Optimization of L-Asparaginase Production from Aspergillus caespitosus: Solid-State and Submerged Fermentation Using Low-Cost Substrates and Partial Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cultural Conditions
2.2. Solid-State Fermentation (SSF)
2.2.1. Ora-Pro-Nobis Fiber (OPNF) Used as a Substrate for SSF
2.2.2. Optimization of L-ASNase Enzyme Production by SSF
2.3. Submerged Fermentation (SmF)
2.3.1. Whey Protein
2.3.2. Optimization of L-ASNase Enzyme Production by SmF
2.4. Determination of L-ASNase Activity
2.5. Partial Purification of L-ASNase Obtained by SmF
2.5.1. Batch Adsorption Tests
2.5.2. Partial Purification of L-ASNase
2.6. Statistical Analyzes
3. Results
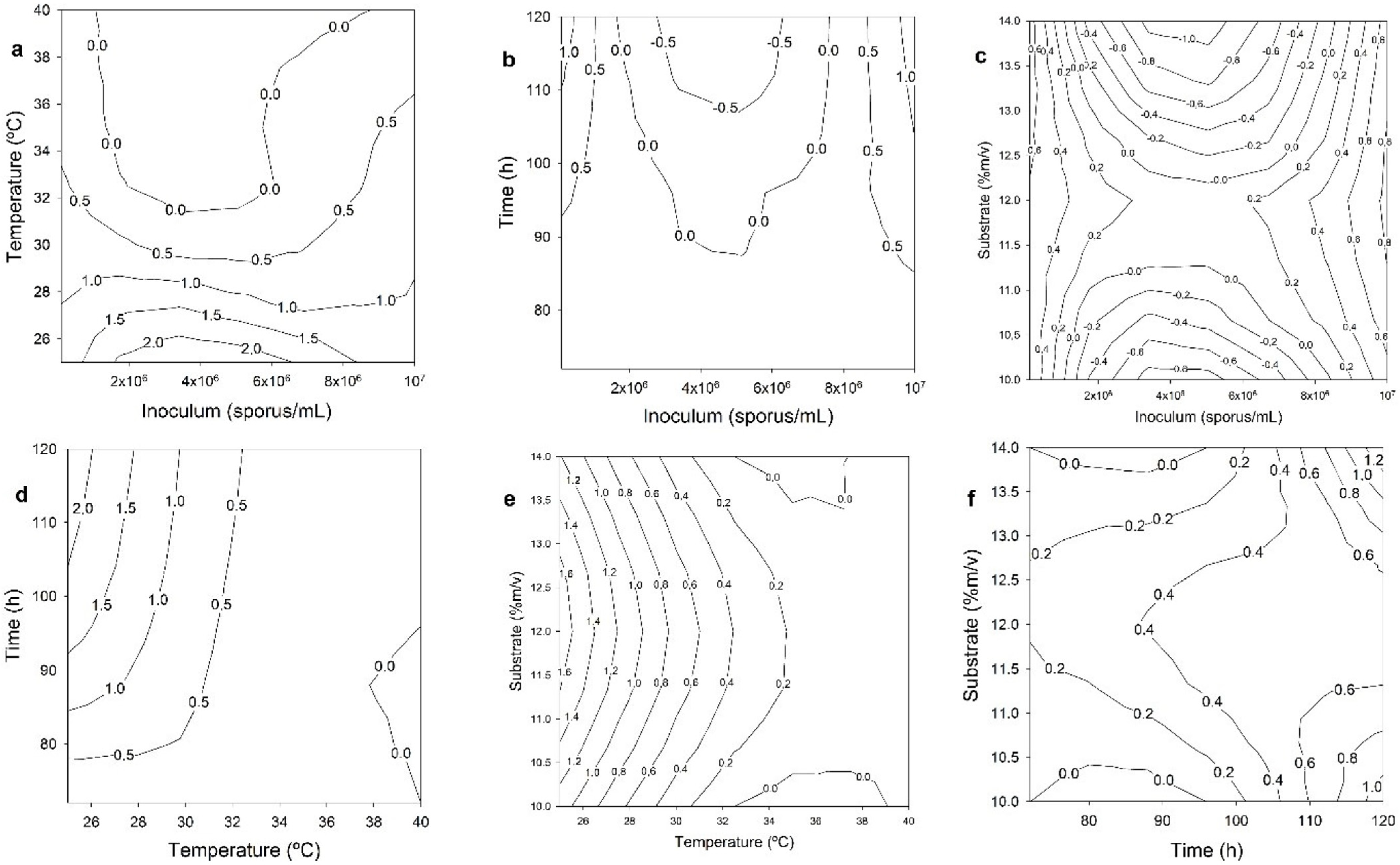
3.1. Solid Fermentation
Optimization of the Parameters Used in the SSF Process for the Production of L-Asnase
+ (0.12032465 × x3) + (2.61479370 × x4)
+ (0.0000000 × x1 × x1) + (0.00755849 × x2 × x2)
− (0.10773418 × x4 × x4) − (0.00294059 × x2× x3)
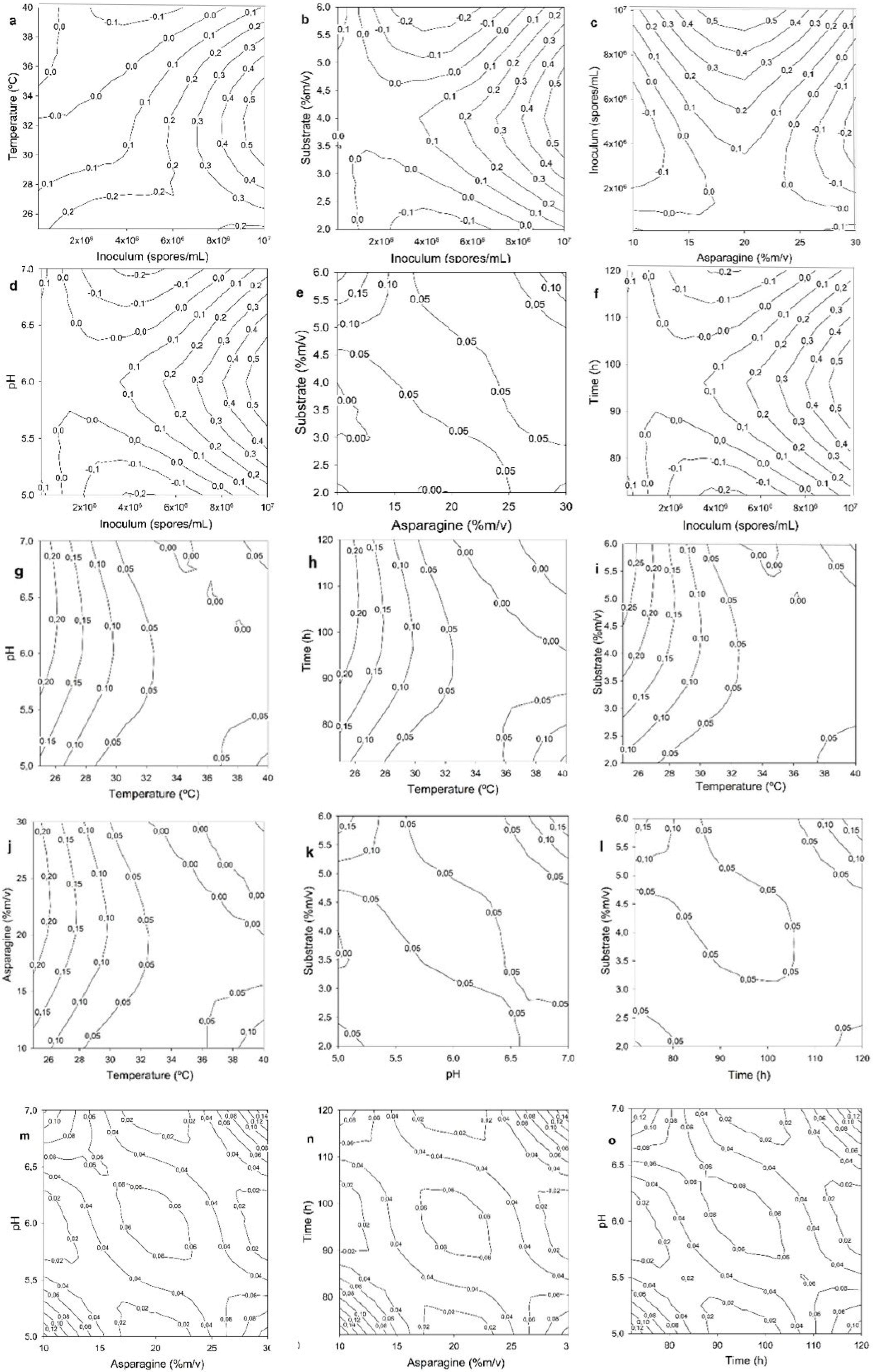
3.2. Submerged Fermentation
Optimization of the Parameters in the SmF for L-ASNase
+ (0.0218688758 × x2) + (0.0000000034 × x3)
+ (0.083746041 × x4) + (0.355620550 × x5)
− (0.007607069 × x6) − (0.000000002 × x3 × x1)
− (0.000000002 × x3 × x4) + (0.000000012 × x3 × x5)
+ (0.000000006 × x3 × x2) − (0.006119979 × x4 × x2)
+ (0.000334705 × x6 × x1) − (0.010805979 × x4 × x5)
3.3. Purification of the L-ASNase Enzyme
3.3.1. Adsorption Tests
3.3.2. Purification Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ganesh, A.; Sevda, S. Asparaginase: Production, Harvest, Recovery, and Potential Industrial Application. In Industrial Microbiology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 213–239. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Al-Ghamdi, M.A.; Khan, J.A. Studies on the recombinant production in E. coli and characterization of pharmaceutically important thermostable L-asparaginase from Geobacillus thermodenitrificans. Pak. J. Zool. 2019, 51, 1235. [Google Scholar] [CrossRef]
- Agrawal, S.; Kango, N. Development and catalytic characterization of L-asparaginase nano-bioconjugates. Int. J. Biol. Macromol. 2019, 135, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, W.; Costa-Silva, T.A.; Agamez-Montalvo, G.S.; Feitosa, V.A.; Machado, S.; de Souza Lima, G.; Pessoa, A., Jr.; Alves, H.d.S. Screening and optimizing fermentation production of l-asparaginase by Aspergillus terreus strain S-18 isolated from the Brazilian Caatinga Biome. J. Appl. Microbiol. 2019, 126, 1426–1437. [Google Scholar] [CrossRef]
- Patel, P.G.; Panseriya, H.Z.; Vala, A.K.; Dave, B.P.; Gosai, H.B. Exploring current scenario and developments in the field of microbial L-asparaginase production and applications: A review. Process Biochem. 2022, 121, 529–541. [Google Scholar] [CrossRef]
- Swapnil, S.J.; Arpana, H.J. Isolation, Identification and Susceptibility Testing of Potential Lipase producing Bacterial and Fungal Strains from soil. Res. J. Biotechnol. 2019, 14, 8. [Google Scholar]
- Srinivasan, N.; Thangavelu, K.; Sekar, A.; Sanjeev, B.; Uthandi, S. Aspergillus caespitosus ASEF14, an oleaginous fungus as a potential candidate for biodiesel production using sago processing wastewater (SWW). Microb. Cell Factories 2021, 20, 179. [Google Scholar] [CrossRef]
- Guimarães, L.; Júnior, A.; Jorge, J.; Terenzi, H.; Polizeli, M. Purification and biochemical characterization of a mycelial alkaline phosphatase without DNAase activity produced by Aspergillus caespitosus. Folia Microbiol. 2007, 52, 231–236. [Google Scholar] [CrossRef]
- Kasirajan, L.; Kamaraj, K.; Maupin-Furlow, J.A.; Uthandi, S. Isolation, purification, and identification of novel lignin-degrading Aspergillus caespitosus strain S2. Biomass Convers. Biorefinery 2022, 14, 28685–28699. [Google Scholar] [CrossRef]
- Alegre, A.C.P.; Polizeli, M.d.L.T.d.M.; Terenzi, H.F.; Jorge, J.A.; Guimarães, L.H.S. Production of thermostable invertases by Aspergillus caespitosus under submerged or solid state fermentation using agroindustrial residues as carbon source. Braz. J. Microbiol. 2009, 40, 612–622. [Google Scholar] [CrossRef]
- Vimal, A.; Kumar, A. Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol. Genet. Eng. Rev. 2017, 33, 40–61. [Google Scholar] [CrossRef]
- Fernandes, M.L.P.; Veríssimo, L.A.A.; de Souza, A.C.; Schwan, R.F.; Dias, D.R. Low-cost agro-industrial sources as a substrate for the production of l-asparaginase using filamentous fungi. Biocatal. Agric. Biotechnol. 2021, 34, 102037. [Google Scholar] [CrossRef]
- Bharathi, S.; Yogesh, B.; Manasa, Y.; Pramod, T. Biotechnological potential of microbial L-asparaginases: Recent approach. In Enzymes-Mechanisms and Action; Jaya Publishing House: Delhi, India, 2023; pp. 1–23. [Google Scholar]
- Tiwari, A.; Khawas, R. Food waste and agro by-products: A step towards food sustainability. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Sitanggang, A.B. Production of lactulose from cheese whey. In Enzymes Beyond Traditional Applications in Dairy Science and Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 403–423. [Google Scholar] [CrossRef]
- Duan, F.; Zhao, R.; Yang, J.; Xiao, M.; Lu, L. Integrated utilization of dairy whey in probiotic β-galactosidase production and enzymatic synthesis of galacto-oligosaccharides. Catalysts 2021, 11, 658. [Google Scholar] [CrossRef]
- Clerici, N.J.; Lermen, A.M.; Daroit, D.J. Agro-industrial by-products as substrates for the production of bacterial protease and antioxidant hydrolysates. Biocatal. Agric. Biotechnol. 2021, 37, 102174. [Google Scholar] [CrossRef]
- Martin, A.A.; de Freitas, R.A.; Sassaki, G.L.; Evangelista, P.H.L.; Sierakowski, M.R. Chemical structure and physical-chemical properties of mucilage from the leaves of Pereskia aculeata. Food Hydrocoll. 2017, 70, 20–28. [Google Scholar] [CrossRef]
- Junior, F.A.L.; Conceição, M.C.; de Resende, J.V.; Junqueira, L.A.; Pereira, C.G.; Prado, M.E.T. Response surface methodology for optimization of the mucilage extraction process from Pereskia aculeata Miller. Food Hydrocoll. 2013, 33, 38–47. [Google Scholar] [CrossRef]
- Khanna, P.; Sundari, S.S.; Kumar, N.J. Production, isolation and partial purification of xylanases from an Aspergillus sp. World J. Microbiol. Biotechnol. 1995, 11, 242–243. [Google Scholar] [CrossRef]
- da Cunha, M.C.; Silva, L.C.; Sato, H.H.; de Castro, R.J.S. Using response surface methodology to improve the L-asparaginase production by Aspergillus niger under solid-state fermentation. Biocatal. Agric. Biotechnol. 2018, 16, 31–36. [Google Scholar] [CrossRef]
- Pandey, A.; Selvakumar, P.; Soccol, C.R.; Nigam, P. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 1999, 77, 149–162. [Google Scholar]
- Gulati, R.; Saxena, R.; Gupta, R. A rapid plate assay for screening l-asparaginase producing micro-organisms. Lett. Appl. Microbiol. 1997, 24, 23–26. [Google Scholar] [CrossRef]
- Cachumba, J.J.M.; Antunes, F.A.F.; Peres, G.F.D.; Brumano, L.P.; Santos, J.C.D.; Silva, S.S.D. Current applications and different approaches for microbial L-asparaginase production. Braz. J. Microbiol. 2016, 47, 77–85. [Google Scholar] [CrossRef]
- Dias, F.F.G.; Ruiz, A.L.T.G.; Della Torre, A.; Sato, H.H. Purification, characterization and antiproliferative activity of L-asparaginase from Aspergillus oryzae CCT 3940 with no glutaminase activity. Asian Pac. J. Trop. Biomed. 2016, 6, 785–794. [Google Scholar] [CrossRef]
- Imada, A.; Igarasi, S.; Nakahama, K.; Isono, M. Asparaginase and glutaminase activities of micro-organisms. Microbiology 1973, 76, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- de Oliveira, A.C.F.; Neves, I.C.O.; Saraiva, J.A.M.; de Carvalho, M.F.F.; Batista, G.A.; Veríssimo, L.A.A.; Resende, J.V.d. Capture of lysozyme on macroporous cryogels by hydrophobic affinity chromatography. Sep. Sci. Technol. 2019, 55, 2012–2024. [Google Scholar] [CrossRef]
- Neves, I.C.O.; Rodrigues, A.A.; Valentim, T.T.; de Oliveira Meira, A.C.F.; Silva, S.H.; Veríssimo, L.A.A.; de Resende, J.V. Amino acid-based hydrophobic affinity cryogel for protein purification from ora-pro-nobis (Pereskia aculeata Miller) leaves. J. Chromatogr. B 2020, 1161, 122435. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, T.; Camacho-Córdova, D.; Agamez-Montalvo, G.; Parizotto, L.d.A.; Sánchez-Moguel, I.; Pessoa-Jr, A. Optimization of culture conditions and bench-scale production of anticancer enzyme L-asparaginase by submerged fermentation from Aspergillus terreus CCT 7693. Prep. Biochem. Biotechnol. 2018, 49, 95–104. [Google Scholar] [CrossRef]
- Doriya, K.; Kumar, D.S. Optimization of solid substrate mixture and process parameters for the production of L-asparaginase and scale-up using tray bioreactor. Biocatal. Agric. Biotechnol. 2018, 13, 244–250. [Google Scholar] [CrossRef]
- Ding, K.; Liu, D.; Chen, X.; Zhang, H.; Shi, S.; Guo, X.; Zhou, L.; Han, L.; Xiao, W. Scalable lignocellulosic biorefineries: Technoeconomic review for efficient fermentable sugars production. Renew. Sustain. Energy Rev. 2024, 202, 114692. [Google Scholar] [CrossRef]
- Muso Cachumba, J.; Terán Hilares, R.; Brumano, L.; Marcelino, P.; Antunes, F.; Santos, J.; da Silva, S. Extracellular L-asparaginase production in solid-state fermentation by using sugarcane bagasse as support material. Prep. Biochem. Biotechnol. 2019, 49, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Uzma, F.; Narasimha Murthy, K.; Srinivas, C. Optimization of physiological conditions for L-asparaginase production by endophytic fungi (Fusarium solani) isolated from Tinospora cordifolia (Willd.) Hook. F & Thomson. Eur. J. Exp. Biol. 2016, 6, 37–45. [Google Scholar]
- Jaronski, S.T. Chapter 11: Mass production of entomopathogenic fungi—State of the art. Mass Prod. Benef. Org. Second Edition 2023, 317–357. [Google Scholar] [CrossRef]
- Chinnadurai, V.; Govindasamy, C. L-Asparaginase producing ability of Aspergillus species isolated from tapioca root soil and optimized ideal growth parameters for L-Asparaginase production. Environ. Res. 2024, 259, 119543. [Google Scholar] [CrossRef]
- Dias, F.F.G.; Santos Aguilar, J.G.d.; Sato, H.H. L-Asparaginase from Aspergillus spp.: Production based on kinetics, thermal stability and biochemical characterization. 3 Biotech 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Gurunathan, B.; Sahadevan, R. Optimization of Culture Conditions and Bench-Scale Production of $ _L $-Asparaginase by Submerged Fermentation of Aspergillus terreus MTCC 1782. J. Microbiol. Biotechnol. 2012, 22, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Abbas Ahmed, M.; Dahad, F.; Taha, M.T.; Hassan, S.F. Production, purification and characterization of L-asparaginase from marine endophytic Aspergillus sp. ALAA-2000 under submerged and solid state fermentation. J. Microb. Biochem. Technol. 2015, 7, 165–172. [Google Scholar] [CrossRef]
- Patro, K.R.; Basak, U.C.; Mohapatra, A.K.; Gupta, N. Development of new medium composition for en d production of L-asparaginase by Aspergillus f. J. Environ. Biol. 2013, 35, 295–300. [Google Scholar]
- Lincoln, L.; Niyonzima, F.N.; More, S.S. Purification and properties of a fungal L-asparaginase from Trichoderma viride pers: SF GREY. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 310–316. [Google Scholar]
- Patro, K.R.; Gupta, N. Extraction, purification and characterization of L-asparaginase from Penicillium sp. by submerged fermentation. Int. J. Biotechnol. Mol. Biol. Res. 2012, 3, 30–34. [Google Scholar] [CrossRef]
- Isaac, G.; Abu-Tahon, M. Production of extracellular anti-leukemic enzyme L-asparaginase from Fusarium solani AUMC 8615 grown under solid-state fermentation conditions: Purification and characterization of the free and immobilized enzyme. Egypt. J. Bot. 2016, 56, 799–816. [Google Scholar] [CrossRef]
- Veríssimo, L.A.A.; Paganoto, F.S.; Mol, P.C.G.; Ilheu Fontan, R.d.C.; Minim, V.P.R.; Minim, L.A. Preparation of an affinity cryogel column for lysozyme purification. Sep. Sci. Technol. 2017, 52, 1973–1982. [Google Scholar] [CrossRef]
- Kumar, A.; Bansal, V.; Andersson, J.; Roychoudhury, P.K.; Mattiasson, B. Supermacroporous cryogel matrix for integrated protein isolation: Immobilized metal affinity chromatographic purification of urokinase from cell culture broth of a human kidney cell line. J. Chromatogr. A 2006, 1103, 35–42. [Google Scholar] [CrossRef]
| Independent Variables | Code | Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Inoculum concentration (spores/g) | x1 | 105 | 106 | 107 |
| Temperature (°C) | x2 | 25 | 32.5 | 40 |
| Time (h) | x3 | 72 | 96 | 120 |
| OPNF concentration (% m/v) | x4 | 2 | 4 | 6 |
| L-asparagine concentration (% m/v) | x5 | 1 | 2 | 3 |
| Independent Variables | Code | Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| L-asparagine concentration (% m/v) | x1 | 10 | 20 | 30 |
| Whey protein concentration (% m/v) | x2 | 2 | 4 | 6 |
| Inoculum concentration (spores/mL) | x3 | 105 | 106 | 107 |
| Temperature (°C) | x4 | 25 | 32.5 | 40 |
| pH | x5 | 5 | 6 | 7 |
| Time (h) | x6 | 72 | 96 | 120 |
| Treatments | Sodium Phosphate Concentration (mg/mL) | pH | q (Mgproteín/Gcryogel) |
|---|---|---|---|
| 1 | 0.025 | 7.0 | 21.91 ± 2.38 b |
| 2 | 0.050 | 7.0 | 23.83 ± 0.42 ab |
| 3 | 0.100 | 7.0 | 14.65 ± 1.59 c |
| 4 | 0.025 | 3.0 | 24.38 ± 1.19 ab |
| 5 | 0.050 | 3.0 | 26.62 ± 0.51 a |
| 6 | 0.100 | 3.0 | 26.12 ± 0.51 a |
| Samples | Total Protein (mg/mL) | Specific Activity (U/mg) | Enzymatic Activity (U/mL) |
|---|---|---|---|
| Initial solution | 0.088 | 2.78 | 0.22 |
| Eluted | 0.008 | 4.40 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo, N.G.; Simões, L.A.; Fernandes, N.d.A.T.; Souza, A.C.; Fernandes, M.L.P.; Veríssimo, L.A.A.; Schwan, R.F.; Dias, D.R. Optimization of L-Asparaginase Production from Aspergillus caespitosus: Solid-State and Submerged Fermentation Using Low-Cost Substrates and Partial Purification. Appl. Microbiol. 2025, 5, 19. https://doi.org/10.3390/applmicrobiol5010019
Rabelo NG, Simões LA, Fernandes NdAT, Souza AC, Fernandes MLP, Veríssimo LAA, Schwan RF, Dias DR. Optimization of L-Asparaginase Production from Aspergillus caespitosus: Solid-State and Submerged Fermentation Using Low-Cost Substrates and Partial Purification. Applied Microbiology. 2025; 5(1):19. https://doi.org/10.3390/applmicrobiol5010019
Chicago/Turabian StyleRabelo, Natana Gontijo, Luara Aparecida Simões, Natália de Andrade Teixeira Fernandes, Angélica Cristina Souza, Maysa Lima Parente Fernandes, Lizzy Ayra Alcântara Veríssimo, Rosane Freitas Schwan, and Disney Ribeiro Dias. 2025. "Optimization of L-Asparaginase Production from Aspergillus caespitosus: Solid-State and Submerged Fermentation Using Low-Cost Substrates and Partial Purification" Applied Microbiology 5, no. 1: 19. https://doi.org/10.3390/applmicrobiol5010019
APA StyleRabelo, N. G., Simões, L. A., Fernandes, N. d. A. T., Souza, A. C., Fernandes, M. L. P., Veríssimo, L. A. A., Schwan, R. F., & Dias, D. R. (2025). Optimization of L-Asparaginase Production from Aspergillus caespitosus: Solid-State and Submerged Fermentation Using Low-Cost Substrates and Partial Purification. Applied Microbiology, 5(1), 19. https://doi.org/10.3390/applmicrobiol5010019






