Abstract
Phosphorus (P) solubilization is one of the major traits for plant growth-promoting rhizobacteria since P is easily rendered insoluble in soil. Phosphate-solubilizing microorganisms can be harnessed as an environment-friendly strategy to enhance the mobilization and acquisition of P by crops. Utilization of such microorganisms as microbial inoculants in agriculture serves as an alternative to chemical fertilizers and an approach for more efficient P fertilization. Hence, this study aims to characterize a phosphate-solubilizing isolate and evaluate its potential as a microbial inoculant. Morphological, biochemical, and genetic characterization of the isolate were performed. Then, the mineral phosphate solubilization ability of the isolate was evaluated. Lastly, this study evaluated the plant growth promotion of the isolate as a single inoculant in rice or as a co-inoculant with rhizobia in peanuts. On the basis of biochemical and 16S rRNA analysis, the isolate was identified as Enterobacter sp. Also, it can solubilize P from tricalcium phosphate or aluminum phosphate. Simultaneous with P solubilization, medium acidification, and gluconic acid secretion were observed. Lastly, the Enterobacter sp. isolate could potentially be developed as a biofertilizer in reducing P resource input or to enhance the performance of a rhizobia inoculant.
1. Introduction
Plant growth emerges from the tripartite interaction of soil, microbiota, and plant [1,2]. The soil substrate provides the starting inoculum and is regulated by the plant, resulting in the composition and function of plant microbiota. Understanding this tripartite interaction serves as a guide for the development of sustainable agricultural management practices and products such as microbial inoculants or biofertilizers. One strategy is through manipulation of the structure and function of rhizosphere microbiota through microbial inoculants. Microbial inoculant contains beneficial microorganism to be applied in seed, soil, or compost with an aim to increase the population of the specific microorganism, thus enhancing the ability for the nutrient acquisition of plants [3]. Studies under laboratory conditions indicated that plant growth-promoting rhizobacteria could clearly improve plant growth through mineral acquisition from soil and/or protection against pathogens [4]. This was the basis for targeted isolation from root and rhizosphere specimens to be applied and marketed as microbial inoculants in agriculture [5].
Microbiota that promote mineral availability for the host plant are an attractive avenue for crop nutrition and yield [6]. Microbial solubilization of insoluble inorganic phosphate was first reported by [7]. Certain species of bacteria or fungi referred to as phosphate-solubilizing microorganisms (PSM) can grow in a culture media with insoluble phosphate compounds as the sole phosphorus (P) source. Phosphate solubilization can be attributed to the production of organic acids, which are products of microbial metabolism, such as a direct oxidation pathway on the outer side of the cytoplasmic membrane [8]. Many Gram-negative bacteria involve the secretion of gluconic acid generated by a direct oxidation pathway of glucose via the membrane-bound quinoprotein glucose dehydrogenase [9,10,11]. A study on the mineral phosphate solubilization ability of Serratia marcescens (Gram-negative bacteria) showed a significant drop in pH and secretion of gluconic acid during phosphate solubilization [12]. The PSM not only assimilates phosphorus but also releases a large portion of soluble phosphorus in excess of its own requirements; hence, it can contribute to plant P acquisition [13]. PSM application in agriculture is usually as a biofertilizer, and in field trials it exhibited a 0% to 20% increase in crop yield [14].
The concentration of available soil P is around 10 µM [15], which is much lower in comparison to plant tissue P (5 to 20 mM) [16]. By reason of the low concentration and poor mobility of plant-available P in soils, it is imperative to apply chemical P fertilizers to improve crop growth and yield. Most soluble inorganic phosphates as chemical fertilizers can be bound to metal ions after application in soil [17]. Soils in the tropics are commonly characterized by high P fixation capacity with an estimate of 1018 million hectares [18]. P is easily rendered insoluble as complexes of different types of phosphates such as variscite (AlPO4·2H2O) and strengite (FePO4·2H2O) in acidic agricultural soils [19] or bound to calcium, which is predominant in alkaline soils [20]. Thus, the efficiency of P fertilizer used on the crop is less and the farmers tend to increase chemical P fertilizer application higher than the crop’s need. Consequently, there is an excess of nutrients, which poses a threat to the environment, specifically water pollution [21]. In contrast, mineral P used for the production of P fertilizer is limited and has poor access in some areas, impacting food security targets [22]. P demand for crop production may double by 2050 [23], and by 2040, global P requirements will overtake the global P supply [24]. P access issues are a threat to food security in economically underdeveloped countries [25]. An estimated 1 out of 7 farmers could not afford sufficient fertilizers to meet crop production needs affecting their ability to produce food [26]. The increasing price of fertilizers is of great concern, as farmers may not be able to access sufficient P to produce food using the existing farming systems [27]. PSM can be harnessed as an environment-friendly strategy to enhance mobilization and acquisition of P by crops, further reducing P resource input.
Increased availability of P to crop plants has been documented by various workers when PSMs are present in rhizosphere soils. Solubilizing bound phosphates by PSM, thereby increasing soil P availability for rice, is a possible mechanism for growth promotion under field conditions [28]. An additional alternative approach to using PSM as a microbial inoculant is by using mixed cultures or co-inoculation with other microorganisms. The combination of PSM with other plant-growth-promoting rhizobacteria improved the biomass and root lengths of rice grown in organic paddy fields across India [29]. Another example is the co-inoculation of phosphate solubilizers with Rhizobium, resulting in growth promotion and enhanced N and P uptakes of chickpeas [30]. Therefore, the development of biofertilizers containing PSM provides an excellent avenue for an efficient and environmentally friendly approach to P fertilization in crops.
This research aims to characterize a phosphate-solubilizing isolate and evaluate its potential as a microbial inoculant. Morphological, biochemical, and genetic characterization of the isolate were performed. Then, the mineral phosphate solubilization ability of the isolate was evaluated. Lastly, this study demonstrated the plant growth promotion of the isolate as a single inoculant in rice or as a co-inoculant with rhizobia in peanuts.
2. Materials and Methods
2.1. Bacterial Strain
A phosphate-solubilizing bacterium named strain LG7 was originally isolated from a bulk soil sample in Negros Occidental, Philippines (10°24′31.0″ N 122°58′58.8″ E; 143 m asl; tropical monsoon climate). The isolate was cultured in nutrient agar (NA) (Condalab, Madrid, Spain) and phosphate solubilization ability was determined using a National Botanical Research Institute P (NBRIP) medium containing (pH 7.0) (g L–1) 10 glucose, 0.1 (NH4)2SO4, 0.2 KCl, 0.25 MgSO4·7H2O, 5 MgCl2·6H2O, 1.8% agar, and 5 Ca3(PO4)2. It was preserved and kept as a pure culture with accession number BIOTECH 10607 in the Philippine National Collection of Microorganisms (PNCM), National Institute of Molecular Biology and Biotechnology (BIOTECH), University of the Philippines Los Baños (UPLB).
2.2. Morphological and Biochemical Characterization
The cultural characteristics of isolate LG7 were determined by growing it on tryptic soy agar (TSA) (Condalab, Madrid, Spain) and incubated at 30 °C under aerobic conditions. Cellular traits were visualized using a compound light microscope (Olympus, Tokyo, Japan). Vitek® 2 Identification System GN card (Biomerieux, Craponne, France) was used to evaluate the substrate utilization/assimilation and identity of LG7. The card contained 47 biochemical tests and one negative control well.
2.3. DNA Extraction, Amplification, and Sequencing
The bacterial isolate was grown in nutrient broth (NB) (Condalab, Madrid, Spain) at 30 °C for 24 h. Cells were collected by centrifugation at 13,000× g for 2 min and washed with sterile distilled water. Genomic DNA was extracted following the modified bacterial genomic DNA isolation method [31].
A polymerase chain reaction (PCR) and sequencing of the 16S rRNA gene were performed using the bacterial universal primers 8F (5′ AGAGTTTGATCCTGGCTCAG 3′) and reverse primer 1512R (5′ ACGGCTACCTTGTTACGACT 3′) [32]. The gene was amplified using PCR mix (Vivantis, Selangor, Malaysia) containing (µL) 1 DNA template; 4 MgCl2; 0.5 dNTP; 1 forward primer; 1 reverse primer; 0.2 Taq polymerase; 2 buffer; and 10.3 nuclease-free water. Then, the PCR product was sequenced using Sequencing by Oligonucleotide Ligation and Detection platform (SOLiD) (Applied Biosystems). The sequences obtained were compared to the 16S rRNA gene sequences deposited in the GenBank database using BLAST online software (http://www.ncbi.nlm.nih.gov/BLAST; accessed on 17 March 2018). The phylogenetic tree based on the nucleotide sequence of the 16S rRNA gene sequences was aligned using Genetyx version 11 (Genetics). The phylogenetic tree was constructed based on the neighbor-joining algorithm with 1000 replications using the bootstrap method and the maximum composite likelihood model without topology. These processes were conducted using Molecular Evolutionary Genetics Analysis (MEGA) software (version 6.0; Pennsylvania State University, State College, PA, USA) [33]. The DNA sequence was deposited in the DNA Data Bank of Japan (DDBJ) under the accession number LC818941.
2.4. Effect of Carbon Source on Mineral Phosphate Solubilization
The effect of varying carbon sources for mineral phosphate solubilization (MPS) was observed by culturing LG7 of 107 colony forming unit mL−1 (CFU mL−1) in solid and liquid NBRIP with 5 g L−1 Ca3(PO4)2 or AlPO4 without any carbon source added or in the presence of 10 g L−1 glucose, mannitol, or sucrose. The carbon source supporting the maximal MPS was chosen to be used in the succeeding estimation of MPS ability. The phosphate solubilizing ability of the isolate on the solid NBRIP was determined by the presence of the halo zone around the colony after 7 days. Moreover, qualitative estimation was determined in NBRIP broth with pH indicators [34]: bromocresol purple for Ca3(PO4)2 and bromophenol blue for AlPO4. Color change indicates phosphate solubilization.
For the quantitative estimation, LG7 inoculated NBRIP broth was incubated at 30 °C under shaking at 160 rpm. After 7-day incubation, the supernatant was collected by centrifugation at 13,000× g for 10 min. Soluble P released in the supernatant was quantified using the P method [35]. The experiment was performed in triplicate. Uninoculated samples served as negative controls. The positive control bacterium Pseudomonas aeruginosa BIOTECH 1335, which is a known phosphate solubilizer [36], was supplied by the PNCM, BIOTECH, and UPLB.
2.5. Estimation of Phosphate-Solubilizing Ability and Gluconic Acid
The carbon source that supported the maximum phosphate solubilization was used in this experiment. The bacterial isolate was grown in NB at 30 °C for 24 h. The bacterial cells with a density of 107 CFU ml−1 were transferred to a solid NBRIP medium supplemented with 5 g L−1 Ca3(PO4)2 or AlPO4. The phosphate solubilizing ability of the isolate on the solid NBRIP was measured by the halo zone, phosphate solubilizing (PS) index was calculated as previously described [37].
In addition, a quantitative estimation of the PS ability of LG7 was performed. The bacterial cells with a density of 107 CFU mL−1 were put into a liquid NBRIP medium supplemented with 5 g L−1 Ca3(PO4)2 or AlPO4. After 3 and 7-day incubation at 30 °C under shaking at 160 rpm, the supernatant was collected by centrifugation at 13,000× g for 10 min. Soluble P released in the supernatant was quantified [35], and pH was also noted. In addition, the quantification of secreted gluconic acid in the supernatant was quantified colorimetrically following the protocol of the D-gluconic acid assay kit (Sigma-Aldrich, St. Louis, MO, USA). The experiment was performed in triplicate. Uninoculated samples served as negative controls, while BIOTECH 1335 served as the positive control bacterium.
2.6. Plant Growth Assay as Single Inoculant
The effectiveness of LG7 on the P nutrition of rice was evaluated under screenhouse conditions. The pot experiment was conducted in a screenhouse at BIOTECH, UPLB, Laguna, Philippines (14°08′58.7″ N 121°15′42.4″ E; 22 m asl). Pre-germinated rice ‘NSIC Rc222’ seeds were soaked in LG7 inoculated NB (107 CFU ml−1) for 1 h before sowing in a pot (20 × 10 × 15 cm) containing 2 kg of unsterile Ultisol soil with pH of 5.7 and low available P (4.8 mg kg−1) (Supplementary Table S1). NSIC Rc222 was chosen for this assay because it is one of the most popular rice varieties cultivated by farmers in the Philippines [38]. This rice variety developed by the International Rice Research Institute has an average yield of 6.1 tons ha−1.
The experiment was set up using factorial in a completely randomized design with three replicates, with each pot containing two plants. The first factor is on varying rates of chemical P fertilizer application while the second factor is inoculation treatment. In this study, solophos containing 18% of P2O5, 10% of S, and 18% of CaO were used as P sources. The amount of solophos fertilizer applied was based on the recommended rate of phosphorus fertilizer (RRP) on rice, which is 21 mg P2O5 dm3−1. In particular, the P fertilizer application treatments were no application (no; native soil P without external P addition), half recommended rate of P fertilizer (1/2 RRP; addition at 4.5 mg P dm3−1), and recommended rate of P fertilizer (RRP; addition at 9 mg P dm3−1). Nitrogen fertilizer, which is urea (73 mg N dm3−1), was applied in all the samples. Uninoculated plants served as negative control. Each pot was irrigated with tap water at 100% water-holding capacity throughout the cultivation period.
The plants were harvested eight weeks after sowing, and plant height was measured. The aboveground parts were then dried at 80 °C until constant weight to determine dry weight. Furthermore, the dried aboveground parts were ground. The plant samples were then subjected to dry ashing. Then, HCl was added to dehydrate SiO2 followed by diluting the sample to make aliquot filtrate. The P concentration was determined using ammonium molybdate according to the total P analysis [35] with three replicates.
2.7. Plant Growth Assay as Co-Inoculant with Rhizobium
2.7.1. Inoculant Production
For the inoculant preparation, LG7 was cultured in NB while the rhizobium was inoculated in yeast extract mannitol broth (YEM) containing (pH 6.5) (g L–1) 10 mannitol, 0.5 KH2PO4, 0.2 Mg SO4·7H2O, 0.1 NaCl, and 0.5 yeast extract. The LG7 and rhizobia were incubated at 30 °C under constant shaking at 24 and 72 h, respectively. The bacterial cultures’ cell density was adjusted to 107 CFU mL−1 and were inoculated in soil–charcoal carrier separately. Equal portions of rhizobia and LG7 inoculants were then mixed and used for the plant assays.
2.7.2. Viability and Competence of LG7-Rhizobia Inoculant
The viability of LG7 in combination with the rhizobia inoculant on peanut seeds and roots was monitored by the spread plate method under growth room conditions. The LG7-rhizobia inoculant was applied onto slightly moistened surface sterilized peanut seeds at the rate of 100 g of inoculant: 50 kg of seeds. Three subsamples of 25 seeds were suspended in 25 mL of sterile distilled water and shaken vigorously. The remaining peanut seeds were sowed into an autoclavable container (7.6 × 7.6 × 10.2 cm) with 40 g vermiculite. The plants were grown in a growth chamber room under controlled environmental conditions with 16 h light (5000–7000 lx) and 8 h darkness and maintained at 28 ± 2 °C. Each container was irrigated with half-strength N-free solution [39] at 100% water-holding capacity for vermiculite throughout the cultivation period. The population of LG7 and rhizobia on the seed and roots was counted by spread plate into NA with a 24 h incubation period and yeast extract mannitol agar (YEMA) incubated at 72 h. The experiment was a completely randomized design with three replicates for each treatment.
2.7.3. Screenhouse Experiment of LG7-Rhizobia Inoculant
Finally, the effectiveness of the rhizobia inoculant with LG7 as co-inoculants on peanut ‘Biyaya’ was evaluated in a screenhouse at the BIOTECH, UPLB, Laguna, Philippines. Biyaya is a high-yielding and early-maturing peanut variety [40]. The peanut seeds were coated with the inoculants before sowing in a pot (20 × 10 × 15 cm) containing 2 kg unsterile Ultisol soil.
The pot experiment was set up using factorial in a completely randomized design with four replicates, with each pot containing two plants. The first factor is varying rates of chemical P fertilizer application, while the second factor is inoculation treatment. A commercial fertilizer solophos was used as a P source, and the amount applied was based on the recommended P rate of 16 mg P2O5 dm3−1. Specifically, the P fertilizer application treatments were no application (no; no external P addition), half recommended rate of P fertilizer (1/2 RRP; addition at 3.5 mg P dm3−1), and recommended rate of P fertilizer (RRP; addition at 7 mg P dm3−1). Plants inoculated with rhizobium alone served as negative control. Each pot was irrigated with tap water at 100% water-holding capacity throughout the cultivation period. Plant height and number of nodules were determined six weeks after sowing. Aboveground dry weight was measured after the plant samples were dried at 80 °C until constant weight. N and P concentrations in the tissues were determined with four replicates [35]. The dried plant samples were ground and subjected to dry ashing. For P analysis, HCl was added to dehydrate SiO2 followed by diluting the sample to make aliquot filtrate. The P concentration was determined using the ammonium molybdate method. The remaining plant tissue samples were used for N determination following the Kjeldahl method. Post-harvest soil P content was also measured with four replicates [35]. Air-dried soil samples were extracted for phosphate using 0.5 M NaHCO3 (pH 8.5). P concentration was determined using the ammonium molybdate method.
2.8. Statistical Analyses
Experiment data were statistically analyzed using SAS software (SAS Institute Inc., Cary, NC, USA). Specifically, Tukey’s test, Dunnett’s test, and Spearman’s rank correlation were carried out using SAS software. Redundancy analysis was visualized using R (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria). Lastly, a phylogenetic tree was constructed using MEGA (version 6.0; Pennsylvania State University, State College, PA, USA).
3. Results
3.1. Morphological, Biochemical, and Genetic Characterization of LG7
Isolate LG7 produced translucent, circular, shiny, and convex colonies with entire margins on TSA. The colony diameter was estimated at 1.5–2.0 mm. For cellular characteristics, LG7 is Gram-negative and observed as coccoidal rods arranged in singles, pairs and clumps. Lastly, the size of each cell was 0.5–1.0 µm in length and 0.5 µm in width.
The biochemical profile of isolate LG7 is presented in Table 1. To name a few, it can utilize glucose, mannitol, or sucrose. LG7 also has resistance to 0/129 compound. Referring to the database of Vitek® 2 Compact Identification System for GN Card, the identification results reflected the closest phenotypic match, which was Enterobacter sp. with 97% percentage probability.

Table 1.
Biochemical tests were used to characterize LG7 with the GN card of the VITEK® 2 system.
The closest reference type strains for LG7 were summarized in the phylogenetic analysis and were strengthened by using an outgroup (Thermococcus gammatolerans EJ3) (Figure 1). In parallel with the biochemical identification, LG7 was classified as Enterobacter sp. using 16S rRNA gene sequencing.
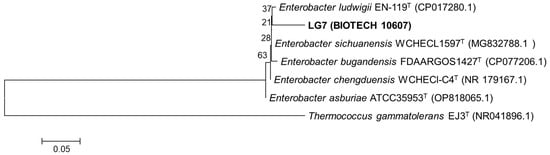
Figure 1.
Phylogenetic tree based on 16S rRNA gene sequencing of isolate LG7 (BIOTECH 10607) and type strains. Numbers at the nodes indicate the level of bootstrap support (%) based on a 1500 bp DNA fragment and neighbor-joining analysis with 1000 replications. The scale bar indicates 0.05 changes per site.
3.2. Effect of Varying Carbon Sources on Mineral Phosphate Solubilization
The effect of carbon sources on the solubilization of P from Ca3(PO4)2 (Ca-P) or AlPO4 (Al-P) was qualitatively estimated by noting clearing around the colonies on solid P growth medium and change in color of pH indicator on liquid P medium are summarized in Supplementary Table S2. Based on the screening on a solid medium, isolates were only able to solubilize P from Ca-P with glucose as a carbon source. On the other hand, P solubilization from Ca-P and Al-P was observed in isolate LG7 with glucose, sucrose, and mannitol as carbon sources based on liquid medium assessment (Supplementary Figure S1A,B). Isolate BIOTECH 1335 was only able to solubilize P from Ca-P with glucose as a C source in a liquid medium, while no discernible color change was observed in Al-P (Supplementary Figure S1C,D).
In addition, quantitative estimation revealed that glucose supported the maximum Ca-P solubilization for LG7, followed by sucrose, then mannitol (Figure 2A). Aside from glucose, it can also utilize mannitol and sucrose for Al-P solubilization (Figure 2B).
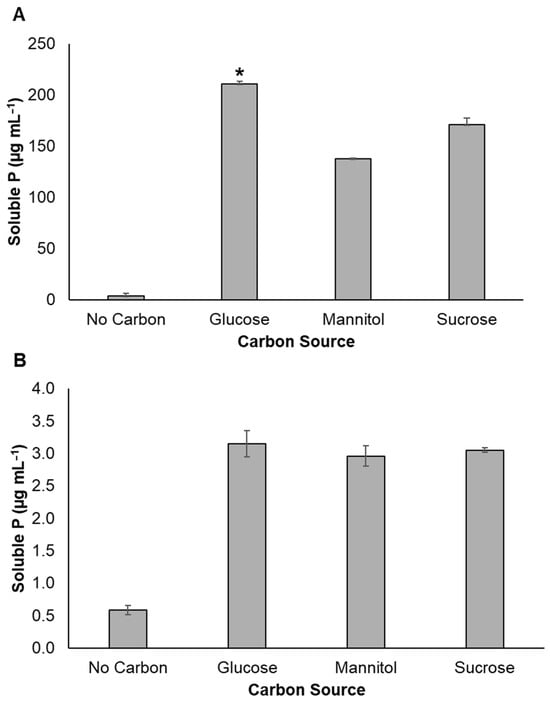
Figure 2.
Quantitative estimation of phosphate solubilization of isolate LG7 on P growth media amended with tricalcium phosphate (A) or aluminum phosphate (B) with varying carbon sources. The values represent the net soluble P by deducting the value of uninoculated samples. Statistical differences were determined by Tukey’s test. One asterisk indicates p < 0.05 among glucose and other treatments. Means and standard deviations (n = 3) are shown.
3.3. Estimation of Phosphate-Solubilizing Ability and Gluconic Acid
Estimation of Ca-P or Al-P solubilizing ability and secreted gluconic acid by LG7 was performed using glucose as a carbon source. The concentration of soluble P in the medium released from Ca-P by LG7 increased gradually and reached 171.9 µg mL−1 and 210.4 µg mL−1 after a growth period of 3 and 7 days after inoculation (DAI), respectively (Figure 3A). Positive control coded as BIOTECH 1335 released soluble P from Ca-P with concentrations of 183.9 µg mL−1 and 275.8 µg mL−1, respectively. Moreover, acidification of the medium was noted after the incubation period. Significant pH drops from uninoculated sample pH of 6.2 to pH of 4.5 and 4.3 by LG7 and BIOTECH 1335, respectively.
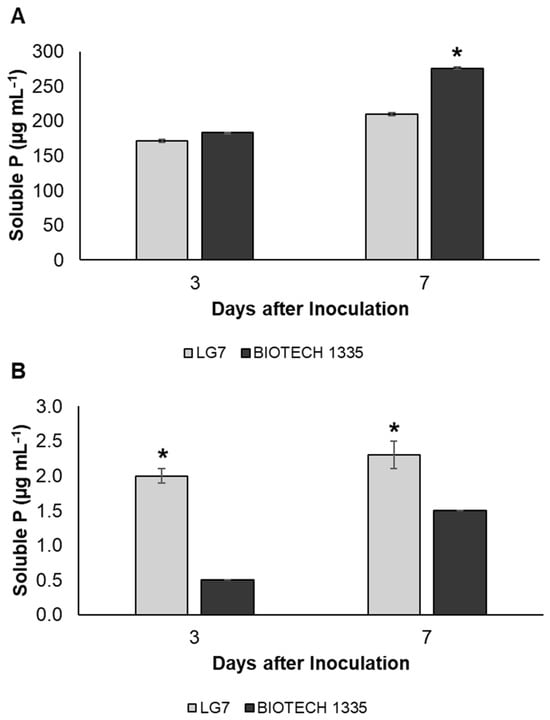
Figure 3.
Quantitative estimation of phosphate solubilization of isolate LG7 on P growth media amended with tricalcium phosphate (A) or aluminum phosphate (B). The values represent the net soluble P by deducting the value of uninoculated samples. BIOTECH 1335 served as a positive control. Significant differences were determined by Tukey’s test. One asterisk indicates p < 0.05 between LG7 and BIOTECH 1335. Means and standard deviations (n = 3) are shown.
In Al-P solubilization (Figure 3B), concentrations of soluble P released by LG7 were 2.0 µg mL−1 and 2.3 µg mL−1 during the periods at 3 and 7 DAI. BIOTECH 1335 gave lower solubilized P with concentrations of 0.5 µg mL−1 and 1.5 µg mL−1, respectively. Likewise, in Ca-P solubilization, there is a significant decrease in the pH of the P growth medium with Al-P after incubation. The pH 5.3 of the uninoculated sample declined in samples inoculated with LG7 (pH: 3.8) and BIOTECH 1335 (pH:4.0).
Gluconic acid (GA) was detected in the inoculated P growth media with different inorganic P sources. Estimation of gluconic acid simultaneous with P release from Ca-P showed that the isolate LG7 (Figure 4A) produced 383 ng µL−1 and 375 ng µL−1 of GA with consequent P solubilized at 171.9 µg mL−1 and 210.4 µg mL−1 at 3 and 7 DAI, respectively. BIOTECH 1335 (514.4 µg mL−1) secreted significantly higher gluconic acid compared to LG7 at 7 DAI. For Al-P solubilization, a decreasing trend was observed in the GA produced by the isolates, while an increasing trend was shown in P solubilization. LG7 produced 983.9 ng µL−1 and 676.3 ng µL−1 of GA with P release at 2.0 µg mL−1 and 2.3 µg mL−1 at 3 and 7 DAI, respectively. In parallel to Al-P solubilization, BIOTECH 1335 secreted lower amounts of GA in comparison to LG7.
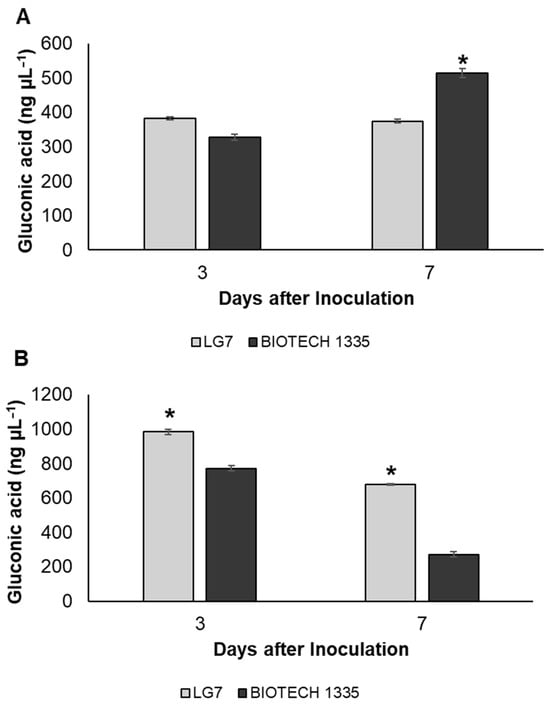
Figure 4.
Gluconic acid secreted by isolate LG7 on P growth media amended with tricalcium phosphate (A) or aluminum phosphate (B). BIOTECH 1335 served as a positive control. Significant differences were determined by Tukey’s test. One asterisk indicates p < 0.05 between LG7 and BIOTECH 1335. Means and standard deviations (n = 3) are shown.
3.4. Effect of Single Inoculation of LG7 on Rice Growth Promotion
The effectiveness of single inoculation on rice growth promotion and P nutrition was evaluated under screenhouse conditions. Irrespective of P fertilizer rates, inoculation treatment significantly affects the plant height of rice ‘NSIC Rc222’. LG7 inoculated plants with ½ RRP fertilizer gave maximal plant height (Figure 5A). In ½ RRP, LG7 inoculated plants gave a significantly increased aboveground dry weight compared to uninoculated rice (Figure 5B). Lastly, inoculation with LG7 and ½ RRP improved P uptake over uninoculated rice (Figure 5C).
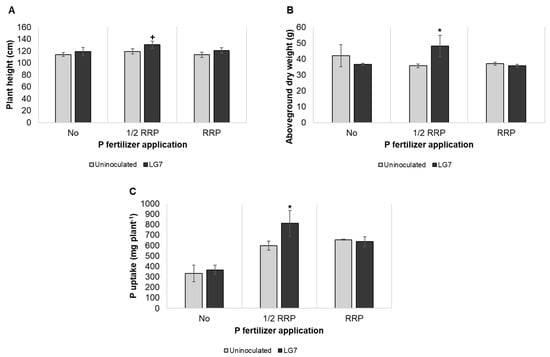
Figure 5.
Plant growth promotion of LG7 on rice ‘NSIC Rc222’ at eight weeks after sowing. Plant length (A), Aboveground dry weight (B), P uptake (C). Significant differences between uninoculated and LG7 were determined by Dunnett’s test (* p < 0.05; + p < 0.10). The error bar indicates the standard deviation of three replications.
Redundancy analysis was performed to assess the relationship between P solubilizing ability and the plant-growth traits determined (Supplementary Figure S3). Spearman’s rank correlation was also performed to evaluate the significance of the relationships (Supplementary Table S3). The first two RDA components (RDA 1 and RDA 2) explained 78% and 6% of the total variance of the determined plant growth traits, respectively. Positive relationships among P solubilizing ability, GA secretion, and plant-growth traits under ½ RRP were observed. It was found that a positive relationship of plant height with P solubilization (ρ = 0.76) and gluconic acid secretion (ρ = 0.94). Also, GA secretion in Al-P was in a positive relationship (ρ = 0.76) with the aboveground dry weight and P uptake, likewise positive correlation of these traits with GA production in CA-P medium (ρ = 0.88).
3.5. Effect of Co-Inoculation of LG7 and Rhizobia on Peanut Growth Promotion
The viability of LG7 in combination with rhizobia was first monitored on peanut seeds and roots under growth room conditions. LG7 and rhizobia are viable on the seeds with 105 CFU g−1 seed after coating. Furthermore, the population count of LG7 on peanut roots increased steadily, reaching 107 CFU g−1 dry root until the 6th week of growth (Supplementary Figure S2). The isolate was able to colonize the peanut roots and survive for several weeks while the population of rhizobia was maintained at 106 CFU g−1 dry root.
Based on the pot experiment, the number of nodules was significantly increased by co-inoculation of LG7 and rhizobia under the recommended rate of P fertilization (Figure 6A). Also, co-inoculation improved the aboveground dry weight in comparison to rhizobia under RRP (Figure 6B). Irrespective of P fertilization, no significant increase in plant height by inoculation treatment (Supplementary Figure S4A). Co-inoculation augmented the N (50%) and P (42%) uptake with RRP in comparison to single inoculation of rhizobia (Supplementary Figure S4B,C). Lastly, co-inoculation gave a substantial increase in post-harvest soil P content than rhizobia alone under RRP (Figure 6C).
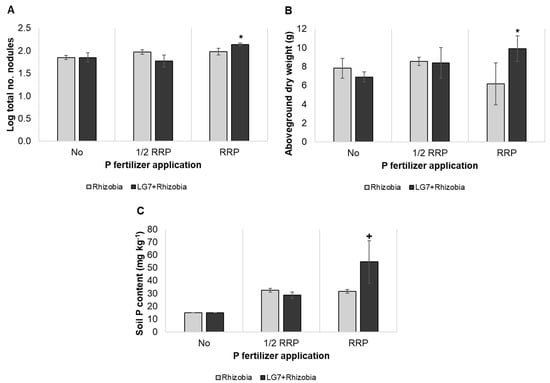
Figure 6.
Plant growth promotion and post-harvest soil P content of combined LG7 and rhizobia inoculant on peanut ‘Biyaya’ at six weeks after sowing. Total nodule number (A), Aboveground dry weight (B), soil P content (C). Significant differences between rhizobia alone and LG7 + rhizobia were determined by Dunnett’s test (* p < 0.05; + p < 0.10). The error bar indicates the standard deviation of four replications.
4. Discussion
The strain LG7, which has been assigned BIOTECH 10607 (deposited in PNCM, BIOTECH, UPLB), was isolated in tropical soil with plant growth-promoting (PGP) traits. On the basis of biochemical characterization and 16S rRNA analysis, LG7 was identified as Enterobacter sp. A similar bacterial genus was isolated from postmining soil samples in the Philippines with PGP characters [41], from semi-arid tropics [42,43,44], or rice paddy fields [45]. Tropical soils are often regarded as acidic, have low fertility, and have high P fixation capacity [18]; thus, phosphate solubilization is one important trait for the development of microbial inoculants in the tropics. Mineral phosphate solubilization of the isolate was characterized using a selective medium with no soluble P. Two insoluble P sources were used (Ca3[PO4]2 and AlPO4) in the characterization since P forms are dependent on soil pH, and there is no universal metal phosphate compound used in the screening of PSM [46]. Enterobacter sp. BIOTECH 10607 exhibited solubilization of P from both tricalcium phosphate and aluminum phosphate in parallel with the findings of studies on MPS of the genus Enterobacter [47,48,49,50]. This suggests that this isolate has the potential to solubilize different P forms in soils with different pH.
Medium acidification was observed in concomitant with MPS of Enterobacter sp. BIOTECH 10607. The decline of pH in MPS of BIOTECH 10607 was inversely correlated with the amount of released P, as reported in other studies [12,48,51]. The significant decrease in pH may be due to the secretion of organic acids such as gluconic acid. GA secretion in both Al-P and Ca-P solubilization was observed. GA secretion is a well-known mechanism of MPS in Gram-negative bacteria, especially in the Enterobacteriaceae family [9,10,52]. The bacterial genus Enterobacter was regarded as a good GA-producing bacteria [53]. This low-molecular-weight organic acid results from the extracellular oxidation of glucose [54]; in relation, glucose as a carbon source supported the maximum phosphate solubilization of Enterobacter sp. BIOTECH 10607. The Ca-P solubilization of BIOTECH 10607 was brought upon by a significant decrease in pH caused by GA secretion. However, the amount of secreted GA is inversely proportional to solubilized P from aluminum phosphate in this study, although medium acidification was still observed. This is in agreement with the result of the study that no significant relationship was observed with organic acids but had associations with a decrease in medium pH [55]. This suggests that Enterobacter sp. BIOTECH 10607 possibly exudes other types of organic acids or other compounds, such as inorganic acids or H+, that can change the pH of the medium for the dissolution of AlPO4. It is imperative to study other exudates from the isolate to fully understand its mechanism on MPS.
The bacterial genus Enterobacter has demonstrated its plant growth improvement on various crops such as tomato [56], maize [57], or Jathropa [58]. In this study, BIOTECH 10607 demonstrated plant growth promotion as a single inoculant in rice ‘NSIC Rc222’ and as a co-inoculant with rhizobia to peanut ‘Biyaya’ in Ultisol soil. In rice, an increase in aboveground biomass and P uptake was observed in the inoculation of BIOTECH 10607 with the application of ½ RRP. Similarly, a significant increase in total P uptake was observed in the inoculation of Pantoea cypripedii, which belongs to the Enterobacteriaceae family, along with rock phosphate fertilization compared with control [59]. Interestingly, significant positive correlations were observed among PS ability, GA secreted, and plant-growth traits under ½ RRP. Enterobacter sp. BIOTECH 10607 may aid in rice growth promotion through the secretion of GA that might mobilize the soil P for plant uptake. It was also demonstrated that inoculation of BIOTECH 10607 can improve rice growth even with lesser P input. Reduction of P fertilizer input in crop production is important especially in underdeveloped areas which are vulnerable to rising prices of fertilizers.
Another approach for PSM as a microbial inoculant is mixed cultures or combinations with other plant-growth-promoting microorganisms. The aboveground biomass and N and P uptakes of peanut were augmented by inoculation with combined BIOTECH 10607-rhizobia inoculant in comparison to single inoculation of rhizobia. P is essential in legumes in the form of adenosine triphosphate for nodule formation and biological nitrogen fixation [60,61,62]. BIOTECH 10607 as PSB can assist in the P nutrition of peanuts, thereby in nodule formation and N2 fixation. Furthermore, enhanced P content in post-harvest soil was observed in co-inoculation with BIOTECH 10607, in congruence to the findings that mixed culture of Rhizobium and phosphate solubilizer increased available P content in soil [30]. BIOTECH 10607 increases the availability of P in the soil for plant uptake, thus helping in the P nutrition of peanuts. The adequate P then aids in the nodule formation and N2 fixation by the rhizobia. A study on the synergistic interaction of BIOTECH 10607 and rhizobia follows through.
In conclusion, a phosphate-solubilizing Enterobacter sp. BIOTECH 10607, which secretes gluconic acid has the potential as a microbial inoculant. Also, it can enhance the performance of rhizobia inoculant in peanuts. However, further studies are needed to confirm its efficacy as a microbial inoculant in high P fixation areas, elucidation of its aluminum phosphate solubilization mechanism, and interaction studies with rhizobia.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/applmicrobiol4030080/s1, Figure S1. Change in color intensity of selective P growth media with tricalcium phosphate (A,C) or aluminum phosphate (B,D) in different carbon sources inoculated with LG7 and BIOTECH 1335. No C: No carbon; Glu: Glucose; Man: Mannitol; Suc: Sucrose; Figure S2. Population of LG7 and rhizobia on peanut ‘Biyaya’ roots under growth room conditions for six weeks. Means (n = 3) are shown. Figure S3. Redundancy analysis plot of the correlation among phosphate solubilization ability, gluconic acid secreted, and rice growth traits. AlPS: aluminum phosphate solubilization; CaPS: tricalcium phosphate solubilization; Al_GA: gluconic acid secreted from AlPS; Ca_GA: gluconic acid secreted from CaPS; NP_PHT: plant height under no P application; NP_ADW: aboveground dry weight under no P application; NP_Pup: P uptake under no P application; HP_PHT: plant height under half P application; HP_ADW: aboveground dry weight under half P application; HP_Pup: P uptake under half P application; FP_PHT: plant height under full P application; FP_ADW: aboveground dry weight under full P application; FP_Pup: P uptake under full P application. Figure S4. Promotion of plant growth and nutrient uptake of combined LG7 and rhizobia inoculant on peanut ‘Biyaya’ at six weeks after sowing. Plant height (A), P uptake (B), N uptake (C). Significant differences between rhizobia alone and LG7 + rhizobia were determined by Dunnett’s test (* p < 0.05; + p < 0.10). The error bar indicates the standard deviation of four replications. Table S1. Chemical analysis of soil used in the plant growth assay of LG7. Table S2. Qualitative estimation of Ca3(PO4)2 and AlPO4 solubilization with different carbon sources in solid and liquid P growth media. Table S3. Spearman’s rank correlation coefficients of the phosphate solubilizing ability, gluconic acid secretion, and rice growth traits.
Author Contributions
J.L.C.D. and M.L.S. designed the experiments; J.L.C.D. and M.P. performed experiments and analyzed the data; M.L.S. and M.P. supervised the experiments; J.L.C.D., M.P. and M.L.S. contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Research Program to J.L.C.D. and the BIOTECH Core Fund to M.L.S. from the University of the Philippines Los Baños (UPLB).
Data Availability Statement
The accession number for the gene used in this manuscript is deposited in the DDBJ.
Acknowledgments
This work was supported by the UPLB Basic Research Program and BIOTECH Core Fund. We would also like to express our gratitude to the Institute of Plant Breeding-UPLB for the peanut seeds and the Philippine Rice Institute for the rice seeds.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; van Veen, J.; van Elsas, J. MICROBIAL DIVERSITY IN SOIL: Selection of Microbial Populations by Plant and Soil Type and Implications for Disease Suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Boraste, A.; Vamsi, K.K.; Jhadav, A.; Khairnar, Y.; Gupta, N.; Trivedi, S.; Patil, P.; Gupta, G.; Gupta, M.; Mujapara, A.K.; et al. Biofertilizers: A novel tool for agriculture. Int. J. Microbiol. Res. 2009, 1, 23–31. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, R.A.; Giles, C.; Paterson, E.; Robertson-Albertyn, S.; Cesco, S.; Mimmo, T.; Pii, Y.; Bulgarelli, D. Plant-microbiota interactions as a driver of the mineral Turnover in the Rhizosphere. Adv. Appl. Microbiol. 2016, 95, 1–67. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital capacity of source microbial species. Microbiologia 1948, 17, 362–370. [Google Scholar]
- Zaidi, A.; Khan, M.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; McDonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar] [CrossRef]
- Lin, T.-F.; Huang, H.-I.; Shen, F.-T.; Young, C.-C. The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Bioresour. Technol. 2006, 97, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Archana, G.; Kumar, G.N. Variation in the Nature of Organic Acid Secretion and Mineral Phosphate Solubilization by Citrobacter sp. DHRSS in the Presence of Different Sugars. Curr. Microbiol. 2008, 56, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, M.; Farhat, A.; Bejar, W.; Kammoun, R.; Bouchaala, K.; Fourati, A.; Antoun, H.; Bejar, S.; Chouayekh, H. Characterization of the mineral phosphate solubilizing activity of Serratia marcescens CTM 50650 isolated from the phosphate mine of Gafsa. Arch. Microbiol. 2009, 191, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Jones, D.; Oburger, E. Solubilization of phosphorus by soil microorganism. In Phosphorus in Action; Buenemann, E., Oberson, A., Frossard, E., Eds.; Springer: New York, NY, USA, 2011; pp. 169–198. [Google Scholar]
- Bieleski, R.L. Phosphate Pools, Phosphate Transport, and Phosphate Availability. Annu. Rev. Plant Physiol. 1973, 24, 225–252. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.; Logan, T. Myths and science about the chemistry and fertility of soils in the tropics. In Myths and Science of Soils of the Tropics; Lal, R., Sanchez, P., Eds.; Soil Science Society of America: Madison, WI, USA, 1992; pp. 35–46. [Google Scholar]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Goldstein, A.H.; Braverman, K.; Osorio, N. Evidence for mutualism between a plant growing in a phosphate-limited desert environment and a mineral phosphate solubilizing (MPS) rhizobacterium. FEMS Microbiol. Ecol. 2006, 30, 295–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective: Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. Bioscience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Brownlie, W.J.; Sutton, M.A.; Reay, D.S.; Heal, K.V.; Hermann, L.; Kabbe, C.; Spears, B.M. Global actions for a sustainable phosphorus future. Nat. Food 2021, 2, 71–74. [Google Scholar] [CrossRef]
- Mogollón, J.M.; Bouwman, A.F.; Beusen, A.H.W.; Lassaletta, L.; van Grinsven, H.J.M.; Westhoek, H. More efficient phosphorus use can avoid cropland expansion. Nat. Food 2021, 2, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact to 2050. Glob. Food Secur. 2020, 26, 100426. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, W.J.; Sutton, M.A.; Cordell, D.; Reay, D.S.; Heal, K.V.; Withers, P.J.A.; Vanderbeck, I.; Spears, B.M. Phosphorus price spikes: A wake-up call for phosphorus resilience. Front. Sustain. Food Syst. 2023, 7, 1088776. [Google Scholar] [CrossRef]
- IAASTD. Global Report International Assessment of Agriculture at a Crossroads; IAASTD: Washington, DC, USA, 2009. [Google Scholar]
- Barbieri, P.; MacDonald, G.K.; Bernard de Raymond, A.; Nesme, T. Food system resilience to phosphorus shortages on a telecoupled planet. Nat. Sustain. 2021, 5, 114–122. [Google Scholar] [CrossRef]
- Verma, S.C.; Ladha, J.K.; Tripathi, A.K. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J. Biotechnol. 2001, 91, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, M.T.; Sharma, L.; Bag, N.; Das, S. Isolation, Characterization, and Evaluation of Native Rhizobacterial Consortia Developed From the Rhizosphere of Rice Grown in Organic State Sikkim, India, and Their Effect on Plant Growth. Front. Microbiol. 2021, 12, 713660. [Google Scholar] [CrossRef] [PubMed]
- Alagawadi, A.R.; Gaur, A.C. Associative effect of Rhizobium and phosphate-solubilizing bacteria on the yield and nutrient uptake of chickpea. Plant Soil 1988, 105, 241–246. [Google Scholar] [CrossRef]
- Cheng, H.-R.; Jiang, N. Extremely Rapid Extraction of DNA from Bacteria and Yeasts. Biotechnol. Lett. 2006, 28, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Nautiyal, C.S. An Efficient Method for Qualitative Screening of Phosphate-Solubilizing Bacteria. Curr. Microbiol. 2001, 43, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Philippine Council for Agriculture and Resources Research. Standard Methods of Analysis for Soil, Plant Tissue, Water and Fertilizer, 22nd ed.; Philippine Council for Agriculture and Resources Research, Farm Resources and Systems Research Division: Los Baños, Philippines, 1991; Volume 120. [Google Scholar]
- Ahemad, M.; Khan, M.S. Phosphate-Solubilizing and Plant-Growth-Promoting Pseudomonas aeruginosa PS1 Improves Greengram Performance in Quizalafop-p-ethyl and Clodinafop Amended Soil. Arch. Environ. Contam. Toxicol. 2010, 58, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Edi Premono, M.; Moawad, A.M.; Vlek, P.L.G. Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 1996, 11, 13–23. [Google Scholar]
- Philippine Rice Research Institute. Rc 222 Boosts Yield by 20 Cavans. Available online: https://www.philrice.gov.ph (accessed on 17 March 2018).
- Tejima, K.; Arima, Y.; Yokoyama, T.; Sekimoto, H. Composition of amino acids, organic acids, and sugars in the peribacteroid space of soybean root nodules. Soil Sci. Plant Nutr. 2003, 49, 239–247. [Google Scholar] [CrossRef]
- Rhoades, R.; Nazarea, V. Peanut in Local and Global Food Systems Series Report No. 1, 1998.
- Zarate, J.T.; Aquino, G.M.B.; Cruz, J.M.C.; Villa, N.O.; Rosana, A.R.R. Draft Genome Sequence of Enterobacter sp. Strain AD2-3, Isolated from a Postmining Site in Benguet, Philippines. Microbiol. Resour. Announc. 2019, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Kirui, C.K.; Njeru, E.M.; Runo, S. Diversity and Phosphate Solubilization Efficiency of Phosphate Solubilizing Bacteria Isolated from Semi-Arid Agroecosystems of Eastern Kenya. Microbiol. Insights 2022, 15, 117863612210889. [Google Scholar] [CrossRef] [PubMed]
- Muindi, M.M.; Muthini, M.; Njeru, E.M.; Maingi, J. Symbiotic efficiency and genetic characterization of rhizobia and non rhizobial endophytes associated with cowpea grown in semi-arid tropics of Kenya. Heliyon 2021, 7, e06867. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, O.A.; Oladipo, E.K.; Kwenda, S.; Khumalo, Z.; Ismail, A.; Oloke, J.K.; Oyawoye, O.M.; Onyeaka, H. Whole genomic sequence of Enterobacter sichuanensis AJI 2411—A plant growth promoting rhizobacteria. Gene 2023, 887, 147725. [Google Scholar] [CrossRef] [PubMed]
- Chinachanta, K.; Shutsrirung, A.; Herrmann, L.; Lesueur, D. Isolation and characterization of KDML105 aromatic rice rhizobacteria producing indole-3-acetic acid: Impact of organic and conventional paddy rice practices. Lett. Appl. Microbiol. 2022, 74, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Monroy Miguel, R.; Carrillo González, R.; Rios Leal, E.; González-Chávez, M.d.C.A. Screening bacterial phosphate solubilization with bulk-tricalcium phosphate and hydroxyapatite nanoparticles. Antonie Leeuwenhoek 2020, 113, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.Y.A.; de Oliveira, A.L.M.; Valentinuzzi, F.; Jayme, N.S.; Monterisi, S.; Fattorini, R.; Cesco, S.; Pii, Y. An insight into the role of the organic acids produced by Enterobacter sp. strain 15S in solubilizing tricalcium phosphate: In situ study on cucumber. BMC Microbiol. 2023, 23, 184. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Palta, J.A.; Li, Y.; Fan, X. An Enterobacter cloacae strain NG-33 that can solubilize phosphate and promote maize growth. Front. Microbiol. 2022, 13, 1047313. [Google Scholar] [CrossRef] [PubMed]
- Damo, J.L.C.; Ramirez, M.D.A.; Agake, S.-I.; Pedro, M.; Brown, M.; Sekimoto, H.; Yokoyama, T.; Sugihara, S.; Okazaki, S.; Ohkama-Ohtsu, N. Isolation and Characterization of Phosphate Solubilizing Bacteria from Paddy Field Soils in Japan. Microbes Environ. 2022, 37, ME21085. [Google Scholar] [CrossRef]
- Li, X.L.; Zhao, X.Q.; Dong, X.Y.; Ma, J.F.; Shen, R.F. Secretion of Gluconic Acid from Nguyenibacter sp. L1 Is Responsible for Solubilization of Aluminum Phosphate. Front. Microbiol. 2021, 12, 784025. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.; Archana, G.; Naresh, K. Metabolic chanelling of glucose towards gluconate in phosphate solubilizing Pseudomonas aeruginosa P4 under phosphorus deficiency. Res. Microbiol. 2008, 159, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Stella, M.; Halimi, M.S. Gluconic acid production by bacteria to liberate phosphorus from insoluble phosphate complexes. J. Trop. Agric. Food Sci. 2015, 43, 41–53. [Google Scholar]
- Goldstein, A.H. Recent Progress in Understanding the Molecular Genetics and Biochemistry of Calcium Phosphate Solubilization by Gram Negative Bacteria. Biol. Agric. Hortic. 1995, 12, 185–193. [Google Scholar] [CrossRef]
- Wang, D.; Zhan, J.; Sun, Q.-Y. Phosphate solubilization of Aureobasidium pullulan F4 and its mechanism. Ying Yong Sheng Tai Xue Bao 2014, 25, 2079–2084. [Google Scholar] [PubMed]
- Ranawat, B.; Bachani, P.; Singh, A.; Mishra, S. Enterobacter hormaechei as Plant Growth-Promoting Bacteria for Improvement in Lycopersicum esculentum. Curr. Microbiol. 2021, 78, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Anzuay, M.S.; Prenollio, A.; Ludueña, L.M.; Morla, F.D.; Cerliani, C.; Lucero, C.; Angelini, J.G.; Taurian, T. Enterobacter sp. J49: A Native Plant Growth-Promoting Bacteria as Alternative to the Application of Chemical Fertilizers on Peanut and Maize Crops. Curr. Microbiol. 2023, 80, 85. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Peng, N.; Te, N.S.; Hsin I, C.; Lin, C.; Lin, F.; Reddy, C.; Yan, H.; Ji, L. Improvement of plant growth and seed yield in Jatropha curcas by a novel nitrogen-fixing root associated Enterobacter species. Biotechnol. Biofuels 2013, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Reddy, M.S. Phosphate solubilizing rhizobacteria from an organic farm and their influence on the growth and yield of maize (Zea mays L.). J. Gen. Appl. Microbiol. 2013, 59, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Tran, L.-S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Ryan, M.H.; Lambers, H.; Siddique, K.H. Phosphorus acquisition and utilisation in crop legumes under global change. Curr. Opin. Plant Biol. 2018, 45, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ljones, T. Nitrogen fixation and bioenergetics: The role of ATP in nitrogenase catalysis. FEBS Lett. 1979, 98, 981–988. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
