1. Introduction
Chronic endometritis (CE) is an infectious and inflammatory disease of the uterine lining, which is characterized by endometrial stromal plasmacyte (ESPC) infiltration. Many of the women with CE are asymptomatic or oligosymptomatic only with subtle and nondescript manifestations such as vaginal spotting, leukorrhea, and pelvic discomfort [
1]. However, recent studies demonstrated the association between CE and infertility of unknown etiology (28%), repeated implantation failure (RIF) following in vitro fertilization–embryo transfer cycles (14–41%), and recurrent pregnancy loss (8–28%) [
1,
2,
3,
4]. Moreover, chronic deciduitis, the persistent form of CE during the gestational period, is thought to relate to preterm labor and several neonatal complications, including periventricular leukomalacia and cerebral palsy in premature infants [
1].
Antibiotic agents, such as doxycycline, metronidazole, ciprofloxacin, azithromycin, and moxifloxacin, have been effective and prescribed for the treatment of CE [
1,
4,
5]. Multi-drug resistance (MDR), however, is an emerging issue, as in other medical fields. We recently reported the prevalence of CE in RIF women and MDR (defined as resistance to the first-line antibiotic treatment with oral doxycycline 200 mg/day, for 14 days and then second-line antibiotic treatment with a combination of oral metronidazole 500 mg/day and oral ciprofloxacin 400 mg/day, both agents for 14 days) in the whole RIF/CE women in the last decade (from April 2010 to March 2020) as 31.4% and 7.8%, respectively [
5]. While the yearly prevalence of CE in RIF women was steady throughout the ten years and showed no marked fluctuation (30.2% between April 2010 and March 2015 versus 31.7% between April 2015 and March 2020, odds ratio 1.07, 95% confidence interval 0.90–1.28,
p trend value > 0.05), that of MDR in these women significantly (odds ratio 8.27, 95% confidence interval 2.58–26.43,
p trend value < 0.001) increased from 1.3% (between April 2010 and March 2015) to 9.6% (between April 2015 and March 2020).
To determine the potential antibiotic regimen that would be effective and feasible in the next treatment for these RIF/MDR-CE women, we performed a microbiota analysis using their paired vaginal secretions/endometrial fluid samples [
5]. Unfortunately, we could not identify any local bacterial genera and/or microbial communities unique to MDR-CE. We thereby conducted a pilot study comparing the effectiveness and safety of the third-line empiric oral antibiotic treatments against MDR-CE using two regimens (oral moxifloxacin, 400 mg/day, 10 days versus oral azithromycin, 500 mg/day, 3 days). Compared with the older-generation fluoroquinolones, moxifloxacin displays a broader antimicrobial spectrum with improved activity against Gram-negative bacteria and anaerobes. Some recent studies demonstrated that moxifloxacin was superior to metronidazole in treatment against two bacterial vaginosis-associated species,
Atopobium vaginae and
Gardnerella vaginalis [
6,
7]. Meanwhile, azithromycin belongs to the acid-stable macrolide group and structurally resembles erythromycin but widely covers pelvic, genital, and/or sexually transmitted infectious diseases, including female urethritis and cervicitis, with its broader antimicrobial spectrum [
8]. Following the completion of these oral antibiotic treatments, the cure rate of histopathologic CE was similar between the two regimens (moxifloxacin group 79.2% versus azithromycin group 75.0%), as well as the live birth rate in the immediate subsequent cycle and cumulative three embryo transfer cycles (31.6% and 57.9% versus 33.3% and 61.1%, odds ratio 0.92 and 0.88, 95% confidence interval 0.23–3.66 and 0.23–3.26,
p-value of 0.91 and 0.84, respectively). This resulted in 11 out of 48 (22.9%) RIF/MDR-CE women remaining resistant to a total of three courses of oral antibiotic regimens [
5].
The aim of this study was to follow up on these 11 patients suffering from RIF/MDR-CE and provide treatment strategies that lead to successful reproductive outcomes.
2. Case Reports
Following the failure of the third-line oral antibiotic treatment, all 11 RIF/MDR-CE women desired the histopathologic cure of CE prior to proceeding to the subsequent infertility treatment cycles. To find the effective antibiotic regimens for these women, we performed the secondary microbiota analysis again in their paired vaginal secretions and endometrial fluid for the identification of the specific bacterial species and/or microbial communities associated with MDR-CE under written informed consent. The microbiota analysis was a part of an ongoing prospective case-control study approved by the Ethical Committee of Institutional Review Board of Reproduction Clinic Osaka (Approval Institutional Review Board Number 20172, 20 September 2017) and registered on clinical trial registration on 6 October 2017 (Clinical Trial Registration Number UMIN-CTR 000029449) and conducted from October 2017 (
Figure 1).
As described previously [
9], the microbiota analysis was performed using the paired vaginal secretions and endometrial fluid samples obtained in the mid-secretory phase. The samples were carefully aspirated and soaked separately into collection tubes. Following pretreatment with lysozyme solution (Sigma-Aldrich, Darmstadt, Germany), genomic DNA isolation, and double-stranded DNA concentration measurement, the variable region 4 of the bacterial 16S rRNA gene was amplified using the modified primer pairs 515f and 806rB. Polymerase chain reaction (PCR) was then performed with the appropriate buffer solution containing 25 ng DNA, 200 μmol/L 4-deoxynucleotide triphosphates, 400 nmol/L of primer pairs, 2.5 U of FastStart HiFi polymerase, 4% of 20 mg/mL bovine serum albumin, 0.5 mol/L betaine, and MgCl
2 (Sigma-Aldrich, Darmstadt, Germany). Thermal cycling conditions are as follows: initial denaturation (94 °C, 2 min), 30 cycles of denaturation (94 °C, 20 s), annealing (50 °C, 30 s), and extension (72 °C, 1 min), along with final extension (72 °C, 5 min). Following amplicon mixture purification and PCR products multiplex, the indexing PCR was performed, and the products were purified again. The final library was paired-end sequenced at 2 × 200 bp. The ZymoBIOMICS Microbial Community Standard (Zymo Research, Orange, CA, USA) that contains a mixture of
Pseudomonas, Escherichia, Salmonella, Lactobacillus, Enterococcus, Listeria, Bacillus, and two yeast species
Saccharomyces and
Cryptococcus was adopted as a positive control. UltraPure™ DNase/RNase-Free Distilled Water (Thermo Fisher Scientific Inc., Waltham, MA, USA) was utilized as a blank control.
A median 291-base pair merged sequence length was obtained using EA-Utils fastq-join [
10]. The quality control of the merged sequence was performed with USEARCH v10.0.240 [
11] to remove PhiX reads, truncate primer-binding sequences, and discard sequences with <100 bp length and sequence quality < Q20. Using Quantitative Insights Into Microbial Ecology 1.9.1 [
12], the sequences were clustered into operational taxonomic units with the UCLUST method based on 97% sequence identity. Ribosomal Database Project Classifier [
13] was utilized for the taxonomy assignment with a 0.50 confidence threshold against the Greengenes database version 13_8 [
14]. The following bacterial taxa (
Acidovorax,
Acinetobacter,
Chryseobacterium,
Citrobacter,
Elizabethkingia,
Escherichia,
Flavobacterium,
Janthinobacterium,
Leptothrix,
Methylobacterium,
Pseudomonas,
Rhodococcus,
Sphingomonas,
Stenotrophomonas, and
Yersinia), known as contaminants found in a blank control [
14,
15,
16,
17], were excluded from endometrial fluid samples using Quantitative Insights Into Microbial Ecology 1.9.1.
Permutational multivariate analysis of the variance test was used for the calculation of the α- and β-diversity values and a comparison between the groups. Pearson’s correlation coefficient was applied to the comparison between the microbiota in the endometrial fluid and vaginal secretions within the same individual. Fisher’s exact test was used to compare taxon-relative abundances between the subjects and controls. A
p-value less than 0.05 was regarded as statistically significant. Unfortunately, in this secondary microbiota analysis, we failed to identify any unique bacterial communities as well as unique bacterial genera again in these 11 women (
Supplementary Table S1).
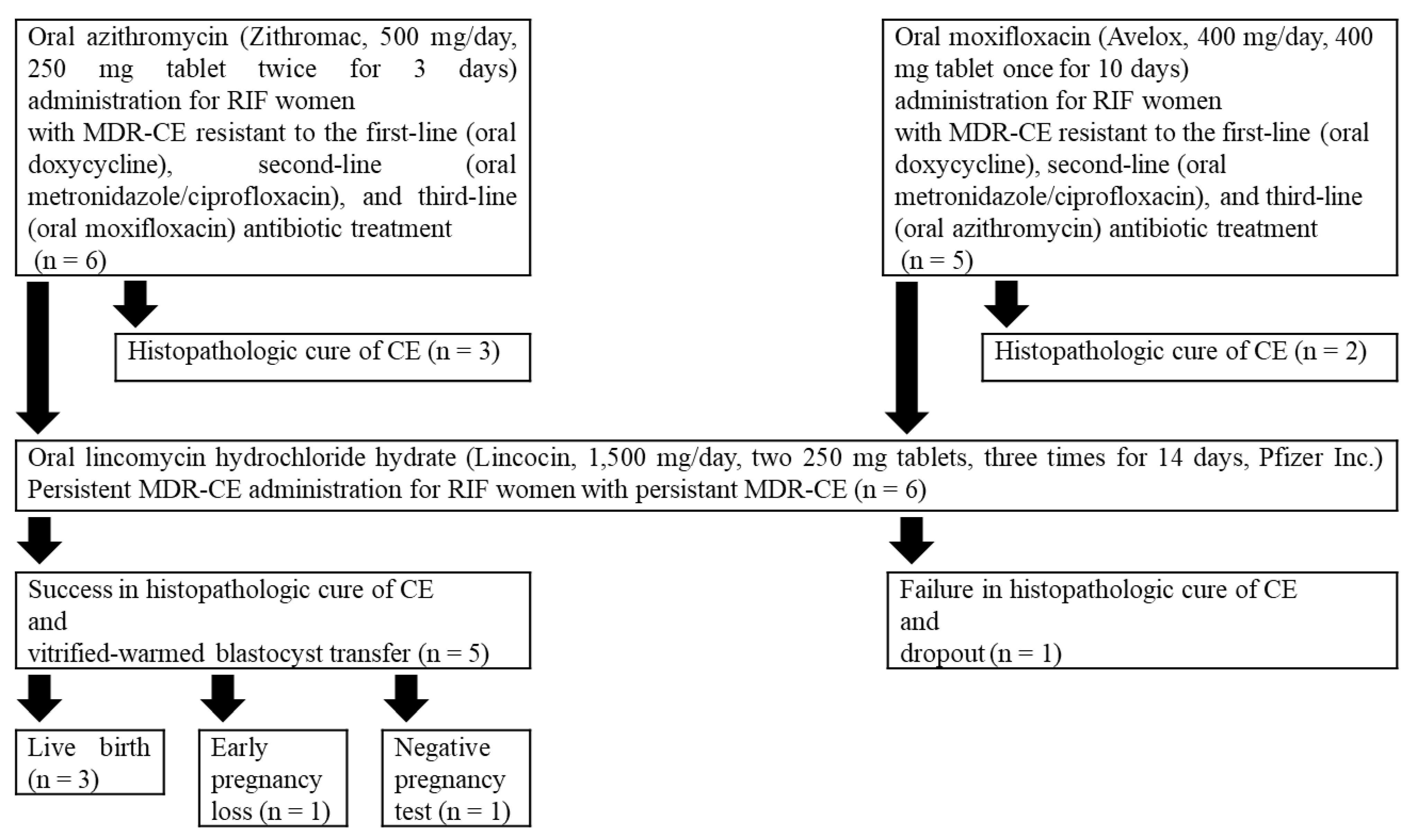
As a fourth-line antibiotic treatment, oral azithromycin (Zithromac, 500 mg/day, 250 mg tablet twice for 3 days, Pfizer Inc., Tokyo, Japan) was administered to RIF/MDR-CE women resistant to the third-line moxifloxacin (Avelox, 400 mg/day, 400 mg tablet once for 10 days, Bayer Healthcare Co., Osaka, Japan), and oral moxifloxacin was administered to RIF/MDR-CE women resistant to azithromycin. Histopathologic cure of CE was examined in endometrial specimens obtained by a 3-mm curette (Atom-Medical, Tokyo, Japan) biopsy using immunohistochemistry with mouse anti- monoclonal IgG antibody against an ESPC marker CD138 (B-A38; Nichirei, Tokyo, Japan) and hematoxylin counterstaining. Under a light microscope (400 × magnification), the sections were evaluated for ESPC (stromal CD138+ cells with nucleic heterochromatin pattern) in 20 or more high-power fields by an experienced gynecologic pathologist. As described previously, the ESPC density index (ESPDI) was calculated, and histopathologic CE was diagnosed with an ESPDI of 0.25 or more [
5].
While histopathologic cure of CE was achieved in five women (three in the azithromycin group and two in the moxifloxacin group), these treatments failed six women. MDR-CE that was resistant to these five different antibiotic agents was defined here as persistent MDR-CE.
All of them desired alternative treatment for persistent MDR-CE before proceeding to the subsequent transfer cycles and agreed to the following antibiotic treatment. Oral lincomycin hydrochloride hydrate (Lincocin, 1500 mg/day, two 250 mg tablets, three times for 14 days, Pfizer Inc., Tokyo, Japan) [
15] was administered to all six women with persistent MDR-CE. There were no reports on the serious adverse effects that required discontinuation and/or additional treatments. Histopathologic cure of persistent MDR-CE was confirmed in the endometrial biopsy sections obtained in the following menstrual cycle in five out of six RIF women. Meanwhile, one woman who failed in histopathologic cure of persistent MDR-CE dropped out of treatment.
In the immediate vitrified-warmed blastocyst transfer cycles following confirmation of histopathologic cure of persistent MDR-CE, three (Patient 1, 3, and 5) out of five RIF women had a successful live birth. Patient 2 had an early pregnancy loss in the first-trimester pregnancy, and Patient 4 resulted in a negative pregnancy test. At the time point of December 2021, both of them are waiting for authorization of a preimplantation genetic test for aneuploidy in 2022 in our nation. Their demographics are shown in
Table 1.
3. Discussion
CE is no exception regarding antibiotic resistance. Cicinelli et al. reported that less than 20% of CE was resistant to single-course oral doxycycline treatment in 2008, but 24.6% of CE was untreatable with three courses of antibiotic administration [
2,
18]. More recently, Xiong et al. reported that 11.0% of CE was resistant to two courses of the combined antibiotic treatments for 14 days [
19]. However, to the best of the authors’ knowledge, no studies demonstrated the prevalence and management of persistent MDR-CE.
Following the failure of the third-line oral antibiotic treatment, all 11 RIF/MDR-CE women desired the histopathologic cure of CE prior to proceeding to the subsequent infertility treatment cycle. Under informed consent, we performed the secondary microbiota analysis in the vaginal secretions and endometrial fluid of these women but failed to identify specific microbial genera and/or bacterial communities associated with MDR-CE again. As a fourth-line antibiotic treatment, oral azithromycin was administered to RIF/MDR-CE women resistant to the third-line moxifloxacin, and oral moxifloxacin was administered to RIF/MDR-CE women resistant to azithromycin. In the following cycle, the histopathologic cure of CE was achieved in five women, whereas these treatments failed for six women. MDR-CE that was resistant to these five different antibiotic agents was defined here as persistent MDR-CE.
Of them, five RIF women were finally treatable with oral lincomycin administration (1500 mg/day, 14 days). One RIF woman who was yet resistant to this antibiotic agent regrettably dropped out of the infertility treatment. In the cycles following confirmation of histopathologic cure of persistent MDR-CE, four out of five RIF women had a clinical pregnancy in the immediate vitrified-warmed blastocyst transfer cycle, whereas one woman resulted in a negative pregnancy test. While one out of four pregnant women resulted in an early pregnancy loss, three women had a successful live birth.
Lincomycin is a narrow spectrum lincosamide antibiotic agent that originates in
Streptomyces lincolnensis [
20]. The effectiveness of this antibiotic is estimated to be 19–38%. Lincomycin binds the bacterial 50 S ribosomal subunits, which inhibits protein synthesis and leads to bactericides. The macrolide agent clindamycin is a derivative of lincomycin, of which the 7-hydroxy group is replaced with a chlorine atom with inversed chirality. In common with clindamycin, lincomycin exerts the bioactivity and antibacterial spectrum against gram-positive and anaerobic bacteria and has been utilized for more than 50 years. Lincomycin further displays an antibiotic effect against some pathogens such as
Actinomycetes and
Mycoplasma, both of which potentially cause CE and are often resistant to macrolides [
21]. However, to the best knowledge of the authors, there have been no reports that proved the therapeutic effects of lincomycin against CE. As all of these women desired more cycles of antibiotic treatment, we chose and administered this agent against persistent MDR-CE under informed consent. The treatment resulted in favorable outcomes in the cure of histopathologic CE, although the mechanisms underlying the therapeutic effect of lincomycin remain unexplained. One possible explanation for the effect of lincomycin on our cases may be due to the rare use of and rather lower resistance against this antibiotic agent in clinical practice. In the immediate vitrified-warmed blastocyst transfer cycles, three out of five RIF women had a successful pregnancy that resulted in a live birth.
The potential biases of this study are as follows: (i) There are no control antibiotic agents to compare the effectiveness against persistent MDR-CE. The cautions are needed to determine if oral lincomycin is the best choice of antibiotic agent against this pathologic condition. (ii) The sample size is very small, although the findings warrant further investigations to validate the effectiveness and safety of this agent. (iii) Some women self-reported the use of oral and/or vaginal prebiotics and/or probiotics (freeze-dry Lactobacillus products and/or lactoferrin supplements) during the study period. These supplementations may have intervened in the results and helped the improvement of persistent MDR-CE in these women.
Despite these limitations, our findings suggest that oral lincomycin is an antibiotic agent worth listing in the line-up for the treatment against persistent MDR-CE. The effectiveness and safety of this agent should be compared with another antibiotic regimen.