Abstract
Bacterial-fungal interactions are important in the functioning of natural ecosystems. We examined possible synergistic or antagonistic effects during the degradation of polycyclic aromatic hydrocarbons (PAHs) by a fungal–bacterial co-culture. Bacteria and fungi were grown in a liquid nutrient medium supplemented with PAH substrates. The degradation of PAHs and the identification of metabolites were checked by HPLC. Enzyme activities were spectrophotometrically measured with test substrates. Compared to monocultures, the co-culture yielded higher mycelium dry weights and higher numbers of bacterial colony-forming units (CFUs). Both organisms and their co-culture transformed three- and four-ring PAHs into the corresponding quinones. The degradation of PAHs was accompanied by the production of fungal extracellular laccase and versatile peroxidase, whose activities were higher in the co-culture than they were in the monocultures. The presence of exogenous indole-3-acetic acid (IAA) boosted PAH degradation and enzyme production. The xylotrophic basidiomycete Pleurotus ostreatus Florida and the plant-growth-promoting rhizobacterium Azospirillum brasilense exerted a positive mutual effect, including increases in mycelium dry weight, number of CFUs, degradation of PAHs, and production of fungal extracellular enzymes. IAA may be a factor in the interactions of P. ostreatus Florida with A. brasilense.
1. Introduction
Fungi and bacteria coexist in a variety of environments, from soil and food to plants and animals. Bacterial-fungal interactions are important for habitat colonization and for survival and pathogenesis [1], and they are an essential component of microbial communities in terrestrial ecosystems. In the past decade, research attention to these interactions has increased tremendously. Information is available on the mechanisms and functional responses of partners in a wide range of such interactions [2].
Bacteria, which move by flagella along the continuous liquid film on the surface of fungal hyphae [3], can use hyphae as pathways to reach inaccessible habitats [4]. Unlike bacteria, fungi can easily spread in water-deficient environments, facilitating the access of pollutant-degrading bacteria to pollutants [5]. In addition, fungal hyphae may promote the attachment of bacteria to plant roots, facilitating their entry into the rhizosphere or tissues [6]. Bacteria and fungi can form a mixed biofilm on the surface of plants, which protects them from adverse environmental conditions [7].
Most research has focused on bacterial–fungal interactions under controlled conditions [2]. How relevant this research is to the importance of bacterial–fungal interactions for the functioning of natural microbial communities in actual ecosystems is still an open question. Most often, in vitro models have explored bacterial–fungal interactions related to food microbiology, soil ecology, bacterial control of pathogenic fungi, and mycorrhiza formation [1,8].
Studies on bacterial–fungal interactions during the degradation of natural substrates and xenobiotics are few. There exists a niche differentiation between saprotrophic fungi and bacteria. Fungi mainly degrade hard lignocellulosic materials (such as wood debris), whereas bacteria mainly utilize easy-to-degrade soluble organic compounds (such as sugars). This is evidence for a relationship between the degrading activities of fungi and bacteria [2].
Some authors believe that indole-3-acetic acid (IAA), which is a phytohormone of the auxin class, can also be a universal physiological code not only for the plants but also for bacteria and fungi. Increasing evidence shows IAA as a diffusible signal that is used for interspecies communications. This phenomenon suggests the existence of a framework, widely evolved in both eukaryotes and prokaryotes, allowing the production, transfer, and perception of IAA signals between distantly related organisms across the branches of phylogenetically diverse groups of the tree of life [9]. The production of IAA by bacteria is well-studied. Although, IAA-producing fungi are known [10], their biosynthetic pathways and role in fungal ecology have not been widely studied.
The interaction of soil bacteria with fungi is very important for the degradation of various man-made pollutants, as well as for the metabolism of natural substrates, such as cellulose, hemicellulose, and lignin. Cooperation and antagonism have been described between bacterial microflora and fungi during the degradation and mineralization of pollutants. The antagonistic interactions between soil bacteria and fungi may be caused by competition for an available carbon source [11]. In the degradation of some pollutants, fungi act synergistically with bacteria [7,12,13]. In such a case, the fungal and bacterial degradation pathways may complement each other. The cometabolic degradation leads to complete pollutant utilization, because metabolites formed by one organism are metabolized by the other. Co-cultures of bacteria and fungi have been used in the bioremediation of soils polluted by polycyclic aromatic hydrocarbons (PAHs) [14,15,16,17]. Using a bacterial-fungal culture, Boonchan et al. [18] observed a decrease in the mutagenicity of soil organic extracts, as well as an increase in the soil degradation of PAHs, as compared to the data for the native microflora and for soil inoculated with separate cultures. Similarly, soil remediation with co-cultures of Rhodococcus sp. and Aspergillus terreus or Penicillium sp. resulted in decreased soil toxicity and complete degradation of anthracene, phenanthrene, and pyrene [19]. This synergism may be related to the ability of fungi to transform PAHs into polar compounds that are utilized by the soil microflora. This polarity reversal leads to pollutant redistribution between the soil and water phases, which also makes polar products more accessible to bacteria [20,21].
As a promising option, fungal enzymes are regarded as a powerful choice for the degradation of PAHs. Ligninolytic fungi have been extensively studied because they produce extracellular enzymes with extremely reduced substrate specificity. The ligninolytic system contains three peroxidases (lignin peroxidase, Mn-peroxidase, and versatile peroxidase) and laccase. The involvement of these enzymes in the degradation of PAHs was shown many studies. The first attack of the molecule of PAH resulted in the formation of corresponding quinones. In addition to the mentioned other fungal enzymes, such as cytochrome P450 monooxygenase and epoxide hydrolase, some dioxygenases participate in PAH degradation. Finally, the fungal degradation of PAHs resulted in the mineralization of parent compounds [22]. More often in bacterial degradation pathways, the PAHs are activated by directly incorporating molecular oxygen with the help of mono- or dioxygenases [23]. Usually, the first step in the aerobic bacterial degradation of PAHs is the hydroxylation of an aromatic ring via a dioxygenase, with the formation of a cis-dihydrodiol, which gets rearomatized to a diol intermediate by the action of a dehydrogenase. These diol intermediates may then be cleaved by intradiol or extradiol ring-cleavage dioxygenases, leading to intermediates that can be involved in the TCA cycle [24].
Wood-inhabiting basidiomycetes of the genus Pleurotus belong to ligninolytic fungi, which are some of the most active degraders of lignin in nature. They can degrade a wide range of persistent aromatics and produce a complex of extracellular nonspecific enzymes including Mn-peroxidases (EC 1.11.1.13), versatile peroxidases (EC 1.11.1.16), and laccases (EC 1.10.3.2). The ability of Pleurotus fungi to survive in soil, compete with the indigenous microflora, and degrade soil pollutants makes them promising candidates for mycoremediation [25].
Bacteria belonging to the genus Azospirillum are free-living microbes that promote plant growth (PGPR). The most accepted theory regarding the mechanism of action of Azospirillum is its growth promotion, which includes nitrogen fixation and phytohormone (including IAA), polyamine, and trehalose production. There are reports on using Azospirillum strains in the pollutant (phenol, benzoate, protocatechuate, catechol, phenanthrene, and crude oil) degradation process [26,27]. The production of laccase and ligninolytic peroxidases, such as fungi, by Azospirillum are also known [28,29].
The study of the interaction mechanisms operating in bacterial–fungal systems is an integral part of research into the functioning of natural ecosystems in polluted settings and is necessary for the design of bioremediation technologies. Here, we sought to identify possible synergistic or antagonistic effects during PAH degradation by a co-culture of the basidiomycete Pleurotus ostreatus and the rhizobacterium Azospirillum brasilense.
2. Materials and Methods
P. ostreatus f. Florida (IBPPM 540) and A. brasilense SR80 (IBPPM 24) were obtained from the IBPPM RAS Collection of Rhizosphere Microorganisms (http://collection.ibppm.ru, accessed on 23 July 2022).
The fungus was grown on a modified basidiomycetes-rich medium [30] composed as follows (g/L): NH4NO3, 0.724; KH2PO4, 1.0; MgSO4×7H2O, 1.0; KCl, 0.5; yeast extract, 0.5; FeSO4×7H2O, 0.01; ZnSO4×7H2O, 0.0028; CaCl2×2H2O, 0.033; glucose, 10.0; peptone, 10.0; agar, 15.0; (pH 6.0). The growth of P. ostreatus mycelium was controlled by the weight method [31].
Bacteria were grown on R2A medium [32] composed as follows (g/L): yeast extract, 0.5; peptone, 0.5; casein hydrolysate, 0.5; glucose, 0.5; soluble starch, 0.5; sodium pyruvate, 0.3; K2HPO4, 0.3; MgSO4×7H2O, 0.05; agar, 15.0. Growth was controlled by direct plating of multiple dilutions of the culture medium on R2A agar and by counting the CFUs.
Phenanthrene (∼90%), pyrene (∼90%), anthracene (≥95%), fluoranthene (≥97%), fluorene (≥99%), and indole-3-acetic acid (IAA; Pestanal®, analytical standard) were all purchased from Fluka (Buchs, Switzerland).
For degradation studies, we used a medium of the following composition (g/L): glucose, 4.5; fructose, 4.5; L-asparagine, 1.5 [33]. A mixture of PAHs (anthracene, phenanthrene, fluorene, pyrene, and fluoranthene; 1 mg of each) was added to a final total PAH concentration of 5 mg per 100 mL. P. ostreatus (20 mg of dry weight/about 200 mg of wet weight) and A. brasilense (3 × 10−5 CFUs), both singly and in combination, were grown at 24, 30, or 37 °C in 0.3-L Erlenmeyer flasks on a shaker New Brunswick™ Excella® E24/E24R Shaker (Eppendorf, Nürtingen, Germany) at 130 rpm. At certain time intervals, an aliquot (2 mL) was taken from the flasks and the activity of extracellular enzymes and the concentration of IAA were determined as described below.
After 14 days, the contents of the flasks were extracted three times with 5 mL of chloroform, and the extracts were evaporated. The sorption of PAHs by fungal mycelium did not exceed 5–7%. PAH content was analyzed by HPLC on an Agilent Technologies 1220 Infinity II LC chromatograph (Agilent Technology, Waldbronn, Germany) equipped with a 4.6 × 150-mm ZORBAX Eclipse PAH 5-Micron column and a 254-nm UV detector. The solvent system was H2O:acetonitrile, the linear gradient was 40–100% acetonitrile, and the time was 15 min. The PAHs and their metabolites were analyzed by comparing the retention times with those of standard compounds.
IAA content was analyzed by HPLC on an Agilent Technologies 1220 Infinity II LC chromatograph (Agilent Technology, Waldbronn, Germany) equipped with a 4.6 × 50-mm ZORBAX Eclipse Plus C18 5-Micron column and a 254-nm UV detector. The solvent system was H2O (acidified with H3PO4 to pH 2.5):acetonitrile, the linear gradient was 10–90% acetonitrile, and the time was 15 min.
Laccase activity was measured by the oxidation rate for 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt (ABTS) at 436 nm (ε = 29,300 M−1cm−1), according to Niku-Paavola et al. [34]. Versatile peroxidase was measured by the oxidation rate for 2,6-dimethoxyphenol (DMP) with H2O2 and Mn2+ at 468 nm (ε = 14,800 M−1cm−1), according to Martinez et al. [35]. One unit of enzyme activity was defined as the amount required to catalyze the formation of 1 µmol of product per minute and is expressed in arbitrary units (µmol/min/mL of enzyme preparation (U/mL)).
In separate experiments the effect of IAA on PAH degradation and extracellular enzyme production was investigated by supplementing the culture medium with IAA to a final concentration of 1 mg/100 mL. At certain time intervals, an aliquot (2 mL) was taken from the flasks and the activity of extracellular enzymes and the concentration of IAA were determined too.
All experimental treatments and all analyses were repeated at least three times. Each experiment was repeated at least three times. Results were analyzed with Excel 2003 (Microsoft Office 2003, USA) and Origin 7 software.
3. Results
3.1. Degradation of the PAH Mixture
Before A. brasilense SR80 and P. ostreatus Florida were co-cultured in the liquid medium, their antagonistic activity on R2A agar was investigated by the double-culture method [36,37]. The results showed an absence of mutual antagonism (data not shown). The optimal temperatures to grow the organisms were 37 °C (A. brasilense SR80), 24 °C (P. ostreatus Florida), and 30 °C (co-culture).
At 24 and 30 °C, co-culturing of P. ostreatus Florida and A. brasilense SR80 in liquid medium increased the dry weight of the fungal mycelium and the bacterial CFU number (Table 1). Increasing the temperature to 37 °C inhibited mycelium growth.

Table 1.
Effect of PAHs and co-culture on growth variables of P. ostreatus and A. brasilense.
The presence of the PAH mixture significantly reduced mycelium dry weight, as compared to the control, possibly as the result of inhibition of fungal growth by the PAHs and/or their metabolites. The CFU number in A. brasilense SR80 also decreased in the presence of the PAHs but increased in the dual culture (Table 1). It may be that, in this case, fungal metabolites of PAHs may be an additional source of carbon for the bacteria. This hypothesis will be the subject of further research.
During the disappearance of PAHs, we evaluated the activity of extracellular oxidative enzymes. A. brasilense SR80 did not produce extracellular laccase or peroxidase; by contrast, P. ostreatus Florida did produce laccase and versatile peroxidase in all treatments. At 30 and 37 °C, the activities of both enzymes were significantly lower than they were at 24 °C. The PAHs and/or their metabolites induced the production of versatile peroxidase. Laccase and versatile peroxidase activities were the highest in the co-culture (Table 2).

Table 2.
Effect of growth conditions on activities of P. ostreatus extracellular enzymes.
Both organisms, alone and in combination, utilized the PAHs. Fluorene was the most accessible of all PAHs used, because it is relatively well-soluble (0.39–1.98 mg/L) and has a low ionization potential (7.88–8.2 eV) [38]. Phenanthrene (at 30 °C), fluoranthene, and pyrene were hard to use by bacteria (Figure 1); by contrast, all PAHs were available for fungal utilization. At an elevated temperature, however, only phenanthrene was subject to fungal utilization, and fluorene and phenanthrene were subject to bacterial utilization. This may have been the result of poor fungal growth at high temperatures. Although A. brasilense SR80 grew well, it degraded the PAHs to a worse degree at 37 °C than it did at 24 and 30 °C.
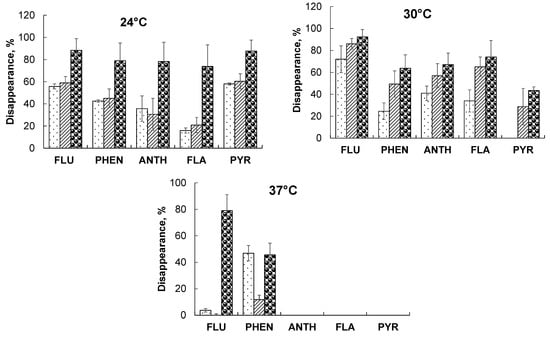
Figure 1.
PAH degradation by A. brasilense SR80 ( ), P. ostreatus Florida (
), P. ostreatus Florida ( ), and A. brasilense + P. ostreatus (
), and A. brasilense + P. ostreatus ( ) at different temperatures: FLU—fluorene, PHEN—phenanthrene, ANTH—anthracene, FLA—fluoranthene, PYR—pyrene.
) at different temperatures: FLU—fluorene, PHEN—phenanthrene, ANTH—anthracene, FLA—fluoranthene, PYR—pyrene.
 ), P. ostreatus Florida (
), P. ostreatus Florida ( ), and A. brasilense + P. ostreatus (
), and A. brasilense + P. ostreatus ( ) at different temperatures: FLU—fluorene, PHEN—phenanthrene, ANTH—anthracene, FLA—fluoranthene, PYR—pyrene.
) at different temperatures: FLU—fluorene, PHEN—phenanthrene, ANTH—anthracene, FLA—fluoranthene, PYR—pyrene.
When the co-culture was used, synergism was noted in the utilization of anthracene, fluoranthene (at 24 °C), pyrene (at 30 °C), and fluorene (at 37 °C). Pyrene, inaccessible to A. brasilense SR80 at 30 °C, was metabolized by 43%, and fluorene, inaccessible to P. ostreatus at 37 °C, was metabolized by 78% (Figure 1).
The fungal degradation products of fluorene (9-fluorenone), phenanthrene (9,10-phenanthrenquinone), and anthracene (9,10-anthraquinone) were identified by HPLC. The product of the bacterial degradation of fluorene was 9-fluorenone. The same quinone metabolites were detected during PAH degradation by the co-culture.
3.2. Degradation of Fluorene and Its Main Metabolite, 9-Fluorenone
According to the obtained data, fluorene and its degradation product, 9-fluorenone, were chosen for further study of the metabolism by co-cultures. The chosen temperature of 30 °C was optimal for the growth of both cultures. PAH degradation by the fungus and the co-culture was accompanied by yellow coloration of the growth medium, which may indicate the formation of quinone metabolites [39].
The presence of fluorene or 9-fluorenone in the growth medium decreased the mycelium dry weight, as compared to the PAH-free control (Table 1). The co-culturing of P. ostreatus Florida and A. brasilense SR80 increased the mycelium dry weight (Table 3). The CFU value of A. brasilense SR80 alone also decreased in the presence of 9-fluorenone, but it increased by many times in the co-culture.

Table 3.
Growth and enzymatic activity of P. ostreatus Florida and A. brasilense SR80 monocultures and the co-culture during biodegradation of fluorene and 9-fluorenone.
We also evaluated the activity of extracellular oxidative enzymes during the degradation of fluorene and 9-fluorenone (Table 3). A. brasilense SR80 was not found to produce extracellular laccase or peroxidase. The production of laccase and versatile peroxidase by P. ostreatus Florida was lower in the presence of 9-fluorenone than in the presence of fluorene. This was expected, because the inhibition of these enzymes by PAH quinones is well-known. In the co-culture, the activities of both enzymes were higher than those in the fungus alone.
HPLC showed that fluorene and its fungal degradation intermediate 9-fluorenone were metabolized by both organisms alone and in combination (Figure 2). The degradation of fluorene by A. brasilense SR80 was as high as 74%, whereas that of 9-fluorenone was 40%.
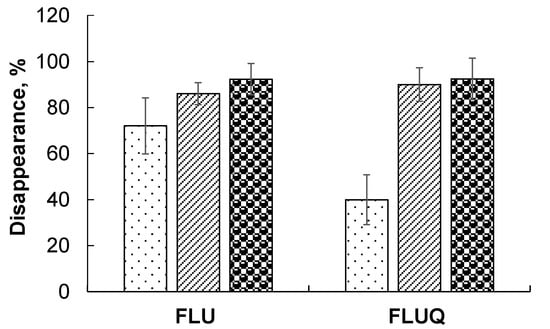
Figure 2.
Degradation of fluorene and 9-fluorenone by A. brasilense SR80 ( ), P. ostreatus Florida (
), P. ostreatus Florida ( ), and A. brasilense + P. ostreatus (
), and A. brasilense + P. ostreatus ( ).
).
 ), P. ostreatus Florida (
), P. ostreatus Florida ( ), and A. brasilense + P. ostreatus (
), and A. brasilense + P. ostreatus ( ).
).
Comparison of the retention times (RTs) of the identified metabolites with those of standard compounds showed that P. ostreatus Florida metabolized fluorene to 9-fluorenone and two unidentified metabolites with RTs of 0.817 and 2.668 min. In addition, some compounds with RTs of 1.304 and 12.375 min were detected in the co-culture (Figure S1).
The degradation 9-fluorenone by P. ostreatus Florida yielded two unidentified metabolites with RTs of 0.817 and 2.668 min. The same metabolites were detected after 9-fluorenone was degraded by the co-culture (Figure S2).
Thus, P. ostreatus Florida, separately and in combination with A. brasilense SR80, metabolized fluorene to provide 9-fluorenone and several unidentified metabolites, two of which, with RTs of 0.817 and 2.668 min, were detected after 9-fluorenone utilization and, apparently, are products of deeper degradation of the starting compounds.
3.3. Effect of IAA on Fungal Growth, Enzyme Activity, and PAH Degradation
The accelerated mycelium growth and the consequent increase in fungal biomass may have been the result of bacterial production of IAA.
We examined the effect of exogenous IAA on extracellular enzyme production and PAH degradation by the P. ostreatus component of the co-culture. The addition of exogenous IAA into the growth medium did not significantly affect mycelium production by the fungus, regardless of the presence of PAHs. Two weeks into the experiment, the amounts of IAA were 0.23 μg/mL in the absence of PAHs and 0.13 μg/mL in the presence of PAHs.
The presence of IAA in the culture medium increased the degradation of all PAHs but fluorene by 24–41% (Figure 3).

Figure 3.
Effect of IAA on PAH degradation by P. ostreatus Florida:  —control (without IAA);
—control (without IAA);  —with IAA.
—with IAA.
 —control (without IAA);
—control (without IAA);  —with IAA.
—with IAA.
A similar picture emerged for extracellular enzyme activity (Figure 4). IAA caused laccase activity to increase by about 1.5 times, regardless of the presence of PAHs in the culture medium.
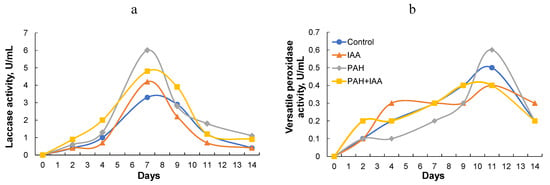
Figure 4.
Effect of IAA on production of ligninolytic enzymes by P. ostreatus Florida: (a) laccase; (b) versatile peroxidase; ●—control (without PAHs or IAA); ▲—IAA; ♦—PAHs; ■—PAHs + IAA.
We also evaluated IAA production by A. brasilense, both as a monoculture and as a component of the co-culture (Figure 5).
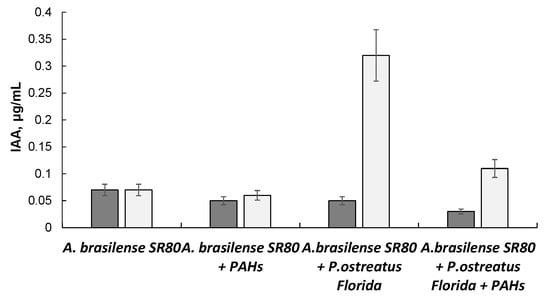
Figure 5.
Effect of PAHs and P. ostreatus Florida on IAA production by A. brasilense SR80:  —7 days;
—7 days;  —14 days.
—14 days.
 —7 days;
—7 days;  —14 days.
—14 days.
The concentration of IAA produced by A. brasilense alone was as high as 0.05–0.07 μg/mL and was barely affected by PAHs. A significant increase in IAA production (up to 0.32 μg/mL) was noted in the co-culture at the end of Week 2 of growth.
4. Discussion
In nature, fungi interact with other organisms (in particular, with rhizosphere bacteria), which may affect their degradative activity. P. ostreatus Florida was chosen as a model object for study because it is a potent degrader of a wide range of pollutants, including oil and PAHs [40], and because it can be used in mycoremediation [41]. Bacteria associated with P. ostreatus mycelium are mainly in the genera Curvibacter, Pseudomonas, Bacillus, Cupriavidus, Pelomonas, and Propionbacterium [42].
Azospirillum bacteria are well-known for their associative plant-growth-promoting characteristics [43,44] and have recently been proposed for use in environmental biotechnology [45,46]. The oil-oxidizing activity of Azospirillum was previously shown, with A. brasilense SR80 [26,47] as an example. In addition, strain SR80 was found to have ligninolytic activity, which manifested as the production of laccase and a number of peroxidases [29].
Plant-growth-promoting rhizobacteria (PGPR) are beneficial to mycorrhizal communities [48]. However, the co-culturing of Azospirillum with xylotrophic basidiomycetes that do not form mycorrhizae was not reported before the pioneering research at the Microbiology Laboratory of our institute [33,49]. That research made a strong case for the use of bacterial-fungal cultures for the effective production of mycelium biomass and fruiting of basidiomycetes [33].
An interesting aspect of such co-cultures is their degradative activity toward various kinds of pollutants. There are few reports in this area and only one that deals with the effect of bacteria on the degradative activity of P. ostreatus [50]. The objective of the work [50], however, was to explore bacterial effects on the fungal degradation of the anthraquinone dye Remazol Brilliant Blue R. Pseudomonas fluorescens and Bacillus licheniformis, known for their antagonism to fungi, did not strongly affect the growth of mycelium or the degradative activity of P. ostreatus [50]. If, however, a PGPR and a degradative fungus are used simultaneously, the interaction between them is inevitable, which is what we have examined in our model system.
The co-culturing of P. ostreatus Florida with A. brasilense SR80 at 24 and 30 °C increased the dry weight of mycelium and the number of CFUs. A similar picture was observed by Tsivileva et al. [33], who showed a significant increase in fungal dry biomass in a co-culture of P. ostreatus and A. brasilense SR80. In our experiments, increasing the temperature further to 37 °C inhibited fungal growth.
The presence of PAHs and/or their metabolites in the culture medium inhibited the growth of P. ostreatus and A. brasilense SR80 as monocultures. However, the CFU value of A. brasilense SR80 increased in the co-culture, which may have resulted from the bacterial utilization of the fungal metabolites of the PAHs as an additional carbon source.
Both organisms degraded the PAHs under cometabolism conditions. It is well-known that ligninolytic fungi are degradative to PAHs [22]. By contrast, this study is the first to have found that A. brasilense can transform or degrade PAHs. Fluorene, which is relatively well-soluble and has a low ionization potential, was intensely utilized by both P. ostreatus and A. brasilense SR80. The co-culture acted synergistically, increasing the degradation of anthracene, fluoranthene, pyrene, and fluorene. At 30 °C, pyrene was unavailable to A. brasilense SR80 alone but was metabolized by 43% in the co-culture. In turn, fluorene was unavailable for fungal degradation at 37 °C but was metabolized by 78% in the co-culture. These data also indicate a positive interaction between the xylotrophic basidiomycete and the PGPR in a PAH-polluted setting.
PAH quinones (9-fluorenone, 9,10-phenanthrenquinone, and 9,10-anthraquinone), detected by HPLC as resulting from PAH degradation, were identical for both the fungal monoculture and co-culture. The formation of the corresponding quinones at the first stage of PAH degradation by ligninolytic fungi is well-known. The reactions of ligninolytic peroxidases and laccases lead to the formation of these products [22,24]. In our experiments, the formation of quinone metabolites in the fungal monoculture and co-culture with A. brasilense SR80 can also be the result of the catalytic action of laccase and versatile peroxidase produced by this fungus. 9-fluorenone revealed as a product of the bacterial degradation of fluorene can be an indirect confirmation of the participation of bacterial laccase in the degradation of this PAH. The study of metabolism of PAHs in A. brasilense SR80 and the involvement of laccases will be the subject of further research.
P. ostreatus Florida, singly and as combined with A. brasilense SR80, metabolized not only fluorene but also its metabolite, 9-fluorenone, yielding a number of unidentified metabolites, two of which (RTs 0.817 and 2.668 min) were apparently products of deeper degradation of the starting compounds. They appeared on a later day of cultivation and disappeared with an increase in cultivation time. In the presence of 9-fluorenone, the CFU number of A. brasilense SR80 in the co-culture increased many times, indicating utilization of the formed metabolites. One from the key metabolites of PAH degradation by fungi is phthalic acid, which, through a number of stages, is included in the main metabolism [22,24]. However, the retention times of the metabolites, revealed in our experiments, do not correspond to phthalic acid. At this stage of the study, we were unable to identify these compounds. Their identification will be the subject of further research.
The formation of PAH quinones is associated with the fungal production of ligninolytic enzymes (laccase, Mn peroxidase, versatile peroxidase, and lignin peroxidase) [22]. A. brasilense SR80, too, is a producer of ligninolytic enzymes [29]. This may suggest that P. ostreatus and A. brasilense use similar mechanisms to initially attack PAH molecules. This hypothesis will be the subject of further research.
The ability of Azospirillum bacteria to metabolize aromatic compounds can be regarded as the need for adaptation to the rhizosphere, a habitat saturated with various organic substrates [51]. However, information on the metabolism of aromatic compounds (especially PAHs) by Azospirillum has not yet been systematized. A few authors have reported that Azospirillum can utilize benzoate, salicylate, gentisate, naphthalene, and some other aromatic substrates as carbon sources [52,53,54]. In A. brasilense and A. lipoferum, Chen et al. [52] detected the activities of catechol 2,3-dioxygenase (growth with benzoate and catechol) and protocatechuate 4,5-dioxygenase (growth with 4-hydroxybenzoate and protocatechuate), and in A. lipoferum, they also detected the activities of catechol 1,2-dioxygenase (growth with benzoate, catechol, and naphthalene) and protocatechuate 3,4-dioxygenase (growth with 4-hydroxybenzoate, protocatechuate, and 4-toluate). Cruz-Hernandez et al. [54] showed that Azospirillum growth with aromatic substrates is accompanied by the production of biosurfactants. In addition, bioinformatic analysis of the genome of A. brasilense strain CBG-497 led the authors to record 19 sequences encoding the degradation of aromatic compounds. Of these, 11 were associated with the metabolism of central aromatic intermediates and 5 were involved in peripheral catabolic pathways, whose function is associated with the degradation pathways for quinate, benzoate, salicylate, gentisate, and toluene [54]. Our studies of PAH degradation by A. brasilense SR80 show that this strain can degrade phenanthrene, fluorene, and anthracene, yielding the corresponding quinones (unpublished data). Full genome analysis of strain SR80 did not reveal any genetic determinants corresponding to the dioxygenase conversion of these PAHs, but laccase genes were found. The laccase activity of A. brasilense SR80 was described earlier [29]. Considering that quinones are the products of the primary attack on the PAH molecules by laccases, including bacterial ones [55], we believe that PAH conversion by A. brasilense SR80 is mediated by laccases and yields quinones.
Examination of extracellular laccase and peroxidase activities showed that, in all experimental treatments, P. ostreatus Florida produced laccase and versatile peroxidase. These extracellular enzymes, however, were not found in A. brasilense SR80. This may be because the SR80 enzymes are localized either intracellularly or on the cell wall surface, such as laccase from A. lipoferum as described by Diamantidis [28]. Laccase and versatile peroxidase activities were the highest in the co-culture. A similar increase in extracellular laccase production was found by Flores et al. [56], who co-cultured P. ostreatus with some species of Trichoderma. The authors suggested that the production of lytic enzymes by Trichoderma leads to the destruction of P. ostreatus cells, which, in turn, increases the amount of extracellular laccase. The increased production of extracellular enzymes in the co-culture may be explained in several ways: (1) P. ostreatus responded to the stress caused by the presence of Azospirillum. (2) The Azospirillum metabolites, including PAH degradation products, could induce these enzymes. (3) The lytic enzymes of Azospirillum destroyed the P. ostreatus cell wall and promoted enzyme release. All these assumptions require further research.
The accelerated growth of mycelium and, consequently, the increase in fungal biomass may have resulted from the bacterial production of IAA, as shown for dual cultures of Pleurotus eryngii and Pseudomonas sp. P7014 [57] and of P. ostreatus and Bacillus cereus, Bacillus aryabhattai, or Acinetobacter pitti [58]. Bacteria that promote the growth of higher fungi have recently been designated mushroom-growth-promoting bacteria (MGPB) [59]. Adding Pseudomonas sp. P7014 and its supernatant liquid to the growth medium of P. eryngii increased the mycelium growth rate by 1.6 times [8]. Analysis of ethyl acetate extracts of the culture liquid of this bacterium showed the presence of compounds that promote fungal growth, including IAA. The IAA concentration needed to accelerate fungal growth is less than 10 nM [57].
In this study, the addition of exogenous IAA to the culture medium did not noticeably affect the growth of P. ostreatus mycelium, regardless of the presence of PAHs. However, it increased the degradation of all PAHs but fluorene by 24–41% and promoted the production of fungal extracellular laccase. In this case, apparently, IAA and/or the products of its utilization by P. ostreatus induced the laccase. In turn, the increased laccase production could lead to increased PAH degradation.
To test whether IAA affected growth, PAH degradation, and enzyme production, we determined its content in the A. brasilense monoculture and in the co-culture with P. ostreatus. The IAA concentration in the A. brasilense SR80 monoculture was low (0.05–0.07 μg/mL), although this strain produces up to 20–30 μg/mL of IAA in the Nfb medium supplemented with tryptophan [26]. As a rule, IAA production in Azospirillum is tryptophan-dependent, but a minor, tryptophan-independent IAA synthetic pathway is also present in these bacteria [60]. In this study, the medium did not contain exogenous tryptophan. Apparently, the synthesis of IAA in the A. brasilense SR80 monoculture proceeds by way of the minor pathway, which explains the low IAA concentration. In the co-culture (Week 2 of growth), IAA production increased significantly (up to 0.32 μg/mL). In this case, fungi may contribute to IAA synthesis, as shown for another Pleurotus member, P. pulmonarius [10].
5. Conclusions
Bacterial–fungal interactions in the presence of PAHs were investigated in a model system. A positive mutual influence was found between the xylotrophic basidiomycete P. ostreatus Florida and the PGPR A. brasilense SR80. This influence manifested as an increase in the production of fungal mycelium, the number of bacterial CFUs, the degradation of PAHs, and the production of fungal extracellular enzymes. The identified PAH quinones were the same for both fungal and co-cultures. The product of the bacterial degradation of fluorene was 9-fluorenone, in whose presence the CFUs of A. brasilense SR80 increased in the co-culture. This increase may indicate the utilization of the resulting metabolites. One factor of mutual influence between P. ostreatus Florida and A. brasilense SR80 is their produced IAA.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol2040056/s1, Figure S1: Chromatograms of culture liquid extracts after fluorene degradation; Figure S2: Chromatograms of culture liquid extracts after 9-fluorenone degradation.
Author Contributions
Conceptualization, N.P. and A.M.; methodology, N.P. and A.M.; software, N.P. and A.M.; validation, N.P. and A.M.; formal analysis, O.T.; investigation, N.P. and A.M.; resources, N.P. and A.M.; data curation, N.P. and A.M.; writing—original draft preparation, N.P. and A.M.; writing—review and editing, N.P., A.M. and O.T; visualization, N.P. and A.M.; supervision, O.T.; project administration, O.T.; funding acquisition, O.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out under research theme no. 121031700141-7 and was supported in part by a grant from the Russian Foundation for Basic Research (project no. 18-29-05062\18).
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Dmitry N. Tychinin for his assistance in preparation of the English text of this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wargo, M.; Hogan, D. Fungal-bacterial interactions: A mixed bag of mingling microbes. Curr. Opin. Microbiol. 2006, 9, 359–364. [Google Scholar] [CrossRef]
- de Boer, W. Upscaling of fungal-bacterial interactions: From the lab to the field. Curr. Opin. Microbiol. 2017, 37, 35–41. [Google Scholar] [CrossRef]
- Kohlmeier, S.; Smits, T.H.M.; Ford, R.M.; Keel, C.; Harms, H.; Wick, L.Y. Taking the fungal highway: Mobilization of pollutant-degrading bacteria by fungi. Environ. Sci. Technol. 2005, 39, 4640–4646. [Google Scholar] [CrossRef]
- van Overbeek, L.S.; Saikkonen, K. Impact of bacterial-fungal interactions on the colonization of the endosphere. Trends Plant Sci. 2016, 21, 230–242. [Google Scholar] [CrossRef]
- Furuno, S.; Päzolt, K.; Rabe, K.; Neu, T.R.; Harms, H.; Wick, L.Y. Fungal mycelia allow chemotactic dispersal of polycyclic aromatic hydrocarbon-degrading bacteria in water-unsaturated systems. Environ. Microbiol. 2010, 12, 1391–1398. [Google Scholar] [CrossRef]
- Minerdi, D.; Bianciotto, V.; Bonfante, P. Endosymbiotic bacteria in mycorrhizal fungi: From their morphology to genomic sequences. Plant Soil 2002, 244, 211–219. [Google Scholar] [CrossRef]
- Jambon, I.; Thijs, S.; Weyens, N.; Vangronsveld, J. Harnessing plant-bacteria-fungi interactions to improve plant growth and degradation of organic pollutants. J. Plants Interact. 2018, 13, 119–130. [Google Scholar] [CrossRef]
- Kim, M.K.; Math, R.K.; Cho, K.M.; Shin, K.J.; Kim, J.O.; Ryu, J.S.; Lee, Y.H.; Yun, H.D. Effect of Pseudomonas sp. P7014 on the growth of edible mushroom Pleurotus eringii in bottle culture for commercial production. Bioresour. Technol. 2008, 99, 3306–3308. [Google Scholar] [CrossRef]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Pham, M.T.; Huang, C.-M.; Kirschner, R. The plant growth-promoting potential of the mesophilic wood-rot mushroom Pleurotus pulmonarius. J. Appl. Microbiol. 2019, 127, 1157–1171. [Google Scholar] [CrossRef]
- McErlean, C.; Marchant, R.; Banat, I. An evaluation of soil colonization potential of selected fungi and their production of ligninolytic enzymes for use in soil bioremediation applications. Antonie Van Leeuwenhoek 2006, 90, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.; Cajthaml, T.; Šašek, V. Mycoremediation of PAH-contaminated soil. Folia Microbiol. 2002, 47, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Thion, C.; Cebron, A.; Beguiristain, T.; Leyval, C. Inoculation of PAH-degrading strains of Fusarium solani and Arthrobacter oxydans in rhizospheric sand and soil microcosms: Microbial interactions and PAH dissipation. Biodegradation 2013, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nomura, N.; Nakajima, T.; Uchiyama, H. Case study of the relationship between fungi and bacteria associated with high-molecular-weight polycyclic aromatic hydrocarbon degradation. J. Biosci. Bioeng. 2012, 113, 624–630. [Google Scholar] [CrossRef]
- Zafra, G.; Absalon, A.; Cuevas, M.; Cortes-Espinosa, D. Isolation and selection of a highly tolerant microbial consortium with potential for PAH biodegradation from heavy crude oil-contaminated soil. Water Air Soil Pollut. 2014, 225, 1826. [Google Scholar] [CrossRef]
- Ma, X.; Ding, N.; Peterson, E.C.; Daugulis, A.J. Heavy metals species affect fungal-bacterial synergism during the bioremediation of fluoranthene. Appl. Microbiol. Biotechnol. 2016, 100, 7741–7750. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Das, A.; Palaniswamy, M.; Angayarkanni, J. Degradation of benzo[a]pyrene by Pleurotus ostreatus PO-3 in the presence of defined fungal and bacterial co-cultures. J. Basic Microbiol. 2017, 57, 95–103. [Google Scholar] [CrossRef]
- Boonchan, S.; Britz, M.L.; Stanley, G.A. Degradation and mineralization of high-molecular-weight polycyclic aromatic hydrocarbons by defined fungal-bacterial cocultures. Appl. Environ. Microbiol. 2000, 66, 1007–1019. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C. Microbial degradation of polycyclic aromatic hydrocarbon in soil by bacterium-fungus co-cultures. Biotechnol. Bioprocess Eng. 2007, 12, 410–416. [Google Scholar] [CrossRef]
- Brodkorb, T.; Legge, R. Enhanced biodegradation of phenanthrene in oil tar-contaminated soils supplemented with Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1992, 58, 3117–3121. [Google Scholar] [CrossRef]
- Zeng, J.; Lin, X.; Zhang, J.; Zhu, H.; Chen, H.; Wong, M.H. Successive transformation of benzo[a]pyrene by laccase of Trametes versicolor and pyrene-degrading Mycobacterium strains. Appl. Microbiol. Biotechnol. 2013, 97, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumar, M. Biodegradation of polycylic aromatic hydrocarbons (PAHs): A sustainable approach. In Sustainable Green Technologies for Environment; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Springer: Singapore, 2019; pp. 111–139. [Google Scholar]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.; Balandina, S.; Turkovskaya, O. Degradative properties of Pleurotus fungi. In Pleurotus Mushrooms: Ecology, Cultivation and Uses; Mervyn, P., Gwynn, I., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 1–56. [Google Scholar]
- Muratova, A.Y.; Turkovskaya, O.V.; Antonyuk, L.P.; Makarov, O.E.; Pozdnyakova, L.I.; Ignatov, V.V. Oil-oxidizing potential of associative rhizobacteria of the genus Azospirillum. Microbiology 2005, 74, 210–215. [Google Scholar] [CrossRef]
- Gruz-Hernandez, M.A.; Mendoza-Herrera, A.; Bocanegra-Garcia, V.; Rivera, G. Azospirillum spp. From plant growth-promoting bacteria to their use in bioremediation. Microorganisms 2022, 10, 1057. [Google Scholar] [CrossRef]
- Diamantidis, G.; Eosse, A.; Potier, P.; Bally, R. Purifcation and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol. Biochem. 2000, 32, 919–927. [Google Scholar] [CrossRef]
- Kupriashina, M.A.; Petrov, S.V.; Ponomareva, E.G.; Nikitina, V.E. Ligninolytic activity of bacteria of the genera Azospirillum and Niveispirillum. Microbiology 2015, 84, 791–795. [Google Scholar] [CrossRef]
- Bezalel, L.; Hadar, Y.; Cerniglia, C. Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 1997, 63, 2495–2501. [Google Scholar] [CrossRef]
- Nikiforova, S.; Pozdnyakova, N.; Makarov, O.; Chernyshova, M.; Turkovskaya, O. Chrysene bioconversion by the white rot fungus Pleurotus ostreatus D1. Microbiology 2010, 79, 456–460. [Google Scholar] [CrossRef]
- Reasoner, D.J.; Geldreich, E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 1985, 49, 1–7. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Shaternikov, A.N.; Nikitina, B.E. Bakterii roda Azospirillum v optimizatsii iskusstvennogo kul’tivirovaniya vysshikh gribov-ksilotrofov (Bacteria of the Azospirillum genus for the optimization of the artificial culture of xylotrophic mushrooms). Biotekhnologiya (Biotechnology) 2020, 36, 16–25, (In Russian, abstract in English). [Google Scholar]
- Niku-Paavola, M.; Karhunen, E.; Salola, P.; Raunio, V. Ligninolytic enzymes of the white rot fungus Phlebia radiata. Biochem. J. 1988, 254, 877–884. [Google Scholar] [CrossRef]
- Martinez, M.; Ruiz-Duenas, F.; Guillen, F.; Martinez, A. Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur. J. Biochem. 1996, 237, 424–432. [Google Scholar] [CrossRef]
- Royse, D.J.; Ries, S.M. The influence of fungi isolated from peach twigs on the pathogenicity of Cytospora cincta. Phytopathology 1978, 68, 603–607. [Google Scholar] [CrossRef]
- Kalaiselvi, S.; Panneerselvam, A. In vitro assessment of antagonistic activity of Trichoderma sp. against Sarocladium oryzae causing sheath rot disease in paddy. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 179–183. [Google Scholar]
- Haritash, A.; Kaushik, C. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Lambert, M.; Kremer, S.; Sterner, O.; Anke, H. Metabolism of pyrene by the basidiomycete Crinipellis stipitaria and identification of pyrene quinones and their hydroxylated precursors in strain JK375. Appl. Environ. Microbiol. 1994, 60, 3597–3601. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Balandina, S.; Turkovskaya, O. Degradativnaya aktivnost’ gribov po otnosheniyu k uglevodorodam nefti v usloviyakh povyshennoy temperatury (Degradative activity of fungi towards oil hydrocarbons under high temperature). Teor. I Prikl. Ekologiya. (Theor. Appl. Ecol.) 2019, 4, 30–36, (In Russian, abstract in English). [Google Scholar]
- Pozdnyakova, N.N.; Dubrovskaya, E.V.; Grinev, V.S.; Turkovskaya, O.V. Perspektivy ispol’zovaniya ksilotrofnykh gribov Pleurotus ostreatus Florida i Schizophyllum commune dlya mikoremediatsii pochv, zagryaznennykh neftyanymi uglevodorodami i poverkhnostno-aktivnymi veshchestvami (Prospects for the use of xylotrophic fungi Pleurotus ostreatus Florida and Schizophyllum commune for mycoremediation of soils contaminated with oil hydrocarbons and surfactants). Biotekhnologiya (Biotechnology) 2021, 37, 108–116, (In Russian, abstract in English). [Google Scholar]
- Adamski, M.; Pietr, S. Biodiversity of bacteria associated with eight Pleurotus ostreatus (Fr.) P. Kumm. strains from Poland, Japan and the USA. Pol. J. Microbiol. 2019, 68, 71–81. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Gałązka, A.; Król, M.; Perzyński, A. The efficiency of rhizosphere bioremediation with Azospirillum sp. and Pseudomonas stutzeri in soils freshly contaminated with PAHs and diesel fuel. Pol. J. Environ. Stud. 2012, 21, 345–353. [Google Scholar]
- Kulkarni, K.; Bhogale, G.M.; Nalawade, R. Adsorptive removal of fluoride from water samples using Azospirillum biofertilizer and lignite. Korean J. Chem. Eng. 2017, 35, 153–163. [Google Scholar] [CrossRef]
- Bondarenkova, A.D.; Muratova, A.Y.; Turkovskaya, O.V. Influence of oil on the associative rhizobacterium Azospirillum brasilense. Bull. Saratov State Agrar. Univ. N.I. Vavilov 2009, 9, 5–10, (In Russian, abstract in English). [Google Scholar]
- Obase, K. Bacterial community on ectomycorrhizal roots of Laccaria laccata in a chestnut plantation. Mycoscience 2019, 60, 40–44. [Google Scholar] [CrossRef]
- Loshchinina, E.A.; Nikitina, V.E.; Tsivileva, O.M.; Stepanova, L.V.; Ponomareva, E.G.; Sheludko, A.V. Morphological and cultural characteristics of the basidiomycete Lentinus edodes in co-cultivation with bacteria of the genus Azospirillum. Bull. Saratov State Agrar. Univ. N.I. Vavilov 2006, 6, 24–26, (In Russian, abstract in English). [Google Scholar]
- Valkova, H.; Novotny, C.; Malachova, K.; Slovarcikova, P.; Fojtik, J. Effect of bacteria on the degradation ability of Pleurotus ostreatus. Sci. Total Environ. 2017, 584–585, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski-Dyé, F.; Lozano, L.; Acosta-Cruz, E.; Borland, S.; Drogue, B.; Prigent-Combaret, C.; Rouy, Z.; Barbe, V.; Mendoza Herrera, A.; González, V.; et al. Genome sequence of Azospirillum brasilense CBG497 and comparative analyses of Azospirillum core and accessory genomes provide insight into niche adaptation. Genes 2012, 3, 576–602. [Google Scholar] [CrossRef]
- Chen, J.P.; Lopez-de-Victoria, G.; Lowell, C.R. Utilization of aromatic compounds as carbon and energy sources during growth and N2-fixation by free-living nitroxen fixing bacteria. Arch. Microbiol. 1993, 159, 207–212. [Google Scholar] [CrossRef]
- Krol, M.J.; Perzynski, A. The utilization of polycyclic aromatic hydrocarbons (PAHs) as the sole carbon source in fixation of nitrogen-free by Azospirillum spp. strains of bacteria. Pol. J. Agron. 2002, 131, 69–80. [Google Scholar]
- Cruz-Hernandez, M.A.; Jimenez-Andrade, J.M.; Mendoza-Herrera, A. Characterization of the degradation potential of xenobiotic compounds by the rhizobacteria Azospirillum brasilense. Mex. J. Biotechnol. 2019, 4, 10–22. [Google Scholar] [CrossRef]
- Zeng, J.; Lin, X.; Zhang, J.; Li, X.; Wong, M.H. Oxidation of polycyclic aromatic hydrocarbons by the bacterial laccase CueO from E. coli. Appl. Microbiol. Biotechnol. 2011, 89, 1841–1849. [Google Scholar] [CrossRef]
- Flores, C.; Vidal, C.; Trejo-Hernandez, M.R.; Galino, E.; Serrano-Carreon, L. Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J. Appl. Microbiol. 2009, 106, 249–257. [Google Scholar] [CrossRef]
- Kang, Y.M.; Cho, K.M. Identification of auxin from Pseudomonas sp. P7014 for the rapid growth of Pleurotus eryngii mycelium. Korean J. Microbiol. 2014, 50, 15–21. [Google Scholar] [CrossRef]
- Febriansyah, E.; Saskiawan, I.; Mangunwardoyo, W.; Sulistiyani, T.R.; Widhiya, E.W. Potency of growth promoting bacteria on mycelial growth of edible mushroom Pleurotus ostreatus and its identification based on 16S rDNA analysis. AIP Conf. Proc. 2018, 2002, 020023. [Google Scholar]
- Young, L.-S.; Chu, J.-N.; Hameed, A.; Young, C.-C. Cultivable mushroom growth-promoting bacteria and their impact on Agaricus blazei productivity. Pesq. Agropecu. Bras. 2013, 48, 636–644. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–430. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).