1. Introduction
Due to the public health risks posed by enteric viruses in water, reliable indicators for their presence in drinking and recreational waters have long been sought. There has been an increased focus on the molecular detection of viruses in wastewater as a monitor of pathogen occurrence and spread since the COVID-19 pandemic. Although there are culture and molecular methods available for the detection of enteric viruses in water, the application of these methods is difficult, expensive and slow to yield results when attempting to simultaneously detect all types of human enteric viruses.
Coliphages, viruses infecting
Escherichia coli, have been proposed as potential viral indicators because they are structurally similar to enteric viruses, abundant in sewage and readily detectable by relatively simple, rapid culture methods. Coliphages are classified as male-specific and somatic. Male-specific (F+) coliphages infect male or F+ strains of
E. coli via attachment to F-pili, while somatic coliphages infect via direct attachment to receptors on the outer surface of the cell. Male-specific coliphages have been primarily studied as potential viral indicators in water due to their structural similarity to many enteric viruses [
1,
2,
3,
4,
5,
6]. However, F+ coliphages have limitations due to their low prevalence and concentrations in feces, variable concentrations in sewage, and rapid die-off in warmer water [
7].
Somatic coliphages have also been proposed as possible alternative viral indicators in sewage and water [
5,
8,
9,
10]. New and significant changes to the taxonomy of bacterial viruses have abolished the morphological families of
Myoviridae, Siphoviridae and
Podoviridae [
11,
12]. Despite these changes, somatic coliphages remain extremely diverse, with variations in DNA content, size, structure, and replication cycle, ranging from the relatively large, tailed, double-stranded DNA phages of the order
Caudoviriales to those of small size and cubic symmetry, containing single-stranded DNA in the
Microviridae family.
Previous studies have assessed the use of somatic coliphages as indicators of enteric viruses but have not systematically considered their taxonomic diversity [
5,
13,
14]. Despite their abundance, somatic coliphages found in fecal material have not been genetically characterized, and it is not clear if the relationships between the presence of somatic coliphages and enteric viruses in water are different based on the genetic group or strain of somatic coliphage. Previous studies of somatic coliphages as viral indicators treat somatic coliphages as a single homogeneous group despite their known genetic diversity [
14]. Due to their diversity, the presence and behavior of somatic coliphages in environmental water systems are potentially also variable based on their family and strain presence. Therefore, it is understandable why somatic coliphages, as a broad and diverse group, may have so far shown little significant association with enteric virus presence in water [
15].
To better determine whether somatic coliphages are useful indicators for human enteric viruses in water, the prevalence and comparative environmental persistence of members of the somatic taxonomic groups need to be evaluated. Characterization of the relative abundance in human fecal waste of specific virus families within the broad somatic coliphage group may enable the identification of individual families, groups or strains that are more feces-specific than the heterogeneous somatic coliphage group, as
Escherichia coli is a feces-specific bacterium within the larger coliform bacteria group [
16]. This approach is supported by previous studies that reported somatic coliphages from the
Myoviridae family in human sewage and somatic coliphages from the
Siphoviridae family are the most common group in surface waters [
17,
18,
19]. Comparative survival studies based on using and detecting different coliphage strains representative of each virus family or group can also help determine which coliphages are the most environmentally persistent, a key criterion for an ideal indicator [
20].
In order to examine the importance of the taxonomic identity of somatic coliphages as indicators, methods are needed to genetically distinguish and characterize them. Rapid nucleic acid-based molecular methods are promising not only for genetically characterizing somatic phages but also for routine environmental monitoring for the presence of these specific phage taxa in water and wastewater. Our goal in this study is to identify a candidate somatic coliphage family for use as an indicator of the presence of human enteric viruses in surface water based on their detection, occurrence and survival. To achieve this, we evaluated the comparative occurrence, prevalence and persistence of the main taxonomic group(s) among the various culturable somatic coliphages present in municipal sewage, followed by their molecular identification using newly developed nucleic acid-based molecular methods for their group-specific detection.
2. Materials and Methods
2.1. Coliphage Propagation and Purification
The type strains of somatic coliphage families used in this study were PhiX174 (
Microviridae), T4 and Mu (
Myoviridae), T7 and N4 (
Podoviridae), and T1, Lambda and HK97 (
Siphoviridae). While the taxonomy of the latter three of these four distinct groups has changed recently [
11,
12], we retained their distinct separation in this study, in part because of their morphological and genetic differences. Phages and their bacterial hosts were obtained from the Felix d’Hérelle Reference Center for Bacterial Viruses, University of Laval, Quebec, Canada. Bacteriophages were propagated as follows: T1, T4 and T7 in
E. coli B and PhiX174, Lambda, HK97 and N4 in
E. coli C,
E. coli K12S Lederberg,
E. coli Y mel mel-1 supF58 and
E. coli W3350, respectively. Each host strain was grown in tryptic soy broth (TSB; Difco). Coliphages were propagated in the appropriate host strain in TSB on a shaker platform (100 RPM) overnight at 36 °C. Overnight broth culture lysates were vigorously mixed with fluorocarbon (1,1,2-trichloro-1,2,2-trifluoroethane, FCCl
2CClF
2, Fisher Scientific product #T180) for 2 min and then centrifuged at 2600×
g for 15 min. This semi-purified virus supernatant was retained as virus stock. Each strain was further purified by filtering using a 0.22 µm pore size syringe filter. Host bacteria and prepared coliphage stocks were stored at −80 °C. Phage titers were determined by a single agar layer (SAL) plaque assay [
21].
2.2. Detection and Survival of Wild-Type Somatic Coliphages in Water
The detection, occurrence and survival of wild-type somatic coliphages were determined for those in primary effluent from the Orange County Water and Sewer Authority (OWASA), the wastewater treatment plant serving Chapel Hill, NC, a largely residential academic community with no major industrial waste sources. Primary effluent was collected and transported to the laboratory. A control sample to measure the initial somatic coliphage concentration at time zero (0) was taken and assayed immediately. One aliquot of effluent was held at room temperature (23–25 °C), and one was held at refrigerator temperature (4 °C). Samples were taken and assayed for virus infectivity by a single agar layer (SAL) plaque assay using E. coli CN-13 as the host at the time intervals of 10, 15, 29, 47 and 65 days. Duplicate samples were assayed at each time point.
At each time point, a representative number of somatic coliphage plaques (20–50) were chosen randomly (considering different plaque sizes and morphologies and were not spatially biased in one location or on one plate) from SAL plates for further molecular characterization. Plaques were picked using the tip of a micropipettor (20–200 µL capacity) set at a 100 µL volume, suspended in 100 µL of TSB and assayed for infectivity using a spot plate plaque assay technique with individual plaques in each spot counted to determine the sample titer [
22]. Individual plaques from each spot were picked, re-suspended and re-enriched for 24 h in TSB using the host bacterium
E. coli CN-13. After incubation, the enriched phage isolates were filtered to remove cell debris using 0.45 µm pore size filters and frozen at −80 °C for further molecular characterization.
2.3. Conventional PCR for Family- (or Group-) Level Identification
To determine which families or groups of somatic coliphages were present and detectable by molecular methods in primary sewage effluent, oligo-nucleotide primers targeting the members of each somatic coliphage family or group were developed using bioinformatics tools. Sequence analysis of all four families or groups was conducted by applying bioinformatic analysis of the full genome sequences of strains from the previously designated
Myoviridae (9 strains),
Microviridae (44 strains), the previously designated
Podoviridae (8 strains), and the previously designated
Siphoviridae (9 strains) archived in the National Center for Biotechnology Information (NCBI) website. Sequence analysis was performed using Vector NTI (ver.10, Invitrogen), MEGA (version 4, [
23]) and Jalview (version 2, [
24]). Multiple alignments were carried out with ClustalW2, and blast searches for protein–protein in the previously designated
Myoviridae and
Siphoviridae families were conducted to find conserved protein regions among strains in each family to target with family-specific primers.
The following positive control coliphages from each family or group were used to optimize PCR conditions: PhiX174 (Microviridae), T4 and Mu (Myoviridae), T7 and N4 (Podoviridae) and T1, Lambda and HK97 (Siphoviridae). Viral DNA was extracted from stocks of these positive control coliphages using a QIAamp viral mini kit (QIAGEN Inc., Hilden, Germany). After serial 10-fold dilution of positive control DNA, PCR was carried out using different sets of amplification conditions, including different annealing temperatures and cycle numbers. These conditions were developed based on information provided in the manufacturer’s instructions.
To determine the lower detection limit of the group-specific conventional PCR method, conventional PCR and a plaque assay (SAL) were applied to serial 10-fold dilutions of all positive somatic coliphage strains. Somatic coliphage stocks from each family were serially diluted ten-fold, coliphage DNA was extracted from each dilution, and the optimized conventional PCR procedure was applied to the extracted DNA. When viral DNA was not detected in the PCR reactions past a certain serial 10-fold dilution, this was taken as the detection limit endpoint of PCR detection relative to the plaque assay titers (SAL) of somatic coliphage strains.
2.4. Sequencing and Phylogenetic Analysis
For further characterization of somatic coliphage environmental isolates from primary sewage effluent, PCR products were purified (QIAquick PCR Purification kit; QIAGEN Inc.) and sequenced at the Genome Analysis Facility (University of North Carolina, Chapel Hill). Multiple sequence alignments and clustering of the sequenced isolates along with
Microviridae full genome sequences from the NCBI website were conducted with the ClustalW2 program at the European Bioinformatics Institute (EBI) website. Phylogenic analysis was conducted using the Neighbor-Joining method [
25]. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) was displayed next to the branches [
26]. The evolutionary distances were computed using the Kimura 2-parameter method as the substitution model in the nucleotide category in the MEGA4 program [
27]. Phylogenetic analyses were conducted in MEGA4 (Version 4, 21). Statistical analyses were conducted in SAS (version 9.2).
3. Results
3.1. Survival of Somatic Coliphages Isolated in Sewage Effluent
The infectivity concentrations of total somatic coliphages (all detectable genetic groups) in primary sewage at 4 °C and 23–25 °C are shown in
Table 1. The concentrations of total somatic coliphages decreased over time at both temperatures. However, the decline over time at 4 °C was less than at 23–25 °C, with 32 PFU/mL and 0.1 PFU/mL of an initial 400 PFU/mL remaining at 4 and 23–25 °C, respectively. At both temperatures, some phages underwent substantial declines after day 28, with 6-fold and 11-fold declines in phage titers between days 15 and 29 at 4 and 23–25 °C, respectively. The number of somatic coliphage isolates collected at each time point is also shown in
Table 1. After day 65, the infectivity concentration of total somatic coliphages at 23–25 °C had declined to 0.1 PFU/mL; thus, three somatic coliphage isolates were collected at this time point. From this time–course study of somatic coliphage survival in primary sewage effluent incubated at 23–25 °C and 4 °C, a total of 275 presumptive somatic coliphage isolates were collected and archived for subsequent taxonomic characterization by PCR.
3.2. Primer Design and Optimization of Multiplex PCR
The approach used for finding conserved regions, either by nucleotide or protein level analysis by viral family or group, is shown in
Table 2. After multiple alignment analysis by the ClustalW2 tool, strains were grouped by conserved regions of nucleotides or proteins in each family to find candidate target regions for primers.
The candidate taxonomic group-specific primers designed are shown in
Table 3. The
Microviridae and the
Podoviridae families or groups showed conserved regions for primer design in multiple alignment analysis at the nucleotide sequence level. The
Myoviridae and
Siphoviridae groups showed greater diversity at the nucleotide level, so a protein-level approach was needed to design subgroup-specific primers. The subgroup was chosen based on the conserved protein region identified in each family, with the primer location of the family selected through subgroup multiple alignment of the target group (
Table 3).
Once primers were selected, PCR conditions were optimized by comparing the results of varying annealing temperatures and amplification cycles. The PCR conditions that yielded a gradual decrease in the band thickness with serial dilution of the sample were selected as optimal and used for the further characterization of isolates. For the
Myoviridae group, the optimized PCR conditions were as follows: 95 °C—5 min, [95 °C—30 s, 58 °C—30 s, 72 °C—30 s] × 40 cycles, 72 °C—10 min and 4 °C—10 min. For the
Microviridae group, the optimized PCR conditions were as follows: 95 °C—5 min, [95 °C—30 s, 61 °C—30 s, 72 °C—30 s] × 40 cycles, 72 °C—10 min and 4 °C—10 min. For the HK97 and Lambda subgroup in the
Siphoviridae and N4 in the
Podoviridae, the optimized PCR conditions were as follows: 95 °C—5 min, [95 °C—30 s, 55 °C—30 s, 72 °C—30 s] × 40 cycles, 72 °C—10 min and 4 °C—10 min. For the JK subgroup of
Siphoviridae and the K1F subgroup of
Podoviridae, the optimized annealing temperatures were 51 °C and 63 °C, respectively. The other conditions were identical across subgroups.
Figure 1 is an electrophoresed agarose gel stained with ethidium bromide, showing all positive controls amplified at their optimized conditions. Cross-reaction or non-specific amplification within the primers of each subgroup and family was not found for any of the virus taxonomic groups studied.
3.3. Detection Limits of Taxonomic Group-Specific Conventional PCR
The lower detection limits of the group-specific conventional PCR of positive strains of each somatic coliphage family were determined, and the detection limits ranged from 0.4 to 2 × 10
5 PFU (
Table 4).
3.4. Somatic Coliphage Family Identification Using Family- or Group-Specific Conventional PCR
All environmental isolates collected from sewage survival experiments were tested using taxonomic group-specific primers and the optimized PCR conditions described above. As shown in
Table 5, the taxonomic diversity of the somatic coliphage population in environmental sewage samples changed over time. On day 0 and day 1, two of the four somatic coliphage families, Microviridae and Myoviridae, were detected among the isolates. In samples from days 47 and 65, only one of the four families, Microviridae, was detected. At later time points, the
Microviridae family became most prevalent in the surviving somatic coliphage population. At day 47 (31 positives/36 isolates) and day 65 (three positives/three isolates) at 23–25 °C, all PCR amplifiable isolates were from the Microviridae family, and none were from other taxonomic groups (
Table 5). There are relatively few
Microviridae among the day 1 samples (10 of 48 isolates), but they predominate at day 65 (31 of 47 at 4 °C and 3 of 3 at 25 °C). In addition, there were three samples positive for Myoviridae on days 0–1 and day 29, but no Siphoviridae or Podoviridae environmental isolates were detected (
Table 4). On day 0, the taxonomies of the majority of somatic coliphage isolates (approximately 70%) could not be determined by the group-specific conventional PCR method developed. The archived strains of each family from the NCBI website were enterobacteriophages with full genome sequences and not those with partial genome sequences. The developed group-specific PCR methods could detect the strains of each taxonomic group described in
Table 2. However, many strains in each group with partial genome sequences could not be detected by this method.
3.5. Sequencing and Survival Modeling
All PCR-positive environmental isolates cultured from primary sewage effluent were sequenced and further characterized. Myoviridae-positive isolates were mapped to T4 in the NCBI blast database. The best BLAST match for the positive isolates in the
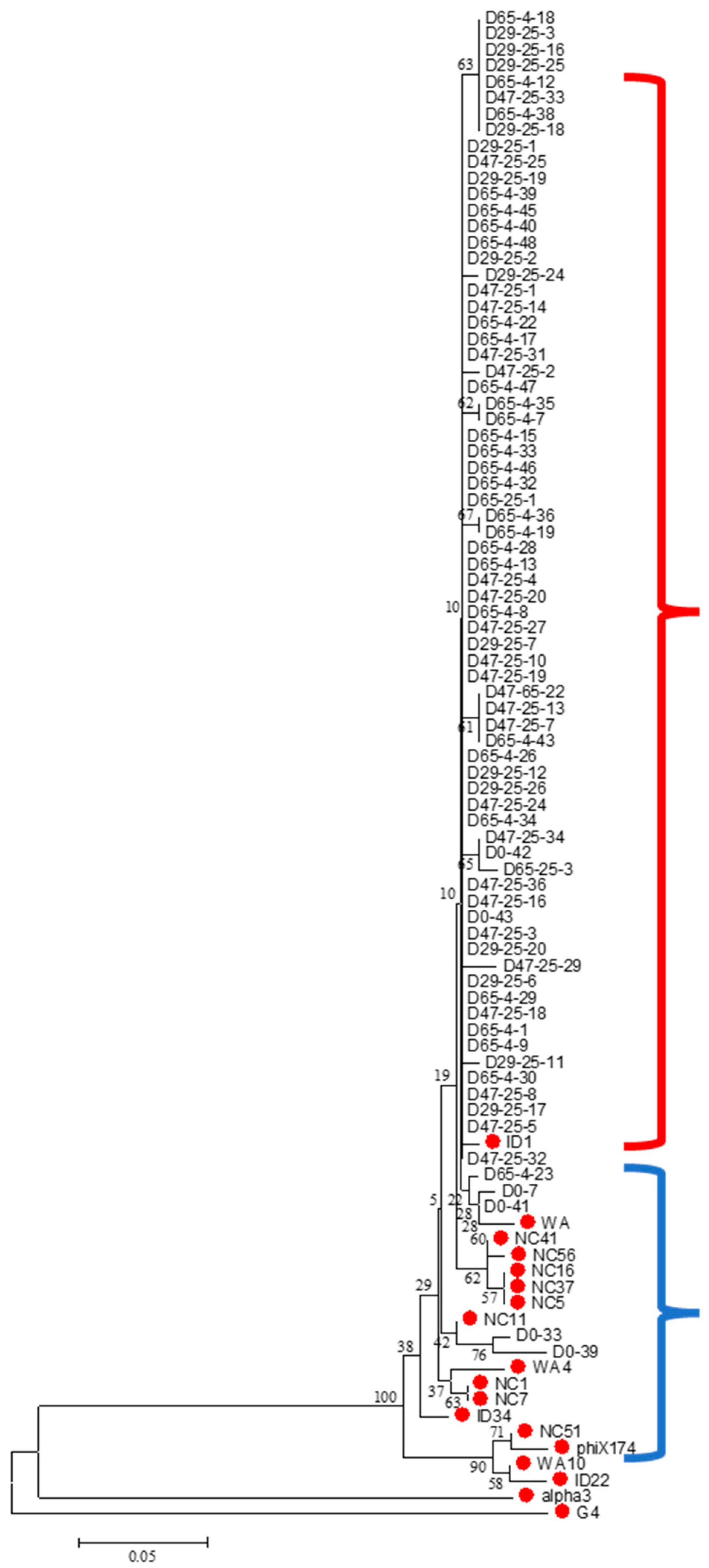
Microviridae family mapped to ID1, Enterobacteria phage PhiX174 sensu lato. The sequenced Microviridae-positive isolates were clustered to better describe the phylogenetic relationships among them (
Figure 1). As described in Rokyta et al. (2006) [
28], the Microviridae were grouped based on genome analysis into three subgroups: PhiX174-like, G4-like and Alpha-3-like. These three representative strains of this family and ID1 were included in the clustering. Most of the isolates were clustered with the PhiX174-like group.
The two different incubation temperatures (4 °C and 23–25 °C) were not considered for examining the association of Microviridae groups in this analysis because of the limited number of isolates per temperature. The associations of elapsed time in days and taxonomic groups of surviving phages were investigated using modeling approaches. A generalized linear model using a Poisson response was used to investigate the association of elapsed time in days and the taxonomic groups of surviving phages. Day variables include day 0, day 29, day 47 and day 65, and group variables include Group 1 and Group 2. Group 1 represents phage ID1, while Group 2 represents all the strains in the PhiX174 group of the Microviridae family except for ID1. The goodness-of-fit test of this model is significant (p < 0.001), so this regression model is significant. The day variable is a significant predictor of the average count of surviving phages in each group (p = 0.0002). Also, the group variable is a significant (p < 0.0001) predictor of the average count of surviving phages. A frequency test was conducted between taxonomic groups and days elapsed. The Chi-square test for the frequency of taxonomic groups by days elapsed showed less than 0.05 (p < 0.0001); therefore, the association between taxonomic groups and the elapsed time (day) was significant. In other words, the elapsed time in days is a significant variable to estimate the presence of each taxonomic group.
Also, a nominal logistic regression analysis was conducted to estimate the probability of the taxonomic groups of phages detected in the samples at each point in time. The p-value of the likelihood ratio of the regression model is less than 0.05 (p = 0.0006), so the logistic regression model is significant. The variable day in this model is significant (p = 0.0061); the time elapsed is a significant predictor of the surviving taxonomic groups detected in water. However, the missing values for Group 2 on day 29 make the results from the nominal logistic regression analysis questionable.
Using a generalized linear model with a Poisson response and a frequency test, the elapsed time in days is a significant predictor of the taxonomic groups of phages detected in the OWASA sewage samples.
4. Discussion
In this study, one particular somatic coliphage family,
Microviridae, is identified as a potential viral indicator of sewage because of its presence, persistence and increased relative abundance over time in stored sewage effluent when assayed by infectivity. Most studies to investigate somatic coliphage as viral indicators have treated them as a single microbial group, even though they are taxonomically heterogeneous and highly diverse [
5,
9,
10]. Although previous evidence suggests that there is a lack of correlation between enteric viruses and somatic coliphages taken as a whole group [
15], an individual family of somatic coliphages could potentially have a significant association with the occurrence of enteric viruses in water. Also, the conventional PCR method developed here for specifically identifying members of each somatic coliphage family or group could be used as a tool for characterizing coliphages of environmental waters using infectivity to detect the individual members of each somatic coliphage family or group in a total collection or pool of isolates. Our results were analyzed prior to the 2022 change in somatic coliphage taxonomy [
11,
12]; however, our results indicate that the
Microviridae family is the most prevalent and persistent group.
The infectious isolates from the survival study of somatic coliphages in municipal wastewater treatment plant primary sewage effluent were classified using optimized conventional group-specific PCR amplification methods for each individual taxonomic group. A total of 160 somatic coliphage isolates obtained by culture were used to investigate the genetic diversity of somatic coliphage environmental isolates from primary sewage effluent. The Microviridae family was the most prevalent among the identifiable isolates from sewage effluent. In addition, the surviving proportion of isolates belonging to the Microviridae family increased over time at 23–25 °C. This result suggests that somatic coliphages of the Microviridae family present in sewage are the most persistent members of the somatic coliphage population using the culture and detection methods we employed. However, further studies are needed to determine if this family might consistently be the most abundant and persistent in sewage from different human and animal sources under different conditions in environmental waters.
Most of the identifiable somatic coliphage isolates from primary sewage effluent belonged to the Microviridae and Myoviridae families or groups; no isolates were identified from the Siphoviridae or Podoviridae groups. Further studies are needed to determine if these results are characteristic of the culturable somatic coliphage make-up of primary sewage effluent or if there could be differences in those taxonomic groups present based on regional differences and seasonal effects and the presence of human and various animal sources.
It is also possible that the observed results are truly representative of the culturable somatic coliphage population as our conventional group-specific PCR method did not test all members of the Siphoviridae and Podoviridae groups, as they were not detected in our sample of sewage. There may have been culturable or non-culturable members of these groups present in the primary effluent that were not detected by the primers used; however, until recently, no molecular methods have been available to readily detect those coliphages. The representative somatic coliphage family type strains used in this study for finding conserved regions in each family or group were complete genomes that were archived from the NCBI website. Partial genomes present in the database were not used for the development of the family group-specific PCR. Therefore, it is possible that all strains in a family were not detected by this method because the culturable strains were not sufficiently representative of the genetic diversity of the family. It is also possible that the lower detection limit of the PCR was not low enough to detect the low numbers of somatic coliphages in the samples subjected to PCR despite their enrichment propagation. The lower detection limits of PCR ranged from as low as 0.4 PFU per reaction for the Lambda subgroup of Siphoviridae to as high as 2 × 105 PFU per reaction for the N4 subgroup of Podoviridae. The possibility that the lower detection limit of PCR amplification is related to the size (bp) of the target amplicon was tested using Spearman rank correlation analysis and found to be positive (r = 0.82) and significant (p = 0.034). The lack of PCR amplification and identification of enriched coliphage isolates has been reported by others in our laboratory, and the reasons for such findings remain uncertain. In addition, positive control coliphages were not available to optimize the PCR conditions for the subgroup of the 933 set in Podoviridae. Therefore, the selected candidate primers of the 933 set might not detect all possible members of Podoviridae.
Limitations of the work presented here include the lack of identification of some culturable coliphage isolates at the molecular level in the sewage effluent survival test, as some isolates could not be genetically classified. However, the Microviridae family appears to be both the most persistent and most abundant of the identifiable coliphage isolates of the four taxonomic groups detectable in the culturable somatic coliphage population of primary sewage effluent samples analyzed. These results suggest that the Microviridae family could serve as a candidate somatic coliphage viral indicator based on its observed abundance and persistence, as observed in the survival time course study of culturable somatic coliphages in primary sewage effluent.
Other work on somatic coliphage typing was based on the electron microscopic examination of viral particle morphology [
18,
29]. This type of electron microscopic analysis is usually tedious, slow and, at times, unreliable. Therefore, the new nucleic acid molecular-based typing methods for culturable somatic coliphage group detection developed in this study have the potential to provide a more convenient, reliable and rapid analytical approach to understanding the genetic composition of culturable environmental somatic coliphages detected and quantified in environmental samples. Additional research on further developing and applying nucleic acid-based detection and characterization methods will assist future studies to evaluate culturable somatic coliphages as enteric viral indicators for water quality assessments. These newly developed methods can be used to expand the body of evidence on the utility of culturable somatic coliphages as practical viral indicators by applying them in future studies of virological water quality and health. The optimized conventional group-specific PCR methods developed in this study make possible further investigation of the occurrence of the culturable and non-culturable somatic coliphage
Microviridae family, other somatic coliphage families and human enteric viruses to gain a better understanding of their relationships and their potential human health risk in environmental waters.