Prebiotic Galacto-Oligosaccharide and Xylo-Oligosaccharide Feeds in Pig Production: Microbiota Manipulation, Pathogen Suppression, Gut Architecture and Immunomodulatory Effects
Abstract
1. Introduction
2. The Development of Prebiotic Applications
2.1. The Prebiotic Concept
2.2. Prebiotic Oligosaccharides
2.3. Porcine Oligosaccharides
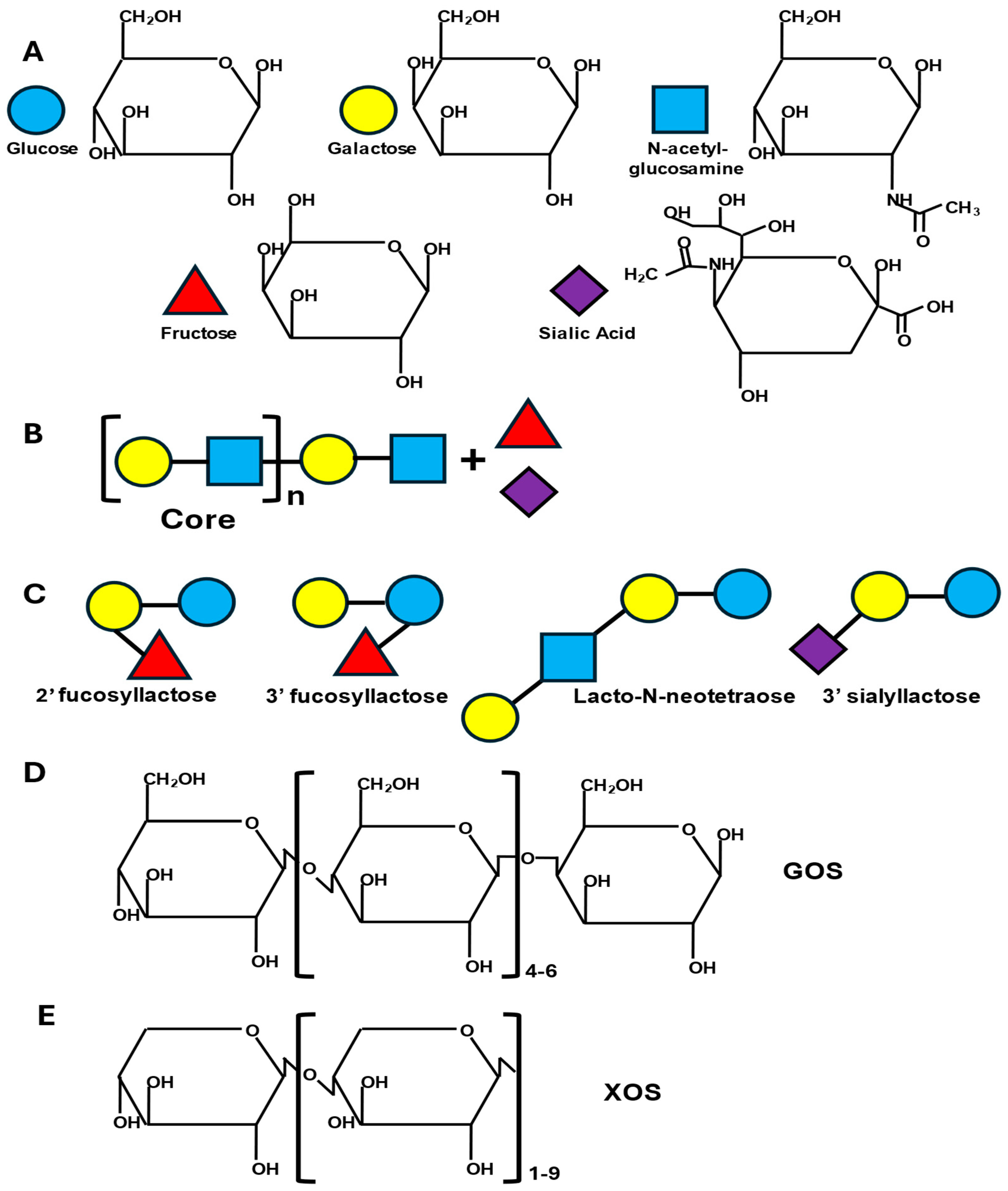
2.4. Commercially Available Galacto- and Xylo-Oligosaccharides
3. Biological Effects of Galacto- and Xylo-Oligosaccharides
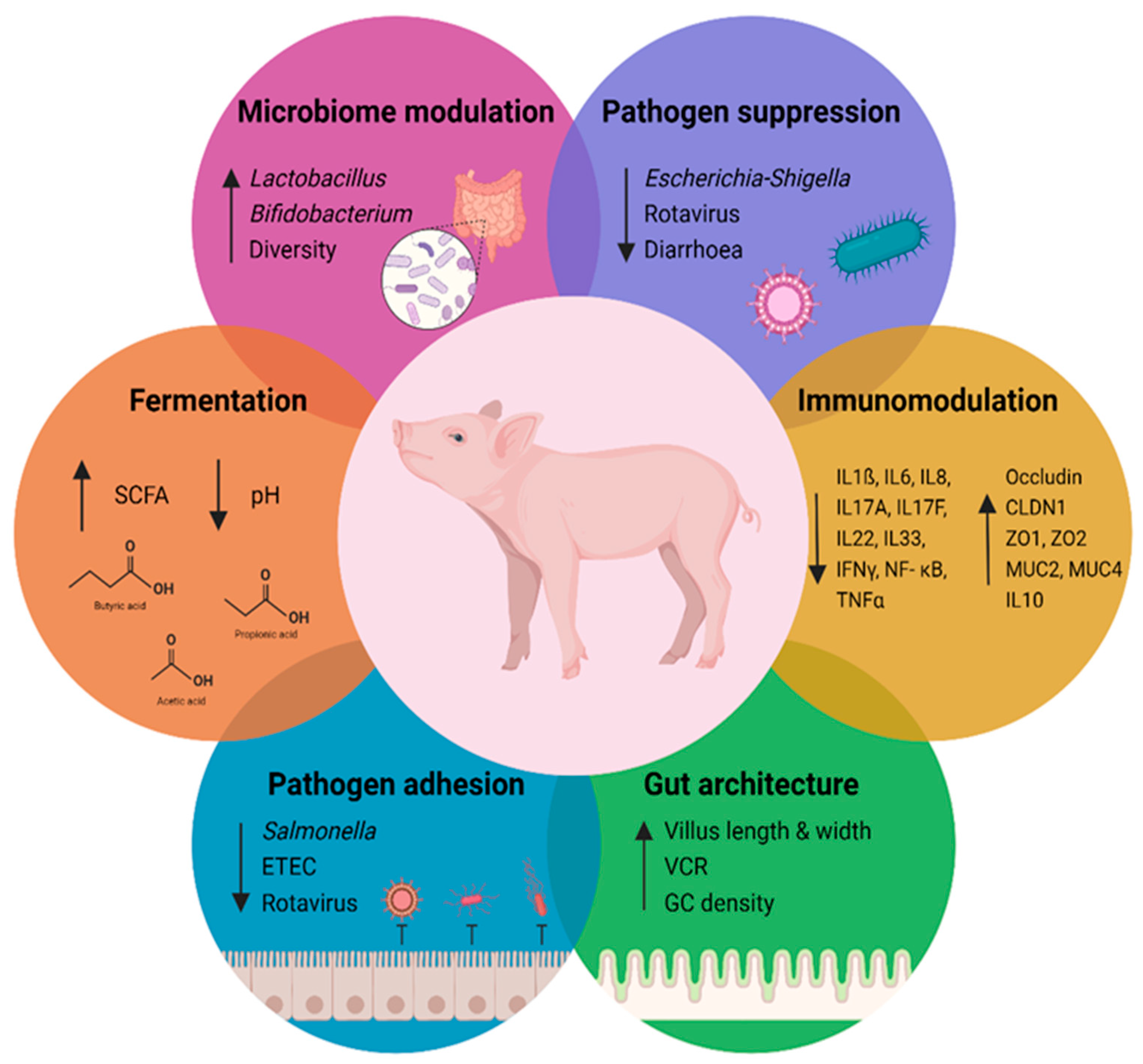
3.1. Preventing Pathogen Adhesion
3.2. Galacto- and Xylo-Oligosaccharides Are Substrates for Fermentation
3.3. Microbiota Mediated Beneficial Effects of Galacto- and Xylo-Oligosaccharides
| Growth Stage | In-Feed XOS, % | Bacterial Source | Effect of XOS on 16S rRNA Community Taxa † | Reference |
|---|---|---|---|---|
| Weaning | 0.025 | Ileum | Acinetobacter ↑, Herbaspirillum ↑, Xanthobacter ↑ | Ding et al. [135] |
| Colon | Bifidobacterium ↑, Sharpea ↑, Slackia ↑, Veillonella ↑ | |||
| Weaning | 0.1 | Ileum | Streptococcus ↑, Bifidobacterium ↑, Ruminococcus ↓ | Gao et al. [134] |
| Weaning | 1 | Ileum | Lactobacillus ↑, Bifidobacterium ↑ | Sun et al. [128] |
| Weaning | 0.05 | Ileum | Lactobacillus ↑, Escherichia-Shigella ↓, Clostridium sensu stricto 1 ↓ | Chen et al. [129] |
| Caecum | Lactobacillus ↑, Clostridium sensus strico 1 ↓, Terrisporobacter ↓ | |||
| Weaning | 0.02 | Caecum | Escherichia-Shigella ↑, Streptococcus ↑ | Wang et al. [131] |
| Weaning | 0.05 | Caecum | Lactobacillus ↑, Intestinibacter ↓, Anaerotruncus ↓, Ruminiclostridium 9 ↓, Clostridium sensu stricto 1 ↓, Turicibacter ↓ | Tang et al. [136] |
| Weaning | 0.01 | Intestinal contents | Streptococcus ↑, Turicibacter ↑, Lactobacillus ↓ | Yin et al. [15] |
| Growing/fattening | 0.01 | Intestinal contents | Lactobacillus ↑, Citrobacter ↓ | Pan et al. [137] |
| Weaning | 0.02 | Faecal | Lactobacillus ↑, Escherichia coli ↓ | Liu et al. [73] |
| Weaning | 1.5 | Faecal | Lactobacillus ↑, Bifidobacterium↑, Fusicatenibacter ↑, Ruminococcus ↓, Eubacterium coprostanoligenes ↓, Clostridia UCG-014 ↓ | Pang et al. [74] |
| Weaning | 0.01/0.025 /0.05 | Faecal | Lactobacillus ↑, Bifidobacterium ↑ | Su et al. [138] |
| Growing | 0.02 | Faecal | Prevotellaceae_NK3B1 ↑, Muribaculaceae ↑ | Sutton et al. [139] |
| Pre-weaning | 5 | Faecal | Cloacibacillus porcorum ↑, Clostridium sensus strico 1 ↓, Parabacteroides goldsteinii ↓ | Bai et al. [140] |
3.4. The Effects of Galacto- and Xylo-Oligosaccharides on the Gut Architecture
| Growth Stage | XOS Purity, % (w/w) | In-Feed XOS, % | Effect of XOS, % Difference to Control † | Reference | |||
|---|---|---|---|---|---|---|---|
| Jejunum | Ileum | ||||||
| VH | VCR | VH | VCR | ||||
| Weaning | 95 | 0.01 | - | - | 6.2 | 7.2 | Chen, et al. [72] |
| 0.05 | 5.1 | 7.5 | 9.2 | 11.4 | |||
| 0.1 | - | - | - | - | |||
| Weaning | ≥35 | 0.01 | - | - | - | - | Su et al. [138] |
| 0.025 | - | - | - | - | |||
| 0.05 | - | 14.6 | 17.6 | - | |||
| Weaning | 95 | 0.05 | ↑ | ↑ | Chen et al. [129] | ||
| Weaning | 70 | 0.1 | ↑ | ↑ | Gao et al. [134] | ||
| Weaning | 70 | 1.0 | ↑ | - | Sun et al. [128] | ||
| Weaning | 35 | 0.02 | - | 12.4 | 19.8 | 8.7 | Wang et al. [131] |
| Weaning | ≥35 | 0.025 | 12.2 | 10 | Ding et al. [135] | ||
| Weaning | 50 | 0.02 | - | 11.3 | - | - | Liu et al. [73] |
3.5. Immunomodulatory Effects of Galacto- and Xylo-Oligosaccharides
3.6. Late Gestational Effects of Galacto-Oligosaccharides on Sows and Piglets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| AGPs | antimicrobial growth promoters |
| GOS | galacto-oligosaccharides |
| XOS | xylo-oligosaccharides |
| FOS | fructo-oligosaccharides |
| GIT | gastrointestinal tract |
| PWD | post-weaning diarrhoea |
| SCFA | short-chain fatty acids |
| LAB | lactic acid bacteria |
| RVA | Rotavirus A |
| BW | body weight |
| ADG | average daily gain |
| G:F | gain-to-feed ratio |
References
- Dourmad, J.Y.; Ryschawy, J.; Trousson, T.; Bonneau, M.; Gonzàlez, J.; Houwers, H.W.J.; Hviid, M.; Zimmer, C.; Nguyen, T.L.T.; Morgensen, L. Evaluating environmental impacts of contrasting pig farming systems with life cycle assessment. Animal 2014, 8, 2027–2037. [Google Scholar] [CrossRef]
- Giraldi-Díaz, M.R.; Castillo-González, E.; De Medina-Salas, L.; Velásquez-De la Cruz, R.; Huerta-Silva, H.D. Environmental Impacts Associated with Intensive Production in Pig Farms in Mexico through Life Cycle Assessment. Sustainability 2021, 13, 11248. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Vondruskova, H.; Slamova, R. Alternatives to antibiotic growth promotors in prevention of diarrhea in weaned piglets: A review. Vet. Med. 2010, 55, 199–224. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Hasman, H.; Aarestrup, F.M. Zinc resistance of Staphylococcus aureus of animal origin is strongly associated with methicillin resistance. Vet. Microbiol. 2011, 150, 344–348. [Google Scholar] [CrossRef]
- Jensen, J.; Kyvsgaard, N.C.; Battisti, A.; Baptiste, K.E. Environmental and public health related risk of veterinary zinc in pig production-using Denmark as an example. Environ. Int. 2018, 114, 181–190. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA/394961/2017. In European Medicines Agency, Questions and Answers on Veterinary Medicinal Products Containing Zinc Oxide to be Administered Orally to Food-Producing Species Outcome of a Referral Procedure Under Article 35 of Directive 2001/82/EC (EMEA/V/A/118); European Medicines Agency: London, UK, 2017; Available online: https://www.ema.europa.eu/en/documents/referral/zinc-oxide-article-35-referral-questions-answers-veterinary-medicinal-products-containing-zinc-oxide_en.pdf (accessed on 28 February 2025).
- Proorocu, M.; Petrescu, D.C.; Burny, P.; Petrescu-Mag, R.M. Pork meat consumption, from statistics to consumer behavior: A review. Porc. Res. 2021, 11, i–vi. Available online: https://porc.bioflux.com.ro/home/volume-11-1-2021/ (accessed on 28 February 2025).
- Kim, S.W.; Gormley, A.; Jang, K.B.; Duarte, M.E. Invited Review—Current status of global pig production: An overview and research trends. Anim. Biosci. 2024, 37, 719–729. [Google Scholar] [CrossRef]
- Canibe, N.; Højberg, O.; Kongsted, H.; Vodolazska, D.; Lauridsen, C.; Nielsen, T.S.; Schönherz, A.A. Review on Preventive Measures to Reduce Post-Weaning Diarrhoea in Piglets. Animals 2022, 12, 2585. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Pi, Y.; Han, D.; Feng, C.; Zhao, J.; Chen, L.; Che, D.; Bao, H.; Xie, Z.; et al. Maternal galactooligosaccharides supplementation programmed immune defense, microbial colonization and intestinal development in piglets. Food Funct. 2021, 12, 7260–7270. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Wang, M.; Monaco, M.H.; Alexander, L.S.; Mudd, A.T.; Chichlowski, M.; Waworuntu, R.V.; Berg, M.B.; Miller, M.J.; Dilger, R.N.; et al. Prebiotics and Bioactive Milk Fractions Affect Gut Development, Microbiota, and Neurotransmitter Expression in Piglets. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 688–697. [Google Scholar] [CrossRef]
- Yin, J.; Li, F.; Kong, X.; Wen, C.; Guo, Q.; Zhang, L.; Wang, W.; Duan, Y.; Li, T.; Tan, Z.; et al. Dietary Xylo-Oligosaccharide Improves Intestinal Functions in Weaned Piglets. Food Funct. 2019, 10, 2701–2709. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Zhang, Y.; Lv, Y.; Heng, J.; Min, T.; Li, L.; Guan, W. Recent progress of porcine milk components and mammary gland function. J. Anim. Sci. Biotechnol. 2018, 9, 77. [Google Scholar] [CrossRef]
- Baker, J.T.; Duarte, M.E.; Holanda, D.M.; Kim, S.W. Friend or Foe? Impacts of Dietary Xylans, Xylooligosaccharides, and Xylanases on Intestinal Health and Growth Performance of Monogastric Animals. Animals 2021, 11, 609. [Google Scholar] [CrossRef]
- Bauer, E.; Metzler-Zebeli, B.U.; Verstegen, M.W.; Mosenthin, R. Intestinal Gene Expression in Pigs: Effects of Reduced Feed Intake during Weaning and Potential Impact of Dietary Components. Nutr. Res. Rev. 2011, 24, 155–175. [Google Scholar] [CrossRef]
- Mulder, I.E.; Schmidt, B.; Lewis, M.; Delday, M.; Stokes, C.R.; Bailey, M.; Aminov, R.I.; Gill, B.P.; Pluske, J.R.; Mayer, C.D.; et al. Restricting Microbial Exposure in Early Life Negates the Immune Benefits Associated with Gut Colonization in Environments of High Microbial Diversity. PLoS ONE 2011, 6, e28279. [Google Scholar] [CrossRef]
- Merrifield, C.A.; Lewis, M.C.; Berger, B.; Cloarec, O.; Heinzmann, S.S.; Charton, F.; Krause, L.; Levin, N.S.; Duncker, S.; Mercenier, A.; et al. Neonatal environment exerts a sustained influence on the development of the intestinal microbiota and metabolic phenotype. ISME J. 2016, 10, 145–157. [Google Scholar] [CrossRef]
- Gibson, G.R. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J. Nutr. 1999, 129, 1438S–1441S. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; de Souza Vandenberghe, L.P.; Vieira, S.; Goyzueta-Mamani, L.D.; de Mattos, P.B.G.; Manzoki, M.C.; Soccol, V.T.; Soccol, C.R. The Potential of Xylooligosaccharides as Prebiotics and Their Sustainable Production from Agro-Industrial by-products. Foods 2023, 12, 2681. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. S2), S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef]
- Albrecht, S.; Lane, J.A.; Marino, K.; Al Busadah, K.A.; Carrington, S.D.; Hickey, R.M.; Rudd, P.M. A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 2014, 111, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Saito, T.; Nakamura, T.; Messer, M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 2001, 18, 357–371. [Google Scholar] [CrossRef]
- Urashima, T.; Taufik, E.; Fukuda, K.; Asakuma, S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [Google Scholar] [CrossRef]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69 (Suppl. S2), 42–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lane, J.A.; Chen, J.; Zheng, Y.; Wang, H.; Fu, X.; Huang, Q.; Dhital, S.; Liu, F.; Zhang, B. In Vitro Infant Fecal Fermentation Characteristics of Human Milk Oligosaccharides Were Controlled by Initial Microbiota Composition More than Chemical Structure. Mol. Nutr. Food Res. 2022, 66, 2200098. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Oh, J.K.; Vasquez, R.; Kim, S.H.; Hwang, I.-C.; Song, J.H.; Park, J.H.; Kim, I.H.; Kang, D.-K. Multispecies Probiotics Alter Fecal Short-Chain Fatty Acids and Lactate Levels in Weaned Pigs by Modulating Gut Microbiota. J. Anim. Sci. Technol. 2021, 63, 1142–1158. [Google Scholar] [CrossRef]
- Nepelska, M.; Cultrone, A.; Beguet-Crespel, F.; Le Roux, K.; Dore, J.; Arulampalam, V.; Blottiere, H.M. Butyrate produced by commensal bacteria potentiates phorbol esters induced AP-1 response in human intestinal epithelial cells. PLoS ONE 2012, 7, e52869. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacious, G.M.; Morrow, A.L. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef]
- Yang, H.; Fan, X.; Mao, X.; Yu, B.; He, J.; Yan, H.; Wang, J. The protective role of prebiotics and probiotics on diarrhea and gut damage in the rotavirus-infected piglets. J. Anim. Sci. Biotechnol. 2024, 15, 61. [Google Scholar] [CrossRef]
- Holscher, H.D.; Davis, S.R.; Tappenden, K.A. Human milk oligosaccharides influence maturation of human intestinal Caco-2Bbe and HT-29 cell lines. J. Nutr. 2014, 144, 586–591. [Google Scholar] [CrossRef]
- Suligoj, T.; Vigsnaes, L.K.; Abbeele, P.V.D.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef]
- Forder, R.E.; Howarth, G.S.; Tivey, D.R.; Hughes, R.J. Bacterial Modulation of small intestinal goblet cells and mucin composition during early post hatch development of poultry. Poult. Sci. 2007, 86, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; McDonald, K.G.; McCrate, S.; McDole, J.R.; Newberry, R.D. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015, 8, 198–210. [Google Scholar] [CrossRef]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef]
- Bhatia, S.; Nagendra Prabhu, P.; Benefiel, A.C.; Miller, M.J.; Chow, J.; Davis, S.R.; Gaskins, H.R. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol. Nutr. Food. Res. 2015, 59, 566–573. [Google Scholar] [CrossRef]
- Cheng, L.; Kong, C.; Walvoort, M.T.C.; Faas, M.M.; de Vos, P. Human milk oligosaccharides differently modulate goblet cells under homeostatic, proinflammatory conditions and ER Stress. Mol. Nutr. Food Res. 2020, 64, e1900976. [Google Scholar] [CrossRef]
- Zuurveld, M.; van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Stahl, B.; Van’t Land, B.; Willemsen, L.E.M. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Z.A.; Wang, B.; Jahan, M.; Wang, Z.; Wynn, P.C.; Du, Y. Characterization of porcine milk oligosaccharides over lactation between primiparous and multiparous female pigs. Sci. Rep. 2018, 8, 4688. [Google Scholar] [CrossRef]
- Cavalcante, T.; Medeiros, M.M.; Mule, S.N.; Palmisano, G.; Stolf, B.S. The Role of Sialic Acids in the Establishment of Infections by Pathogens, With Special Focus on Leishmania. Front. Cell. Infect. Microbiol. 2021, 11, 671913. [Google Scholar] [CrossRef]
- Maraz, A.M.; Kovacs, Z.; Benjamins, E.; Pazmandi, M. Recent developments in microbial production of high-purity galacto-oligosaccharides. World J. Microbiol. Biotechnol. 2022, 38, 95. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.; Alonso, J.; Domínguez, H.; Parajó, J. Xylooligosaccharides: Manufacture and applications. Trends Food Sci. Technol. 2000, 11, 387–393. [Google Scholar] [CrossRef]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xu, Q.; Yang, K.; He, J.; Chen, D.; Du, Y.; Yin, H. Annotation of porcine milk oligosaccharides throughout lactation by hydrophilic interaction chromatography coupled with quadruple time of flight tandem mass spectrometry. Electrophoresis 2016, 37, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Difilippo, E.; Pan, F.; Logtenberg, M.; Willems, R.; Braber, S.; Fink-Gremmels, J.; Schols, H.A.; Gruppen, H. Milk Oligosaccharide Variation in Sow Milk and Milk Oligosaccharide Fermentation in Piglet Intestine. J. Agric. Food Chem. 2016, 64, 2087–2093. [Google Scholar] [CrossRef]
- Mudd, A.T.; Salcedo, J.; Alexander, L.S.; Johnson, S.K.; Getty, C.M.; Chichlowski, M.; Berg, B.M.; Barile, D.; Dilger, R.N. Porcine Milk Oligosaccharides and Sialic Acid Concentrations Vary Throughout Lactation. Front. Nutr. 2016, 3, 39. [Google Scholar] [CrossRef]
- Salcedo, J.; Frese, S.; Mills, D.; Barile, D. Characterization of porcine milk oligosaccharides during early lactation and their relation to the fecal microbiome. J. Dairy Sci. 2016, 99, 7733–7743. [Google Scholar] [CrossRef]
- Intanon, M.; Arreola, S.L.; Pham, N.H.; Kneifel, W.; Haltrich, D.; Nguyen, T.H. Nature and biosynthesis of galacto-oligosaccharides related to oligosaccharides in human breast milk. FEMS Microbiol. Lett. 2014, 353, 89–97. [Google Scholar] [CrossRef]
- Selvendran, R.R. Developments in the chemistry and biochemistry of pectic and hemicellulosic polymers. J. Cell Sci. 1985, 2, 51–88. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G.J.; Safety, F. Xylooligosaccharides (XOS) as an emerging prebiotic: Microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, X.; Hua, X.; Yong, Q.; Liu, D.; Xu, Y. Advances and prospection in preparations, bio-actives and applications of functional xylo-oligosaccharide. Biocatal. Agric. Biotechnol. 2024, 60, 103297. [Google Scholar] [CrossRef]
- de Freitas, C.; Carmona, E.; Brienzo, M. Xylooligosaccharides Production Process from Lignocellulosic Biomass and Bioactive Effects. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100184. [Google Scholar] [CrossRef]
- European Food Safety Authority. Safety of the extension of use of galacto-oligosaccharides as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06844. [Google Scholar] [CrossRef]
- European Food Safety Authority. Safety of xylo-oligosaccharides (XOS) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2018, 16, e05361. [Google Scholar] [CrossRef]
- Boston, T.E.; Wang, F.; Lin, X.; Kim, S.W.; Fellner, V.; Scott, M.F.; Ziegler, A.L.; Van Landeghem, L.; Blikslager, A.T.; Odle, J. Prebiotic galactooligosaccharide improves piglet growth performance and intestinal health associated with alterations of the hindgut microbiota during the peri-weaning period. J. Anim. Sci. Biotechnol. 2024, 15, 88. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Effects of galacto-oligosaccharides on growth and gut function of newborn suckling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 75. [Google Scholar] [CrossRef]
- Xing, Y.-Y.; Li, K.-N.; Xu, Y.-Q.; Wu, Y.-Z.; Shi, L.-L.; Guo, S.-W.; Yan, S.-M.; Jin, X.; Shi, B.-L. Effects of galacto-oligosaccharide on growth performance, feacal microbiota, immune response and antioxidant capability in weaned piglets. J. Appl. Anim. Res. 2020, 48, 63–69. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Wang, J.; Zhu, W. Differential Effects of Early-Life and Postweaning Galacto-oligosaccharide Intervention on Colonic Bacterial Composition and Function in Weaning Piglets. Appl. Environ. Microbiol. 2022, 88, e0131821. [Google Scholar] [CrossRef]
- Lee, A.; Mansbridge, S.C.; Liang, L.; Connerton, I.F.; Mellits, K.H. Galacto-Oligosaccharides Increase the Abundance of Beneficial Probiotic Bacteria and Improve Gut Architecture and Goblet Cell Expression in Poorly Performing Piglets, but Not Performance. Animals 2023, 13, 230. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Zhong, R.; Han, H.; Liu, L.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Effects of Graded Levels of Xylo-Oligosaccharides on Growth Performance, Serum Parameters, Intestinal Morphology, and Intestinal Barrier Function in Weaned Piglets. J. Anim. Sci. 2021, 99, skab183. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Cao, S.C.; Liu, J.; Xie, Y.N.; Zhang, H.F. Effect of Probiotics and Xylo-Oligosaccharide Supplementation on Nutrient Digestibility, Intestinal Health and Noxious Gas Emission in Weanling Pigs. Asian Austr. J. Anim. Sci. 2018, 31, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhou, X.; Ye, H.; Wu, Y.; Wang, Z.; Lu, D.; Wang, J.; Han, D. The High Level of Xylooligosaccharides Improves Growth Performance in Weaned Piglets by Increasing Antioxidant Activity, Enhancing Immune Function, and Modulating Gut Microbiota. Front. Nutr. 2021, 8, 764556. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Wu, D.; Dai, Q. Effects of dietary xylo-oligosaccharide on growth performance, serum biochemical parameters, antioxidant function, and immunological function of nursery piglets. Rev. Bras. Zootec. 2020, 49, e20190170. [Google Scholar] [CrossRef]
- Vaillancourt, J.P.; Stein, T.E.; Marsh, W.E.; Leman, A.D.; Dial, G.D. Validation of producer-recorded causes of preweaning mortality in swine. Prev. Vet. Med. 1990, 10, 119–130. [Google Scholar] [CrossRef]
- Svensmark, B.; Jorsal, S.E.; Nielsen, K.; Willeberg, P. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. I. Pre-weaning diarrhoea. Acta Vet. Scand. 1989, 30, 43–53. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Stokes, C.R. The development and role of microbial-host interactions in gut mucosal immune development. J. Anim. Sci. Biotechnol. 2017, 8, 12. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Moxley, R.A.; Duhamel, G.E. Comparative pathology of bacterial enteric diseases of swine. In Mechanisms in the Pathogenesis of Enteric Diseases 2; Paul, P.S., Francis, D.H., Eds.; Springer: Boston, MA, USA, 1999; Volume 473, pp. 83–101. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porc. Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É. Colibacillosis. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley & Son: Hoboken, NJ, USA, 2019; pp. 807–834. [Google Scholar] [CrossRef]
- Yan, Y.L.; Gänzle, M.G. Structure and function relationships of the binding of β- and α-galactosylated oligosaccharides to K88 fimbriae of enterotoxigenic Escherichia coli. Int. Dairy J. 2018, 81, 104–112. [Google Scholar] [CrossRef]
- Tzortzis, G.; Goulas, A.K.; Gee, J.M.; Gibson, G.R. A Novel Galactooligosaccharide Mixture Increases the Bifidobacterial Population Numbers in a Continuous In Vitro Fermentation System and in the Proximal Colonic Contents of Pigs In Vivo. J. Nutr. 2005, 135, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef]
- Gormley, A.; Garavito-Duarte, Y.; Kim, S.W. The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review. Biology 2024, 13, 663. [Google Scholar] [CrossRef]
- Asadpoor, M.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Differential effects of oligosaccharides on the effectiveness of ampicillin against Escherichia coli in vitro. Pharma Nutr. 2021, 16, 100264. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef]
- Fitzgerald, G.R.; Barker, T.; Welter, M.W.; Welter, C.J. Diarrhea in young pigs: Comparing the incidence of the five most common infectious agents. Vet. Med. 1988, 83, 80–86. [Google Scholar]
- Estes, M.K.; Kapikian, A. Rotaviruses. In Fields Virology; Knipe, D., Griffin, D., Lamb, R., Martin, M., Roizman, B., Straus, S., Eds.; Wolters Kluwer Health; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 1917–1975. [Google Scholar]
- Svensmark, B.; Nielsen, K.; Dalsgaard, K.; Willeberg, P. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. III. Rotavirus infection. Acta Vet. Scand. 1989, 30, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk oligosaccharides inhibit human rotavirus infectivity in MA104 cells. J. Nutr. 2017, 147, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C., Jr.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Rigo-Adrover, M.d.M.; Herrero, L.; Franch, À.; Castell, M.; Vulevic, J.; Pérez-Cano, F.J.; Lagunas, M.J.R. A Galactooligosaccharide Product Decreases the Rotavirus Infection in Suckling Rats. Cells 2022, 11, 1669. [Google Scholar] [CrossRef]
- Kanuganti, S.R.; Wesley, I.V.; Reddy, P.G.; Mckean, J.; Hurd, H.S. Detection of Listeria monocytogenes in Pigs and Pork. J. Food Prot. 2002, 65, 1470–1474. [Google Scholar] [CrossRef]
- Ebersbach, T.; Jorgensen, J.B.; Heegaard, P.M.; Lahtinen, S.J.; Ouwehand, A.C.; Poulsen, M.; Frokiaer, H.; Licht, T.R. Certain dietary carbohydrates promote Listeria infection in a guinea pig model, while others prevent it. Int. J. Food Microbiol. 2010, 140, 218–224. [Google Scholar] [CrossRef]
- Ebersbach, T.; Bo, J.; Bergstro, A.; Hutkins, R.W.; Rask, T. Xylo-oligosaccharides inhibit pathogen adhesion to enterocytes in vitro. Res. Microbiol. 2012, 163, 22–27. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, L.; Zhang, C.; Cao, Q.; Ye, H.; Dong, Z.; Feng, D.; Zuo, J.; Wang, W. Research Note: Xylooligosaccharide directly attenuates Salmonella Typhimurium colonization and its induction of impairments in intestinal barrier and growth performance of broilers. Poult. Sci. 2024, 103, 103184. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Cardarelli, H.R.; Borst, W.; Albrecht, S.; Schols, H.; Gutiérrez, O.P.; Maathuis, A.J.H.; de Melo Franco, B.D.G.; De Martinis, E.C.P.; Zoetendal, E.G.; et al. Effect of galactooligosaccharides and Bifidobacterium animalis Bb-12 on growth of Lactobacillus amylovorus DSM 16698, microbial community structure, and metabolite production in an in vitro colonic model set up with human or pig microbiota. FEMS Microbiol. Ecol. 2013, 84, 110–123. [Google Scholar] [CrossRef]
- Difilippo, E.; Pan, F.; Logtenberg, M.; Willems, R.; Braber, S.; Fink-Gremmels, J.; Schols, H.A.; Gruppen, H. In vitro fermentation of porcine milk oligosaccharides and galactooligosaccharides Using Piglet Fecal Inoculum. J. Agric. Food. Chem. 2016, 63, 10862–10872. [Google Scholar] [CrossRef] [PubMed]
- Tanner, S.A.; Chassard, C.; Zihler Berner, A.; Lacroix, C. Synergistic effects of Bifidobacterium thermophilum RBL67 and selected prebiotics on inhibition of Salmonella colonization in the swine proximal colon PolyFermS model. Gut Pathog. 2014, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Luehrs, H.; Menzel, T. Beneficial health effects of low-digestible carbohydrate consumption. Br. J. Nutr. 2001, 85 (Suppl. S1), S23–S30. [Google Scholar] [CrossRef]
- Macfarlane, G.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Richards, P.J.; Almutrafy, A.; Liang, L.; Flaujac Lafontaine, G.M.; King, E.; Fish, N.M.; Connerton, A.J.; Connerton, P.L.; Connerton, I.F. Prebiotic Galactooligosaccharide Feed Modifies the Chicken Gut Microbiota to Efficiently Clear Salmonella. mSystems 2024, 9, e0075424. [Google Scholar] [CrossRef]
- Kabel, M.A.; Kortenoeven, L.; Schols, H.A.; Voragen, A.G.J. In vitro fermentability of differently substituted xylo-oligosaccharides. J. Agric. Food Chem. 2002, 50, 6205–6210. [Google Scholar] [CrossRef]
- Moura, P.; Cabanas, S.; Lourenço, P.; Gírio, F.; Loureiro-Dias, M.C.; Esteves, M.P. In vitro fermentation of selected xylo-oligosaccharides by piglet intestinal microbiota. LWT-Food Sci. Technol. 2008, 41, 1952–1961. [Google Scholar] [CrossRef]
- Mäkeläinen, H.; Forssten, S.; Saarinen, M.; Stowell, J.; Rautonen, N.; Ouwehand, A.C. Xylo-oligosaccharides enhance the growth of bifidobacterial and Bifidobacterium lactis in a simulated colon model. Benef. Microbes 2010, 1, 81–91. [Google Scholar] [CrossRef]
- Smiricky-Tjardes, M.R.; Flickinger, E.A.; Grieshop, C.M.; Bauer, L.L.; Murphy, M.R.; Fahey, G.C. In vitro fermentation characteristics of selected oligosaccharides by swine fecal microflora1. J. Anim. Sci. 2003, 81, 2505–2514. [Google Scholar] [CrossRef]
- Martin-Pelaez, S.; Gibson, G.R.; Martin-Orue, S.M.; Klinder, A.; Rastall, R.A.; La Ragione, R.M.; Woodward, M.J.; Costabile, A. In vitro fermentation of carbohydrates by porcine faecal inocula and their influence on Salmonella Typhimurium growth in batch culture systems. FEMS Microbiol. Ecol. 2008, 66, 608–619. [Google Scholar] [CrossRef]
- Difilippo, E.; Bettonvil, M.; Willems, R.H.; Braber, S.; Fink-Gremmels, J.; Jeurink, P.V.; Schoterman, M.H.C.; Gruppen, H.; Schols, H.A. Oligosaccharides in urine, blood, and feces of piglets fed milk replacer containing galacto-oligosaccharides. J. Agric. Food Chem. 2015, 64, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Changes in Ileal Microbial Composition and Microbial Metabolism by an Early-Life Galacto-Oligosaccharides Intervention in a Neonatal Porcine Model. Nutrients 2019, 11, 1753. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Tian, S.; Wang, J.; Zhu, W. Galacto-oligosaccharides improve barrier function and relieve colonic inflammation via modulating mucosa-associated microbiota composition in lipopolysaccharides-challenged piglets. J. Anim. Sci. Biotechnol. 2021, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Bouwhuis, M.A.; McDonnell, M.J.; Sweeney, T.; Mukhopadhya, A.; O’Shea, C.J.; O’Doherty, J.V. Seaweed extracts and galacto-oligosaccharides improve intestinal health in pigs following Salmonella Typhimurium challenge. Animal 2017, 11, 1488–1496. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Yu, H.; Wang, J.; Zhu, W. Response of Colonic Mucosa-Associated Microbiota Composition, Mucosal Immune Homeostasis, and Barrier Function to Early Life Galactooligosaccharides Intervention in Suckling Piglets. J. Agric. Food Chem. 2019, 67, 578–588. [Google Scholar] [CrossRef]
- Eudy, B.J.; Odle, J.; Lin, X.; Maltecca, C.; Walter, K.R.; McNulty, N.P.; Fellner, V.; Jacobi, S.K. Dietary prebiotic oligosaccharides and arachidonate alter the fecal microbiota and mucosal lipid composition of suckling pigs. J. Nutr. 2023, 153, 2249–2262. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van‘t Land, B.; Tims, S.; Stahl, B.; Knol, J.; Garssen, J.; Franch, À.; Castell, M.; et al. Oligosaccharides Modulate Rotavirus-Associated Dysbiosis and TLR Gene Expression in Neonatal Rats. Cells 2019, 8, 876. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Boehm, G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 2007, 137, 2420–2424. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef]
- Pandey, S.; Kim, E.S.; Cho, J.H.; Song, M.; Doo, H.; Kim, S.; Keum, G.B.; Kwak, J.; Ryu, S.; Choi, Y.; et al. Swine Gut Microbiome Associated with Non-Digestible Carbohydrate Utilization. Front. Vet. Sci. 2023, 10, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Varel, V.H.; Robinson, I.M.; Jung, H. Influence of dietary fiber on xylanolytic and cellulolytic bacteria of adult pigs. Appl. Environ. Microbiol. 1987, 53, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Swanson, K.S.; Fahey, G.C., Jr.; Dien, B.S.; Beloshapka, A.N.; Bauer, L.L.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. In vitro Fermentation of Xylooligosaccharides Produced from Miscanthus x giganteus by Human Fecal Microbiota. J. Agric. Food Chem. 2016, 64, 262–267. [Google Scholar] [CrossRef]
- Sun, F.; Li, H.; Sun, Z.; Liu, L.; Zhang, X.; Zhao, J. Effect of Arabinoxylan and Xylo-Oligosaccharide on Growth Performance and Intestinal Barrier Function in Weaned Piglets. Animals 2023, 13, 964. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Zhong, R.; Liu, L.; Lin, C.; Xiao, L.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front. Microbiol. 2021, 12, 355. [Google Scholar] [CrossRef]
- González-Solé, F.; Solà-Oriol, D.; Ramayo-Caldas, Y.; Rodriguez-Prado, M.; Ortiz, G.G.; Bedford, M.R.; Pérez, J.F. Supplementation of xylo-oligosaccharides to suckling piglets promotes the growth of fiber-degrading gut bacterial populations during the lactation and nursery periods. Sci. Rep. 2022, 12, 11594. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, K.; Yu, C.; Wang, L.; Liang, T.; Zhu, H.; Xu, X.; Liu, Y. Xylooligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Injury in Piglets via Suppressing Inflammation and Modulating Cecal Microbial Communities. Anim. Nutr. 2021, 7, 609–620. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Guo, L.; Ma, X.; Yang, Y.; Zhuo, Y.; Jiang, X.; Hua, L.; Che, L.; Xu, S.; et al. Xylo-oligosaccharides improve the adverse effects of plant-based proteins on weaned piglet health by maintaining the intestinal barrier and inhibiting harmful bacterial growth. Front. Microbiol. 2023, 14, 1189434. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Hautefort, I.; Thompson, A.; Hinton, J.C.; Van Immerseel, F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 2006, 72, 946–949. [Google Scholar] [CrossRef]
- Gao, H.; Sun, F.; Lin, G.; Guo, Y.; Zhao, J. Molecular actions of different functional oligosaccharides on intestinal integrity, immune function and microbial community in weanling pigs. Food Funct. 2022, 13, 12303–12315. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, X.; Azad, M.A.K.; Ma, C.; Gao, Q.; He, J.; Kong, X. Dietary Supplementation with Bacillus Subtilis and Xylo-Oligosaccharides Improves Growth Performance and Intestinal Morphology and Alters Intestinal Microbiota and Metabolites in Weaned Piglets. Food Funct. 2021, 12, 5837–5849. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Chen, Y.; Deng, F.; Yan, X.; Zhong, R.; Meng, Q.; Liu, L.; Zhao, Y.; Zhang, S.; Chen, L.; et al. Xylooligosaccharide-Mediated Gut Microbiota Enhances Gut Barrier and Modulates Gut Immunity Associated with Alterations of Biological Pro-cesses in a Pig Model. Carbohydr. Polym. 2022, 294, 119776. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yin, J.; Zhang, K.; Xie, P.; Ding, H.; Huang, X.; Blachier, F.; Kong, X. Dietary Xylo-Oligosaccharide Supplementation Alters Gut Microbial Composition and Activity in Pigs According to Age and Dose. AMB Express 2019, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, W.; Ma, C.; Xie, P.; Blachier, F.; Kong, X. Dietary supplementation with xylo-oligosaccharides modifies the intestinal epithelial morphology, barrier function and the fecal microbiota composition and activity in weaned piglets. Front. Vet. Sci. 2021, 8, 680208. [Google Scholar] [CrossRef]
- Sutton, T.A.; Masey O’Neill, H.V.; Bedford, M.R.; McDermott, K.; Miller, H.M. Effect of xylanase and xylo-oligosaccharide supplementation on growth performance and faecal bacterial community composition in growing pigs. Anim. Feed Sci. Technol. 2021, 274, 114822. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Zhou, X.; Zhang, Y.; Ye, H.; Wang, H.; Pi, Y.; Lian, S.; Hana, D.; Wang, J. Ingestion of xylooligosaccharides during the suckling period improve the feed efficiency and hindgut fermentation capacity of piglets after weaning. Food Funct. 2021, 12, 10459–10469. [Google Scholar] [CrossRef]
- Jeurissen, S.H.; Lewis, F.; van der Klis, J.D.; Mroz, Z.; Rebel, J.M.; Ter Huurne, A.A. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr. Issues Intest. Microbiol. 2002, 3, 1–14. [Google Scholar]
- Buddington, R.K.; Sangild, P.T. Companion animals symposium: Development of the mammalian gastrointestinal tract, the resident microbiota, and the role of diet in early life. J. Anim. Sci. 2011, 89, 1506–1519. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Gao, R.; Wang, J.; Zhu, W. Early-life galacto-oligosaccharides supplementation alleviates the small intestinal oxidative stress and dysfunction of lipopolysaccharide-challenged suckling piglets. J. Anim. Sci. Biotechnol. 2021, 13, 70. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, T.; Kim, Y.; Lee, S.; Kim, S.; Kang, S.; Yang, J.-Y.; Baek, I.-J.; Sung, Y.H.; Park, Y.-Y.; et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef]
- Goulet, O.; Ruemmele, F.; Lacaille, F.; Colomb, V. Irreversible intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, C.; Wang, Q.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Yang, H.; Yin, Y. The relationship between villous height and growth performance, small intestinal mucosal enzymes activities and nutrient transporters expression in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 606–615. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Zweimueller-Mayer, J.; Steinbacher, P.; Lametschwandtner, A.; Bauer, H.C. The dual role of zonula occludens (ZO) proteins. J. Biomed. Biotechnol. 2010, 11, 402593. [Google Scholar] [CrossRef]
- Bruewer, M.; Nusrat, A. Regulation of Paracellular Transport across Tight Junctions by the Actin Cytoskeleton. In Tight Junctions; Gonzalez-Mariscal, L., Ed.; Springer: Boston, MA, USA, 2006; pp. 135–145. ISBN 978-0-387-36673-9. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.; Schoterman, M.H.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Jin, Y.; Xiao, Z.; Yaqoob, M.U.; Lin, Y.; Chen, H.; Wang, M. Galactooligosaccharides as a protective agent for intestinal barrier and its regulatory functions for intestinal microbiota. Food Res. Int. 2022, 155, 111003. [Google Scholar] [CrossRef]
- Blyth, G.A.D.; Connors, L.; Fodor, C.; Cobo, E.R. The network of colonic host defense peptides as an innate immune defense against enteropathogenic bacteria. Front. Immunol. 2020, 11, 965. [Google Scholar] [CrossRef]
- Figueroa-Lozano, S.; Ren, C.; Yin, H.; Pham, H.; van Leeuwen, S.; Dijkhuizen, L.; de Vos, P. The impact of oligosaccharide content, glycosidic linkages and lactose content of galacto-oligosaccharides (GOS) on the expression of mucus-related genes in goblet cells. Food Funct. 2020, 11, 3506–3515. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.F. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of geno-type MUC4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor negative. Appl. Environ. Microbiol. 2017, 83, e02747-16. [Google Scholar] [CrossRef]
- O’Neil, L.A.J. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol. Revs. 2008, 226, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Liu, X.; Zhao, Z.; Tao, S.; Xu, Q.; Zhao, J.; Dai, Z.; Zhang, G.; Han, D.; et al. Galactooligosaccharides and Limosilactobacillus reuteri synergistically alleviate gut inflammation and barrier dysfunction by enriching Bacteroides acidifaciens for pentadecanoic acid biosynthesis. Nat. Commun. 2024, 15, 9291. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.J.; Bouwhuis, M.A.; Sweeney, T.; Mukhopadhya, A.; O’Shea, C.J.; O’Doherty, J.V. Effects of dietary supplementation of galactooligosaccharides and seaweed-derived polysaccharides on an experimental Salmonella Typhimurium challenge in pigs. J. Anim. Sci. 2016, 94, 153–156. [Google Scholar] [CrossRef]
- Richards, P.J.; Lafontaine, G.M.F.; Connerton, P.L.; Liang, L.; Asiani, K.; Fish, N.M.; Connerton, I.F. Galacto-oligosaccharides modulate the juvenile gut microbiome and innate immunity to improve broiler chicken performance. mSystems 2020, 5, e00827-19. [Google Scholar] [CrossRef]
- Verheijden, K.A.T.; Akbari, P.; Willemsen, L.E.M.; Kraneveld, A.D.; Folkerts, G.; Garssen, J.; Fink-Gremmels, J.; Braber, S. Inflammation-induced expression of the alarmin interleukin 33 can be suppressed by galacto-oligosaccharides. Int. Arch. Allergy Immunol. 2015, 167, 127–136. [Google Scholar] [CrossRef]
- Dai, Z.; Feng, S.; Liu, A.; Wang, H.; Zeng, X.; Yang, C.S. Anti-inflammatory effects of newly synthesized α-galacto-oligosaccharides on dextran sulfate sodium-induced colitis in C57BL/6J mice. Food Res. Int. 2018, 109, 350–357. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Aljamaei, H.M.; Stadnyk, A.W. The Production and Function of Endogenous Interleukin-10 in Intestinal Epithelial Cells and Gut Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1343–1352. [Google Scholar] [CrossRef]
- Dignass, A.U. Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 2001, 7, 68–77. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Gao, R.; Wang, J.; Zhu, W. Galacto-oligosaccharides directly attenuate lipopolysaccharides-induced inflammatory response, oxidative stress and barrier impairment in intestinal epithelium. J. Funct. Foods. 2022, 91, 105006. [Google Scholar] [CrossRef]
- Lee, A.; Liang, L.; Connerton, P.L.; Connerton, I.F.; Mellits, K.H. Galacto-oligosaccharides fed during gestation increase Rotavirus A specific antibodies in sow colostrum, modulate the microbiome, and reduce infectivity in neonatal piglets in a commercial farm setting. Front. Vet. Sci. 2023, 10, 1118302. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Fernández, L.; Verhasselt, V. The Gut?Breast Axis: Programming Health for Life. Nutrients 2021, 13, 606. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; van‘t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation with 2’-FL and scGOS/lcFOS ameliorates rotavirus-induced diarrhea in suckling rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Greiner, L.L.; Humphrey, D.C.; Holland, S.N.; Anderson, C.J.; Schmitz-Esser, S. The validation of the existence of the entero-mammary pathway and the assessment of the differences of the pathway between first and third parity sows. Transl. Anim. Sci. 2022, 6, txac047. [Google Scholar] [CrossRef]
- Kiernan, D.P.; O’Doherty, J.V.; Sweeney, T. The Effect of Maternal Probiotic or Synbiotic Supplementation on Sow and Offspring Gastrointestinal Microbiota, Health, and Performance. Animals 2023, 13, 2996. [Google Scholar] [CrossRef]
- Macpherson, A.; de Agüero, M.; Ganal-Vonarburg, S. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Amir, M.; Ananthanarayanan, A.; Singaraju, A.; Shiland, N.B.; Hong, H.S.; Kamada, N.; Inohara, N.; Núñez, G.; Zeng, M.Y. Maternal gut microbiome–induced IgG regulates neonatal gut microbiome and immunity. Sci. Immunol. 2022, 7, eabh3816. Available online: https://www.science.org/doi/10.1126/sciimmunol.abh3816 (accessed on 28 February 2025). [CrossRef]
- Usami, K.; Niimi, K.; Matsuo, A.; Suyama, Y.; Sakai, Y.; Sato, S.; Fujihashi, K.; Kiyono, H.; Uchino, S.; Furukawa, M.; et al. The gut microbiota induces Peyer′s-patch-dependent secretion of maternal IgA into milk. Cell Rep. 2021, 36, 109655. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H. Mammary gland immunology and neonate protection in pigs. In Biology of the Mammary Gland: Homing of Lymphocytes into the MG; Advances in Experimental Medicine and Biology; Mol, J.A., Clegg, R.A., Eds.; Springer: Boston, MA, USA, 2002; Volume 480. [Google Scholar] [CrossRef]
- Cortez, V.; Schultz-Cherry, S. The role of goblet cells in viral pathogenesis. FEBS J. 2021, 288, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Xie, C.Y.; Wu, Y. Effects of dietary supplement with xylo-oligosaccharide and active yeast during lactation of sows on reproduction performance and serum biochemical indices of suckling piglets. Chin. J. Anim. Nutr. 2015, 27, 838–844. Available online: https://en.cnki.com.cn/Article_en/CJFDTOTAL-DWYX201503054.htm (accessed on 28 February 2025).
| Growth Stage | BW, kg | Age, Days | Days Fed GOS | GOS Purity, % | In-Feed GOS, % | Effect of GOS, % Difference to Controls † | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| BW | ADG | G:F | |||||||
| Post-wean | 8.8 | 26 | 28 | 95 | 0.8 | - | - | - | Alizadeh et al. [67] |
| Pre-wean | 6.1 | 21 | 21 | 90 | 0.1 | 5.1 | 16.6 | Tian et al. [68] | |
| Post-wean | 18.4 | 56 | 28 | - | 0.1 | 4.5 | 8.8 | 0.33 | Xing et al. [69] |
| Pre-wean | 5.9 | 28 | 7 | 90 | 0.001 | - | - | - | Tian et al. [70] |
| Post-wean | 6.3 | 28 | 7 + 7 | 90 | 2.0 | - | - | - | |
| Pre-wean * | 4.0 | 21 | 21 | 90 | 1.0 | - | - | - | Lee et al. [71] |
| Pre-wean | 7.9 | 31 | 16 | 38 | 5 | - | - | - | Boston et al. [66] |
| Post-wean | 6.3 | 31 | 16 + 8 | 38 | 3.8 | - | 6.1 | 13.1 | |
| Growth Stage | BW, kg | Age, Days | Days Fed XOS | XOS Purity, % | In-Feed XOS, % | Effect of XOS, % Difference to Controls † | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| BW | ADG | G:F | |||||||
| Weaning | 8.8 | 28 | 28 | 95 | 0.01 | - | - | - | Chen, et al. [72] |
| 0.05 | 4.7 | 9.6 | 7.1 | ||||||
| 0.1 | - | - | - | ||||||
| Weaning | 6.3 | 21 | 28 | 50 | 0.02 | - | 16.6 | 14.3 | Liu et al. [73] |
| Weaning | 7.5 | 30 | 28 | 70 | 0.75 | - | 5.9 | - | Pang et al. [74] |
| 1.5 | 8.6 | 15.5 | - | ||||||
| 3 | 5.3 | 9.9 | - | ||||||
| Nursing | 19.94 | ND | 28 | >35 | 0.04 | 10.6 | 33.1 | Hou et al. [75] | |
| Growth Stage | In-Feed GOS, % | Bacterial Source | Effect of GOS on 16S rRNA Community Taxa † | Reference |
|---|---|---|---|---|
| Post-wean | 0.8 | Faecal | Bifidobacterium ↑, Lactobacillus ↑ | Alizadeh et al. [67] |
| Pre-wean | age variable | Colon | Prevotella ↑, Barnesiella ↑, Parabacteroides ↑, Porphyromonada ↑, Dorea ↓ | Wang et al. [119] |
| Pre-wean | 0.001 | Colon | Ruminococcaceae UCG-014 ↑, Faecalibacterium ↑ | Tian et al. [70] |
| Clostridium sensus strico 1 ↑, Terrisporobacter ↑ | ||||
| Post-wean | 2 | Dorea ↑ Phascolarctobacterium ↑ | ||
| Pre-wean * | 1.0 | Caecum | Lactobacillus ↑, Bifidobacterium ↑, Leuconostoc ↑, Streptococcus ↓ | Lee et al. [71] |
| Pre-wean | 0.4 | Faecal | Anaerostipes ↑, Mitsuokella ↑, Prevotella ↑, Clostridium IV ↑, Bulleidia ↑, Bilophila ↓, Clostridium XIVb ↓, Enterococcus ↓ | Eudy et al. [120] |
| Pre-wean | 5 | Caecum | No significant effect | Boston et al. [66] |
| Fusicatenibacter ↑, Collinsella ↑, Agathobacter ↓, | ||||
| Post-wean | 3.8 | Ruminococcaceae ↑, Frisingicoccus ↓, Campylobacter ↓ |
| Growth Stage | GOS Purity, % (w/w) | In-Feed GOS, % | Effect of GOS, % Difference to Control † | Reference | |||
|---|---|---|---|---|---|---|---|
| Jejunum | Ileum | ||||||
| VH | VCR | VH | VCR | ||||
| Post-wean | 59 | 0.8 | 21 | 50 | 16.3 | - | Alizadeh et al. [67] Tian et al. [68] |
| Weaning | 90 | 0.1 | - | - | |||
| Pre-weang * | 90 | 1.0 | 16–64 | 14–76 | 10–13 | 10–22 | Lee et al. [71] |
| Pre-wean | 38 | 5 | - | - | Boston et al. [66] | ||
| Post-wean | 3.8 | - | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Stanley, J.S.; Mellits, K.H.; Connerton, I.F. Prebiotic Galacto-Oligosaccharide and Xylo-Oligosaccharide Feeds in Pig Production: Microbiota Manipulation, Pathogen Suppression, Gut Architecture and Immunomodulatory Effects. Appl. Microbiol. 2025, 5, 42. https://doi.org/10.3390/applmicrobiol5020042
Lee A, Stanley JS, Mellits KH, Connerton IF. Prebiotic Galacto-Oligosaccharide and Xylo-Oligosaccharide Feeds in Pig Production: Microbiota Manipulation, Pathogen Suppression, Gut Architecture and Immunomodulatory Effects. Applied Microbiology. 2025; 5(2):42. https://doi.org/10.3390/applmicrobiol5020042
Chicago/Turabian StyleLee, Adam, James S. Stanley, Kenneth H. Mellits, and Ian F. Connerton. 2025. "Prebiotic Galacto-Oligosaccharide and Xylo-Oligosaccharide Feeds in Pig Production: Microbiota Manipulation, Pathogen Suppression, Gut Architecture and Immunomodulatory Effects" Applied Microbiology 5, no. 2: 42. https://doi.org/10.3390/applmicrobiol5020042
APA StyleLee, A., Stanley, J. S., Mellits, K. H., & Connerton, I. F. (2025). Prebiotic Galacto-Oligosaccharide and Xylo-Oligosaccharide Feeds in Pig Production: Microbiota Manipulation, Pathogen Suppression, Gut Architecture and Immunomodulatory Effects. Applied Microbiology, 5(2), 42. https://doi.org/10.3390/applmicrobiol5020042








