Selected Antimicrobial Peptides Inhibit In Vitro Growth of Campylobacter spp.
Abstract
1. Introduction
2. Materials and Methods
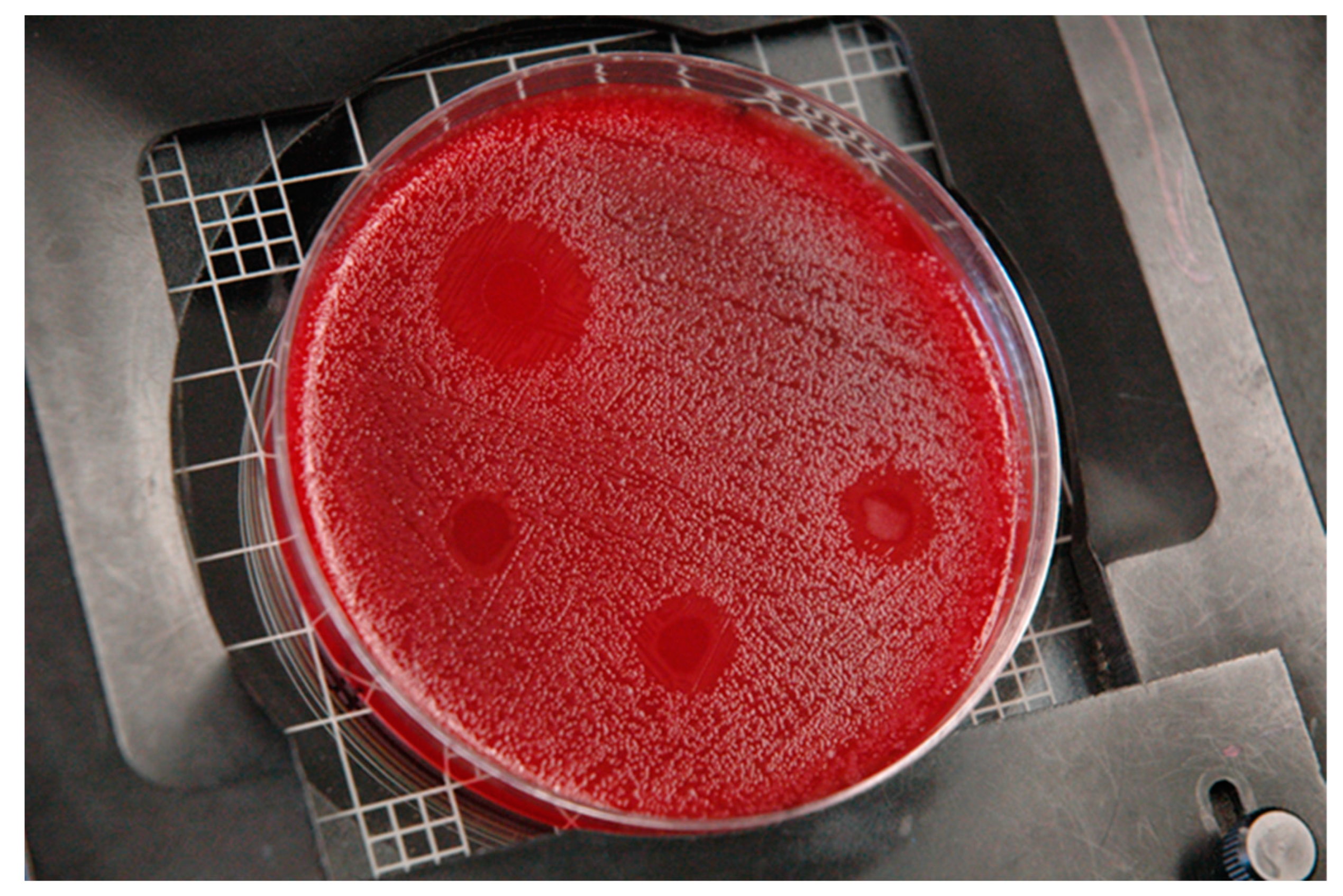
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venugopal, D.; Klapper, D.; Srouji, A.H.; Bhonsle, J.B.; Borschel, R.; Mueller, A.; Russell, A.L.; Williams, B.C.; Hicks, R.P. Novel antimicrobial peptides that exhibit activity against select agents and other drug resistant bacteria. Bioorg. Med. Chem. 2010, 18, 5137–5147. [Google Scholar] [CrossRef] [PubMed]
- Cuperus, T.; Coorens, M.; Van Dijk, A.; Haagsman, H.P. Avian host defense peptides. Dev. Comp. Immunol. 2013, 41, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and reisitance. Pharm. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, R.Q.; Liu, Y.; Zhao, H.X.; Song, L.L.; Cao, Y.; Qiao, D.R. Design, recombinant expression, and antimicrobial activity of the cecropins-melittin hybrid antimicrobial peptides. Curr. Microbiol. 2010, 61, 169–175. [Google Scholar] [CrossRef]
- Aguilera-Mendoza, L.; Marrero-Ponce, Y.; Tellez-Ibarra, R.; Llorente-Quesada, M.T.; Salgado, J.; Barigye, S.J.; Liu, J. Overlap and diversity in antimicrobial peptide databases: Compiling a non-redundant set of sequences. Bioinformatics 2015, 31, 2553–2559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res. 2021, 50, D488–D496. [Google Scholar] [CrossRef]
- Ryan, K.J.; Ray, C.G.; Sherris, J.C. Sherris Medical Microbiology: An Introduction to Infection Diseases, 4th ed.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Ursing, J.B.; Lior, H.; Owen, R.J. Proposal of Minimal Standards for Describing New Species of the Family Campylobacteraceae. Int. J. Syst. Bacteriol. 1994, 44, 842–845. [Google Scholar] [CrossRef][Green Version]
- Altekruse, S.F.; Stern, N.J.; Fields, P.I.; Swerdlow, D.L. Campylobacter jejuni—An Emerging Foodborne Pathogen. Emerg. Infect. Dis. 1999, 5, 28–35. [Google Scholar] [CrossRef]
- Janssen, R.; Krogfelt, K.A.; Cawthraw, S.A.; van Pelt, W.; Wagenaar, J.A.; Owen, R.J. Host-pathogen interactions in Campylobacter infections: The host perspective. Clin. Microbiol. Rev. 2008, 21, 505–518. [Google Scholar] [CrossRef]
- Kubota, K.; Kasuga, F.; Iwasaki, E.; Inagaki, S.; Sakurai, Y.; Komatsu, M.; Toyofuku, H.; Angulo, F.J.; Scallan, E.; Morikawa, K. Estimating the Burden of Acute Gastroenteritis and Foodborne Illness Caused by Campylobacter, Salmonella, and Vibrio parahaemolyticus by Using Population-Based Telephone Survey Data, Miyagi Prefecture, Japan, 2005 to 2006. J. Food Prot. 2011, 74, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Campylobacter (Campylobacteriosis), Questions & Answers. Available online: https://www.cdc.gov/campylobacter/technical.html (accessed on 31 August 2021).
- Sahin, O.; Morishita, T.Y.; Zhang, Q. Campylobacter colonization in poultry: Sources of infection and modes of transmission. Anim. Health Res. Rev. 2002, 3, 95–105. [Google Scholar] [CrossRef]
- Newell, D.G.; Fearnley, C. Sources of Campylobacter Colonization in Broiler Chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.J. Reservoirs for Campylobacter jejuni and approaches for intervention in poultry. In Campylobacter Jejuni: Current Status and Future Trends; Nachamkin, I., Blaser, M.J., Tompkins, L., Eds.; American Society for Microbiology: Washington, DC, USA, 1992; pp. 49–60. [Google Scholar]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-Related Illness and Death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef]
- Jore, S.; Viljugrein, H.; Brun, E.; Heier, B.; Borck, B.; Ethelberg, S.; Hakkinen, M.; Kuusi, M.; Reiersen, J.; Hansson, I.; et al. Trends in Campylobacter incidence in broilers and humans in six European countries, 1997–2007. Prev. Vet. Med. 2010, 93, 33–41. [Google Scholar] [CrossRef]
- Meunier, M.; Guyard-Nicodeme, M.; Dory, D.; Chemaly, M. Control strategies against Campylobacter at the poultry production level: Biosecurity measures, feed additives and vaccination. J. Appl. Microbiol. 2015, 120, 1139–1173. [Google Scholar] [CrossRef]
- Gyles, C.L. Antimicrobial resistance in selected bacteria from poultry. Anim. Health Res. Rev. 2008, 9, 149–158. [Google Scholar] [CrossRef]
- Aarestrup, F.M. The livestock reservoir for antimicrobial resistance: A personal view on changing patterns of risks, effects of interventions and the way forward. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140085. [Google Scholar] [CrossRef]
- Doyle, M.E. Multidrug-Resistant Pathogens in the Food Supply. Foodborne Pathog. Dis. 2015, 12, 261–279. [Google Scholar] [CrossRef]
- Seal, B.S.; Lillehoj, H.S.; Donovan, D.M.; Gay, C.G. Alternatives to antibiotics: A symposium on the challenges and solutions for animal production. Anim. Health Res. Rev. 2013, 14, 78–87. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L. The growing threat of food-borne bacterial enteropathogens of animal origin. Clin. Infect. Dis. 2007, 45, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Gilbert, J.M. Antimicrobial drug delivery in food animals and microbial food safety concerns: An overview of in vitro and in vivo factors potentially affecting the animal gut microflora. Adv. Drug Deliv. Rev. 2004, 56, 1497–1521. [Google Scholar] [CrossRef]
- Regulation EC. No. 1831/2003 of the European Parliament and the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: https://www.eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32003R1831 (accessed on 20 September 2022).
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Treating Infectious Diseases in a Microbial World: Report of Two Workshops on Novel Antimicrobial Therapeutics; The National Academies Press: Washington, DC, USA, 2006; ISBN 0-309-65490-4. Available online: http://www.nap.edu/catalog.php?record_id=11471 (accessed on 20 September 2022).
- Persoons, D.; Dewulf, J.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Butaye, P.; Haesebrouck, F. Prevalence and Persistence of Antimicrobial Resistance in Broiler Indicator Bacteria. Microb. Drug Resist. 2010, 16, 67–74. [Google Scholar] [CrossRef]
- Stern, N.J.; Line, J.E. Campylobacter. In The Microbiological Safety and Quality of Food; Lund, B., Baird-Parker, T., Gould, G., Eds.; International Thomson Publishing: London, UK, 1885; pp. 1040–1056, 2000. [Google Scholar]
- Wagenaar, J.A.; Mevius, D.J.; Havelaar, A.H. Campylobacter in primary animal production and control strategies to reduce the burden of human campylobacteriosis. Rev. Sci. Tech.-Off. Int. Epizoot. 2006, 25, 581–594. [Google Scholar] [CrossRef]
- Nauta, M.; Hill, A.; Rosenquist, H.; Brynestad, S.; Fetsch, A.; van der Logt, P.; Fazil, A.; Christensen, B.; Katsma, E.; Borck, B.; et al. A comparison of risk assessments on Campylobacter in broiler meat. Int. J. Food Microbiol. 2008, 129, 107–123. [Google Scholar] [CrossRef]
- Crim, S.M.; Iwamoto, M.; Huang, J.Y.; Griffin, P.M.; Gilliss, D.; Cronquist, A.B.; Cartter, M.; Tobin-D’Angelo, M.; Blythe, D.; Smith, K.; et al. Centers for Disease Control and Prevention (CDC). Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 328–332. [Google Scholar]
- Crim, S.M.; Griffin, P.M.; Tauxe, R.; Marder, E.P.; Gilliss, D.; Cronquist, A.B.; Cartter, M.; Tobin-D’Angelo, M.; Blythe, D.; Smith, K.; et al. Preliminary incidence and trends of infection with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 u.s. Sites, 2006–2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 495–499. [Google Scholar]
- Line, J.; Hiett, K.; Conlan, A. Comparison of Challenge Models for Determining the Colonization Dose of Campylobacter jejuni in Broiler Chicks. Poult. Sci. 2008, 87, 1700–1706. [Google Scholar] [CrossRef]
- 81 FR 7285. Available online: https://www.govinfo.gov/content/pkg/FR-2016-02-11/pdf/2016-02586.pdf (accessed on 20 September 2022).
- Casteels, P.; Ampe, C.; Jacobs, F.; Vaeck, M.; Tempst, P. Apidaecins: Antibacterial peptides from honey bees. EMBO J. 1989, 8, 2387–2391. [Google Scholar] [CrossRef]
- Makobongo, M.O.; Kovachi, T.; Gancz, H.; Mor, A.; Merrell, D.S. In Vitro Antibacterial Activity of Acyl-Lysyl Oligomers against Helicobacter pylori. Antimicrob. Agents Chemother. 2009, 53, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Quadri, L.; Sailer, M.; Roy, K.; Vederas, J.; Stiles, M. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 1994, 269, 12204–12211. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kang, J.H.; Jang, S.Y.; Kim, Y.; Kim, K.L.; Hahm, K. Effects of the hinge region of cecropin A(1-8)-magainin 2(1-12), a synthetic antimicrobial peptide, on liposomes, bacterial and tumor cells. Biochem. Biophys. Acta 2000, 1463, 209–218. [Google Scholar] [CrossRef]
- Mor, A.; Van Huong, N.; Delfour, A.; Migliore-Samour, D.; Nicolas, P. Isolation, amino acid sequence and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 1991, 30, 8824–8830. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Patrzykat, A.; Gallant, J.W.; Seo, J.-K.; Pytyck, J.; Douglas, S.E. Novel Antimicrobial Peptides Derived from Flatfish Genes. Antimicrob. Agents Chemother. 2003, 47, 2464–2470. [Google Scholar] [CrossRef]
- Park, I.Y.; Park, C.B.; Kim, M.S.; Kim, S.C. Parasin I, antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus. FEBS Lett. 1998, 437, 258–262. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.-L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Zhao, C.; Nguyen, T.; Boo, L.M.; Hong, T.; Espiritu, C.; Orlov, D.; Wang, W.; Waring, A.; Lehrer, R.I. RL-37, an Alpha-Helical Antimicrobial Peptide of the Rhesus Monkey. Antimicrob. Agents Chemother. 2001, 45, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.L.; Barra, D. Temporins, antimicrobial peptides from the European red frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Shafer, W.M. (Ed.) Antibacterial Peptide Protocols: Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 1997; Volume 78, p. 1173. [Google Scholar]
- Clinical and Laboratory Standards Institute. M26-A: Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999; Volume 12. [Google Scholar]
- Wu, M.; Hancock, R.E.W. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 1999, 274, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Makobongo, M.O.; Gancz, H.; Carpenter, B.M.; McDaniel, D.P.; Merrell, D.S. The oligo-acyl lysyl antimicrobial peptide C12K-2β12 exhibits a dual mechanism of action and demonstrates strong in vivo efficacy against Helicobacter pylori. Antimicrob. Agents Chemother. 2012, 56, 378–390. [Google Scholar] [CrossRef]
- Sarkar, T.; Chetia, M.; Chatterjee, S. Antimicrobial Peptides and Proteins: From Nature’s Reservoir to the Laboratory and Beyond. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moghaddam, M.M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Wang, G. The antimicrobial peptide database provides a platform for decoding the design principles of naturally occurring antimicrobial peptides. Protein Sci. 2019, 29, 8–18. [Google Scholar] [CrossRef]
- Waghu, F.H.; Idicula-Thomas, S. Collection of antimicrobial peptides database and its derivitives: Applications and beyond. Protein Sci. 2019, 29, 36–42. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.; Herrebout, M.; Tersteeg-Zijderveld, M.H.; Bokhoven, J.L.T.-V.; Bleumink-Pluym, N.; Jansman, A.J.; Veldhuizen, E.J.; Haagsman, H.P. Campylobacter jejuni is highly susceptible to killing by chicken host defense peptide cathelicidin-2 and suppresses intestinal cathelicidin-2 expression in young broilers. Vet. Microbiol. 2012, 160, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P.; Amri, C. The dermaseptin superfamily: A gene-based combinatorial library of antimicrobial peptides. Biochem. Biophys. Acta 2009, 1788, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Saviello, M.R.; Malfi, S.; Campiglia, P.; Cavalli, A.; Grieco, P.; Novellino, E.; Carotenuto, A. New Insight into the Mechanism of Action of the Temporin Antimicrobial Peptides. Biochemistry 2010, 49, 1477–1485. [Google Scholar] [CrossRef]
- Kragol, G.; Lovas, S.; Varadi, G.; Condie, B.A.; Hoffman, R.; Otvos, L., Jr. The antimicrobial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 2001, 40, 3016–3026. [Google Scholar] [CrossRef]
- Lee, D.G.; Hahm, K.-S.; Shin, S.Y. Structure and fungicidal activity of a synthetic antimicrobial peptide, P18, and its truncated peptides. Biotechnol. Lett. 2004, 26, 337–341. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kang, J.H.; Hahm, K.-S. Structure-antibacterial, antitumor and hemolytic activity relationships of cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Res. 1999, 53, 82–90. [Google Scholar] [CrossRef]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular engineering of antimicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021, 13, 325–369. [Google Scholar] [CrossRef]
- Daneshmand, A.; Kermanshahi, H.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M. Antimicrobial peptide, cLF36, affects performance and intestinal morphology, microflora, junctional proteins, and immune cells in broilers challenged with E. coli. Sci. Rep. 2019, 9, 14176. [Google Scholar] [CrossRef]
- Wickramasuriya, S.S.; Park, I.; Lee, Y.; Kim, W.H.; Przybyszewski, C.; Gay, C.G.; van Oosterwijk, J.G.; Lillehoj, H.S. Oral Delivery of Bacillus subtilis Expressing Chicken NK-2 Peptide Protects Against Eimeria acervulina Infection in Broiler Chickens. Front. Vet. Sci. 2021, 8, 684818. [Google Scholar] [CrossRef]
- Radzishevsky, I.S.; Rotem, S.; Bourdetsky, D.; Navon-Venezia, S.; Carmeli, Y.; Mor, A. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat. Biotechnol. 2007, 25, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Radzishevsky, I.S.; Kovachi, T.; Porat, Y.; Ziserman, L.; Zaknoon, F.; Danino, D.; Mor, A. Structure-Activity Relationships of Antibacterial Acyl-Lysine Oligomers. Chem. Biol. 2008, 15, 354–362. [Google Scholar] [CrossRef]
- Livne, L.; Kovachi, T.; Sarig, H.; Epand, R.F.; Zaknoon, F.; Epand, R.M.; Mor, A. Design and Characterization of a Broad -Spectrum Bactericidal Acyl-lysyl Oligomer. Chem. Biol. 2009, 16, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.-P.S.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Quested, T.; Cook, P.; Gorris, L.; Cole, M. Trends in technology, trade and consumption likely to impact on microbial food safety. Int. J. Food Microbiol. 2010, 139 (Suppl. 1), S29–S42. [Google Scholar] [CrossRef] [PubMed]
| AMP | Source | AA # | Structure and Characteristics | Reference |
|---|---|---|---|---|
| Apidaecin 1B | Honeybee lymph | 18 | Cationic, no α-helix formation, high proline content, stable at high temp and low pH, small mol wt 2100 | [39] |
| C12K-2β12 | Synthetic oligo-acyl-lysyl (OAK) hexamer | 8 | Peptidomimetic, stable at high temp and low pH | [40] |
| Carnobacteriocin B2 | Carnobacterium piscicola | 48 | Class II bacteriocin, cationic, single alpha-helices involved in coiled-coils or other helix–helix interfaces | [41] |
| Cecropin A–Magainin 2 hybrid | Cecropia moth/African clawed frog | 20 | Cationic, short helix–flexible–amphipathic helix, antibacterial as well as antitumor activity | [42] |
| Dermaseptin | Skin of frog (Phyllomedusa) | 34 | Cationic, amphipathic α-helix | [43] |
| Dermcidin DCD | Human sweat glands | 48 | Forms cation-stabilized oligomeric ion channels in lipid bilayers | [44] |
| NRC-13 Pleurocidin | American plaice-winter flounder | 23 | Amphipathic α-helix | [45] |
| Parasin I | Skin mucus of wounded catfish (Parasilurus asotus) | 19 | Amphipathic α-helix | [46] |
| Pyrrhocoricin | European sap-sucking bug (Pyrrhocoris apterus) | 20 | Cyclic, proline-rich peptide | [47] |
| RL-37 | Bone marrow of Rhesus monkey (Macaca mulatta) | 37 | Cathelicidin, α-helix | [48] |
| Temporin L | European red frog skin (Rana temporaria) | 13 | Stable α-helix, secondary amphipathicity, shortest natural AMP found to date | [49] |
| AMP | AA Sequence | Net Charge | Proposed Modes of Action | Hemolysis |
|---|---|---|---|---|
| Apidaecin 1B | GNNRP VYIPQ PRPPH PRL | 3.1 | binding and irreversible combination with a periplasmic receptor/docking molecule, devoid of pore-forming activity | non-hemolytic |
| C12K-2β12 | C12K-KIK-KIK (C12 represents dodecanoic acid) | 4 | rapid membrane depolarization and cell permeabilization | non-hemolytic at 1:64 (1.56 mcg/mL) |
| Carnobacteriocin B2 | VNYGN GVSCS KTKCS VNWGQ AFQER YTAGI NSFVS GVASG AGSIG RRP | 3.9 | cationic membrane-permeabilizing bacteriocin (Class II) | not expected |
| Cecropin A–Magainin 2 hybrid | KWKLFKKIGIGKFLHSAKKF | 7.1 | trp2 insertion, Lys binding, alpha-helix membrane spanning due to flexible hinge | moderate 1:2 (50 mcg/mL) to 1:32 (3.125 mcg/mL) |
| Dermaseptin | ALWKT MLKKL GTMAL HAGKA ALGAA ADTIS QGTQ | 3.1 | forms amphipathic helices when integrated with membrane lipid bilayer | yes |
| Dermcidin DCD | SSLLE KGLDG AKKAV GGLGK LGKDA VEDLE SVGKG AVHDV KDVLD SVL | −1.9 | transmembrane potential formed with nanopore formation upon insertion | not expected |
| NRC-13 Pleurocidin | GWRTLLKKAEVKTVGKLALKHYL | 5.1 | forms ion channels (probable toroidal pore) in planar lipid bilayers. Inhibits nucleic acid and protein synthesis | moderate 1:2 (50 mcg/mL) to 1:256 (0.39 mcg/mL) |
| Parasin I | KGRGK QGGKV RAKAK TRSS | 8 | binds to DnaK, inhibiting its major two functions: ATPase activity and misfolding proteins with inactivation by acting on internal targets | non-hemolytic |
| Pyrrhocoricin | VDKGS YLPRP TPPRP IYNRN | 3 | binds to 70 kDa heat-shock protein DnaK, inhibiting protein folding | undetermined |
| RL-37 | RLGNFFRKVKEKIGGGLKKVGQKIKDFLGNLVPRTAS | 8 | amphipathic alpha-helical structure, pore formation | no |
| Temporin L | FVQWF SKFLG RIL | 2 | allows Temporin A and B to bypass LPS and access the cytoplasmic membrane by preventing their oligomerization to LPS | yes |
| AMP | Cj1 | Cj2 | Cp1 | Cp2 | S1 | S2 | Lm1 | Lm2 | Ec |
|---|---|---|---|---|---|---|---|---|---|
| Apidaecin 1B | - | - | - | - | + | + | - | - | + |
| C12K-2β12 | + | + | + | + | + | + | + | + | + |
| Carnobacteriocin B2 | - | - | - | - | - | - | - | - | - |
| Cecropin A–Magainin 2 hybrid | + | + | - | + | + | + | + | + | + |
| Dermaseptin | + | - | + | + | - | + | + | + | + |
| Dermcidin DCD | - | - | - | - | - | - | - | ± | - |
| NRC-13 Pleurocidin | + | + | - | + | ± | + | + | + | + |
| Parasin I | - | - | - | - | - | - | - | - | - |
| Pyrrhocoricin | - | - | - | - | - | - | - | - | - |
| RL-37 | ++ | ++ | - | + | + | + | + | + | + |
| Temporin L | + | + | + | ++ | + | + | + | + | + |
| Ampicillin (control) | - | - | ++ | ++ | + | + | + | ++ | ++ |
| Acetic acid (control) | - | - | + | + | + | + | + | + | + |
| Target Bacteria | C12K-2β12 | Cecropin A–Magainin 2 | RL-37 |
|---|---|---|---|
| Campylobacter jejuni 14118 | ++ | + | +++ |
| Campylobacter jejuni 81-116 | ++ | + | ++ |
| Campylobacter jejuni 81-176 a | ++ | + | ++ |
| Campylobacter jejuni 11168 ^a | ++ | + | ++ |
| Campylobacter jejuni RM1221 a | +++ | + | +++ |
| Campylobacter jejuni A74C | +++ | + | ++ |
| Campylobacter jejuni A49943 * | ++ | + | ++ |
| Campylobacter jejuni A33250 * | ++ | + | ++ |
| Campylobacter jejuni A29428 * | ++ | + | ++ |
| Campylobacter coli Epi 33-WT | +++ | + | +++ |
| Campylobacter coli A49941 * | +++ | + | +++ |
| Campylobacter coli A33559 * | ++ | + | +++ |
| Campylobacter lari RM2100 | +++ | + | +++ |
| Campylobacter lari A35221 * | ++ | + | ++ |
| Campylobacter lari “slaughter beach” | ++ | + | ++ |
| Salmonella enterica serovar Typhimurium Epi 3 | ++ | + | + |
| Salmonella enterica serovar Heidelberg Epi 42 | ++ | + | + |
| Lactobacillus acidophilus-WT | ++ | + | + |
| Lactobacillus helveticus-WT | ++ | - | - |
| Clostridium perfringens 39 | + | - | - |
| Clostridium perfringens 509 | + | + | + |
| Listeria monocytogenes A49594 (4b) | + | + | + |
| Listeria monocytogenes 311 WT | + | + | + |
| Escherichia coli O157:H7 | + | + | + |
| Target Campylobacter spp. Isolate | C12K-2β12 MIC (µg/mL) | Cecropin A–Magainin 2 MIC (µg/mL) | RL-37 MIC (µg/mL) |
|---|---|---|---|
| Campylobacter jejuni 14118 | 3.1 | 50 | 1.6 |
| Campylobacter jejuni 81-116 | 1.6 | 12.5 | 3.1 |
| Campylobacter jejuni 81-176 a | 3.1 | 50 | 3.1 |
| Campylobacter jejuni 11168 ^a | 3.1 | 25 | 3.1 |
| Campylobacter jejuni RM1221 a | 1.6 | 50 | 1.6 |
| Campylobacter jejuni A74C | 1.6 | 50 | 3.1 |
| Campylobacter jejuni A49943 * | 3.1 | 25 | 3.1 |
| Campylobacter jejuni A33250 * | 3.1 | 100 | 3.1 |
| Campylobacter jejuni A29428 * | 3.1 | 100 | 6.3 |
| Campylobacter coli Epi 33-WT | 1.6 | 50 | 1.6 |
| Campylobacter coli A49941 * | 1.6 | 100 | 1.6 |
| Campylobacter coli A33559 * | 3.1 | 100 | 1.6 |
| Campylobacter lari RM2100 | 1.6 | 25 | 1.6 |
| Campylobacter lari A35221 * | 3.1 | 100 | 3.1 |
| Campylobacter lari “slaughter beach” | 3.1 | 100 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Line, J.E.; Seal, B.S.; Garrish, J.K. Selected Antimicrobial Peptides Inhibit In Vitro Growth of Campylobacter spp. Appl. Microbiol. 2022, 2, 688-700. https://doi.org/10.3390/applmicrobiol2040053
Line JE, Seal BS, Garrish JK. Selected Antimicrobial Peptides Inhibit In Vitro Growth of Campylobacter spp. Applied Microbiology. 2022; 2(4):688-700. https://doi.org/10.3390/applmicrobiol2040053
Chicago/Turabian StyleLine, John Eric, Bruce S. Seal, and Johnna K. Garrish. 2022. "Selected Antimicrobial Peptides Inhibit In Vitro Growth of Campylobacter spp." Applied Microbiology 2, no. 4: 688-700. https://doi.org/10.3390/applmicrobiol2040053
APA StyleLine, J. E., Seal, B. S., & Garrish, J. K. (2022). Selected Antimicrobial Peptides Inhibit In Vitro Growth of Campylobacter spp. Applied Microbiology, 2(4), 688-700. https://doi.org/10.3390/applmicrobiol2040053






