Rev. Biotechnol. 2007, 27, 21–28. [Google Scholar] [CrossRef]
- Swiontek Brzezinska, M.; Jankiewicz, U.; Burkowska, A.; Walczak, M. Chitinolytic microorganisms and their possible application in environmental protection. Curr. Microbiol. 2014, 68, 71–81. [Google Scholar] [CrossRef]
- Ramírez, M.V.; Calzadíaz, L. Industrial enzymes and metabolites from Actinobacteria in food and medicine industry. In Actinobacteria—Basics and Biotechnological Applications; InTech: Houston, TX, USA, 2016. [Google Scholar] [CrossRef]
- Yang, S.; Fu, X.; Yan, Q.; Guo, Y.; Liu, Z.; Jiang, Z. Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillus barengoltzii. Food Chem. 2016, 192, 1041–1048. [Google Scholar] [CrossRef]
- Reverchon, F.; Diyarza Sandoval, N.A. Potential biological control agents against Fusarium spp. in Mexico: Current situation, challenges and perspectives. Biotecnol. Sustentabilidad 2021, 6, 16–39. [Google Scholar] [CrossRef]
- Liu, D.; Cai, J.; Xie, C.-C.; Liu, C.; Chen, Y.-H. Purification and partial characterization of a 36-kDa chitinase from Bacillus thuringiensis subsp. Colmeri and its biocontrol potential. Enzyme. Microb. Technol. 2010, 46, 252–256. [Google Scholar] [CrossRef]
- Thakur, N.; Nath, A.K.; Chauhan, A.; Gupta, R. Purification, characterization, and antifungal activity of Bacillus cereus strain NK91 chitinase from rhizospheric soil samples of Himachal Pradesh, India. Biotechnol. Appl. Biochem. 2022, 69, 1830–1842. [Google Scholar] [CrossRef]
- Verma, K.; Garg, N. Antifungal activity and protoplast formation by chitinase produced from Bacillus licheniformis NK-7. Biol. Fascicle/Analele Univ. Din Oradea 2022, 29, 61–67. Available online: https://www.bioresearch.ro/2022-1/061-067-AUOFB.29.1.2022-VERMA.K.-Antifungal.activity.and.protoplast.pdf (accessed on 25 January 2025).
- Borgi, M.A.; Boudebbouze, S.; Aghajari, N.; Szukala, F.; Pons, N.; Maguin, E.; Rhimi, M. The attractive recombinant phytase from Bacillus licheniformis: Biochemical and molecular characterization. Appl. Microbiol. Biotechnol. 2014, 98, 5937–5947. [Google Scholar] [CrossRef]
- Gong, C.; Maquat, L.E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 2011, 470, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Jellouli, K.; Ghorbel-Bellaaj, O.; Ayed, H.B.; Manni, L.; Agrebi, R.; Nasri, M. Alkaline-protease from Bacillus licheniformis MP1: Purification, characterization and potential application as a detergent additive and for shrimp waste deproteinization. Process Biochem. 2011, 46, 1248–1256. [Google Scholar] [CrossRef]
- Ben Slimene, I.; Tabbene, O.; Gharbi, D.; Mnasri, B.; Schmitter, J.M.; Urdaci, M.-C.; Limam, F. Isolation of a chitinolytic Bacillus licheniformis S213 strain exerting a biological control against Phoma medicaginis infection. Appl. Biochem. Biotechnol. 2015, 175, 3494–3506. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Won, S.-J.; Moon, J.-H.; Lee, U.; Park, Y.-S.; Maung, C.E.H.; Ajuna, H.B.; Ahn, Y.S. Bacillus licheniformis PR2 controls fungal diseases and increases production of jujube fruit under field conditions. Horticulturae 2021, 7, 49. [Google Scholar] [CrossRef]
- Murray, A. XLI.—List of Coleoptera received from old calabar, on the west coast of Africa. Ann. Mag. Nat. Hist. 1867, 20, 314–323. [Google Scholar] [CrossRef]
- Neumann, P.; Elzen, P.J. The biology of the small hive beetle (Aethina tumida, Coleoptera: Nitidulidae): Gaps in our knowledge of an invasive species. Apidologie 2004, 35, 229–247. [Google Scholar] [CrossRef]
- Hood, W.M. The small hive beetle, Aethina tumida: A review. Bee World 2004, 85, 51–59. [Google Scholar] [CrossRef]
- Richardson, K. Beekeeping role in enhancing food security and environmental public health. Health Econ. Manag. Rev. 2023, 4, 69–79. [Google Scholar] [CrossRef]
- Nikolova, T.; Dimitrova, I.; Teneva, A. The development of beekeeping in Bulgaria and the European Union in the last ten years. An overview. Bulg. J. Anim. Husb./Životnov Dni Nauki. 2023, 60, 37–45. [Google Scholar]
- Lehninger Principles of Biochemistry, 7th ed.; W.H. Freeman: New York, NY, USA, 2017.
- Syrový, I.; Hodný, Z. Staining and quantification of proteins separated by polyacrylamide gel electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1991, 569, 175–196. [Google Scholar] [CrossRef]
- Hsu, S.C.; Lockwood, J.L. Powdered chitin agar as a selective medium for enumeration of Actinomycetes in water and Soil1. Appl. Microbiol. 1975, 29, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Monreal, J.; Reese, E.T. The chitinase of Serratia marcescens. Can. J. Microbiol. 1969, 15, 689–696. [Google Scholar] [CrossRef]
- Gallagher, S.R. SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Curr. Protoc. Essent. Lab. Tech. 2012, 6, 7–10. [Google Scholar] [CrossRef]
- Buchholz, S.; Merkel, K.; Spiewok, S.; Pettis, J.S.; Duncan, M.; Spooner-Hart, R.; Ulrichs, C.; Ritter, W.; Neumann, P. Alternative control of Aethina tumida Murray (Coleoptera: Nitidulidae) with lime and diatomaceous earth. Apidologie 2009, 40, 535–548. [Google Scholar] [CrossRef]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Jayanthi, Nalista; et al. Characterization of thermostable chitinase from Bacillus licheniformis B2. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surabaya, Indonesia, 23–24 August 2018; IOP Publishing: Bristol, UK, 2019; Volume 293. [Google Scholar] [CrossRef]
- Laribi-Habchi, H.; Bouanane-Darenfed, A.; Drouiche, N.; Pauss, A.; Mameri, N. Purification, characterization, and molecular cloning of an extracellular chitinase from Bacillus licheniformis stain LHH100 isolated from wastewater samples in Algeria. Int. J. Biol. Macromol. 2015, 72, 1117–1128. [Google Scholar] [CrossRef]
- Menghiu, G.; Ostafe, V.; Prodanovic, R.; Fischer, R.; Ostafe, R. Biochemical characterization of chitinase A from Bacillus licheniformis DSM8785 expressed in Pichia pastoris KM71H. Protein Expr. Purif. 2019, 154, 25–32. [Google Scholar] [CrossRef]
- Brzezinska, M.S.; Jankiewicz, U. Production of antifungal chitinase by Aspergillus niger LOCK 62 and its potential role in the biological control. Curr. Microbiol. 2012, 65, 666–672. [Google Scholar] [CrossRef]
- Aoki, Y.; Haga, S.; Suzuki, S. Direct antagonistic activity of chitinase produced by Trichoderma sp. SANA20 as biological control agent for grey mould caused by Botrytis cinerea. Cogent Biol. 2020, 6, 1747903. [Google Scholar] [CrossRef]
- Essghaier, B.; Zouaoui, M.; Najjari, A.; Sadfi, N. Potentialities and characterization of an antifungal chitinase produced by a halotolerant Bacillus licheniformis. Curr. Microbiol. 2021, 78, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Ajuna, H.B.; Won, S.J.; Choub, V.; Choi, S.I.; Yun, J.Y.; Hwang, W.J.; Park, S.W.; Ahn, Y.S. The Anti-Termite Activity of Bacillus licheniformis PR2 against the Subterranean Termite, Reticulitermes speratus kyushuensis Morimoto (Isoptera: Rhinotermitidae). Forests 2023, 14, 1000. [Google Scholar] [CrossRef]
- Rajendran, K.; Krishnamoorthy, M.; Karuppiah, K.; Ethiraj, K.; Sekar, S. Chitinase from Streptomyces mutabilis as an Effective Eco-friendly Biocontrol Agent. Appl. Biochem. Biotechnol. 2024, 196, 18–31. [Google Scholar] [CrossRef]
- Micocci, K.C.; Moreira, A.C.; Sanchez, A.D.; Pettinatti, J.L.; Rocha, M.C.; Dionizio, B.S.; Correa, K.C.S.; Malavazi, I.; Wouters, F.C.; Bueno, O.C.; et al. Identification, cloning, and characterization of a novel chitinase from leaf-cutting ant Atta sexdens: An enzyme with antifungal and insecticidal activity. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2023, 1867, 130249. [Google Scholar] [CrossRef]
- Aktas, C.; Ruzgar, D.; Gurkok, S.; Gormez, A. Purificación y caracterización de la quitinasa de Stenotrofomonas maltophilia con propiedades antifúngicas e insecticidas. Bioquímica Biotecnol. Preparativa 2023, 53, 797–806. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, S.S.; Zhong, F.; Zhou, M.; Jiang, X.Y.; Cheng, Y.S.; Dan, Y.H.; Hu, G.; Li, C.; Tang, B.; et al. Chitinase (CHI) of Spodoptera frugiperda affects molting development by regulating the metabolism of chitin and trehalose. Front. Physiol. 2022, 13, 1034926. [Google Scholar] [CrossRef]
| 24 h | 48 h | 72 h | |||||
|---|---|---|---|---|---|---|---|
| Concentration (U/mL) | Live | Dead | Live | Dead | Live | Dead | Mortality |
| Control | 30 | 0 | 30 | 0 | 30 | 0 | 0 |
| 10 | 30 | 0 | 30 | 0 | 30 | 0 | 0 |
| 30 | 26 | 4 | 26 | 4 | 26 | 3 | 13% |
| 45 | 22 | 8 | 21 | 9 | 21 | 7 | 30% |
| 70 | 19 | 11 | 18 | 12 | 18 | 12 | 43% |
| 115 | 15 | 15 | 15 | 15 | 15 | 15 | 50% |
| 24 h | 48 h | 72 h | |||||
|---|---|---|---|---|---|---|---|
| Concentration (U/mL) | Live | Dead | Live | Dead | Live | Dead | Mortality |
| Control | 30 | 0 | 30 | 0 | 30 | 0 | 0 |
| 10 | 30 | 0 | 30 | 0 | 30 | 0 | 0 |
| 30 | 27 | 3 | 27 | 3 | 27 | 3 | 10% |
| 45 | 23 | 7 | 23 | 7 | 23 | 7 | 23% |
| 70 | 19 | 11 | 18 | 12 | 18 | 12 | 40% |
| 115 | 13 | 17 | 13 | 17 | 13 | 17 | 56% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Vique, D.d.J.; Flores-Primo, A.; Rodríguez-Dehaibes, S.; Sánchez-Otero, M.G.; Pardío-Sedas, V.T.; Oliart-Ros, R.M.; Blasco-López, G.; Quintana-Castro, R. Purification and Characterization of the Recombinant Chitinase ChiBlUV02 of Bacillus licheniformis UV01 with a Choleoptericidal Effect on Hive Beetle (Aethina tumida). Appl. Microbiol. 2025, 5, 48. https://doi.org/10.3390/applmicrobiol5020048
Velasco-Vique DdJ, Flores-Primo A, Rodríguez-Dehaibes S, Sánchez-Otero MG, Pardío-Sedas VT, Oliart-Ros RM, Blasco-López G, Quintana-Castro R. Purification and Characterization of the Recombinant Chitinase ChiBlUV02 of Bacillus licheniformis UV01 with a Choleoptericidal Effect on Hive Beetle (Aethina tumida). Applied Microbiology. 2025; 5(2):48. https://doi.org/10.3390/applmicrobiol5020048
Chicago/Turabian StyleVelasco-Vique, Deny de Jesús, Argel Flores-Primo, Sóstenes Rodríguez-Dehaibes, María Guadalupe Sánchez-Otero, Violeta T. Pardío-Sedas, Rosa María Oliart-Ros, Gabriela Blasco-López, and Rodolfo Quintana-Castro. 2025. "Purification and Characterization of the Recombinant Chitinase ChiBlUV02 of Bacillus licheniformis UV01 with a Choleoptericidal Effect on Hive Beetle (Aethina tumida)" Applied Microbiology 5, no. 2: 48. https://doi.org/10.3390/applmicrobiol5020048
APA StyleVelasco-Vique, D. d. J., Flores-Primo, A., Rodríguez-Dehaibes, S., Sánchez-Otero, M. G., Pardío-Sedas, V. T., Oliart-Ros, R. M., Blasco-López, G., & Quintana-Castro, R. (2025). Purification and Characterization of the Recombinant Chitinase ChiBlUV02 of Bacillus licheniformis UV01 with a Choleoptericidal Effect on Hive Beetle (Aethina tumida). Applied Microbiology, 5(2), 48. https://doi.org/10.3390/applmicrobiol5020048









