Phycoremediation Processes for Secondary Effluent from Sewage Treatment Plants Using Photosynthetic Microorganisms: A Review
Abstract
1. Introduction
2. Wastewater Properties and Treatment
2.1. Characteristics of Wastewater
2.2. Primary and Secondary Treatment of Wastewater
2.3. Eutrophication Issue
2.4. Phycoremediation as a Tertiary Treatment of Wastewater
3. Parameters Related to Growth of Microorganisms Applied in Phycoremediation, Types of Bioreactors, and Biomass-Harvesting Methods
3.1. Growth Factors of Microorganisms Applied in Phycoremediation
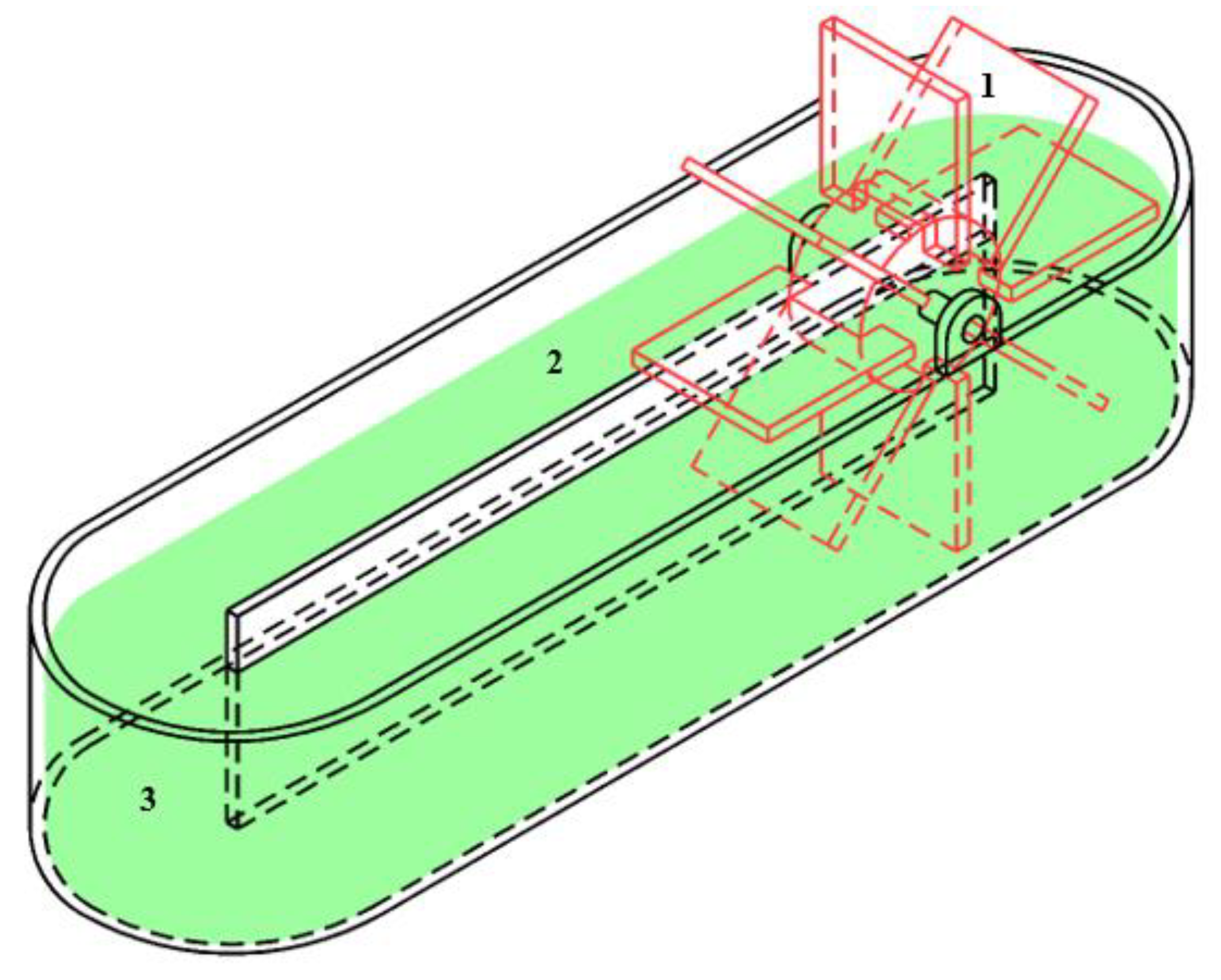
3.2. Types of Bioreactors for Phycoremediation
3.3. Separation Methods for Harvesting Biomass from Tertiary Wastewater Treatment
3.3.1. Filtration
3.3.2. Centrifugation
3.3.3. Sedimentation
3.3.4. Flocculation
3.3.5. Flotation
4. Benefits from the Cultivation of Photosynthetic Microorganisms
4.1. CO2 as Greenhouse Gas and Bio-Fixation
4.2. Biomass Uses
4.3. Benefit Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPA—United States Environmental Protection Agency. Guidelines for Water Reuse. Available online: https://www.epa.gov/waterreuse/guidelines-water-reuse (accessed on 22 August 2021).
- Silva, J.; Torres, P.; Madera, C. Reuso de aguas residuales domésticas en agricultura. Agron. Colomb. 2008, 26, 347–359. [Google Scholar]
- Wiener, M.J.; Jafvert, C.T.; Nies, L.F. The assessment of water use and reuse through reported data: A US case study. Sci. Total Environ. 2016, 539, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal, 1st ed.; IWA Publishing: London, UK, 2017; Volume 1. [Google Scholar]
- Von Sperling, M.; Chernicharo, L. Biological Wastewater Treatment in Warm Climate Regions, 1st ed.; IWA Publishing: London, UK, 2005; Volume 1. [Google Scholar]
- Eriksson, E.; Auffarth, K.; Henze, M.; Ledin, A. Characteristics of grey wastewater. Urban Water 2002, 4, 85–104. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Pisarevsky, A.M.; Polozova, I.P.; Hockridge, P.M. Chemical Oxygen Demand. Russ. J. Appl. Chem. 2005, 78, 101–107. [Google Scholar] [CrossRef]
- Muttamara, S. Wastewater characteristics. Resour. Conserv. Recycl. 1996, 16, 145–159. [Google Scholar] [CrossRef]
- Oswald, W.J. The coming industry of controlled photosynthesis. Am. J. Public Health Nations Health 1962, 52, 235–242. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Perales, J.A. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014, 49, 465–474. [Google Scholar] [CrossRef]
- Neveux, N.; Magnusson, M.; Mata, L.; Whelan, A.; de Nys, R.; Paul, N.A. The treatment of municipal wastewater by the macroalga Oedogonium sp. and its potential for the production of biocrude. Algal Res. 2016, 13, 284–292. [Google Scholar] [CrossRef]
- Grady, C.P.L.; Daigger, G.T.; Love, N.G.; Filipe, C.D. Biological Wastewater Treatment, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Thauer, R.K. Energy metabolism of methanogenic bacteria. Biochim. Biophys. Acta 1990, 1018, 256–259. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Chudoba, J. Quantitative estimation in cod units of refractory organic compounds produced by activated sludge microorganisms. Water Res. 1985, 19, 37–43. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Long term outdoor operation of a tubular airlift pilot photobioreactor and a high rate algal pond as tertiary treatment of urban wastewater. Ecol. Eng. 2013, 52, 143–153. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Montazeri-Najafabady, N.; Shaker, S.; Safari, A.; Kazemi, A.; Mousavi, P.; Mobasher, M.A.; Ghasemi, Y. Removal of nitrogen and phosphorus from wastewater using microalgae free cells in bath culture system. Biocatal. Agric. Biotechnol. 2014, 3, 126–131. [Google Scholar] [CrossRef]
- Pescod, M.B. Wastewater Treatment and Use in Agriculture, 1st ed.; Food and Agriculture organization of the United Nations: Rome, Italy, 1992. [Google Scholar]
- Foley, J.; de Haas, D.; Yuan, Z.; Lant, P. Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res. 2010, 44, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.K.; Khan, S.A.; Ahmad, F.; Gupta, N. Nutrient sequestration and phycoremediation of sewage waste water by selective microalgae. Green Farming 2014, 5, 623–626. [Google Scholar]
- Martínez, M.; Sanchez, S.; Jimenez, J.M.; El Yousfi, F.; Munoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Órpez, R.; Martínez, M.E.; Hodaifa, G.; El Yousfi, F.; Jbari, N.; Sánchez, S. Growth of the microalga Botryococcus braunii in secondarily treated sewage. Desalination 2009, 246, 625–630. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication. Encycl. Inland Waters 2009, 3, 61–73. [Google Scholar] [CrossRef]
- Gold, A.J.; Sims, J.T. Eutrophication. In Encyclopedia of Soils in the Environment, 1st ed.; Daniel, H., Ed.; Elsevier: Oxford, UK, 2005; Volume 1, pp. 486–494. [Google Scholar] [CrossRef]
- Sahoo, D.; Seckbach, J. The Algae World, 1st ed.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Hupfer, M.; Hilt, S. Lake Restoration. In Encyclopedia of Ecology, 1st ed.; Fath, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 1, pp. 2080–2093. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Q.; Wu, G.; Hu, H.-Y. Centralized water reuse system with multiple applications in urban areas: Lessons from China’s experience. Resour. Conserv. Recycl. 2017, 117, 125–136. [Google Scholar] [CrossRef]
- Cole, A.J.; Neveux, N.; Whelan, A.; Morton, J.; Vis, M.; de Nys, R.; Paul, N.A. Adding value to the treatment of municipal wastewater through the intensive production of freshwater macroalgae. Algal Res. 2016, 20, 100–109. [Google Scholar] [CrossRef]
- An, J.-Y.; Sim, S.-J.; Lee, J.S.; Kim, B.W. Hydrocarbon production from secondarily treated piggery wastewater by the green alga Botryococcus braunii. J. Appl. Phycol. 2003, 15, 185–191. [Google Scholar] [CrossRef]
- Golueke, C.G.; Oswald, W.J. Power from solar energy—Via algae-produced methane. Sol. Energy 1963, 7, 86–92. [Google Scholar] [CrossRef]
- Pipes, W.O.; Gotaas, H.B. Utilization of Organic Matter by Chlorella Grown in Sewage. Appl. Microbiol. 1959, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Kao, C.Y.; Chen, T.Y.; Chang, Y.B.; Kuo, C.M.; Lin, C.S. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef]
- Oswald, W.J.; Golueke, C. Eutrophication trends in the United States: A Problem? J. Water Pollut. Control Fed. 1966, 38, 964–975. [Google Scholar]
- Sydney, E.B.; da Silva, T.E.; Tokarski, A.; Novak, A.C.; de Carvalho, J.C.; Woiciecohwski, A.L.; Larroche, C.; Soccol, C.R. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Ji, M.K.; Abou-Shanab, R.A.I.; Kim, S.H.; Salama, E.S.; Lee, S.H.; Kabra, A.N.; Lee, Y.S.; Hong, S.; Jeon, B.H. Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng. 2013, 58, 142–148. [Google Scholar] [CrossRef]
- Malla, F.A.; Khan, S.A.; Rashmi; Sharma, G.K.; Gupta, N.; Abraham, G. Phycoremediation potential of Chlorella minutissima on primary and tertiary treated wastewater for nutrient removal and biodiesel production. Ecol. Eng. 2015, 75, 343–349. [Google Scholar] [CrossRef]
- Blank, C.E.; Parks, R.W.; Hinman, N.W. Chitin: A potential new alternative nitrogen source for the tertiary, algal-based treatment of pulp and paper mill wastewater. J. Appl. Phycol. 2016, 28, 2753–2766. [Google Scholar] [CrossRef]
- Oswald, W.J. Terrestrial approaches to integration of waste treatment. Waste Manag. Res. 1991, 9, 477–484. [Google Scholar] [CrossRef] [PubMed]
- McGinn, P.J.; Dickinson, K.E.; Bhatti, S.; Frigon, J.-C.; Guiot, S.R.; O’Leary, S.J.B. Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: Opportunities and limitations. Photosynth. Res. 2011, 109, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Ferreira, L.S.; Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Converti, A. Metal biosorption onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris: Multi-metal systems. J. Hazard. Mater. 2012, 217–218, 246–255. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Chen, M.-L.; Wu, Y.-H. Total concentrations and fractions of Cd, Cr, Pb, Cu, Ni and Zn in sewage sludge from municipal and industrial wastewater treatment plants. J. Hazard. Mater. 2005, 119, 245–249. [Google Scholar] [CrossRef]
- Flores, E.; Herrero, A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 2005, 33, 164–167. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef]
- Matsudo, M.C.; Moraes, F.A.; Bezerra, R.P.; Arashiro, R.E.; Sato, S.; Carvalho, J.C.M. Use of acetate in fed-batch mixotrophic cultivation of Arthrospira platensis. Ann. Microbiol. 2015, 65, 1721–1728. [Google Scholar] [CrossRef]
- Matsudo, M.C.; Sousa, T.F.; Pérez-Mora, L.S.; Bezerra, R.P.; Sato, S.; Carvalho, J.C.M. Ethanol as complementary carbon source in Scenedesmus obliquus cultivation. J. Chem. Technol. Biotechnol. 2017, 92, 781–786. [Google Scholar] [CrossRef]
- Benítez, M.B.; Champagne, P.; Ramos, A.; Torres, A.F.; Ochoa-Herrera, V. Wastewater treatment for nutrient removal with Ecuadorian native microalgae. Environ. Technol. 2018, 40, 2977–2985. [Google Scholar] [CrossRef]
- Loganathan, B.G.; Orsat, V.; Lefsrud, M. Phycoremediation and valorization of synthetic dairy wastewater using microalgal consortia of Chlorella variabilis and Scenedesmus obliquus. Environ. Technol. 2021, 42, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Sousa, A.; Nunes, I.; Muniz-Junior, A.; Carvalho, J.C.M.; Doria, L.; Matsudo, M. Nitrogen supplementation for the production of Chlorella vulgaris biomass in secondary effluent from dairy industry. Biochem. Eng. J. 2021, 165, 107817. [Google Scholar] [CrossRef]
- Nunes, I.; Inoue, C.; Rodrigues Sousa, A.; Carvalho, J.C.M.; Gomes, A.; Matsudo, M. Tertiary treatment of dairy industry wastewater with production of Chlorella vulgaris biomass: Evaluation of effluent dilution. Braz. J. Environ. Sci. (Online) 2021, 56, 365–373. [Google Scholar] [CrossRef]
- Choi, N.; Nunes, I.V.O.; Ohira, G.O.M.; Carvalho, J.C.M.; Matsudo, M.C. Evaluation of Monoraphidium contortum for the tertiary treatment of dairy industry wastewater and biomass production with nitrogen supplementation. Bioprocess Biosyst. Eng. 2023, 46, 265–271. [Google Scholar] [CrossRef]
- Kamyab, H.; Md Din, M.F.; Ponraj, M.; Keyvanfar, A.; Rezania, S.; Taib, S.M.; Abd Majid, M.Z. Isolation and screening of microalgae from agroindustrial wastewater (POME) for biomass and biodiesel sources. Desalination Water Treat. 2016, 57, 29118–29125. [Google Scholar] [CrossRef]
- Shelef, G. High rate algal ponds for wastewater treatment and protein production. Water Sci. Technol. 1982, 14, 439–452. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J. Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Water Sci. Technol. 2010, 61, 633–639. [Google Scholar] [CrossRef]
- Cromar, N.J.; Fallowfield, H.J.; Martin, N.J. Influence of environmental parameters on biomass production and nutrient removal in high rate algal pond operated by continuous culture. Water Sci. Technol. 1996, 34, 133–140. [Google Scholar] [CrossRef]
- Oswald, W.J. Sewage treatment in tropical high rate ponds. In Proceedings of the National Conference on Environmental Engineering, Lake Buena Vista, FL, USA, 7–9 July 1987. [Google Scholar]
- Ge, S.; Champagne, P. Cultivation of the marine macroalgae Chaetomorpha linum in municipal wastewater for nutrient recovery and biomass production. Environ. Sci. Technol. 2017, 51, 3558–3566. [Google Scholar] [CrossRef]
- Almomani, F.A.; Örmeci, B. Performance of Chlorella vulgaris, Neochloris oleoabundans, and mixed indigenous microalgae for treatment of primary effluent, secondary effluent and centrate. Ecol. Eng. 2016, 95, 280–289. [Google Scholar] [CrossRef]
- Park, K.C.; Whitney, C.G.E.; Kozera, C.; O’Leary, S.J.B.; McGinn, P.J. Seasonal isolation of microalgae from municipal wastewater for remediation and biofuel applications. J. Appl. Microbiol. 2015, 119, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Jesus, H.S.; Cassini, S.T.A.; Pereira, M.V.; Dassoler, A.F.; Gonçalves, R.F. Autochthonous microalgae cultivation with anaerobic effluent: Isolation of strains, survivorship, and characterization of the produced biomass. Rev. Ambiente Água 2019, 14, e2362. [Google Scholar] [CrossRef]
- Wu, Y.; Guan, K.; Wang, Z.; Xu, B.; Zhao, F. Isolation, Identification and Characterization of an Electrogenic Microalgae Strain. PLoS ONE 2013, 8, e73442. [Google Scholar] [CrossRef] [PubMed]
- Florentino, A.P.; Costa, M.C.; Nascimento, J.G.S.; Abdala-Neto, E.F.; Mota, C.R.; Santos, A.B. Identification of microalgae from waste stabilization ponds and evaluation of electroflotation by alternate current for simultaneous biomass separation and cell disruption. Eng. Sanit. Ambient. 2019, 24, 177–186. [Google Scholar] [CrossRef]
- Jeong, D.; Jang, A. Exploration of microalgal species for simultaneous wastewater treatment and biofuel production. Environ. Res. 2020, 188, 109772. [Google Scholar] [CrossRef]
- Chan, A.; Salsali, H.; McBean, E. Heavy Metal Removal (Copper and Zinc) in Secondary Effluent from Wastewater Treatment Plants by Microalgae. ACS Sustain. Chem. Eng. 2013, 2, 130–137. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Ma, Y.; Chen, P.; Zheng, H.; Doan, Y.T.T.; Liu, H.; Chen, C.; et al. Mitigating ammonia nitrogen deficiency in dairy wastewaters for algae cultivation. Bioresour. Technol. 2016, 201, 33–40. [Google Scholar] [CrossRef]
- Carvalho, J.C.M.; Bezerra, R.P.; Matsudo, M.C.; Sato, S. Cultivation of Arthrospira (Spirulina) platensis by Fed-Batch Process. In Advanced Biofuels and Bioproducts, 1st ed.; Lee, J., Ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Carvalho, J.C.M.; Matsudo, M.C.; Bezerra, R.P.; Ferreira-Camargo, L.S. Microalgae bioreactors. In Algal Biorefineries: Cultivation of Cells and Products, 1st ed.; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer: Heidelberg, The Netherlands, 2014; pp. 1–557. [Google Scholar]
- Mutanda, T.; Karthikeyan, S.; Bux, F. The utilization of post-chlorinated municipal domestic wastewater for biomass and lipid production by Chlorella spp. under batch conditions. Appl. Biochem. Biotechnol. 2011, 164, 1126–1138. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.-F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef]
- Tang, E.P.Y.; Vincent, W.F.; Proulx, D.; Lessard, P.; Noüe, J. Polar cyanobacteria versus green algae for tertiary waste-water treatment in cool climates. J. Appl. Phycol. 1997, 9, 371–381. [Google Scholar] [CrossRef]
- Shayan, S.I.; Agblevor, F.A.; Bertin, L.; Sims, R.C. Hydraulic retention time effects on wastewater nutrient removal and bioproduct production via rotating algal biofilm reactor. Bioresour. Technol. 2016, 211, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.M.; Solé-Bundó, M.; Garfí, M.; Ferrer, I.; García, J.; Uggetti, E. Integrating microalgae tertiary treatment into activated sludge systems for energy and nutrients recovery from wastewater. Bioresour. Technol. 2018, 247, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.S.; Tam, N.F.Y.; Wong, Y.S. Wastewater Nutrients Removal by Chlorella vulgaris: Optimization through acclimation. Environ. Technol. 2010, 17, 182–189. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. From laboratory to commercial production: A case study of a Spirulina (Arthrospira) facility in Musina, South Africa. J. Appl. Phycol. 2009, 21, 523–527. [Google Scholar] [CrossRef]
- Tredici, M.R. Mass Production of Microalgae: Photobioreactors. In Handbook of Microalgal Culture, 1st ed.; Richmond, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2004; Chapter 9; pp. 178–214. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy 2011, 88, 3402–3410. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Torzillo, G.; Chini Zittelli, G. Tubular Photobioreactors. In Algal Biorefineries, 1st ed.; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Germany, 2015; pp. 187–212. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef]
- Mirón, A.S.; García, M.C.C.; Gómez, A.C.; Camacho, F.G.; Grima, E.M.; Chisti, Y. Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem. Eng. J. 2003, 16, 287–297. [Google Scholar] [CrossRef]
- Pérez-Mora, L.S.; Matsudo, M.C.; Cezare-Gomes, E.A.; Carvalho, J.C. An investigation into producing Botryococcus braunii in a tubular photobioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 3053–3060. [Google Scholar] [CrossRef]
- Soeder, C.J. Massive cultivation of microalgae: Results and prospects. Hydrobiologia 1980, 72, 197–209. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Molina Grima, E.; Fernandez, F.A.; Medina, A.R. Downstream Processing of Cell-Mass and Products. In Handbook of Microalgal Culture, 1st ed.; Richmond, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 215–252. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J.; Mujumdar, A.S. Advances and challenges on algae harvesting and drying. Dry. Technol. 2015, 33, 386–394. [Google Scholar] [CrossRef]
- Farooq, W.; Moon, M.; Ryu, B.; Suh, W.I.; Shrivastav, A.; Park, M.S.; Mishra, S.K.; Yang, J.-W. Effect of harvesting methods on the reusability of water for cultivation of Chlorella vulgaris, its lipid productivity and biodiesel quality. Algal Res. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Knuckey, R.M.; Brown, M.R.; Robert, R.; Frampton, D.M.F. Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquac. Eng. 2006, 35, 300–313. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from Microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef]
- Schmitz, R.; Magro, C.D.; Colla, L.M. Aplicações ambientais de microalgas. Rev. CIATEC-UPF 2012, 4, 48–60. [Google Scholar] [CrossRef][Green Version]
- Radmann, E.M.; Costa, J.A.V. Conteúdo lipídico e composição de ácidos graxos de microalgas expostas aos gases CO2, SO2 e NO. Quim. Nova 2008, 31, 1609–1612. [Google Scholar] [CrossRef]
- de Morais, M.G.; Costa, J.A.V. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Matsudo, M.C.; Bezerra, R.P.; Converti, A.; Sato, S.; Carvalho, J.C.M. CO2 from alcoholic fermentation for continuous cultivation of Arthrospira (Spirulina) platensis in tubular photobioreactor using urea as nitrogen source. Biotechnol. Prog. 2011, 27, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Matsudo, M.C.; Bezerra, R.P.; Sato, S.; Converti, A.; Carvalho, J.C.M. Photosynthetic efficiency and rate of CO2 assimilation by Arthrospira (Spirulina) platensis continuously cultivated in a tubular photobioreactor. Biotechnol. J. 2012, 7, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Golueke, C.G.; Oswald, W.J. Biological conversion of light energy to the chemical energy of methane. Appl. Microbiol. 1959, 7, 219–227. [Google Scholar] [CrossRef]
- Williams, P.J.; Koros, W.J. Gas Separation by Carbon Membranes. In Advanced Membrane Technology and Applications, 1st ed.; Li, N.N., Fane, A.G., Ho, W.W., Matsuura, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 599–631. [Google Scholar] [CrossRef]
- Chen, C.-C.; Qiu, W.; Miller, S.J.; Koros, W.J. Plasticization-resistant hollow fiber membranes for CO2/CH4 separation based on a thermally crosslinkable polyimide. J. Memb. Sci. 2011, 382, 212–221. [Google Scholar] [CrossRef]
- Wind, J.D.; Paul, D.R.; Koros, W.J. Natural gas permeation in polyimide membranes. J. Memb. Sci. 2004, 228, 227–236. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Kumar, N.A.; Sridhar, S.; Rengasamy, R. A Perspective on the biotechnological potential of microalgae. Crit. Rev. Microbiol. 2008, 34, 77–88. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M.; Garland, C.D. Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J. Appl. Phycol. 1993, 5, 71–83. [Google Scholar] [CrossRef]
- Asano, T.; Burton, F.; Leverenz, H.; Tsuchihashi, H.; Tchobanoglous, G. Water Reuse: Issues, Technologies, and Applications, 1st ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]

| Parameter | Concentration | ||||
|---|---|---|---|---|---|
| Reference source | [35] | [36] | [23] | [37] | [22] |
| 28.98 | 19.33 ± 1.41 | ||||
| 12.76 | 0.4 ± 0.1 | 3.84 ± 4.01 | 27.4 | ||
| 7.99 | 0.62 ± 0.07 | ||||
| 2.63 | |||||
| <0.05 | |||||
| 0.71 | <0.05 | ||||
| 0.71 | <0.05 | ||||
| 0.2 | 8.5 ± 0.4 | 0.9 | 1.29 ± 0.46 | <1 | |
| ) | 0.14 | <1 | |||
| 2.00 | 1.69 ± 0.4 | 11.5 | 11.8 | ||
| 1.7 | 75 ± 0.4 | ||||
| 4.21 | 8.0 ± 0.5 | ||||
| Total carbon | 22.6 ± 1 | 38.43 | |||
| Total inorganic carbon | 14.6 ± 0.1 | 38.36 | |||
| Total organic carbon | 8.1 ± 0.2 | 0.4 | |||
| pH | 7.3 | 7.6 | 7.68 ± 0.14 | 9.3 | |
| Total nitrogen | 8.7 ± 0.5 | 11.9 | |||
| Total phosphorus | 1.71 ± 0.3 | 3.02 ± 0.71 | |||
| Organic matter | 0.11 | ||||
| Mineral matter | 0.47 | ||||
| Photobioreactor | Microorganisms | Process | Light/ Dark Cycle | Temperature | Additional Parameters | Reference |
|---|---|---|---|---|---|---|
| Closed cylindrical photobioreactor. Total volume 30 L. | Mixed culture (microalgae, bacteria, protozoa, and metazoan). Chlorella sp., Scenedesmus sp., and Stigeoclonium sp. (dominant generos) | Semi-continuously (fed once a day) | 12/12 h | 25~29 °C. | Microalgae digestate diluted in secondary effluent at a ratio of 1:50 and operated at 8 days of hydraulic retention time (HRT) and solids retention time (SRT). | [74] |
| Aerated bioreactors made of transparent polyethylene terephthalate (3 L). | Scenedesmus obliquus Chlorella vulgaris | Semi-continuous | 25 °C | Free and immobilized cells. | [15] | |
| Erlenmeyer. Total volume 2 L. Working volume of 1.3 L. | Synechococcus nidulans Chlorella vulgaris Botryococcus braunii Chlorella minutissima | Batch | 12/12 h | 25 °C | Cellular adaptation was evaluated by Neubauer counting chamber. | [35] |
| 11 L BioFlo Fermenter. | Chlorella vulgaris Botryococcus braunii | Batch | 12/12 h | 25 °C | Supply 5% CO2. | [35] |
| Serum bottles. Total volume 500 mL. Working volume of 200 mL. | Chlorella vulgaris Scenedesmus obliquus Ourococcus multisporus | Batch | 12/12 h | 27 °C | Supply 15% CO2. Optical density measurement at 680 nm. | [36] |
| Stirred tank reactor. | Botryococcus braunii | Batch | 12/12 h | 25 °C | Optical density measurement at 600 nm. | [23] |
| Acrylic tanks. Total volume 3.5 L. Working volume of 2 L. | Chlorella vulgaris | Batch | 16/8 h | 24 ± 1 °C | pH = 7.1. Initial density = 3.0 × 106 cells mL−1. Air provided CO2. | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Mora, L.S.; Mejia-da-Silva, L.d.C.; Cezare-Gomes, E.d.A.; Santo, É.d.E.; Gohara-Beirigo, A.K.; Matsudo, M.C.; Nardin, B.M.; Sant’Anna, C.L.; Carvalho, J.C.M.d. Phycoremediation Processes for Secondary Effluent from Sewage Treatment Plants Using Photosynthetic Microorganisms: A Review. Appl. Microbiol. 2023, 3, 400-416. https://doi.org/10.3390/applmicrobiol3020028
Pérez-Mora LS, Mejia-da-Silva LdC, Cezare-Gomes EdA, Santo ÉdE, Gohara-Beirigo AK, Matsudo MC, Nardin BM, Sant’Anna CL, Carvalho JCMd. Phycoremediation Processes for Secondary Effluent from Sewage Treatment Plants Using Photosynthetic Microorganisms: A Review. Applied Microbiology. 2023; 3(2):400-416. https://doi.org/10.3390/applmicrobiol3020028
Chicago/Turabian StylePérez-Mora, Lina Susana, Lauris del Carmen Mejia-da-Silva, Eleane de Almeida Cezare-Gomes, Évellin do Espirito Santo, Aline Kirie Gohara-Beirigo, Marcelo Chuei Matsudo, Bruno Monteiro Nardin, Célia Leite Sant’Anna, and João Carlos Monteiro de Carvalho. 2023. "Phycoremediation Processes for Secondary Effluent from Sewage Treatment Plants Using Photosynthetic Microorganisms: A Review" Applied Microbiology 3, no. 2: 400-416. https://doi.org/10.3390/applmicrobiol3020028
APA StylePérez-Mora, L. S., Mejia-da-Silva, L. d. C., Cezare-Gomes, E. d. A., Santo, É. d. E., Gohara-Beirigo, A. K., Matsudo, M. C., Nardin, B. M., Sant’Anna, C. L., & Carvalho, J. C. M. d. (2023). Phycoremediation Processes for Secondary Effluent from Sewage Treatment Plants Using Photosynthetic Microorganisms: A Review. Applied Microbiology, 3(2), 400-416. https://doi.org/10.3390/applmicrobiol3020028







