Survival Time, Mortality Rate, and Feeding Damage of Adult Myllocerus undecimpustulatus undatus (Coleoptera: Curculionidae) Exposed to Biopesticides in Laboratory Bioassays
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Insects
2.3. Preparation of Commercial Products
2.4. Design of Bioassay Cages and Application of Treatments
2.5. Survival Time and Percentage Mortality
2.6. Mycosis
2.7. Feeding Damage
2.8. Statistical Analysis
3. Results
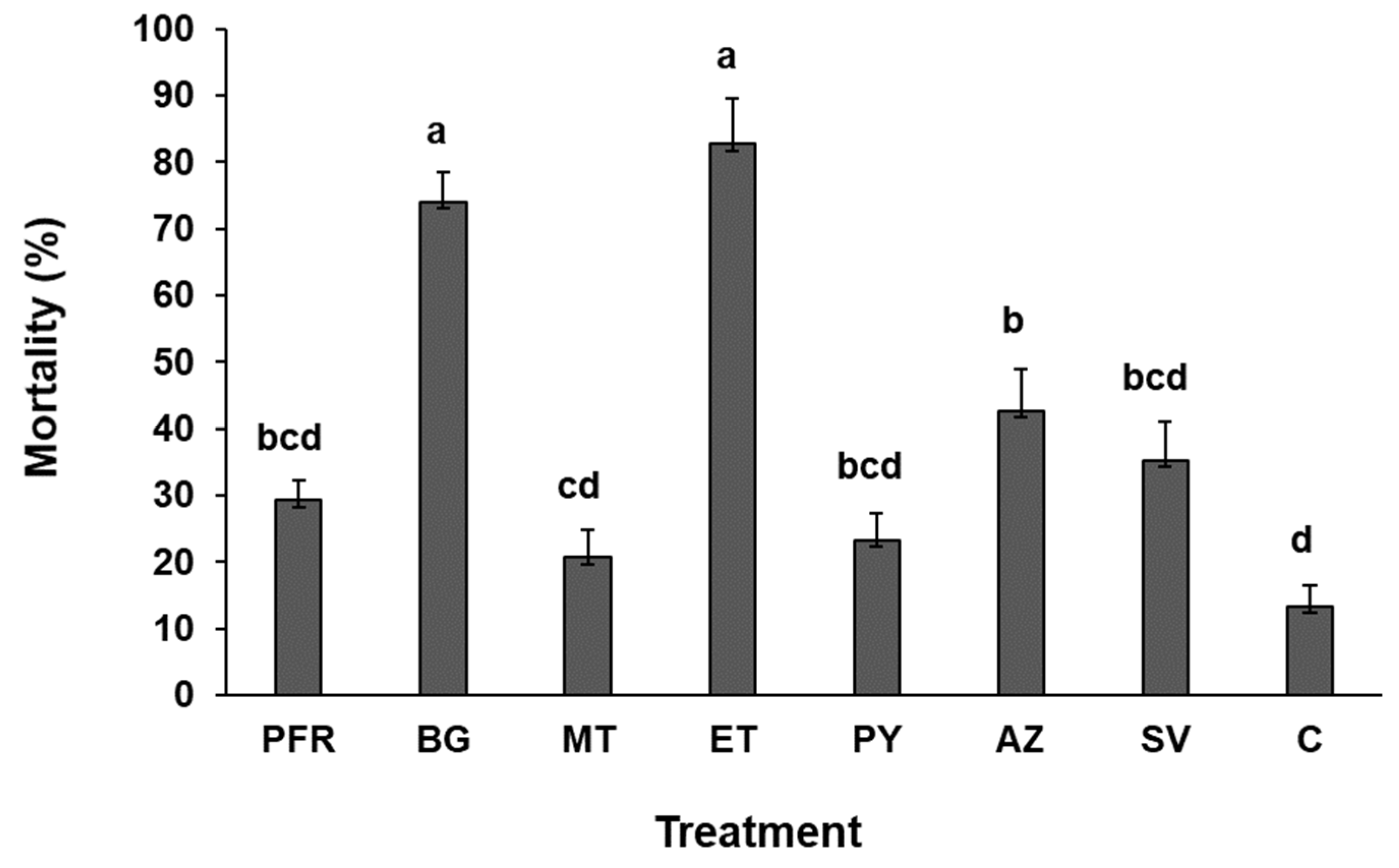
3.1. Survival Time and Percentage Mortality
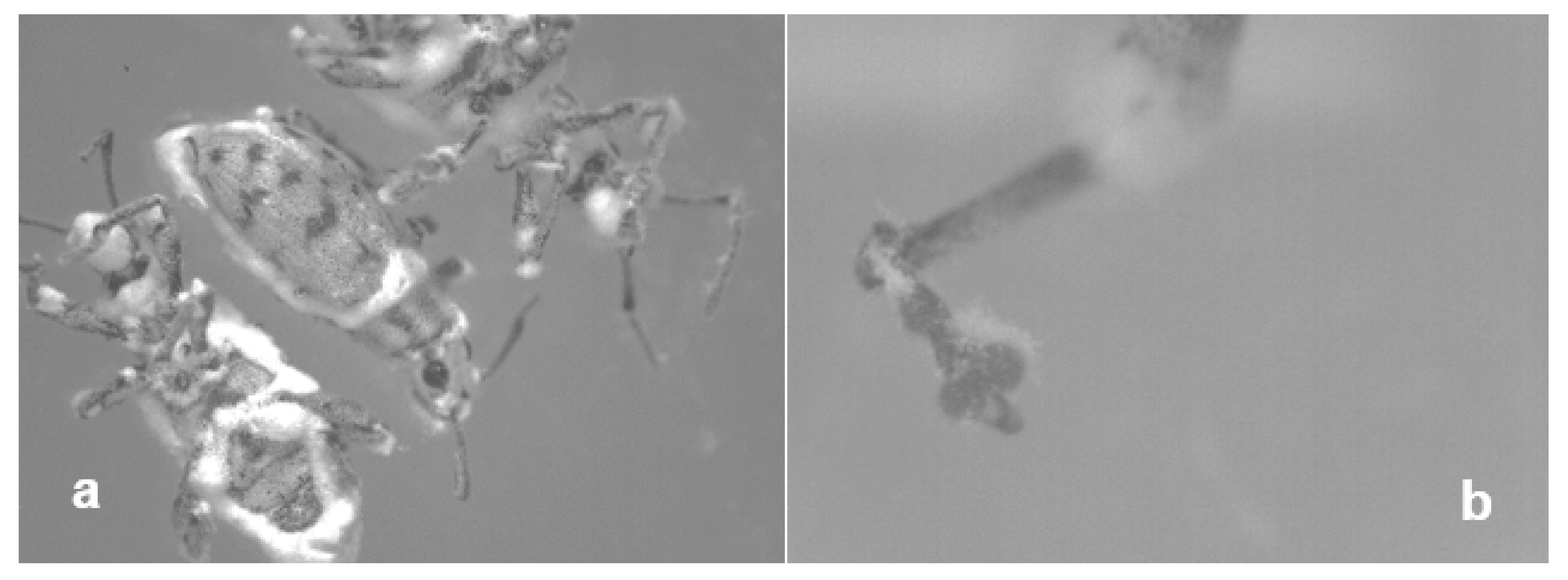
3.2. Mycosis
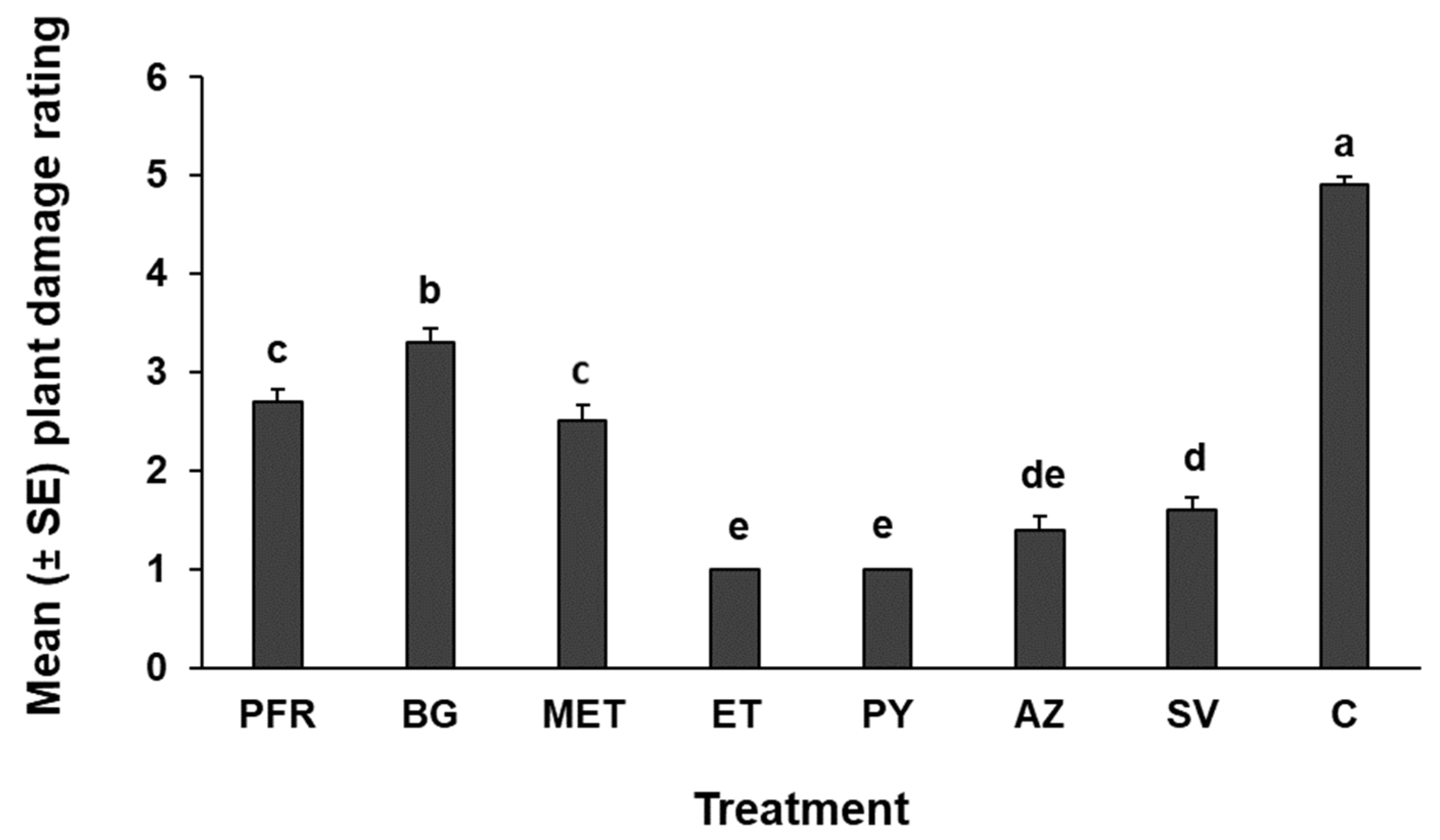
3.3. Feeding Damage Assessment (PDRI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arévalo, H.A.; Stansly, P.A. Suppression of Myllocerus undatus (Coleoptera: Curculionidae) in Valencia orange with chlorpyrifos sprays directed at ground and foliage. Fla. Entomol. 2009, 92, 150–152. [Google Scholar] [CrossRef]
- Larson, N.A.; Barerra, C.J.R.; Owens, D.; Nuessly, G.S. Evaluation of foliar insecticide applications for control of Sri Lanka weevils on cocoplum. AMT 2017, 42, 1–2. [Google Scholar]
- Leibee, G.L.; Capinera, J.L. Pesticide resistance in Florida insects limits management options. Fla. Entomol. 1995, 78, 386–399. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B 1998, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Sporleder, M.; Lacey, L.A. Biopesticides. In Insect Pests of Potato: Global Perspectives on Biology and Management; Giordanengo, P., Vincent, C., Alyokhin, A., Eds.; Elsevier: Oxford, UK, 2013; pp. 463–497. [Google Scholar]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2007, 113, 336–341. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A review of biopesticides and their mode of action against insect pests. In Environmental Sustainability; Thangavel, P., Sridevi, G., Eds.; Springer: New Delhi, India, 2015; pp. 49–63. [Google Scholar]
- Mertz, F.P.; Yao, R.C. Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. Int. J. Syst. Bacteriol. 1990, 40, 34–39. [Google Scholar] [CrossRef]
- Shanthipriya, B.; Misra, H.P. Management of cotton grey weevil, Myllocerus maculosus Desb. using bio-pesticides. J. Plant Prot. Environ. 2007, 4, 159–160. [Google Scholar]
- Avery, P.B.; Bojorque, V.; Gámez, C.; Duncan, R.E.; Carrillo, D.; Cave, R.D. Spore acquisition and survival of ambrosia beetles associated with the laurel wilt pathogen in avocados after exposure to entomopathogenic fungi. Insects 2018, 9, 49. [Google Scholar] [CrossRef]
- Kumar, V.; Avery, P.B.; Ahmed, J.; Cave, R.D.; McKenzie, C.L.; Osborne, L.S. Compatibility and efficacy of Isaria fumosorosea with horticultural oils for mitigation of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 2017, 8, 119. [Google Scholar] [CrossRef]
- McKenzie, C.L.; Hunter, W.B.; Lapointe, S.L.; Dang, P. Iridovirus infection and topical application in whitefly. Proc. Annu. Meet. Fla. Hort. Soc. 2001, 114, 644–667. [Google Scholar]
- Lacey, L.A.; Brooks, W.M. Initial handling and diagnosis of diseased insects. In Manual of Techniques in Insect Pathology; Lacey, L., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1997; p. 5. [Google Scholar]
- Maletta, M.; Tietjen, W.; Ghidiu, G.; Holmstrom, K.; Cowgill, W. Evaluation of controls for flea beetle on eggplant in an organic production system. Acta Hortic. 2004, 638, 341–346. [Google Scholar] [CrossRef]
- Eger, J.E., Jr.; Lindenberg, L.B. Utility of spinosad for insect control in Florida vegetables. Proc. Annu. Meet. Fla. Hort. Soc. 1998, 111, 55–57. [Google Scholar]
- Balusu, R.R.; Fadamiro, H.Y. Evaluation of organically acceptable insecticides as stand-alone treatments and in rotation for managing yellowmargined leaf beetle, Microtheca ochroloma (Coleoptera: Chrysomelidae), in organic crucifer production. Pest Manag. Sci. 2012, 68, 573–579. [Google Scholar] [CrossRef] [PubMed]
- McLeod, P.; Rashid, T. Laboratory toxicity of an organic formulation of spinosad against the eggplant flea beetle, Epitrix fuscula Crotch. J. Biofertil. Biopestic. 2010, 2, 103. [Google Scholar]
- Bažok, R.; Šatvar, M.; Radoš, I.; Drmić, Z.; Lemić, D.; Čačija, M.; Virić Gašparić, H. Comparative efficacy of classical and biorational insecticides on sugar beet weevil, Bothynoderes punctiventris Germar (Coleoptera: Curculionidae). Plant Prot. Sci. 2016, 52, 134–141. [Google Scholar] [CrossRef]
- Ondiaka, S.; Maniana, N.K.; Nyamasyo, G.H.N.; Nderitu, J.H. Virulence of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae to sweet potato weevil Cylas puncticollis and effects on fecundity and egg viability. Ann. Appl. Biol. 2008, 153, 41–48. [Google Scholar] [CrossRef]
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: A SEM study. Microsc. Res. Tech. 2010, 73, 714–725. [Google Scholar]
- Martin, P.; Michal, L.; Michal, N.; Juraj, S. Testing of entomopathogenic fungi in biological control against pine weevil. Biol. Chem. Res. 2015, 3, 1–11. [Google Scholar]
- Lacey, L.; Martins, A.; Ribeiro, C. The pathogenicity of Metarhizium anisopliae and Beauveria bassiana for adults of Japanese beetle, Popilla japonica (Coleoptera: Scarabaeidae). Eur. J. Entomol. 1994, 91, 313–319. [Google Scholar]
- Leng, P.H.; Reddy, G.V.P. Bioactivity of selected eco-friendly pesticides against Cylas formicarius (Coleoptera: Brentidae). Fla. Entomol. 2012, 95, 1040–1047. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.A.; Biresaw, G.; Jackson, M.A. Hydrophobic and electrostatic cell surface properties of blastospores of the entomopathogenic fungus Paecilomyces fumosoroseus. Biointerfaces 2005, 46, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Prior, C.; Jollands, P.; Le Patourel, G. Infectivity of oil and water formulations of Beauveria bassiana (Deuteromycotina: Hyphomycetes) to the cocoa weevil pest Pantorhytes plutus (Coleoptera: Curculionidae). J. Invertebr. Pathol. 1988, 52, 66–72. [Google Scholar] [CrossRef]
- Bateman, R.; Carey, M.; Moore, D.; Prior, C. The enhanced infectivity of Metarhizium flavoviride in oil formulations to desert locusts at low humidities. Ann. Appl. Biol. 1993, 122, 145–152. [Google Scholar] [CrossRef]
- Brown, S.H.; Frank, M.S. Cocoplum (Chrysobalanus icaco L.) Identification and Uses. University of Florida/IFAS Extension Publication ENH1289. Available online: http://edis.ifas.ufl.edu/pdffiles/EP/EP55300.pdf (accessed on 2 April 2018).
- Barrick, W.E. Salt tolerant plants for Florida landscapes. Proc. Annu. Meet. Fla. Hort. Soc. 1978, 91, 82–84. [Google Scholar]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Cory, J.S.; Ericsson, J.D. Fungal entomopathogens in a tritrophic context. BioControl 2010, 55, 75–88. [Google Scholar] [CrossRef]
- Silva, J.P.; Peres, A.M.; Paixão, T.P.; Silva, A.S.; Baetas, A.C.; Barbosa, W.L.; Monteiro, M.C.; Andrade, M.A. Anifungal activity of hydroalcoholic extract of Chrysobalanus icaco against oral clinical isolates of Candida species. Pharmacogn. Res. 2017, 9, 96–100. [Google Scholar]
- Xiao, G.; Ying, S.-H.; Zheng, P.; Wang, Z.-L.; Zhang, S.; Xie, X.-Q.; Shang, Y.; St. Leger, R.J.; Zhao, G.-P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef]
- Feng, M.; Poprawski, T.J.; Khachatourians, G.G. Production, formulation, and application of the entomopathogenic fungus Beauveria bassiana for insect control: Current status. Biocontrol. Sci. Technol. 1994, 4, 3–34. [Google Scholar] [CrossRef]
- Vega, F.E.; Posada, F.; Aime, M.C.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic fungal endophytes. Biol. Control 2008, 46, 72–82. [Google Scholar] [CrossRef]
- Akello, J.; Dubois, T.; Coyne, D.; Kyamanywa, S. Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomol. Exp. Appl. 2008, 129, 157–165. [Google Scholar] [CrossRef]
- Barry, J.D.; Sciarappa, W.J.; Texeria, L.A.F.; Polavarpu, S. Comparative effectiveness of different insecticides for organic management of blueberry maggot (Diptera: Tephritidae). J. Econ. Entomol. 2005, 98, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Trdan, S.; Andrej, A.; Bergant, K.; Andjus, I.; Milica, K.; Vidrih, M.; Ludvik, R. Effect of temperature on efficacy of three natural substances to Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Acta Agric. Scand. B Soil. Plant Sci. 2007, 57, 293–296. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Saitanis, C.J.; Kontodimas, D.C.; Roussos, A.N.; Tsoutsa, M.S.; Anastassopoulou, U.A. Effect of two azadirachtin formulations against adults of Sitophilus oryzae and Tribolium confusum on different grain commodities. J. Food Prot. 2007, 70, 1627–1632. [Google Scholar] [CrossRef]
- Neilson, A.L.; Pote, J.; Buehrer, K.; Grieshop, M.J. The reemergence of an old pest, Orchestes pallicornis (Coleoptera: Curculioidae). J. Integr. Pest Manag. 2012, 3, D1–D4. [Google Scholar]
- Long, R.; Summers, C.; Godfrey, L. Conventional and organic methods for insect pest management in alfalfa. In Proceedings of the Western Alfalfa and Forage Conference, Reno, NV, USA, 2–4 December 2009; UC Cooperative Extension, Plant Sciences Department, University of California: Davis, CA, USA; pp. 1–6. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neal, A.S.; Avery, P.B.; Cave, R.D. Survival Time, Mortality Rate, and Feeding Damage of Adult Myllocerus undecimpustulatus undatus (Coleoptera: Curculionidae) Exposed to Biopesticides in Laboratory Bioassays. Appl. Microbiol. 2023, 3, 388-399. https://doi.org/10.3390/applmicrobiol3020027
Neal AS, Avery PB, Cave RD. Survival Time, Mortality Rate, and Feeding Damage of Adult Myllocerus undecimpustulatus undatus (Coleoptera: Curculionidae) Exposed to Biopesticides in Laboratory Bioassays. Applied Microbiology. 2023; 3(2):388-399. https://doi.org/10.3390/applmicrobiol3020027
Chicago/Turabian StyleNeal, Anita S., Pasco B. Avery, and Ronald D. Cave. 2023. "Survival Time, Mortality Rate, and Feeding Damage of Adult Myllocerus undecimpustulatus undatus (Coleoptera: Curculionidae) Exposed to Biopesticides in Laboratory Bioassays" Applied Microbiology 3, no. 2: 388-399. https://doi.org/10.3390/applmicrobiol3020027
APA StyleNeal, A. S., Avery, P. B., & Cave, R. D. (2023). Survival Time, Mortality Rate, and Feeding Damage of Adult Myllocerus undecimpustulatus undatus (Coleoptera: Curculionidae) Exposed to Biopesticides in Laboratory Bioassays. Applied Microbiology, 3(2), 388-399. https://doi.org/10.3390/applmicrobiol3020027





