Abstract
This study aimed at evaluating the effects of culture media and environmental factors (temperature and water potential (Ψw)) on the growth of the pathogenic fungus Phytopythium vexans (de Bary) associated with root rot and dieback disease in apple trees. Tomato agar, potato dextrose agar (PDA), and soybean agar were the most favourable for rapid mycelial growth, with optimum growth recorded for PDA medium. To determine the environmental conditions that promoted the development of this phytopathogen, the effects of temperature (5–30 °C), water potential (Ψw) (−15.54; −0.67 MPa) (0.89–0.995 aw), and their interaction were evaluated on the in vitro radial growth rates of the five isolates of P. vexans and on their latency phase (time period prior to growth). The results of this study showed that temperature, water potential, and their interaction had significant effects (p < 0.001) on the radial growth rates and latency phases of all tested P. vexans isolates. All isolates were able to grow throughout the temperature range (5 to 30 °C), with the maximum radial growth rate being observed at the highest temperatures, 25–30 °C. Growth was seen to be faster at −0.67 MPa (0.995 aw) at 25 °C and 30 °C. No growth was observed at Ψw < −5.44 MPa (0.96 aw), regardless of the temperature. It was found that the length of the latency phase depended significantly on both environmental factors. The longest latency phases (5 days on average) were recorded at a temperature of 5 °C and Ψw of −0.67 MPa (0.995 aw) and −2.69 MPa (0.98 aw), while the shortest latency phases were observed at a temperature of 30 °C and a Ψw of −0.67 MPa (0.995 aw), with an average of 0.2 days. The findings from this study could help to understand the impact of these environmental factors on the occurrence of diseases caused by P. vexans and more likely to design a reliable preventive control strategy based on the avoidance of conditions that play in favour of the phytopathogen.
1. Introduction
The apple tree (Malus domestica Borkh) is one of the most cultivated fruit species worldwide. It is the subject of a major trade trend [1]. Apple accounts for the third largest fruit production after citrus fruits and banana [2]. The annual world apple production reached 60 million tons in the late 2000s, 62.1 million tons in 2005, 64.2 million tons in 2007, and 87 million tons in 2021 [3]. In Morocco, the apple tree is the species of rosaceous fruit that occupies the largest surface area [1]. Currently, orchards planted with apples are estimated at 52,000 ha, with an annual production of 700,000 tons and an average yield of about 16 T/ha. Middle Atlas, Rif, Saïs, Haouz, and Moulouya are the main apple-growing areas, since more than 56% of the total area planted with apple trees is located in these regions [4].
In recent years, root rot symptoms and dieback have been observed in apple trees in the main growing areas across the country, causing serious damage to the commercial apple plantations [5]. Ubiquitous microorganisms in the soil and especially those belonging to the Pythiaceae family are considered the main causative agents of root rot and dieback diseases on apples [6]. These soilborne pathogens are divided into several genera and species, the most devastating of which are species belonging to the Pythium and Phytophthora genera [7,8,9]. The vast majority of known oomycete pathogens belong to the subgroup of Peronosporales/Peronosporaceae, which includes the two important genera Phytophthora and Pythium, and other genera, such as Phytopythium, Brema, and Peronospora [10,11]. In the 1980s, Phytophthora was recognized as the most widespread oomycete species causing crown rot and root rot diseases in apple trees, whereas in the late 1990s, Pythium was the pathogen responsible for apple replant disease, causing symptoms similar to those of Phytophthora species [12,13,14]. In the 2000s, a new aggressive Phytophthora-Pythium intermediate genus was isolated and identified as Phytopythium vexans (de Bary) Abad, de Cock, Bala, Robideau, Lodhi & Lévesque [15,16,17]. This latter is involved in root rot and apple replant disease [5,16].
Root rot is a disease of plants affecting the root system caused by soil pathogens, which most often are fungi. The diseased plants are characterized by slow growth and exhibit symptoms such as wilting and yellowing leaves, browning roots, sagging brown crown canker, and quick decline if the pathogenic strain is highly aggressive [18,19]. The infection of the host is favoured by wounds inflicted at the level of the crown or on the surface of the secondary root system, which causes the disintegration of the plant organs responsible for water absorption and nutrient uptake. Oomycete pathogens such as Phytophthora spp., Pythium, and P. vexans can survive long in the soil and on diseased plants [17,20]. These pathogens can also grow under conditions of persistent moisture such as frequent watering or excessive irrigation and at temperatures ranging from 15 to 16 °C even in poorly oxygenated soil [21,22]. Their inoculum densities increase more rapidly if there is excess of nitrogen fertilizer and an environmental temperature included between 25 and 28 °C [23,24]. They gradually invade the entire vascular tissue from the root tissue and spread over the soil using mycelia and swimming zoospores through watercourses, watering systems, certain farming practices, and the transport of agricultural products and using contaminated tools from contaminated substrates [5,24,25]. The optimal growth temperature for oomycete pathogens is situated between 15 and 30 °C [23,24]. Phytophthora species grow at temperatures ranging from 13 to 15 °C, but their optimal growth temperature is around 25 °C [26], while Pythium species become more aggressive at temperatures below 18 °C when substrates are full of moisture; thus, Pythium rot occurs more in very wet soils with either little or poor drainage and excessive irrigation during rainy and cool periods (15 to 20 °C) [27]. However, most infections due to Phytopythium species occur at a high temperature; the optimum temperature for growth of most Phytopythium species is about 30 °C [28,29].
In recent decades, P. vexans has become an increasingly major threat to fruit production, in particular apples, as reported in several studies in different parts of the world [30,31,32]. Root rot disease caused by P. vexans is induced by environmental factors such as temperature, soil moisture, nitrogen and phosphorus contents, pH rate, and light intensity. Several studies were conducted to assess the impact of environmental factors on the growth, sporulation, and germination of oomycete species such as Phytophthora and Pythium [24,33,34]. Among these factors, the temperature and the water potential were shown to be the most important environmental factors limiting the growth of these pathogens [24,35]. The impact of these factors on the mycelial growth of Phytopythium species is still undetermined. Therefore, this study aimed at investigating the in vitro effects of the key environmental factors of water potential (aw) and temperature and their interactions on the radial growth and lag time (time prior growth) of P. vexans, the causal agent of dieback in apple trees.
2. Materials and Methods
2.1. Preparation of Oomycete Isolates
Five P. vexans isolates were used in this study: P. vexans I5 (MW815830), E4 (MW815816), M2 (MW815829), S2 (MW815837), and A7 (MW815815). These oomycete pathogens were previously isolated from the soil of the root system of diseased apple trees in five different regions [18]. These pathogens were subcultured and maintained in potato dextrose agar (PDA; Biokar-Diagnostics, Beauvais, France) medium at 4 °C for no more than 6 months. For long-term use, they were stored in 25% glycerol at −80 °C.
2.2. Influence of Culture Media on Phytopythium vexans Radial Growth
The impact of culture media on the mycelial growth of P. vexans species was evaluated. Mycelial plugs (5 mm in diameter) of each isolate were taken from the edge of freshly growing, seven-day-old cultures and placed at the centre of each medium (PDA, tomato agar, and soybean agar) in triplicate for each isolate. The colony diameters were measured daily until the Petri dishes were completely covered, and the radial growth rates (mm/day) were obtained by plotting the radius of colonies against time [36].
2.3. Effect of Temperature on Phytopythium vexans Radial Growth
To determine the effect of temperature on the mycelial radial growth of P. vexans, each isolate was grown in PDA medium for seven days in the dark at 25 °C. Mycelial plugs (5 mm in diameter) were cut from the leading edge of freshly growing colonies in PDA medium and then placed at the centre of Petri dishes containing 20 ml of PDA medium. All plates were then incubated for up to 25 days at 5, 10, 15, 20, 25, and 30 °C with three replicates per temperature treatment.
2.4. Combined Effect of Temperature and Water Potential (Ψw) on Radial Growth and Lag Time
To assess the effects of water potential (Ψw) and temperature on mycelial radial growth of P. vexans isolates, the basic medium used for this experiment was PDA with an aw of 0.995 (Ψw = −0.67 MPa; 0.995 aw). The water potential of this medium was modified by adding an increasing amount of glycerol to reach the Ψw contents of −15.54 (0.89 aw), −12.58 (0.91 aw), −9.68 (0.93 aw), −5.44 (0.96 aw), −2.69 (0.98 aw), and −0.67 (0.995 aw) at 30, 25, 20, 15, 10, and 5 °C [37]. The final media were autoclaved at 120 °C for 20 minutes. Mycelial plugs (5 mm in diameter) were cut from the leading edge of 10-day-old colonies growing in PDA and then placed at the centre of Petri dishes containing a test medium. The Petri dishes were then sealed with Parafilm to prevent water loss and incubated for up to 25 days at 5, 10, 15, 20, 25, and 30 °C. At each incubation temperature, Petri plates were arranged in a completely randomized design with three replicates for each combination of Ψw–temperature [38].
2.5. Radial Growth and Lag Time Assessment
Following the second day of incubation, Petri plates were examined daily without being opened for up to 20 days, and the averaged diameter of each growing mycelial colony was measured in two perpendicular directions using a digital slide stand [39]. Radial growth rates (µ, mm/day) were calculated for each temperature–Ψw combination using linear regression from the linear phase of the growth curve. Simultaneously, the maximum lag time (λ) before growth, the time required (days) for a colony to become visible for each isolate at each temperature–Ψw combination, was also recorded [40,41].
2.6. Statistical Analysis
The effect of temperature, water potential (aw), and their interactions on the radial growth rate of oomycete pathogen P. vexans were determined using the general linear model procedure (GLM) of SPSS Statistical Software (version 20; IBM SPSS Statistics 20). When the effect was found to be statistically significant, Tukey’s multiple range test was used for mean separation at p ≤ 0.05.
3. Results
3.1. Influence of Culture Media on Radial Mycelial Growth
The medium significantly affected the mycelial growth of P. vexans (Figure 1). The obtained results showed that the PDA medium was the best culture medium for the development of the pathogenic fungus P. vexans. The tomato agar medium did not favour a good growth of P. vexans in our case, with variability in the types of colonies of several species of P. vexans in the different media tested, while the lowest growth was obtained with soybean agar medium. Moreover, the abundance of sporangia was the most observed in the PDA medium compared with the other media.
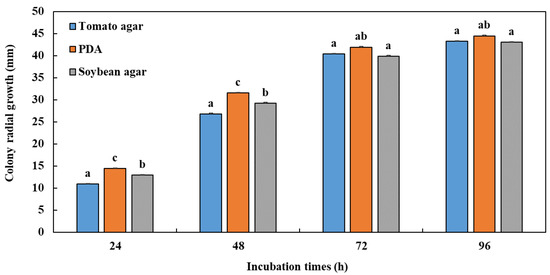
Figure 1.
Colony radial growth (mm) of Phytopythium species as affected by different culture media (tomato agar, PDA medium, and soybean agar) after an incubation period of 96 h at 25 °C. For each incubation time period, treatments having the same letter were not significantly different according to Tukey’s test (p ≤ 0.05). Error bars represent the standard errors (n = 3).
3.2. Effects of Temperature and Water Potential (Ψw) on Mycelial Radial Growth Rate
The effect of temperature was evaluated in the range of 5 to 30 °C. The results obtained in this study asserted a highly significant effect of temperature (p ≤ 0.0001) on the radial growth (µ) of the five P. vexans isolates (Table 1, Figure 2). Temperature had a significant effect on radial growth. All P. vexans isolates were able to grow in temperatures ranging from 5 to 30 °C with significant differences (p ≤ 0.05). Isolate P. vexans E4 had the highest growth rate compared with the other isolates at 15 °C, with a radial growth rate of 5.1 ± 0.04 mm/day, while P. vexans isolate I5 had the highest growth rate compared with the other isolates at 25 °C and 30 °C, with radial growth rates of 5.99 ± 0.09 mm/day and 4.93 ± 0.22 mm/day, respectively. All isolates displayed the lowest growth rate at 5 °C and 10 °C, while their maximum growth rate was observed at 25 °C.

Table 1.
Variance analysis results of the effects of temperature and water potential (aw) on radial growth of Phytopythium vexans isolates.
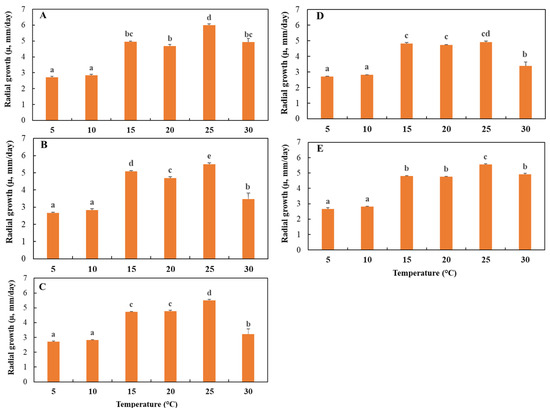
Figure 2.
Radial mycelial growth (mm/day) of Phytopythium vexans isolates ((A), I5 (Imouzzer); (B), E4 (El Hajeb); (C), M2 (Meknes); (D), S2 (Sefrou); and (E), A7 (Azrou)) versus temperature (°C). For each temperature, treatments having the same letter were not significantly different according to Tukey’s test (p ≤ 0.05). Error bars represent the standard errors (n = 3).
As for temperature, the results obtained showed a highly significant effect of water potential Ψw (p ≤ 0.0001) on the mycelial growth of P. vexans isolates (Table 1 and Table 2). The growth of P. vexans isolates was shown to increase with the increase in water availability and temperature. The growth of all isolates was faster at a Ψw of −0.67 MPa (0.995 aw) at 25 and 30 °C. In contrast, no growth was detected at Ψw of −5.44 MPa (0.96 aw), −9.68 MPa (0.93 aw), −12.58 MPa (0.91 aw), and −15.54 MPa (0.89 aw), regardless of the isolate. P. vexans isolates A7 and S2 recorded the maximum radial growth rate at −2.69 MPa (0.98 aw) and at temperatures of 25° and 30 °C, while isolates E4 and M2 registered the highest radial growth at both Ψw of −0.67 MPa (0.995 aw) and −2.69 MPa (0.98 aw) at 25 °C. Exceptionally, isolate I5 reached its maximum radial growth only at a Ψw of −0.67 MPa (0.995 aw) at 25 °C.

Table 2.
Values of radial growth rate (µ) of different studied isolates (Phytopythium vexans I5, E4, M2, S2, and A7) at different temperatures (5–30 °C) and water potentials (0.67; 9.68 MPa) (n = 3).
Using Tukey’s test for mean separation, the three factors studied, temperature, isolates, and water potential, were grouped into different homogeneous groups. The species level provided three different groups, with the first group consisting of P. vexans isolates S2, E4, and M2, while isolate I5 formed the second group, and A7, the third group. In addition, the mean separation for temperatures provided six different groups. Each temperature corresponded to a distinct group in which the highest radial growth rates occurred in a decreasing order at 25, 30, 20, 15, 10, and 5 °C. Regarding the water potential levels, the statistical analysis provided three different groups; each level corresponded to a distinct cluster with the maximum radial growth rates observed at Ψw -0.67 MPa (0.995 aw), followed respectively by −2.69 MPa (0.98 aw) and 5.44 (0.96 aw) (Figure 3).
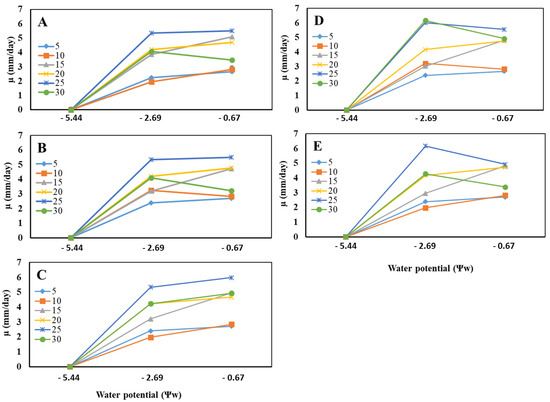
Figure 3.
Effect of water potential Ψw on the radial mycelial growth rate of Phytopythium vexans isolate E4 ((A) El Hajeb), M2 ((B) Meknes), I5 ((C) Imouzzer), A7 ((D) Azrou), and S2 ((E) Sefrou) (n = 3) at different incubation temperatures of 5, 10, 15, 20, 25, and 30 °C.
3.3. Effects of Temperature and Water Potential (Ψw) on Lag Times
The effects of temperature and water potential Ψw on lag times of P. vexans are shown in Table 3. Both key environmental factors, temperature and water potential, had significant effects on the lag time, i.e., the time required to start growth. For all P. vexans isolates, the lag time was considerably decreased with the increases in the temperature and water potential of the medium (Table 3). For all P. vexans isolates, no growth was observed at water potential Ψw of −5.44 MPa (0.96 aw) during the 25 days of the incubation periods regardless of temperature. The longest lag times were recorded at a temperature of 5 °C and Ψw of −0.67 MPa (0.995 aw) and −2.69 MPa (0.98 aw) with an estimated averaged time period of 5 days. Conversely, the shortest lag times were recorded at a temperature of 30 °C and a Ψw of −0.67 MPa (0.995 aw), with an estimated averaged time period of 0.2 days (Table 3).

Table 3.
Water potential effects on the required time (λ) to form a visible colony (in days) of the four Phytopythium vexans isolates (I5 (Imouzzer), E4 (El Hajeb), M2 (Meknès), S2 (Sefrou), and A7 (Azrou)) at different temperatures (n = 3).
4. Discussion
This study highlighted the influence of culture media and environmental factors such as temperature and water potential on the mycelial radial growth of P. vexans, an oomycete pathogen that causes dieback in apple and pear trees in Morocco. In the literature, there are no reports dealing with the impact of these key environmental factors on the growth of Phytopythium species. Therefore, our study is the first investigation evaluating the growth of this pathogen in different temperature and water potential ranges, which might help to determine the range of each factor that plays in favour of the pathogen. The outcome of this study can help to understand the epidemiology of the pathogen and design an integrated control strategy for the managing the disease based on the avoidance and exclusion of the pathogen by optimizing the watering systems in apple plantations. It is known that the presence of water in the soil is crucial for the infection and development of root rot diseases in plants as well as their rapid spread, in particular oomycete pathogens [33,42]. To spread and infect plants, oomycete pathogens need free water, because water can help to release zoospores from sporangia and stimulate the germination of oospores, possibly by creating favourable conditions [43]. In addition, free water must be available in the soil to facilitate the mobility of flagellated zoospores [44].
Culturing P. vexans and producing its infective sporangia for plant inoculation have been a challenge for pathological bioassays on the disease caused by these oomycete pathogens. Therefore, having an appropriate medium for the rapid growth of the mycelium of this pathogen and the production of its sporangia is the first step in passing the pathogenicity test. In our study, all isolates grew on all three media tested; however, the PDA medium was the most favourable for the rapid radial growth of the mycelium of P. vexans. In addition, the highest abundance of sporangia was observed in PDA medium, followed respectively by Tomato agar and Soybean agar, and this was correlated with the appearance of yellow pigmentation. Several studies investigated the influence of culture media on the growth and sporulation of oomycete pathogens [45,46], but only one study was interested in Phytopythium sp. [47]. In this study, the culture medium containing sweet cassava root was found to be the most suitable for mycelial development and pathogen sporulation.
Our results indicated that the environmental factors studied had a significant impact on the radial growth and lag time periods of all P. vexans isolates. It was shown that all isolates were able to grow in the range of temperatures used (5 to 30 °C). These results were in agreement with previous findings that underlined that P. vexans and Pythium irregulare Buisman displayed growth at temperatures ranging from 10 to 35 °C and from 5 to 40 °C, respectively [48]. Similar results were reported for Cylindrocarpan isolates and underlined that fungal isolates had the ability to grow at temperatures between 5 and 30 °C [49]. In addition, the maximum radial growth rate of Cylindrocarpan isolates was observed at temperatures between 25 °C and 30 °C, which was consistent with the results of our study, according to which P. vexans isolates displayed the maximum growth at temperatures ranging from 25 °C to 30 °C. Interestingly, most studies on oomycetes evidenced similar ranges of optimum growth temperature, e.g., 28 °C for Pythium insidiosum de Cock, Mendoza, Padhye, Ajello & Kaufman [50]; 25–30 °C for P. irregular [48]; 30 °C for Pythium aphanidermatum (Edson) Fitz. [51]; and 25 °C for Phythophtora clandestina Taylor, Pascoe & Greenhalgh [52]. Moreover, the genus Cylindrocarpan showed a slight lower optimum growth temperature, between 21.9 and 24.5 °C [49].
The water potential is considered as one of the important factors limiting the growth of phytopathogenic fungi [53,54,55]. In this study, the growth of P. vexans was shown to increase with the increases in water availability and temperature. Surprisingly, no growth of Ph. vexans was observed at Ψw of −5.44 MPa (0.96 aw), −9.68 MPa (0.93 aw), −12.58 MPa (0.91 aw), and −15.54 MPa (0.89 aw), regardless of the temperature. In a similar study, there was no growth at water potentials below −1.2 MPa and at −4.8 MPa for P. clandestina and Phytophthora cinnamomi Rands, respectively [52,56]. Furthermore, the growth of all Phytopythium isolates was faster at −0.67 MPa (0.995 aw) at 25 °C and 30 °C, which was in line with previous findings on P. clandestina according to which this oomycete species registered its maximum growth at 0 MPa and at 25 °C, while for other Phytophthora species, the maximum growth rate was found at Ψw −1 MPa and −1.5 MPa with an optimum growth at −1.08 MPa in P. cinnamomi [56,57]. In addition, the growth of Phytopythium was significantly reduced at a Ψw of −2.69 MPa (0.98 aw). The growth of P. cinnamomi was significantly reduced at Ψw of −3.03 MPa and −3.79 MPa, while the lowest growth of Cylindrocarpan was observed at −5 MPa. Interestingly, the growth of P. clandestina and Phytophthora parasitica Breda de Haan decreased with the decrease in the water potential, but it persisted up to −5 MPa and showed to be strongly reduced at a Ψw of −1.2 MPa at 20 and 25 °C [52,58,59].
To the best of our knowledge, there are very few reports on the influence of environmental factors on the latency phase of oomycetes. The results of the effects of temperature and water potential on the latency time (time period prior growth) underlined that each of these two factors had a significant effect (p ≤ 0.0001). In all isolates, the latency phase decreased considerably with the increase in the water potential, regardless of the temperature. At a water potential of −5.44 MPa (0.96 aw) and at all temperatures, no growth was recorded for a growth period of up to 25 days. The longest latency phases were recorded at a temperature of 5 °C and Ψw of −0.67 MPa (0.995 aw) and −2.69 MPa (0.98 aw), with a five-day average. Furthermore, the shortest latency phases were recorded at a temperature of 30 °C and at a Ψw of −0.67 MPa (0.995 aw), with an average of 0.2 days. These results were in agreement with previous findings on different oomycete and fungal pathogens [49,60].
It was suggested that variations in the soil water content may determine the form of inoculum involved in root rot infection. The formation and motility of zoospores in many oomycete species depend on high soil moisture [61,62,63], while mycelial growth occurs at relatively low water potentials [25,56,64]. Therefore, preventing oomycete diseases is mainly based on the parfait knowledge of the disease cycle of the pathogen and its epidemiology. Knowing how these pathogens spread makes it easier to prevent their occurrence by draining the soil and avoiding excess watering, because once these microorganisms are present in soil, it is difficult to eradicate them.
5. Conclusions
Our findings evidenced that temperature, water potential, and their interaction had significant impacts on the growth of this phytopathogen. The growth rates were shown decreased with the increases in the temperature and water potential, and no growth was observed at Ψw ≤ −5.44 MPa (0.96 aw), regardless of the temperature. These results can be very useful in preventing the onset and spread of this pathogen. Therefore, good soil drainage and good water flow might help to prevent soil saturation, but important consideration must be given to installing a subsurface drainage and well-used drip irrigation, which prevent the increase in soil moisture and limit the spreading of the pathogen.
Author Contributions
Conceptualization, S.J. and R.L.; methodology, S.J. and R.L.; software, S.J.; validation, R.L. and M.B.A.; formal analysis, R.L.; investigation, R.L.; resources, R.L.; data curation, S.J. and R.L.; writing—original draft preparation, S.J.; writing—review and editing, S.J., H.E.H. and R.L.; supervision, R.L. and M.B.A.; project administration, R.L.; funding acquisition, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was financially supported by the Phytopathology Unit, Department of Plant Protection, Ecole Nationale d’Agriculture de Meknes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moinina, A.; Lahlali, R.; MacLean, D.; Boulif, M. Farmers’ Knowledge, Perception and Practices in Apple Pest Management and Climate Change in the Fes-Meknes Region, Morocco. Horticulturae 2018, 4, 42. [Google Scholar] [CrossRef]
- Mbovora, S.M.; Musvosvi, C.; Gasura, E. Morphological Diversity among Accessions of Apple Tree (Malus × Domestica Borkh). Adv. Agric. 2021, 2021, 7705856. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef] [PubMed]
- Raada, S.; Mazouz, H.; Boulif, M. Phytosanitary practices of apple growers in the Ifrane province of the Middle Atlas of Morocco and perspectives of improvement. Pratiques phytosanitaires des pomiculteurs de la province d’Ifrane au Moyen Atlas du Maroc et perspectives d’amélioration. Rev. Maroc. Prot. Plantes 2019, 13, 19–33. [Google Scholar]
- Jabiri, S.; Bahra, C.; MacLean, D.; Radouane, N.; Barka, E.A.; Bendriss Amraoui, M.; Lahlali, R. Phytopythium vexans Associated with Apple and Pear Decline in the Saïss Plain of Morocco. Microorganisms 2021, 9, 1916. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, B.; Gold, C.; Kashaija, I.; Ssali, H.; Night, G.; Bwamiki, D. Effects of legume intercrops on soil-borne pests, biomass, nutrients and soil water in banana. Biol. Fertil. Soils 2001, 34, 342–348. [Google Scholar] [CrossRef]
- Lehtijärvi, A.; Aday Kaya, A.G.; Woodward, S.; Jung, T.; Doğmuş Lehtijärvi, H.T. Oomycota species associated with deciduous and coniferous seedlings in forest tree nurseries of Western Turkey. For. Pathol. 2017, 47, e12363. [Google Scholar] [CrossRef]
- Brasier, C.; Scanu, B.; Cooke, D.; Jung, T. Phytophthora: An ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus 2022, 13, 12. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A Molecular Phylogeny of Phytophthora and Related Oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Richards, T.A.; Talbot, N.J. Plant Parasitic Oomycetes Such as Phytophthora Species Contain Genes Derived from Three Eukaryotic Lineages. Plant Signal. Behav. 2007, 2, 112–114. [Google Scholar] [CrossRef][Green Version]
- Lang-Yona, N.; Pickersgill, D.A.; Maurus, I.; Teschner, D.; Wehking, J.; Thines, E.; Pöschl, U.; Després, V.R.; Fröhlich-Nowoisky, J. Species Richness, rRNA Gene Abundance, and Seasonal Dynamics of Airborne Plant-Pathogenic Oomycetes. Front. Microbiol. 2018, 9, 2673. [Google Scholar] [CrossRef] [PubMed]
- Sewell, G.W.F. Effects of Pythium species on the growth of apple and their possible causal role in apple replant disease. Ann. Appl. Biol. 1981, 97, 31–42. [Google Scholar] [CrossRef]
- Harris, D.C. The Phytophthora diseases of apple. J. Hortic. Sci. 1991, 66, 513–544. [Google Scholar] [CrossRef]
- Bala, K.; Robideau, G.P.; Desaulniers, N.; de Cock, A.W.; Levesque, C.A. Taxonomy, DNA barcoding and phylogeny of three new species of Pythium from Canada. Persoonia 2010, 25, 22–31. [Google Scholar] [CrossRef]
- Nam, B.; Choi, Y.-J. Phytopythium and Pythium Species (Oomycota) Isolated from Freshwater Environments of Korea. Mycobiology 2019, 47, 261–272. [Google Scholar] [CrossRef]
- Jabiri, S.; Lahlali, R.; Bendriss Amraoui, M.; Tahiri, A.; Amiri, S. First report of Phytopythium vexans associated with dieback disease of apple trees in Morocco. J. Plant Pathol. 2020, 102, 1319. [Google Scholar] [CrossRef]
- Rodriguez-Padron, C.; Siverio, F.; Perez-Sierra, A.; Rodriguez, A. Isolation and pathogenicity of Phytophthora species and Phytopythiumvexans recovered from avocado orchards in the Canary Islands, including Phytophthoraniederhauserii as a new pathogen of avocado. Phytopathol. Mediterr. 2018, 57, 89–106. [Google Scholar]
- Polat, Z.; Awan, Q.N.; Hussain, M.; Akgül, D.S. First Report of Phytopythium vexans Causing Root and Collar Rot of Kiwifruit in Turkey. Plant Dis. 2017, 101, 1058. [Google Scholar] [CrossRef]
- Williamson-Benavides, B.A.; Dhingra, A. Understanding Root Rot Disease in Agricultural Crops. Horticulturae 2021, 7, 33. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, C.; Rodríguez, A.; Siverio, F. Survey in Nurseries and Irrigation Water Reservoirs as Sources of Oomycetes Found in Avocado Orchards in the Canary Islands. Plant Dis. 2019, 103, 1264–1274. [Google Scholar] [CrossRef]
- Jones, L.A.; Worobo, R.W.; Smart, C.D.; Elkins, C.A. Plant-Pathogenic Oomycetes, Escherichia coli Strains, and Salmonella spp. Frequently Found in Surface Water Used for Irrigation of Fruit and Vegetable Crops in New York State. Appl. Environ. Microbiol. 2014, 80, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Redekar, N.R.; Bourret, T.B.; Eberhart, J.L.; Johnson, G.E.; Pitton, B.J.L.; Haver, D.L.; Oki, L.R.; Parke, J.L. The population of oomycetes in a recycled irrigation water system at a horticultural nursery in southern California. Water Res. 2020, 183, 116050. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.; Lee, D.-J.; Choi, Y.-J. High-Temperature-Tolerant Fungus and Oomycetes in Korea, Including Saksenaea longicolla sp. nov. Mycobiology 2021, 49, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Breccia, M.; Bruni, N.; Tomassini, A.; Vettraino, A.M. Behaviour and survival of Phytophthora cambivora inoculum in soil-like substrate under different water regimes. For. Pathol. 2012, 42, 362–370. [Google Scholar] [CrossRef]
- Halsall, D.M.; Williams, J.D. Effect of Root Temperature on the Development of Phytophthora cinnamomi Root Rot in Eucalyptus Seedlings. Aust. J. Bot. 1984, 32, 521–528. [Google Scholar] [CrossRef]
- Martin, F.N.; Loper, J.E. Soilborne Plant Diseases Caused by Pythium Spp.: Ecology, Epidemiology, and Prospects for Biological Control. Crit. Rev. Plant Sci. 1999, 18, 111–181. [Google Scholar] [CrossRef]
- de Cock, A.W.A.M.; Lodhi, A.M.; Rintoul, T.L.; Bala, K.; Robideau, G.P.; Abad, Z.G.; Coffey, M.D.; Shahzad, S.; Lévesque, C.A. Phytopythium: Molecular phylogeny and systematics. Persoonia 2015, 34, 25–39. [Google Scholar] [CrossRef]
- Baten, M.A.; Asano, T.; Motohashi, K.; Ishiguro, Y.; Rahman, M.Z.; Inaba, S.; Suga, H.; Kageyama, K. Phylogenetic relationships among Phytopythium species, and re-evaluation of Phytopythium fagopyri comb. nov., recovered from damped-off buckwheat seedlings in Japan. Mycol. Prog. 2014, 13, 1003. [Google Scholar] [CrossRef]
- Prencipe, S.; Savian, F.; Nari, L.; Ermacora, P.; Spadaro, D.; Martini, M. First Report of Phytopythium vexans causing decline syndrome of Actinidia deliciosa ‘Hayward’ in Italy. Plant Dis. 2020, 104, 2032. [Google Scholar] [CrossRef]
- Thao, L.D.; Hien, L.T.; Liem, N.V.; Thanh, H.M.; Khanh, T.N.; Binh, V.T.P.; Trang, T.T.T.; Anh, P.T.; Tu, T.T. First report of Phytopythium vexans causing root rot disease on durian in Vietnam. New Dis. Rep. 2020, 41, 2. [Google Scholar] [CrossRef]
- Boari, A.D.J.; Cunha, E.M.; Quadros, A.F.F.; Barreto, R.W.; Fernandes, A.F. First Report of Phytopythium sp. Causing Storage Root Rot and Foliage Blight of Cassava in Brazil. Plant Dis. 2018, 102, 1042–1104. [Google Scholar] [CrossRef]
- Wielgoss, A.; Nechwatal, J.; Bogs, C.; Mendgen, K. Host plant development, water level and water parameters shape Phragmites australis-associated oomycete communities and determine reed pathogen dynamics in a large lake. FEMS Microbiol. Ecol. 2009, 69, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Derevnina, L.; Petre, B.; Kellner, R.; Dagdas, Y.F.; Sarowar, M.N.; Giannakopoulou, A.; De la Concepcion, J.C.; Chaparro-Garcia, A.; Pennington, H.G.; van West, P.; et al. Emerging oomycete threats to plants and animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150459. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Serrhini, M.N.; Friel, D.; Jijakli, M.H. Predictive modelling of temperature and water activity (solutes) on the in vitro radial growth of Botrytis cinerea Pers. Int. J. Food Microbiol. 2007, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Serrhini, M.N.; Friel, D.; Jijakli, M.H. In vitro effects of water activity, temperature and solutes on the growth rate of P. italicum Wehmer and P. digitatum Sacc. J. Appl. Microbiol. 2006, 101, 628–636. [Google Scholar] [CrossRef]
- Lahlali, R.; Bajji, M.; Serrhini, M.N.; Jijakli, M.H. Modelling the effect of temperature, Water activity and solute on the in vitro growth of the biocontrol yeast Pichia anomala strain K. Biotechnol. Agron. Soc. Environ. 2008, 12, 353–359. [Google Scholar]
- Lahlali, R.; Serrhini, M.N.; Jijakli, M.H. Studying and modelling the combined effect of temperature and water activity on the growth rate of P. expansum. Int. J. Food Microbiol. 2005, 103, 315–322. [Google Scholar] [CrossRef]
- Romero, S.M.; Patriarca, A.; Fernández Pinto, V.; Vaamonde, G. Effect of water activity and temperature on growth of ochratoxigenic strains of Aspergillus carbonarius isolated from Argentinean dried vine fruits. Int. J. Food Microbiol. 2007, 115, 140–143. [Google Scholar] [CrossRef]
- Patriarca, A.; Vaamonde, G.; Fernández Pinto, V.; Comerio, R. Influence of water activity and temperature on the growth of Wallemia sebi: Application of a predictive model. Int. J. Food Microbiol. 2001, 68, 61–67. [Google Scholar] [CrossRef]
- Weiland, J.E.; Santamaria, L.; Grünwald, N.J. Sensitivity of Pythium irregulare, P. sylvaticum, and P. ultimum from Forest Nurseries to Mefenoxam and Fosetyl-Al, and Control of Pythium Damping-off. Plant Dis. 2014, 98, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Hord, M.J.; Ristaino, J.B. Effects of Physical and Chemical Factors on the Germination of Oospores of Phytophthora capsici in vitro. Phytopathology 1991, 81, 1541–1546. [Google Scholar] [CrossRef]
- Delmas, C.E.L.; Mazet, I.D.; Jolivet, J.; Delière, L.; Delmotte, F. Simultaneous quantification of sporangia and zoospores in a biotrophic oomycete with an automatic particle analyzer: Disentangling dispersal and infection potentials. J. Microbiol. Methods 2014, 107, 169–175. [Google Scholar] [CrossRef]
- Shivakumar, K.S.; Somasekhara, Y.M. Influence of Culture Media on Growth and Sporulation of Phytophthora capsici the Cause of Quick Wilt of Black Pepper. Mysore J. Agric. Sci. 2018, 52, 369–374. [Google Scholar]
- Medina, M.V.; Platt, H.W. Comparison of different culture media on the mycelial growth, sporangia and oospore production ofPhytophthora infestans. Am. J. Potato Res. 1999, 76, 121–125. [Google Scholar] [CrossRef]
- de Sousa Silva, J.L.; Ishida, A.K.N.; Cunha, R.L.; Lima, A.M.; Moura, E.F. Culture medium and inoculation methodology for the study of soft root rot caused by Phytopythium sp. Cienc. Rural 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Cantrell, H.F.; Dowler, W.M. Effects of Temperature and ph on Growth and Composition of Pythium Irregulare and Pythium Vexans. Mycologia 1971, 63, 31–37. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Armengol, J. Effects of temperature, pH and water potential on mycelial growth, sporulation and chlamydospore production in culture of Cylindrocarpon spp. associated with black foot of grapevines. Phytopathol. Mediterr. 2012, 51, 37–50. [Google Scholar] [CrossRef]
- Krajaejun, T.; Chongtrakool, P.; Angkananukul, K.; Brandhorst, T. Effect of temperature on growth of the pathogenic oomycete Pythium insidiosum. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1462–1466. [Google Scholar]
- Bolton, A.T. Effects of temperature and pH of soilless media on root rot of poinsettia caused by Pythium aphanidermatum. Can. J. Plant Pathol. 1980, 2, 83–85. [Google Scholar] [CrossRef]
- Wong, D.; Sivasithamparam, K.; Barbetti, M. Influence of environmental factors on the growth and survival of Phytophthora clandestina. Can. J. Microbiol. 2011, 32, 553–556. [Google Scholar] [CrossRef]
- Cook, R.J.; Duniway, J.M. Water Relations in the Life-cycles of Soilborne Plant Pathogens. Water Potential Relat. Soil Microbiol. 1981, 9, 119–139. [Google Scholar]
- Duniway, J.M. Water Relations of Water Molds. Annu. Rev. Phytopathol. 1979, 17, 431–460. [Google Scholar] [CrossRef]
- Gill, J.S.; Sivasithamparam, K.; Smettem, K.R.J. Effect of soil moisture at different temperatures on Rhizoctonia root rot of wheat seedlings. Plant Soil 2001, 231, 91–96. [Google Scholar] [CrossRef]
- Malajczuk, N.; Theodorou, C. Influence of water potential on growth and cultural characteristics of Phytophthora cinnamomi. Trans. Br. Mycol. Soc. 1979, 72, 15–18. [Google Scholar] [CrossRef]
- Harris, R.F.; Dalton, F.N.; Gardner, W.R. Water potential relations of three root-infecting Phytophthora species. Phytopathology 1970, 60, 932–934. [Google Scholar]
- Shew, H.D. Effects of Soil Matric Potential on Infection of Tobacco by Phytophthora parasitica var. nicotianae. Phytopathology 1983, 73, 1160. [Google Scholar] [CrossRef]
- Hord, M.J. Effect of the Matric Component of Soil Water Potential on Infection of Pepper Seedlings in Soil Infested with Oospores of Phytophthora capsici. Phytopathology 1992, 82, 792. [Google Scholar] [CrossRef]
- Begoude, B.A.D.; Lahlali, R.; Friel, D.; Tondje, P.R.; Jijakli, M.H. Response surface methodology study of the combined effects of temperature, pH, and aw on the growth rate of Trichoderma asperellum. J. Appl. Microbiol. 2007, 103, 845–854. [Google Scholar] [CrossRef]
- Judelson, H.S.; Ah-Fong, A.M. Exchanges at the Plant-Oomycete Interface That Influence Disease. Plant Physiol. 2019, 179, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Bassani, I.; Larousse, M.; Tran, Q.D.; Attard, A.; Galiana, E. Phytophthora zoospores: From perception of environmental signals to inoculum formation on the host-root surface. Comput. Struct. Biotechnol. J. 2020, 18, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.G. Interrelationships of Seedling Age, Inoculum, Soil Moisture Level, Temperature, and Host and Pathogen Genotype in Phytophthora Root Rot of Alfalfa. Phytopathology 1976, 66, 81. [Google Scholar] [CrossRef]
- Sterne, R.E. The Effect of Matric and Osmotic Potential of Soil on Phytophthora Root Disease of Persea indica. Phytopathology 1977, 77, 1491. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).