Abstract
Despite great efforts have been made worldwide, the coronavirus disease 19 (COVID-19) still has not a definitive cure, although the availability of different vaccines are slowing down the transmission and severity. It has been shown that surfactin, a cyclic lipopeptide produced by Bacillus subtilis, is a molecule able to counteract both SARS-CoV-1, MERS-CoV and HCoV-229E coronaviruses. In this study the potential antiviral activity of surfactin against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was tested in vitro in a cellular model of infection. Our results show that 2 h treatment with surfactin is able to reduce SARS-CoV-2 infectivity on Vero E6 cells both at 24 h and after 7 days from viral inoculation, probably impairing the viral membrane integrity. Moreover, surfactin, at the concentrations used in our experimental settings, is not cytotoxic. We suggest surfactin as a new potential molecule against SARS-CoV-2, to be employed at least as a disinfectant.
1. Introduction
The coronavirus disease 19 (COVID-19) was reported for the first time in Wuhan (province of Hubei, China) in December 2019 and later spread worldwide, becoming a severe public health issue [1,2]. Despite presenting a lower mortality rate (2.3%) in comparison to severe acute respiratory syndrome coronavirus (SARS-CoV-1, ~9%) [3] and Middle East Respiratory Syndrome (MERS-CoV, ~35%) [4], SARS-CoV-2 shows greater infectivity probably due to the higher binding affinity of the spike (S) protein to the host angiotensin-converting enzyme 2 (ACE2) cell receptor, contributing significantly to the increased transmissibility [5]. SARS-CoV-2 infection manifests with different clinical severity, ranging from asymptomatic or mild, to moderate and severe cases [6]. Advanced age and some comorbidities such as cardiopathy, pneumopathy, diabetes, kidney disease, hypertension, immunosuppression and obesity have been reported as risk factors associated with the increase of COVID-19 severity and mortality rates [7]. The current COVID-19 pandemic has demanded significant efforts to the international scientific and technological community on several fronts of action, to face the serious growth of infected patients and deaths worldwide. The introduction of SARS-CoV-2 vaccine strategies greatly contributed to diminish the number of infected people as well as deaths in several countries, in which the vaccines have been rapidly acquired and widely distributed in adults and elderly, as well as in children, being the pediatric age the most affected during the first period of 2022 [8]. So, even if the prevention strategies are increasing in efficacy, there is still the need for the discovery of compounds with broad spectrum antiviral activity, that will not be affected by the emerging of new variants [9] and will keep being able to inhibit SARS-CoV-2 infection and replication in host cells, thus providing adjuvant tools to fight the virus.
In this context, it has been highlighted that molecules derived from bacteria are able to interact with viruses and to modulate the viral infectivity. A few months before the declaration of the pandemic by WHO, it was found that surfactin, a cyclic lipopeptide associated with peptidoglycans (PG) from Bacillus subtilis, reduced the infectivity of different coronaviruses as SARS-CoV-1, MERS-CoV and Human coronavirus 229E (HCoV-229E) by 10.000 times in a dose and temperature dependent manner. It was demonstrated that surfactin disrupted the integrity of the CoVs virion, and prevented SARS-CoV-1 in vivo infection in mice when the molecule was mixed with the virus prior to the inoculation [10].
Surfactin is thought to have a broad inhibitory effect against enveloped viruses including influenza, Zika, Ebola, Nipah, Chikungunya, Una, Mayaro and Dugbe viruses [10], as well as against herpes-simplex-virus (HSV-1, HSV-2), vesicular stomatitis virus (VSV), simian immunodeficiency virus (SIV), Newcastle disease virus, and porcine epidemic diarrhea virus [11,12,13].
Moreover, surfactin possesses several other features; it has antimicrobial, fungicidal and bactericidal properties combined with very low cytotoxicity. Anti-tumor, anti-mycoplasmic, anticoagulant and antiviral activity have already been demonstrated both in in vitro [11,12] and in in vivo animal models [13]. It can be used as a vehicle for pulmonary drug administration, but it can be also found in cosmetics, food and industrial products; also, it is a protective probiotic agent able to improve gut microbiome functioning [14,15].
Noteworthy, recent in silico docking approaches showed that surfactin could bind SAR-CoV-2 Spike glycoprotein, thus preventing the bonding between spike and ACE2 and the subsequent fusion of virions with the host cell lipid membrane [16], as well as it was able to bind the RNA-dependent RNA polymerase (RdRP, i.e., nonstructural protein 12—nsp12) in the same aminoacidic region where remdesivir (an U.S.A. Food and Drug Administration approved antiviral drug) acts, so blocking the transcription of the genome and the viral replication machinery [17]. Nevertheless, this interesting data required to be confirmed by in vitro functional assays.
In this study, the potential antiviral activity of surfactin was tested against SARS-CoV-2 in vitro on Vero E6 cells. The direct effect of surfactin on virion integrity was assessed as well as the residual infectivity of the treated virus.
2. Materials and Method
2.1. Test of Surfactin against SARS-CoV-2
A previously isolated SARS-CoV-2 strain was used for the experiment [18]. Vero E6 cells (epithelial cells from Cercopithecus aethiops, ATCC CRL-1586) were employed as a cellular model for their high susceptibility towards SARS-CoV-2 infection.
Vero E6 cells were maintained in Minimum essential medium (MEM) supplemented with fetal bovine serum (FBS) 10%, 2 mM glutamine and 100 U⋅mL−1 penicillin/streptomycin (Euroclone, Pero, Italy). For the experiments where Vero E6 cells were challenged with SARS-CoV-2, the concentration of FBS was reduced to 2%.
Based on the study by Johnson et al. [10] two settings were tested:
- (a)
- The direct antiviral effect was examined with incubation of surfactin and the virus prior to cells inoculation.
SARS-CoV-2 (1012 viral copies) was diluted in 100 μL of infection medium and treated with 1, 0.5, 0.1, 0.05, 0.001, 0.005, or 0.0001 μg⋅μL−1 of surfactin (S3523, Merck Sigma-Aldrich, Darmstadt, Germany) and incubated for 2 h at 37 °C, then the mix was added to Vero cells for 1 h at 37 °C in 24 multi-well plate (100.000 cells, at a multiplicity of infection MOI of 0.01).
- (b)
- The effect of 2 h treatment of surfactin on SARS-CoV-2 already infected cells (1 h) was assessed.
SARS-CoV-2 (1012 viral copies) was diluted in 100 μL of infection medium and inoculated to the cells for 1 h, then the cells were treated with surfactin at 1, 0.5, 0.1, 0.05, 0.001, 0.005, or 0.0001 μg⋅μL−1 of surfactin (S3523, Merck Sigma-Aldrich) for 1 h at 37 °C in 24 multi-well plate (100.000 cells, at a MOI of 0.01).
After the treatments the cells were washed in PBS and new medium added and the cells monitored for 7 days at the EVOS XL Core Cell Imaging System (Thermo Fisher Scientific, Waltham, MA, USA).
2.2. Infectivity Assessment
After 24 h and 7 days from the treatment/infection the viral replication was assessed in the supernatants. The viral RNA was extracted with thermolysis of 15 μL of supernatant medium mixed with 45 μL of water (98 °C for 3′, 4 °C for 5′).
At the same time point, the cells were trypsinized for 2 min to remove the attached viruses not internalized, then the reaction was blocked with complete medium (MEM + 10% FBS). The cells were washed twice in PBS and then lysed in 50 μL of PBS + Triton X100 0.2% for 5 min. The liquid was totally aspirated from the wells and maintained at −80 °C until analysed.
2.3. Viral RNA Quantification
Viral RNA quantification was performed by using as target the N gene (nucleocapsid, CDC primers probe [19]: 500 nM forward primer 5′-GGG AGC CTT GAA TAC ACC AAA A-3′, 500 nM reverse primer 5′-TGT AGC ACG ATT GCA GCA TTG-3′, 125 nM probe 5′-FAM-AYC ACA TTG GCA CCC GCA ATC CTG-BHQ1-3′) on the 7500 Fast Real-Time PCR instrument (Thermo Fisher Scientific) with the Luna Universal Probe One-Step RT-qPCR kit (New England Biolabs, Ipswich, MA, USA) as previously described [18]. The standard curve for virus quantification was generated by using a nCoV-CDC-Control Plasmid (Eurofins, Luxembourg).
2.4. RNAse Protection Assay
RNAse protection assay was employed to assess the virion integrity after surfactin treatment, to disclose if the molecule could have impaired the external lipidic envelope. Fifteen μL of mix virus-surfactin were treated with 1 μg of Ribonuclease A (RNAse A, R4875, Merck Sigma Aldrich) for 30 min at 37 °C, then 45 μL of water were added to the mix and submitted to thermolysis and the viral load quantified as above described.
2.5. Statistical Analysis
Statistical analysis was performed with R software by using Mann-Whitney test (KW) test for the comparison of the experimental conditions [18]. The experiments were performed in six replications, in two independent days.
3. Results
The treatment of SARS-CoV-2 with surfactin was able to inhibit SARS-CoV-2 in the setting 1, i.e., the pre-treatment of the virus with surfactin for 2 h prior to the inoculation to the Vero E6 cells.
To assess the effect of surfactin on SARS-CoV-2 after the treatment, the cells were monitored for 7 days. The RNA viral load in the supernatants and intracellularly was quantified at 24 h and 7 days post infection/treatment to determine the possible virus replication. The concentration at 1 μg⋅μL−1 was able to inhibit virus amplification, while the other ones (0.5, 0.1, 0.05, 0.001, 0.005, or 0.0001 μg⋅μL−1) tested were not effective in the antiviral activity (data not shown).
After 24 h the viral load in the cells infected with 1 μg⋅μL−1 surfactin treated virus was significantly lower (105 viral copies⋅mL−1) related to the cells inoculated with the not treated virus (108 viral copies⋅mL−1) (MW-test p-value = 0.002, Figure 1A).
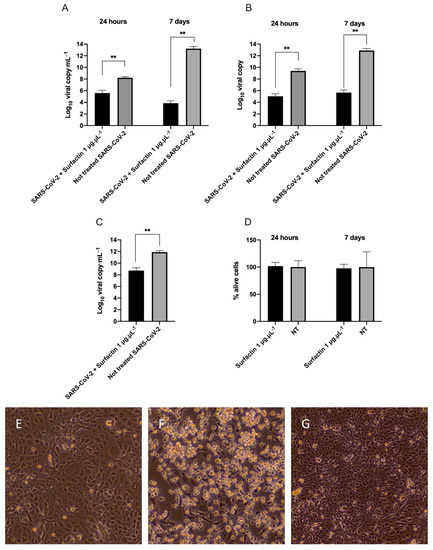
Figure 1.
Surfactin antiviral activity. Effect of surfactin (1 μg⋅μL−1) on SARS-CoV-2. (A) RNA viral load in the supernatants (expressed as Log10 viral copies⋅mL−1) of cells infected with the not treated virus and in the cells infected with surfactin treated virus (1 μg⋅μL−1) after 24 h or 7 days post infection/treatment. (B) Intracellular RNA viral copies (expressed as Log10 viral copies) in the cells infected with the not treated virus and in the cells infected with surfactin treated virus (1 μg⋅μL−1) at 24 h or 7 days post infection/treatment. (C) Assessment of the virion integrity (RNAse protection assay) after treatment of the virus with 1 μg⋅μL−1 surfactin and virion integrity assessment in the not treated virus. The viral loads of the RNAse protection assay are expressed as Log10 viral copies⋅mL−1. (D) Viability of the cells after treatment with surfactin 1 μg⋅μL−1 at 24 h and 7 days. The results are reported as percentage of alive cells related to the untreated cells (NT). The statistical results from Mann-Whitney test are reported: ** p-value < 0.01. (E–G) Representative images of surfactin treated cells after 7 days after infection at 40×. (E) not treated cells, (F) SARS-CoV-2 treated cells, (G) Cells inoculated with SARS-CoV-2 treated with surfactin at 1 μg⋅μL−1. The experiments were performed in six replications, in two independent days.
At 7th day, in the cells infected with 1 μg⋅μL−1 surfactin treated virus the viral load was reduced compared to day 1 (103 viral copies⋅mL−1), while in the cells infected with not treated virus, the RNA viral load strongly increased (1013 viral copies⋅mL−1) (MW-test p-value = 0.002, Figure 1A).
When looking at the intracellular level of SARS-CoV-2 RNA, similar results were found: after 24 h 109 viral copies⋅mL−1 were measured in the cells infected with the not treated virus versus 105 viral copies⋅mL−1 in the cells infected with virus treated with 1 μg⋅μL−1 surfactin (MW-test p-value = 0.002; Figure 1B). At 7th day, 1012 viral copies⋅mL−1 were determined in the cells inoculated with the not treated virus, while only 105 viral copies⋅mL−1 were registered in the lysates of the cells infected with virus treated with 1 μg⋅μL−1 surfactin (MW-test p-value = 0.002; Figure 1B).
Moreover, surfactin (1 μg⋅μL−1) was able to significantly decrease the RNA viral load in the RNAse protection assay, performed immediately after the 2 h of treatment, reporting 1011 viral copies⋅mL−1 in the not treated virus versus 108 viral copies⋅mL−1 in the virus treated with 1 μg⋅μL−1 surfactin leading to a reduction greater than 99% (MW-test adjusted p-value = 0.004, Figure 1C). Notably, this concentration is not cytotoxic (1 μg⋅μL−1) as observed with crystal violet staining of surfactin treated cells at 24 h and 7 days post treatment (Figure 1D).
At the optical microscope, the images corroborated our findings, indeed, evident cytopathic effect (characterized by cell degeneration, rounding and detachment) was observed in the cells inoculated with the not treated virus, while the cells treated with 1 μg⋅μL−1 surfactin appeared similar to the not infected controls (Figure 1E–G).
In the setting 2, i.e., the surfactin treatment of the already infected cells (1 h), no antiviral affect was registered, indicating, that probably when the virus was protected inside the cells, surfactin was not effective in its anti-SARS-CoV-2 action (data not shown).
4. Discussion
Despite the available treatments for COVID-19 infection such as remdesivir, lopinavir, ritonavir, chloroquine, hydroxychloroquine, favipiravir, umifenovir, camostat and ribavirin, or anti-inflammatory drugs, therapeutic antibodies and convalescent plasma, COVID-19 continues to be a significant health risk for humans worldwide, even among the vaccinated population and amid children [7,20]. More serious symptoms such as those occurring in the acute respiratory distress syndrome (ARDS) are managed with mechanical ventilators in the intensive care units [7]. Therefore, novel interventions able to counteract the virus are strongly envisaged.
Our results showed the potentiality of surfactin as an antiviral agent against SARS-CoV-2. Indeed, surfactin was able to inhibit SARS-CoV-2 in vitro after 2 h of treatment. The RNA viral load in the cells infected with the virus treated with surfactin at 1 μg⋅μL−1 was significantly lower compared to the cells inoculated with not treated virus, after 24 h both in the supernatant and intracellularly, as well as after 7 days of infection. This second time point shows that the RNA viral quantity remains at the same basal level of the first day, suggesting a block of viral replication while not treated SARS-CoV-2 greatly amplified inside the Vero E6 cells. The residual presence of not infective SARS-CoV-2 in the cells inoculated with surfactin treated virus was probably due the binding of viruses or viral components to the treated plastic dish or with extracellular proteins on cellular Vero E6 membrane. The washing steps performed are probably not sufficient to eliminate all the viral particles. Indeed, in the supernatants a further reduction of the RNA level in the cells inoculated with surfactin treated virus can be observed between day 1 and day 7, probably due to the degradation of nucleic acid from the partially or totally disrupted virions.
These findings suggest that surfactin (at a concentration of 1 μg⋅μL−1) is able to block the viral ability for replication in vitro, but only when the compound and the virus were pre-mixed and then inoculated on the Vero E6 cells, since our experiments failed in detecting an antiviral effect when the compound was administered in already infected cells. We can hypothesize different scenarios. Surfactin, being a peptide, may be not able to efficiently enter the cells, therefore, the virions are protected by the host cellular membrane. If some molecules penetrate, the virions could be disassembled and the surfactin acting on external envelope or spike protein may be not able to interact with the replication machinery. Finally, the virions could be assembled but they may be protected by the membrane of exocytic vesicles [5]. In all these situations surfactin is probably not playing its direct antiviral role.
In attempting to define the action of surfactin, the RNAse protection assay was performed: RNAse A eliminates the RNA release from disrupted virions, meanwhile RNA from intact virus was protected by the envelope. Surfactin treatment significantly decreased the SARS-CoV-2 RNA viral load when compared to the not treated virus, possibly suggesting a direct disrupting effect on virion envelope leading to virus inactivation. Nevertheless, only a reduction of the RNA viral load was determined; although it was greater than 99%, it was not a complete elimination, indicating that other mechanisms may concur in the antiviral effect of surfactin.
Indeed, the in silico studies conducted so far showed that surfactin may interact with S protein, so inhibiting the binding of virions to host cell receptors [16] and RdRP, thus interfering with the replication machinery [17]. Moreover, this data is in agreement with the study by Johnson et al. [10] where the TEM analysis showed the presence of both intact and disrupted virions after the treatment.
Johnson et al. [10] already described the ability of surfactin to counteract different strains of coronavirus, i.e., HCoV-229E, SARS-CoV-1 and MERS-CoV in vitro [10], as well as a list of other enveloped viruses, although with different grades of efficacy. Surfactin presented also effectiveness in vivo when administered together with SARS-CoV intranasally in mice [10], although prophylactic delivery is not efficient. Other cyclic lipopeptides (i.e., iturin A, fengycin, polymyxin B, colistin, ramoplanin, and daptomycin) were also previously analyzed by Johnson et al. [10], but they showed no antiviral effect, evidencing the specificity and unicity of surfactin activity, possibly due to its mechanisms of action. Indeed, surfactin is able to penetrate the lipid layers of the membrane, causing their solubilization and permeabilization, while the effect on established infection is avoided, suggesting a direct interaction of surfactin with virions [21,22]. Notably, the enriched presence of phosphatidylcholine, particularly sensitive to surfactin, on the microorganisms’ membrane could enhance its virucidal property. Since SARS-CoV-2 exploits endogenous Golgi apparatus and endoplasmic reticulum of host cells for virion assembly, and these organelles are phosphatidylcholine abundant cell sites [5], it has been supposed that SARS-CoV-2 could be especially susceptible to surfactin as our results also indicated. Another possible mechanism of action of surfactin can be the inhibition of membrane fusion between target cells and virus, through the insertion of surfactin in the envelope and the stabilization of the positive membrane curvature, as observed for porcine epidemic diarrhea virus (PEDV), influenza A virus and MERS [13,21,23].
5. Conclusions
Our results, although conducted in an in vitro cellular model of infection, showed a significant antiviral effect exerted by surfactin against SARS-CoV-2, probably resulting from a direct disruptive activity on the virion structures. Despite the fact that antiviral activity of surfactin against enveloped viruses is widely known [10,11,12,13], it has not already been tested on SARS-CoV-2 before our study. Our data corroborates and renews the previous findings by Johnson et al. [10] conducted on three closely related coronavirus (i.e., SARS-CoV-1, MERS-CoV and HCoV-229E) and validate with functional studies the in silico analysis conducted by Xia et al. [17]. Being surfactin active directly on the viruses, a possible employment of surfactin as a low cost disinfectant can be proposed since it is not corrosive and acting only on the membrane integrity [21], therefore allowing its application in several settings including but not limited to, hospital (hosting both adult and pediatric patients), school, and houses.
Author Contributions
Conceptualization, S.C. and T.C.J.; methodology, L.C.d.F. and L.Z.; formal analysis, L.C.d.F. and L.Z.; investigation, L.C.d.F. and L.Z.; resources, S.C., F.F., M.R. and T.C.J.; data curation, L.Z.; writing—original draft preparation, L.C.d.F., L.Z., S.C. and T.C.J.; writing—review and editing, S.C., F.F., M.R., E.P.N.P., I.O.P. and T.C.J.; visualization, S.C. and T.C.J.; supervision, F.F. and T.C.J.; project administration, S.C. and T.C.J.; funding acquisition, S.C., F.F., M.R. and T.C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was approved by IRCCS Burlo Garofolo (RC 47/2020) and UFPE/PROPESQ, and granted by IRCCS Burlo Garofolo/Italian Ministry of Health (RC 15/2017 and RC 47/2020), UFPE/Brazilian Ministry of Education (PROPESQ Edital 06/2020) and Collaborative Grant from Qatar University (QUCG-CAS-22/23-499). LCF and EPNP were supported by doctoral fellowships from Brazilian Government Agency CAPES (Coordination for the Improvement of Higher Education Personnel).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used to support the findings in this study are included in the article.
Acknowledgments
The authors are grateful to TCR-Tecora S.R.L. for providing instrumentation of BSL3 laboratory. The graphical abstract was created with Biorender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization Declares Global Emergency: A Review of the 2019 Novel Coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, Biology and Novel Laboratory Diagnosis. J. Gene Med. 2021, 23, 3303. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D. The Chronology of the 2002–2003 SARS Mini Pandemic. Paediatr. Respir. Rev. 2004, 5, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Aleanizy, F.S.; Mohmed, N.; Alqahtani, F.Y.; El Hadi Mohamed, R.A. Outbreak of Middle East Respiratory Syndrome Coronavirus in Saudi Arabia: A Retrospective Study. BMC Infect. Dis. 2017, 17, 23. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 Pathophysiology: A Review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782. [Google Scholar] [CrossRef]
- Torjesen, I. Covid-19: Omicron Variant is Linked to Steep Rise in Hospital Admissions of Very Young Children. BMJ 2022, 376, o110. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). SARS-CoV-2 Variants of Concern. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 27 July 2022).
- Johnson, B.A.; Hage, A.; Kalveram, B.; Mears, M.; Plante, J.A.; Rodriguez, S.E.; Ding, Z.; Luo, X.; Bente, D.; Bradrick, S.S.; et al. Peptidoglycan-Associated Cyclic Lipopeptide Disrupts Viral Infectivity. J. Virol. 2019, 93, e01282-19. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Pauli, G.; Ozel, M.; Vater, J. Antimycoplasma Properties and Application in Cell Culture of Surfactin, a Lipopeptide Antibiotic from Bacillus Subtilis. Appl. Environ. Microbiol. 1997, 63, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Lu, Z.; Zhao, H.; Bie, X.; Lü, F.; Yang, S. Antiviral Activity of Antimicrobial Lipopeptide from Bacillus Subtilis Fmbj Against Pseudorabies Virus, Porcine Parvovirus, Newcastle Disease Virus and Infectious Bursal Disease Virus in Vitro. Int. J. Pept. Res. Ther. 2006, 12, 373–377. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Wang, Y.; Li, Y.; Wang, X.; Yang, Q. Surfactin Inhibits Membrane Fusion during Invasion of Epithelial Cells by Enveloped Viruses. J. Virol. 2018, 92, e202000496. [Google Scholar] [CrossRef]
- Zeppa, S.D.; Agostini, D.; Piccoli, G.; Stocchi, V.; Sestili, P. Gut Microbiota Status in COVID-19: An Unrecognized Player? Front. Cell. Infect. Microbiol. 2020, 10, 576551. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.F.C.; de Quadros, C.P.; Júnior, M.R.M.; Pastore, G.M. Surfactina: Propriedades Químicas, Tecnológicas e Funcionais Para Aplicações Em Alimentos. Quím. Nova 2007, 30, 409–414. [Google Scholar] [CrossRef]
- Chowdhury, T.; Baindara, P.; Mandal, S.M. LPD-12: A Promising Lipopeptide to Control COVID-19. Int. J. Antimicrob. Agents 2021, 57, 106218. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Luo, M.; Pang, L.; Liu, X.; Yi, Y. Lipopeptides against COVID-19 RNA-Dependent RNA Polymerase Using Molecular Docking. Biomed. J. 2021, 44, S15–S24. [Google Scholar] [CrossRef]
- Zupin, L.; Gratton, R.; Fontana, F.; Clemente, L.; Pascolo, L.; Ruscio, M.; Crovella, S. Blue Photobiomodulation LED Therapy Impacts SARS-CoV-2 by Limiting Its Replication in Vero Cells. J. Biophotonics 2021, 14, e202000496. [Google Scholar] [CrossRef]
- CDC (Centers for Control Disease and Prevention). Research Use Only 2019-Novel Coronavirus (2019-NCoV) Real-Time RT-PCR Primers and Probes. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html (accessed on 28 February 2022).
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community Transmission and Viral Load Kinetics of the SARS-CoV-2 Delta (B.1.617.2) Variant in Vaccinated and Unvaccinated Individuals in the UK: A Prospective, Longitudinal, Cohort Study. Lancet Infect. Dis. 2021, 22, 183–195. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus Spp.: A Gold Mine of Antibiotic Candidates: BACILLUS AND PAENIBACILLUS LIPOPEPTIDES. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Özel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of Inactivation of Enveloped Viruses by the Biosurfactant Surfactin FromBacillus Subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Lin, D.; Chen, L.; Xie, X.; Shen, X.; Yang, Q.; Wu, Q.; Yang, J.; He, J.; et al. Super Short Membrane-Active Lipopeptides Inhibiting the Entry of Influenza A Virus. Biochim. Biophys. Acta 2015, 1848, 2344–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).