Gallium Resistance in Staphylococcus aureus: Polymorphisms and Morphology Impacting Growth in Metals, Antibiotics and Polyfluorinated Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Growth Conditions
2.3. Minimum Inhibitory Concentration (MIC) of Gallium Nitrate
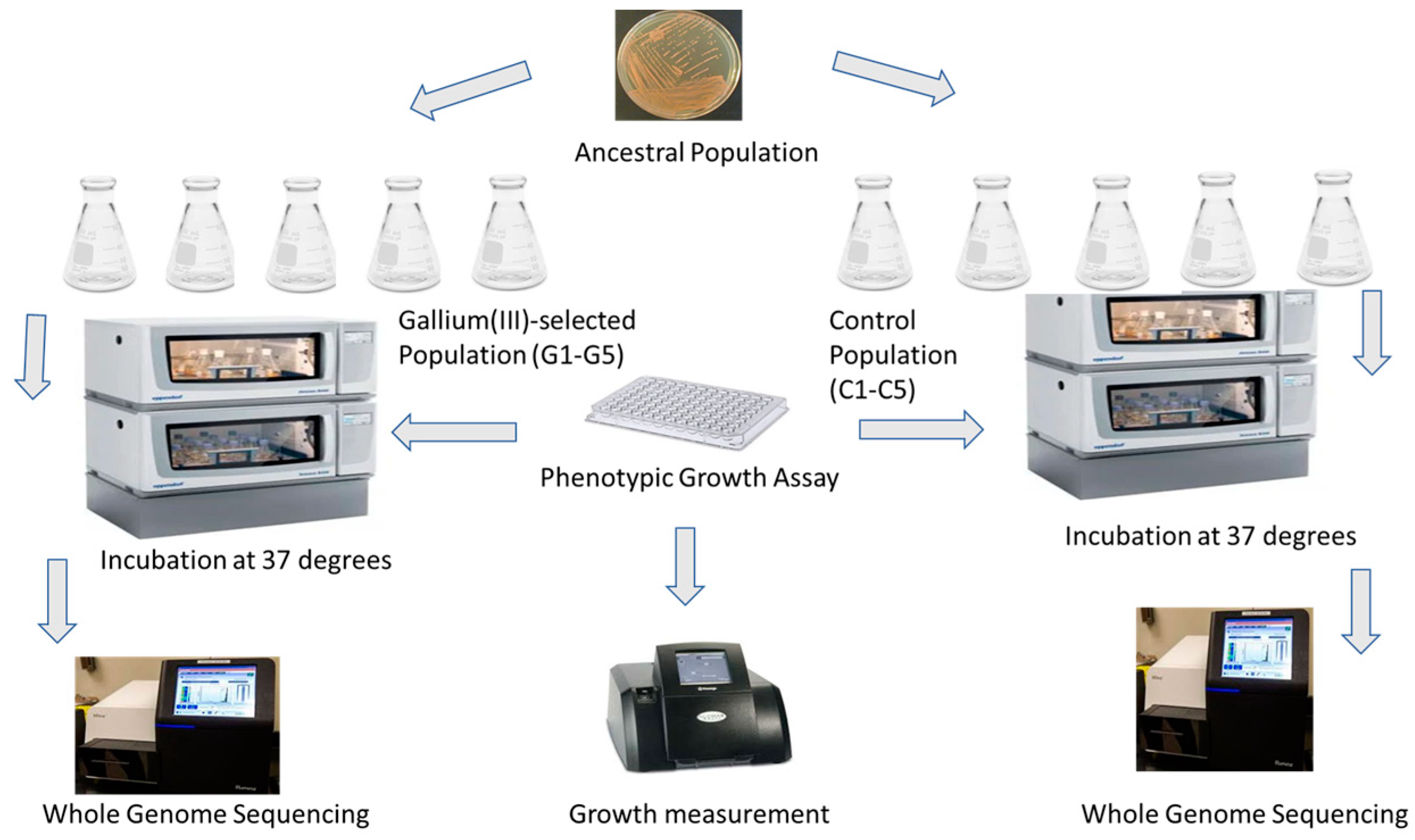
2.4. Experimental Evolution
2.5. Growth Assays: 24 h Growth
2.6. Scanning Electron Microscopy (SEM) Sample Processing
2.7. Whole-Genome Sequencing
2.8. Statistical Analysis
3. Results
3.1. S. aureus Resistance in Gallium (III) Nitrate
3.2. Phenotypic Changes in the Presence of Heavy Metals
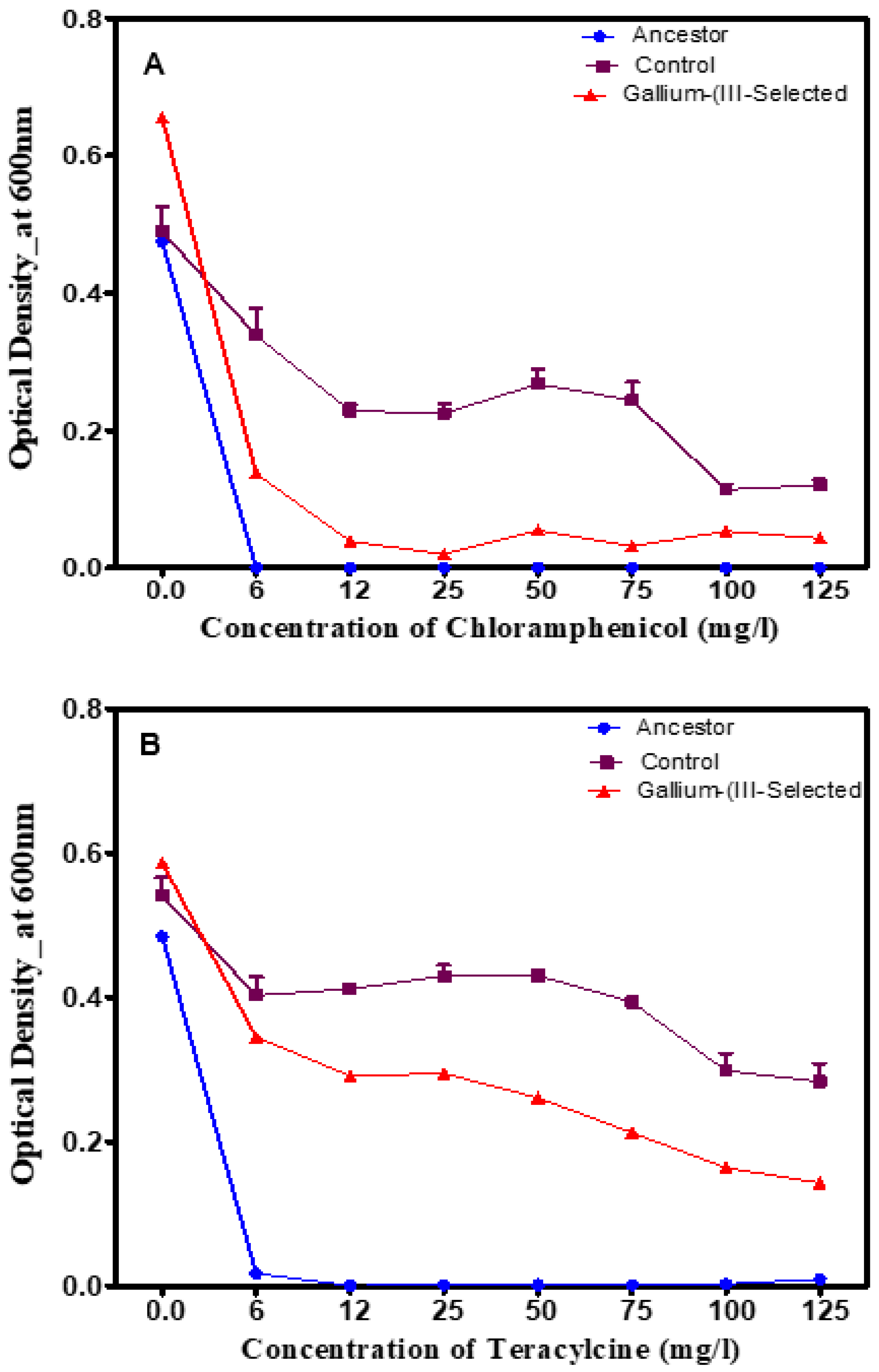
3.3. Phenotypic Changes in the Presence of Traditional Antibiotics
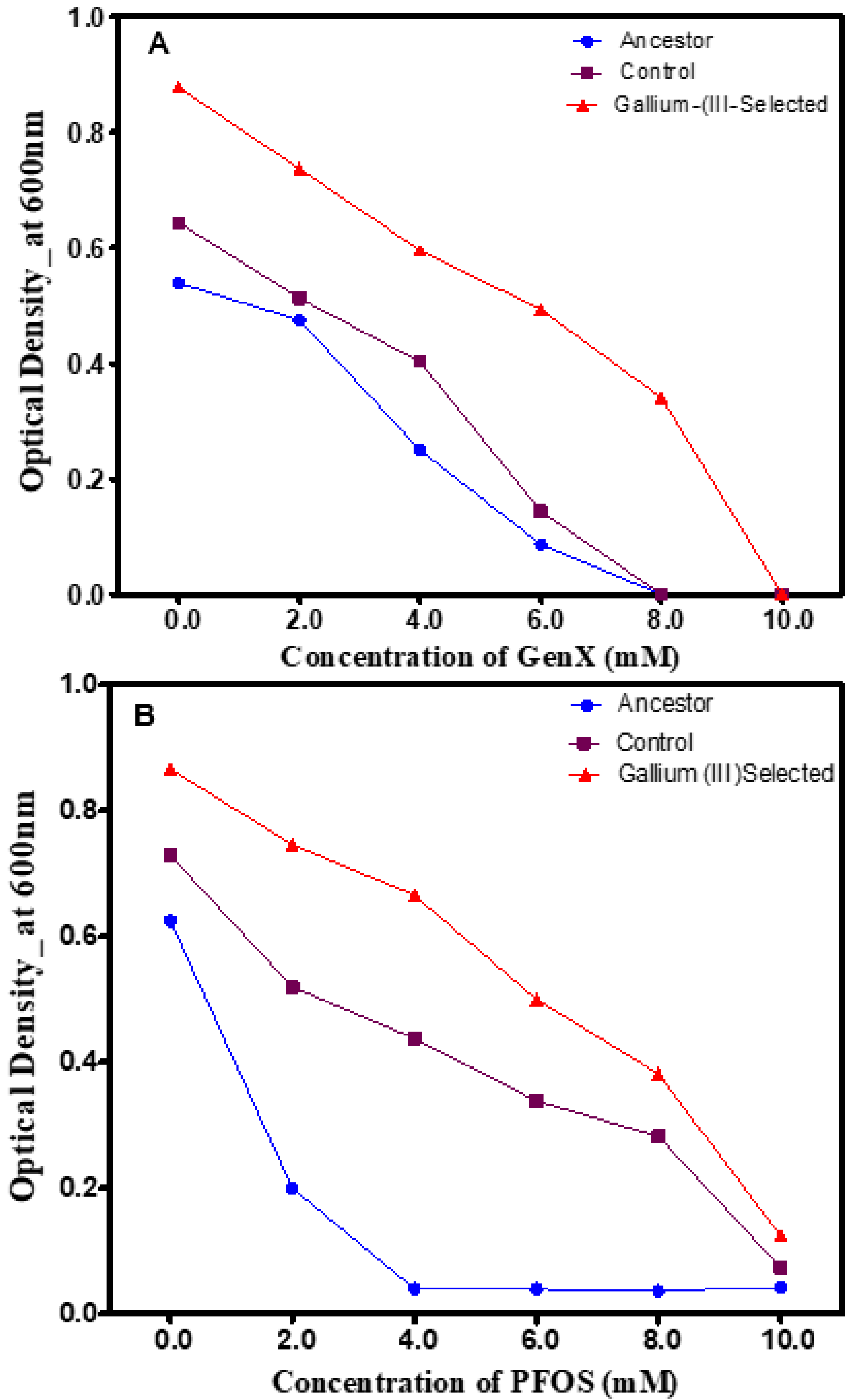
3.4. Phenotypic Changes in the Presence of Polyfluorinated Compounds
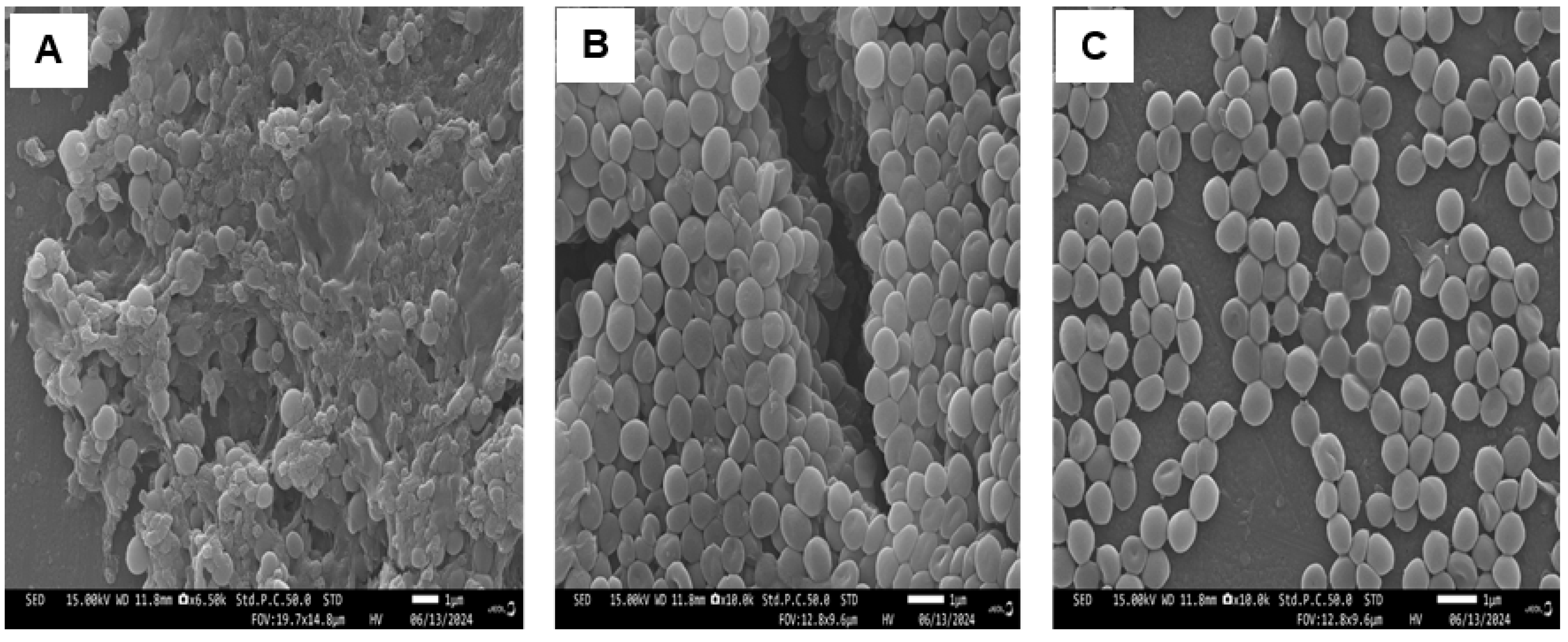
3.5. Scanning Electron Microscopy (SEM)
3.6. Genomic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danis-Wlodarczyk, K.M.; Wozniak, D.J.; Abedon, S.T. Treating bacterial infections with bacteriophage-based enzybiotics: In vitro, in vivo and clinical application. Antibiotics 2021, 10, 1497. [Google Scholar] [CrossRef]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and bacterial resistance—A short story of an endless arms race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar]
- Turner, R.J. The good, the bad, and the ugly of metals as antimicrobials. Biometals 2024, 37, 545–559. [Google Scholar]
- Keren, I.; Wu, Y.; Inocencio, J.; Mulcahy, L.R.; Lewis, K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 2013, 339, 1213–1216. [Google Scholar]
- Rauf, A.; Kiran, S.A.; Raza, H.; Haroon, S.M.; Javed, M.; Jahangir, M.; Hassan, M.; Bahadur, A.; Iqbal, S.; Mahmood, S.; et al. Harnessing the power of multifunctional γ-Fe2O3@ CuO nanocomposites: Effective extraction of heavy metals and bacterial pathogens from contaminated water. Mater. Chem. Phys. 2024, 320, 129411. [Google Scholar]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of gallium and gallium-based compounds as antimicrobial agents. Front. Bio-Eng. Biotechnol. 2022, 10, 827960. [Google Scholar]
- Mohammad Hanifeh, N.; Keyvani-Ghamsari, S.; Khorsandi, K.; Mahmoodi Khaledi, E. Effect of gallium nitrate on the antibacterial activity of vancomycin in methicillin-sensitive and resistant Staphylococcus aureus. Arch. Microbiol. 2024, 206, 304. [Google Scholar]
- Owusu, S.B.; Zaher, A.; Ahenkorah, S.; Pandya, D.N.; Wadas, T.J.; Petronek, M.S. Gallium Uncouples Iron Metabolism to Enhance Glio-blastoma Radiosensitivity. Int. J. Mol. Sci. 2024, 25, 10047. [Google Scholar] [CrossRef]
- Limantoro, C.; Das, T.; He, M.; Dirin, D.; Manos, J.; Kovalenko, M.V.; Chrzanowski, W. Synthesis of antimicrobial gallium nanoparticles using the hot injection method. ACS Mater. Au 2023, 3, 310–320. [Google Scholar] [CrossRef]
- Liu, S.; Ji, Y.; Zhu, H.; Shi, Z.; Li, M.; Yu, Q. Gallium-based metal–organic frameworks loaded with antimicrobial peptides for synergis-tic killing of drug-resistant bacteria. J. Mater. Chem. B 2023, 11, 10446–10454. [Google Scholar] [CrossRef]
- Shi, F.; Ma, S.; Liu, S.; Xin, R.; Chen, B.; Ye, W.; Sun, J. A novel antimicrobial strategy for bacterial infections: Gallium-based materials. Colloid Interface Sci. Commun. 2023, 56, 100735. [Google Scholar] [CrossRef]
- Kulkarni, S.; Pandey, A.; Mutalik, S. Liquid metal based theranostic nanoplatforms: Application in cancer therapy, imaging and biosensing. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102175. [Google Scholar] [CrossRef]
- Peng, X.X.; Gao, S.; Zhang, J.L. Gallium (III) complexes in cancer chemotherapy. Eur. J. Inorg. Chem. 2022, 2022, e202100953. [Google Scholar]
- Mjos, K.D.; Cawthray, J.F.; Polishchuk, E.; Abrams, M.J.; Orvig, C. Gallium (III) and iron (III) complexes of quinolone antimicrobials. Dalton Trans. 2016, 45, 13146–13160. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New antimicrobial strategies based on metal complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, X.; Chen, X.; Chen, Z.; Xia, Z. Antimicrobial effect of gallium nitrate against bacteria encountered in burn wound infections. RSC Adv. 2017, 7, 52266–52273. [Google Scholar] [CrossRef]
- Choi, S.R.; Talmon, G.A.; Hearne, K.; Woo, J.; Truong, V.L.; Britigan, B.E.; Narayanasamy, P. Combination therapy with gallium protoporphyrin and gallium nitrate exhibits enhanced antimicrobial activity in vitro and in vivo against methicillin-resistant Staphylococcus aureus. Mol. Pharm. 2023, 20, 4058–4070. [Google Scholar] [CrossRef]
- Graves, J.L., Jr.; Ewunkem, A.J.; Ward, J.; Staley, C.; Thomas, M.D.; Rhinehardt, K.L.; Han, J.; Harrison, S.H. Experimental evolution of gallium resistance in Escherichia coli. Evol. Med. Public Health 2019, 2019, 169–180. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of per-and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2022, 344, 126223. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Gugliandolo, E.; Cuzzocrea, S.; Crupi, R.; Britti, D. Current review of increasing animal health threat of per-and polyfluoroalkyl substances (pfas): Harms, limitations, and alternatives to manage their toxicity. Int. J. Mol. Sci. 2023, 24, 11707. [Google Scholar] [CrossRef]
- Wackett, L.P. Nothing lasts forever: Understanding microbial biodegradation of polyfluorinated compounds and perfluorinated alkyl substances. Microb. Biotechnol. 2022, 15, 773–792. [Google Scholar] [PubMed]
- Pray, L. Antibiotic resistance, mutation rates and MRSA. Nat. Educ. 2008, 1, 30. [Google Scholar]
- Ewunkem, A.J.; Rodgers, L.; Campbell, D.; Staley, C.; Subedi, K.; Boyd, S.; Graves, J.L., Jr. Experimental evolution of magnetite nanoparticle resistance in Escherichia coli. Nanomaterials 2021, 11, 790. [Google Scholar] [CrossRef]
- Ewunkem, A.J.; Williams, Z.J.; Johnson, N.S.; Brittany, J.L.; Maselugbo, A.; Nowlin, K. Exploring the “Carpenter” as a substrate for green synthesis: Biosynthesis and antimicrobial potential. Gene Protein Dis. 2023, 2, 2155. [Google Scholar]
- Shrestha, S.; Shrestha, B.K.; Tettey-Engmann, F.; Auniq, R.B.; Subedi, K.; Ghimire, S.; Desai, S.; Bhattarai, N. Zein-coated Zn metal particles-incorporated nanofibers: A potent fibrous platform for loading and release of Zn ions for wound healing application. ACS Appl. Mater. Interfaces 2024, 16, 49197–49217. [Google Scholar]
- Ewunkem, A.J.; Johnson, N.; A’lyiha, F.B.; Williams, Z.J.; Tshimanga, I.; Justice, B.; Singh, D.K.; Meixner, J. Green Synthesis–Mediated Nanoparticles: Characterization, Antimicrobial Activity and Genomics Analysis. Available online: https://www.intechopen.com/online-first/1195022 (accessed on 18 March 2025).
- Iyer, D.; Laws, E.; LaJeunesse, D. Escherichia coli adhesion and biofilm formation on polymeric nanostructured surfaces. ACS Omega 2023, 8, 47520–47529. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Kumari, A.; Dellinger, K. Integration of Nanoengineering with Artificial Intelligence and Machine Learning in Surface-Enhanced Raman Spectroscopy (SERS) for the Development of Advanced Biosensing Platforms. Adv. Sens. Res. 2025, 4, 2400155. [Google Scholar]
- Tang, K.W.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar]
- Frangipani, E.; Bonchi, C.; Minandri, F.; Imperi, F.; Visca, P. Pyochelin potentiates the inhibitory activity of gallium on Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 5572–5575. [Google Scholar] [CrossRef]
- Guerrini, M.; d’Agostino, S.; Grepioni, F.; Braga, D.; Lekhan, A.; Turner, R.J. Antimicrobial activity of supramolecular salts of gallium (III) and proflavine and the intriguing case of a trioxalate complex. Sci. Rep. 2022, 12, 3673. [Google Scholar]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; Van Lanen, S.G.; Boros, E. Theranostic gallium siderophore ciprofloxacin conjugate with broad spectrum antibiotic potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar]
- de Assis, A.S.; Pegoraro, G.M.; Duarte, I.C.; Delforno, T.P. Gallium: A decisive “Trojan Horse” against microorganisms. Antonie Van Leeuwenhoek 2025, 118, 3. [Google Scholar]
- Duffin, R.N.; Kelderman, J.T.; Herdman, M.E.; Andrews, P.C. Highly selective organo-gallium hydroxamate mediated inhibition of antibiotic resistant Klebsiella pneumoniae. Dalton Trans. 2025, 54, 649–661. [Google Scholar]
- Poscente, V.; Di Gregorio, L.; Bernini, R.; Bevivino, A. Inhibitory Effects of Nisin and Gallium (III) Nitrate Hydrate on Planktonic and Adhered Cells and Implications for the Viable but Non-Culturable State. Microorganisms 2025, 13, 276. [Google Scholar] [CrossRef]
- Salazar-Alemán, D.A.; Turner, R.J. Escherichia coli growing under antimicrobial gallium nitrate stress reveals new processes of tol-erance and toxicity. Sci. Rep. 2025, 15, 1389. [Google Scholar]
- Abbas, M.; Hayirli, Z.; Drakesmith, H.; Andrews, S.C.; Lewis, M.C. Effects of iron deficiency and iron supplementation at the host-microbiota interface: Could a piglet model unravel complexities of the underlying mechanisms? Front. Nutr. 2022, 9, 927754. [Google Scholar]
- He, Z.; Shen, J.; Li, Q.; Yang, Y.; Zhang, D.; Pan, X. Bacterial metal (loid) resistance genes (MRGs) and their variation and application in environment: A review. Sci. Total Environ. 2023, 871, 162148. [Google Scholar]
- He, J.; Lin, X.; Zhang, D.; Hu, H.; Chen, X.; Xu, F.; Zhou, M. Wake biofilm up to enhance suicidal uptake of gallium for chronic lung infection treatment. Biomaterials 2024, 310, 122619. [Google Scholar]
- Mohamed, D.S.; Abd El-Baky, R.M.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial activity of silver-treated bacteria against other multi-drug resistant pathogens in their environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef]
- Dickinson, A.W.; Power, A.; Hansen, M.G.; Brandt, K.K.; Piliposian, G.; Appleby, P.; O’neill, P.A.; Jones, R.T.; Sierocinski, P.; Koskella, B.; et al. Heavy metal pollution and co-selection for antibiotic resistance: A microbial palaeontology approach. Environ. Int. 2019, 132, 105117. [Google Scholar] [PubMed]
- Yep, A.; McQuade, T.; Kirchhoff, P.; Larsen, M.; Mobley, H.L. Inhibitors of TonB function identified by a high-throughput screen for inhibitors of iron acquisition in uropathogenic Escherichia coli CFT073. mBio 2014, 5, e01089-13. [Google Scholar] [CrossRef]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [PubMed]
- Sun, W.; Qi, M.; Cheng, S.; Li, C.; Dong, B.; Wang, L. Gallium and gallium compounds: New insights into the “Trojan horse” strategy in medical applications. Mater. Des. 2023, 227, 111704. [Google Scholar] [CrossRef]
- Pasqua, M.; Grossi, M.; Zennaro, A.; Fanelli, G.; Micheli, G.; Barras, F.; Colonna, B.; Prosseda, G. The varied role of efflux pumps of the MFS family in the interplay of bacteria with animal and plant cells. Microorganisms 2019, 7, 285. [Google Scholar] [CrossRef]
- Drew, D.; North, R.A.; Nagarathinam, K.; Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 2021, 121, 5289–5335. [Google Scholar]
- Yue, W.W.; Grizot, S.; Buchanan, S.K. Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA. J. Mol. Biol. 2003, 332, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Kedziora, A.; Wernecki, M.; Korzekwa, K.; Speruda, M.; Gerasymchuk, Y.; Lukowiak, A.; Bugla-Ptoskohska, G. Consequences of Long-Term Bacteria’s Exposure To Silver Nanoformulations with Different PhysicoChemical Properties. Int. J. Nanomed. 2020, 15, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H. Mechanisms of bacterial resistance to environmental silver and antimicrobial strategies for silver: A review. Environ. Res. 2024, 248, 118313. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.M.; Gregan, J.; Jarosch, E.; Ragnini, A.; Schweyen, R.J. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J. Biol. Chem. 1999, 274, 20438–20443. [Google Scholar] [CrossRef]
- Preeti Garai, P.G.; Kasturi Chandra, K.C.; Dipshikha Chakravortty, D.C. Bacterial peptide transporters: Messengers of nutrition to virulence. Virulence 2017, 8, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.S.; Kelly, C.L.; Mordaka, P.M.; Heap, J.T. Review of NAD (P) H-dependent oxidoreductases: Properties, engineering and application. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2018, 1866, 327–347. [Google Scholar] [CrossRef]
- Murray, L.M.; Hayes, A.; Snape, J.; Kasprzyk-Hordern, B.; Gaze, W.H.; Murray, A.K. Co-selection for antibiotic resistance by environmental contaminants. Npj Antimicrob. Resist. 2024, 2, 9. [Google Scholar] [CrossRef]
- Pearson, C.R.; Tindall, S.N.; Herman, R.; Jenkins, H.T.; Bateman, A.; Thomas, G.H.; Potts, J.R.; Van der Woude, M.W. Acetylation of surface carbohydrates in bacterial pathogens requires coordinated action of a two-domain membrane-bound acyltransferase. mBio 2020, 11, e01364-20. [Google Scholar] [CrossRef]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef]
- Bradshaw, N.; Levdikov, V.M.; Zimanyi, C.M.; Gaudet, R.; Wilkinson, A.J.; Losick, R. A widespread family of serine/threonine protein phosphatases shares a common regulatory switch with proteasomal proteases. eLife 2017, 6, e26111. [Google Scholar] [CrossRef]
- Niehaus, T.D.; Elbadawi-Sidhu, M.; de Crécy-Lagard, V.; Fiehn, O.; Hanson, A.D. Discovery of a widespread prokaryotic 5-oxoprolinase that was hiding in plain sight. J. Biol. Chem. 2017, 292, 16360–16367. [Google Scholar] [CrossRef]
- Noori, M.T.; Gupta, P.; Hellgardt, K.; Min, B. Per-and poly-fluoroalkyl substances (PFAS): An emerging environmental challenge and (microbial) bioelectrochemical treatment strategies. Curr. Opin. Environ. Sci. Health 2024, 43, 100588. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of environmental wastes: The role of microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar]
- Butcher, R.J.; Tabor, J.J. Real-time detection of response regulator phosphorylation dynamics in live bacteria. Proc. Natl. Acad. Sci. USA 2022, 119, e2201204119. [Google Scholar]
- Lombardo, M.J.; Aponyi, I.; Rosenberg, S.M. General stress response regulator RpoS in adaptive mutation and amplification in Escherichia coli. Genetics. 2004, 166, 669–680. [Google Scholar] [PubMed]
- Naveed, M.; Makhdoom, S.I.; Abbas, G.; Safdari, M.; Farhadi, A.; Habtemariam, S.; Shabbir, M.A.; Jabeen, K.; Asif, M.F.; Tehreem, S. The virulent hypothetical proteins: The potential drug target involved in bacterial pathogenesis. Mini Rev. Med. Chem. 2022, 22, 2608–2623. [Google Scholar] [CrossRef]
- Lima e Silva, A.A.; Carvalho, M.A.; de Souza, S.A.; Dias, P.M.; Silva Filho, R.G.; Saramago, C.S.; Bento, C.A.; Hofer, E. Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz. J. Microbiol. 2012, 43, 1620–1631. [Google Scholar]
- van Teeseling, M.C.; de Pedro, M.A.; Cava, F. Determinants of bacterial morphology: From fundamentals to possibilities for antimicrobial targeting. Front. Microbiol. 2017, 8, 1264. [Google Scholar]
- Chen, J.; Zhou, H.; Huang, J.; Zhang, R.; Rao, X. Virulence alterations in Staphylococcus aureus upon treatment with the sub-inhibitory concentrations of antibiotics. J. Adv. Res. 2021, 31, 165–175. [Google Scholar]
- Chang, J.D.; Foster, E.E.; Thadani, A.N.; Ramirez, A.J.; Kim, S.J. Inhibition of Staphylococcus aureus cell wall biosynthesis by desleucyl-oritavancin: A quantitative peptidoglycan composition analysis by mass spectrometry. J. Bacteriol. 2017, 199, e00278-17. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, D.; Singh, A.K.; Drolia, R.; Bai, X.; Tenguria, S.; Bhunia, A.K. Tunicamycin mediated inhibition of wall teichoic acid affects Staphylococcus aureus and Listeria monocytogenes cell morphology, biofilm formation and virulence. Front. Microbiol. 2018, 9, 1352. [Google Scholar]
- Young, K.D. Bacterial morphology: Why have different shapes? Curr. Opin. Microbiol. 2007, 10, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Z. Structural insights into the transporting and catalyzing mechanism of DltB in LTA D-alanylation. Nat. Commun. 2024, 15, 3404. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hunter, H.N.; Prova, S.; Verma, V.; Qamar, A.; Golemi-Kotra, D. The Staphylococcus aureus methicillin resistance factor FmtA is a d-amino esterase that acts on teichoic acids. mBio 2016, 7, e02070-15. [Google Scholar]
- Schaff, D.A. The adenine phosphoribosyltransferase (APRT) selectable marker system. Plant Sci. 1994, 101, 3–9. [Google Scholar]
- Karpowich, N.K.; Song, J.; Wang, D.N. An aromatic cap seals the substrate binding site in an ECF-type S subunit for riboflavin. J. Mol. Biol. 2016, 428, 3118–3130. [Google Scholar] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar]
- Brenot, A.; King, K.Y.; Janowiak, B.; Griffith, O.; Caparon, M.G. Contribution of glutathione peroxidase to the virulence of Streptococcus pyogenes. Infect. Immun. 2004, 72, 408–413. [Google Scholar]
- Wang, T.Y.; Zhao, J.; Savas, A.C.; Zhang, S.; Feng, P. Viral pseudoenzymes in infection and immunity. FEBS J. 2020, 287, 4300–4309. [Google Scholar] [CrossRef]
- Craney, A.; Dix, M.M.; Adhikary, R.; Cravatt, B.F.; Romesberg, F.E. An alternative terminal step of the general secretory pathway in Staphylococcus aureus. mBio 2015, 6, e01178-15. [Google Scholar]
- Stamsås, G.A.; Myrbråten, I.S.; Straume, D.; Salehian, Z.; Veening, J.W.; Håvarstein, L.S.; Kjos, M. CozEa and CozEb play overlapping and essential roles in controlling cell division in Staphylococcus aureus. Mol. Microbiol. 2018, 109, 615–632. [Google Scholar] [CrossRef]
- Wullich, S.C.; Arranz San Martín, A.; Fetzner, S. An α/β-hydrolase fold subfamily comprising Pseudomonas quinolone signal-cleaving dioxygenases. Appl. Environ. Microbiol. 2020, 86, e00279-20. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, M.T.; Schleif, R.; Bairoch, A.; Hofmann, K.; Ramos, J.L. Arac/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 1997, 61, 393–410. [Google Scholar]
- Conlon, K.M.; Humphreys, H.; O’Gara, J.P. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 2002, 184, 4400–4408. [Google Scholar] [CrossRef]
- Dym, O.; Pratt, E.A.; Ho, C.; Eisenberg, D. The crystal structure of D-lactate dehydrogenase, a peripheral membrane respiratory enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 9413–9418. [Google Scholar] [CrossRef]
- Karzai, A.W.; Sauer, R.T. Protein factors associated with the SsrA⋅ SmpB tagging and ribosome rescue complex. Proc. Natl. Acad. Sci. USA 2001, 98, 3040–3044. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Bremer, E.; Krämer, R. The BCCT family of carriers: From physiology to crystal structure. Mol. Microbiol. 2010, 78, 13–34. [Google Scholar] [CrossRef]
- Dincturk, H.B.; Cunin, R.; Akce, H. Expression and functional analysis of glutamate synthase small subunit-like proteins from archaeon Pyrococcus horikoshii. Microbiol. Res. 2011, 166, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.R.; Moon, K.M.; Chen, M.; Balakrishnan, R.; Foster, L.J.; Fredrick, K. Conserved GTPase LepA (Elongation Factor 4) functions in biogenesis of the 30S subunit of the 70S ribosome. Proc. Natl. Acad. Sci. USA 2017, 114, 980–985. [Google Scholar] [CrossRef]
- Chen, X.; Alonzo, F., III. Bacterial lipolysis of immune-activating ligands promotes evasion of innate defenses. Proc. Natl. Acad. Sci. USA 2019, 116, 3764–3773. [Google Scholar] [CrossRef]
- Nam, D.; Matsumoto, Y.; Uchida, T.; O’Brian, M.R.; Ishimori, K. Mechanistic insights into heme-mediated transcriptional regulation via a bacterial manganese-binding iron regulator, iron response regulator (Irr). J. Biol. Chem. 2020, A295, 11316–11325. [Google Scholar] [CrossRef]
- Kotecka, K.; Kawalek, A.; Kobylecki, K.; Bartosik, A.A. The AraC-type transcriptional regulator GliR (PA3027) activates genes of glycerolipid metabolism in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 5066. [Google Scholar] [CrossRef]
- Bommisetti, P.; Young, A.; Bandarian, V. Elucidation of the substrate of tRNA-modifying enzymes MnmEG leads to in vitro reconstitution of an evolutionarily conserved uridine hypermodification. J. Biol. Chem. 2022, 298, 102548. [Google Scholar]
- Razew, A.; Schwarz, J.N.; Mitkowski, P.; Sabala, I.; Kaus-Drobek, M. One fold, many functions—M23 family of peptidoglycan hydrolases. Front. Microbiol. 2022, 13, 1036964. [Google Scholar]
- Soussan, D.; Salze, M.; Ledormand, P.; Sauvageot, N.; Boukerb, A.; Lesouhaitier, O.; Fichant, G.; Rincé, A.; Quentin, Y.; Muller, C. The NagY regulator: A member of the BglG/SacY antiterminator family conserved in Enterococcus faecalis and involved in virulence. Front. Microbiol. 2023, 13, 1070116. [Google Scholar]
- Chen, J.; Wu, Y.; Zhu, Y.; Zhang, L.; Xu, Y.; Liu, Y. Adaptation for Staphylococcus aureus to hosts via insertion mutation in the accessory gene regulator agrC gene: Decreased viru-lence and enhanced persistence capacity. Microbiol. Spectr. 2025, 13, e01497-24. [Google Scholar]
- Mulat, M.; Eshetie, T.; Getachew, F.; Tesfaye, A.; Jenber, Y.; Tilahun, B.; Damtew, B.; Berhane, N.B. The Association of MECA Gene Polymorphism and Drug Resistance Pattern of Methicillin-Resistant Staphylococcus Aureus Isolated from Keha and Shinta Rivers of Gondar Town, Northwest Ethiopia. Ethiop. J. Nat. Comput. Sci. 2025, 5, 647–660. [Google Scholar]
- Iannuzzi, M.C.; Maliarik, M.; Rybicki, B. Genetic polymorphisms in lung disease: Bandwagon or breakthrough? Respir. Res. 2002, 3, 7. [Google Scholar]
- Hanley, B. Receptors and Natural Products. In Natural and Unnatural Product Chemistry: From Molecules to Systems; Springer Nature: Cham, Switzerland, 2025; pp. 199–221. [Google Scholar]
- Chandrasekaran, P.; Zimmerman, O.; Paulson, M.; Sampaio, E.P.; Freeman, A.F.; Sowerwine, K.J.; Hurt, D.; Alcántara-Montiel, J.C.; Hsu, A.P.; Holland, S.M. Distinct mutations at the same positions of STAT3 cause either loss or gain of function. J. Allergy Clin. Immunol. 2016, 138, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Fakher, S.; Westenberg, D. Properties and antibacterial effectiveness of metal-ion doped borate-based bioactive glasses. Future Microbiol. 2025, 15, 1–7. [Google Scholar]


| Gene | Position | Mutation | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|---|
| sfaA/sfaD | 2,228,365 | Intergenic (−35/−67) | 0.767 | 0.692 | 0.731 | 0.758 | 0.755 |
| KQ76_RS01520 | 342,330 | I180I* (ATT→ATC) | 0.678 | 0.62 | 0.645 | 0.673 | 0.631 |
| fmtA | 1,017,325 | Q304* (CAA→TAA) | 0.583 | 0.653 | 0.599 | 0.624 | 0.539 |
| KQ76_RS08360 | 1,700,478 | G59S* (GGC→AGC) | 0.367 | 0.656 | 0.641 | 0.541 | 0.538 |
| dltB | 860,322 | E344K* (GAA→AAA) | 0.325 | 0.313 | 0.37 | 0.266 | 0.363 |
| hssR | 2,389,192 | R188P* (CGA→CCA) | 0.327 | 0.32 | 0.382 | 0.328 | 0.339 |
| rsp | 2,412,575 | G84D* (GGT→GAT) | 0 | 0 | 0 | 0 | 0.330 |
| KQ76_RS11280/KQ76_RS11285 | 2,252,747 | Intergenic (−54/+157) | 0 | 0.299 | 0 | 0.281 | 0.325 |
| KQ76_RS13020 | 2,574,726 | G69A* (GGC→GCC) ‡ | 0.274 | 0.322 | 0.355 | 0 | 0.318 |
| KQ76_RS13825 | 2,746,925 | A172G* (GCT→GGT) | 0.352 | 0 | 0.36 | 0.246 | 0 |
| KQ76_RS12955 | 2,564,194 | A17P* (GCA→CCA) | 0.298 | 0 | 0 | 0 | 0.281 |
| gltB | 453,752 | Pseudogene (4457/4500 nt) | 0 | 0 | 0 | 0 | 0.281 |
| KQ76_RS13475 | 2,665,478 | E162V* (GAA→GTA) | 0 | 0 | 0 | 0 | 0.278 |
| purS | 1,027,271 | A87P* (GCA→CCA) | 0 | 0.27 | 0.239 | 0.262 | 0.272 |
| KQ76_RS11175 | 2,233,551 | T39S* (ACT→AGT) | 0.27 | 0 | 0 | 0 | 0 |
| KQ76_RS10985 | 2,190,680 | T500S* (ACG→TCG) | 0.255 | 0.275 | 0 | 0.237 | 0.262 |
| KQ76_RS12180 | 2,408,435 | L213I* (TTA→ATA) | 0 | 0.254 | 0 | 0 | 0.262 |
| graR | 673,306 | A185P* (GCA→CCA) | 0.264 | 0 | 0 | 0 | 0.26 |
| KQ76_RS07375 | 1,536,460 | Y112* (TAT→TAA) | 0 | 0.275 | 0 | 0 | 0 |
| KQ76_RS09255 | 1,890,405 | S137T* (AGT→ACT) | 0.254 | 0.266 | 0.265 | 0 | 0 |
| KQ76_RS11185 | 2,236,063 | R52P* (CGT→CCT) | 0.272 | 0 | 0 | 0 | 0.251 |
| KQ76_RS04770 | 986,849 | K56* (AAA→TAA) | 0.281 | 0 | 0.254 | 0 | 0 |
| ylqF | 1,213,003 | E184D* (GAG→GAC) | 0.288 | 0.313 | 0.237 | 0.26 | 0 |
| smpB | 810,694 | M1K* (ATG→AAG) † | 0 | 0.29 | 0 | 0 | 0 |
| vraE | 2,766,496 | I591I* (ATA→ATT) | 0.268 | 0 | 0 | 0 | 0 |
| KQ76_RS02795 | 596,886 | A144V* (GCA→GTA) | 0 | 0 | 0.235 | 0 | 0 |
| KQ76_RS01360/lip2 | 311,944 | Intergenic (−69/−348) | 0 | 0 | 0 | 0.234 | 0 |
| capA | 116,233 | V151M* (GTG→ATG) | 0 | 0 | 0 | 0.233 | 0 |
| KQ76_RS04220 | 871,433 | T26I* (ACA→ATA) | 0 | 0 | 0 | 0.225 | 0 |
| KQ76_RS05175/KQ76_RS05180 | 1,066,987 | Intergenic (−157/+27) | 0.254 | 0 | 0 | 0 | 0 |
| KQ76_RS01815 | 391,361 | S36I* (AGT→ATT) | 0 | 0.291 | 0 | 0 | 0 |
| mnmG | 2,776,116 | H117Q* (CAT→CAA) | 0 | 0.276 | 0 | 0 | 0 |
| thrS | 1,740,864 | L149* (TTA→TAA) | 0 | 0 | 0 | 0.224 | 0 |
| pstC | Phosphate ABC transporter permease subunit |
| KQ76_RS04365 | Acyltransferase family protein |
| hssR | DNA-binding heme response regulator |
| KQ76_RS10525 | PP2C family protein–serine/threonine phosphatase |
| pxpB/greA | 5-oxoprolinase subunit PxpB/transcription elongation factor |
| KQ76_RS13020 | Alpha/beta hydrolase |
| icaR | Ica operon transcriptional regulator |
| KQ76_RS09255 | Hypothetical protein |
| ald/KQ76_RS08720 | Alanine dehydrogenase/universal stress protein |
| mnmG | tRNA uridine-5-carboxymethylaminomethyl(34) synthesis enzyme |
| KQ76_RS04770 | ATP-binding protein |
| cozEb | Cell elongation protein |
| KQ76_RS12955 | D-lactate dehydrogenase |
| KQ76_RS01360/lip2 | YjiH family protein/YSIRK domain-containing triacylglycerol lipase Lip2/Geh |
| KQ76_RS10985 | BglG family transcription antiterminator |
| KQ76_RS13825 | ECF-type riboflavin transporter substrate-binding protein |
| gltB | Glutamate synthase large subunit |
| rsp | AraC family transcriptional regulator |
| ylqF | Ribosome biogenesis GTPase |
| mprF | Bifunctional lysylphosphatidylglycerol flippase/synthetase |
| mutS | DNA mismatch repair protein |
| KQ76_RS11280/KQ76_RS11285 | M23 family metallopeptidase/HAD IIB family hydrolase |
| KQ76_RS12905 | ATP-binding cassette domain-containing protein |
| smpB | SsrA-binding protein |
| KQ76_RS13475 | Glutathione peroxidase |
| KQ76_RS12190/rsp | YbgA family protein/AraC family transcriptional regulator |
| KQ76_RS11175 | BCCT family transporter |
| sfaA/sfaD | StaphyloferrinA export MFS transporter/D-ornithine–citrate ligase |
| KQ76_RS01520 | DUF3169 family protein |
| fmtA | Teichoic acid D-Ala esterase |
| KQ76_RS08360 | Adenine phosphoribosyltransferase |
| dltB | PG:teichoic acid D-alanyltransferase |
| KQ76_RS13825 | ECF-type riboflavin transporter substrate-binding protein |
| KQ76_RS13475 | Glutathione peroxidase |
| purS | Phosphoribosylformylglycinamidine synthase subunit |
| KQ76_RS12180 | Magnesium transporter CorA family protein |
| graR | Response regulator transcription factor GraR/ApsR |
| KQ76_RS07375 | Phage major capsid protein |
| KQ76_RS09255 | Hypothetical protein |
| KQ76_RS11185 | NADP-dependent oxidoreductase |
| vraE | Peptide-resistance ABC transporter permease subunit |
| KQ76_RS02795 | Uracil–DNA glycosylase |
| capA | Capsular polysaccharide-type 5/8 biosynthesis protein |
| KQ76_RS04220 | FAD/NAD(P)-binding protein |
| KQ76_RS05175/KQ76_RS05180 | Nramp family divalent metal transporter/YktB family protein |
| KQ76_RS01815 | General stress protein |
| thrS | Threonine–tRNA ligase |
| Gene | Position | Mutation | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|---|---|
| pstC | 1,384,254 | S178C* (AGT→TGT) | 0 | 0.728 | 0.685 | 0.73 | 0.709 |
| KQ76_RS04365 | 907,688 | N549I* (AAT→ATT) | 0.625 | 0.66 | 0.61 | 0.68 | 0.653 |
| KQ76_RS10525 | 2,102,321 | G175E* (GGA→GAA) | 0.417 | 0.463 | 0.399 | 0.378 | 0.39 |
| KQ76_RS13020 | 2,574,726 | G69A* (GGC→GCC) | 0 | 0.314 | 0.31 | 0.367 | 0.468 |
| ylqF | 1,213,003 | E184D* (GAG→GAC) | 0 | 0.279 | 0 | 0.336 | 0 |
| KQ76_RS13020 | 2,574,727 | G69R* (GGC→CGC) | 0.338 | 0 | 0.36 | 0.316 | 0 |
| hssR | 2,389,188 | E187Q* (GAA→CAA) | 0.349 | 0.30 | 0.41 | 0.316 | 0 |
| pxpB/greA | 1,672,825 | Intergenic (−76/+250) | 0.31 | 0.293 | 0.39 | 0.313 | 0.272 |
| icaR | 2,727,937 | Q79* (CAA→TAA) | 0.278 | 0 | 0.29 | 0 | 0 |
| KQ76_RS10525 | 2,102,321 | G175E* (GGA→GAA) | 0.302 | 0 | 0 | 0 | 0 |
| KQ76_RS06325 | 1,302,347 | D54Y* (GAC→TAC) | 0.293 | 0 | 0 | 0.296 | 0.259 |
| cozEb | 1,352,269 | I247N* (ATT→AAT) | 0 | 0.279 | 0.259 | 0.293 | 0.259 |
| mprF | 1,354,114 | G299D* (GGT→GAT) | 0 | 0 | 0 | 0.292 | 0.253 |
| pepF/yjbH | 941,253 | Intergenic (+205/+255) | 0 | 0 | 0 | 0.292 | 0 |
| KQ76_RS13825 | 2,746,925 | A172G* (GCT→GGT) | 0 | 0.272 | 0 | 0.290 | 0.248 |
| KQ76_RS12190/rsp | 2,412,155 | Intergenic (+1318/170) | 0.246 | 0 | 0 | 0 | 0 |
| KQ76_RS10985 | 2,190,680 | T500S* (ACG→TCG) | 0 | 0.279 | 0 | 0.290 | 0.256 |
| KQ76_RS09255 | 1,890,405 | S137T* (AGT→ACT) | 0 | 0.245 | 0.286 | 0 | 0 |
| KQ76_RS12955 | 2,564,194 | A17P* (GCA→CCA) | 0 | 0.251 | 0 | 0.287 | 0.31 |
| ald/KQ76_RS08720 | 1,776,393 | Intergenic (−57/−84) | 0.349 | 0 | 0.286 | 0 | 0.341 |
| KQ76_RS04275/KQ76_RS04280 | 881,138 | Intergenic (+150/−158) | 0.236 | 0 | 0 | 0.270 | 0.249 |
| mnmG | 2,776,116 | H117Q* (CAT→CAA) | 0 | 0.246 | 0.276 | 0 | 0 |
| ald/KQ76_RS08720 | 1,776,393 | Intergenic (−57/−84) | 0 | 0 | 0.286 | 0.265 | 0 |
| KQ76_RS12955 | 2,564,193 | M16I* (ATG→ATC) | 0 | 0 | 0.25 | 0 | 0 |
| KQ76_RS13475 | 2,665,478 | E162V* (GAA→GTA) | 0.276 | 0 | 0 | 0 | 0 |
| mutS | 1,275,400 | K479N* (AAG→AAC) | 0 | 0 | 0 | 0 | 0.25 |
| rsp | 2,412,863 | C180Y* (TGT→TAT) | 0.246 | 0 | 0 | 0 | 0.251 |
| smpB | 810,694 | M1K* (ATG→AAG) | 0.264 | 0 | 0 | 0 | 0 |
| KQ76_RS01360/lip2 | 311,944 | Intergenic (−69/−348) | 0 | 0 | 0.247 | 0 | 0 |
| KQ76_RS10985 | 2,190,680 | T500S* (ACG→TCG) | 0 | 0 | 0.247 | 0 | 0 |
| KQ76_RS04770 | 986,858 | Q59K* (CAA→AAA) | 0 | 0.245 | 0 | 0 | 0.244 |
| KQ76_RS13825 | 2,746,925 | A172G* (GCT→GGT) | 0 | 0 | 0.245 | 0 | 0 |
| gltB | 453,752 | Pseudogene (4457/4500 nt) | 0 | 0.238 | 0.243 | 0 | 0 |
| KQ76_RS12905 | 2,554,550 | V173L* (GTC→CTC) | 0 | 0 | 0 | 0 | 0.247 |
| KQ76_RS11175 | 2,233,551 | T39S* (ACT→AGT) | 0.23 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewunkem, A.; Simpson, F.; Holland, D.; Bowers, T.; Bailey, A.; Gore, J.; Iloghalu, U.; Williams, V.; Adjei-Fremah, S.; Kiki, L.; et al. Gallium Resistance in Staphylococcus aureus: Polymorphisms and Morphology Impacting Growth in Metals, Antibiotics and Polyfluorinated Compounds. Appl. Microbiol. 2025, 5, 32. https://doi.org/10.3390/applmicrobiol5010032
Ewunkem A, Simpson F, Holland D, Bowers T, Bailey A, Gore J, Iloghalu U, Williams V, Adjei-Fremah S, Kiki L, et al. Gallium Resistance in Staphylococcus aureus: Polymorphisms and Morphology Impacting Growth in Metals, Antibiotics and Polyfluorinated Compounds. Applied Microbiology. 2025; 5(1):32. https://doi.org/10.3390/applmicrobiol5010032
Chicago/Turabian StyleEwunkem, Akamu, Felicia Simpson, David Holland, Tatyana Bowers, Ariyon Bailey, Ja’nyah Gore, Uchenna Iloghalu, Vera Williams, Sarah Adjei-Fremah, Larisa Kiki, and et al. 2025. "Gallium Resistance in Staphylococcus aureus: Polymorphisms and Morphology Impacting Growth in Metals, Antibiotics and Polyfluorinated Compounds" Applied Microbiology 5, no. 1: 32. https://doi.org/10.3390/applmicrobiol5010032
APA StyleEwunkem, A., Simpson, F., Holland, D., Bowers, T., Bailey, A., Gore, J., Iloghalu, U., Williams, V., Adjei-Fremah, S., Kiki, L., & Justice, B. (2025). Gallium Resistance in Staphylococcus aureus: Polymorphisms and Morphology Impacting Growth in Metals, Antibiotics and Polyfluorinated Compounds. Applied Microbiology, 5(1), 32. https://doi.org/10.3390/applmicrobiol5010032






