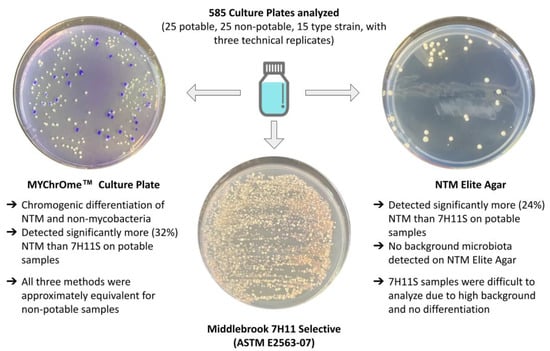
A Comparison of Three Culture Media for the Detection of Rapid-Growing Nontuberculous Mycobacteria in Environmental Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Type Strain Comparison
2.2. Water Sample Analysis
2.3. Colony Confirmation
2.4. Statistical Analysis
3. Results
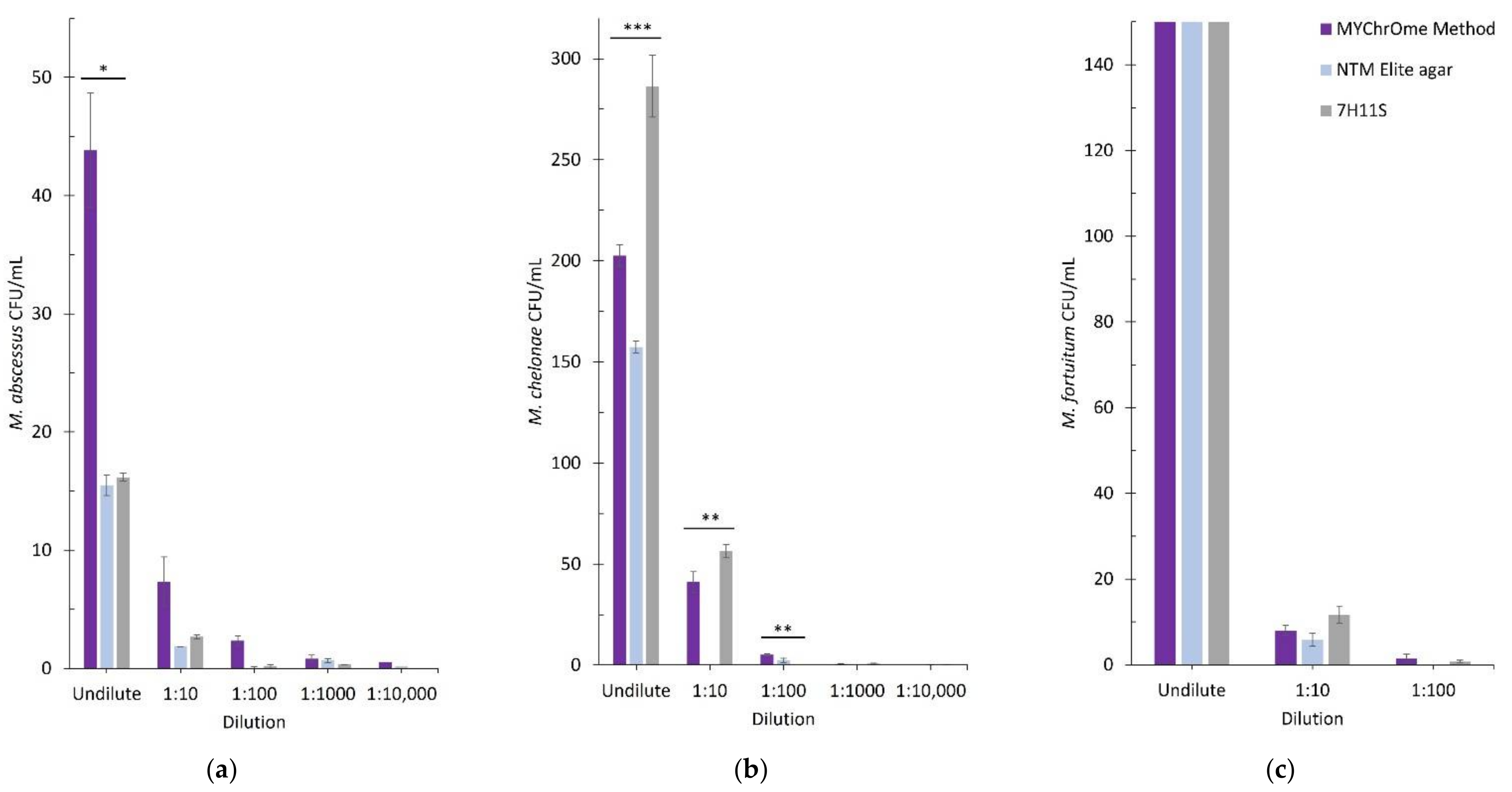
3.1. Type Strain Comparison
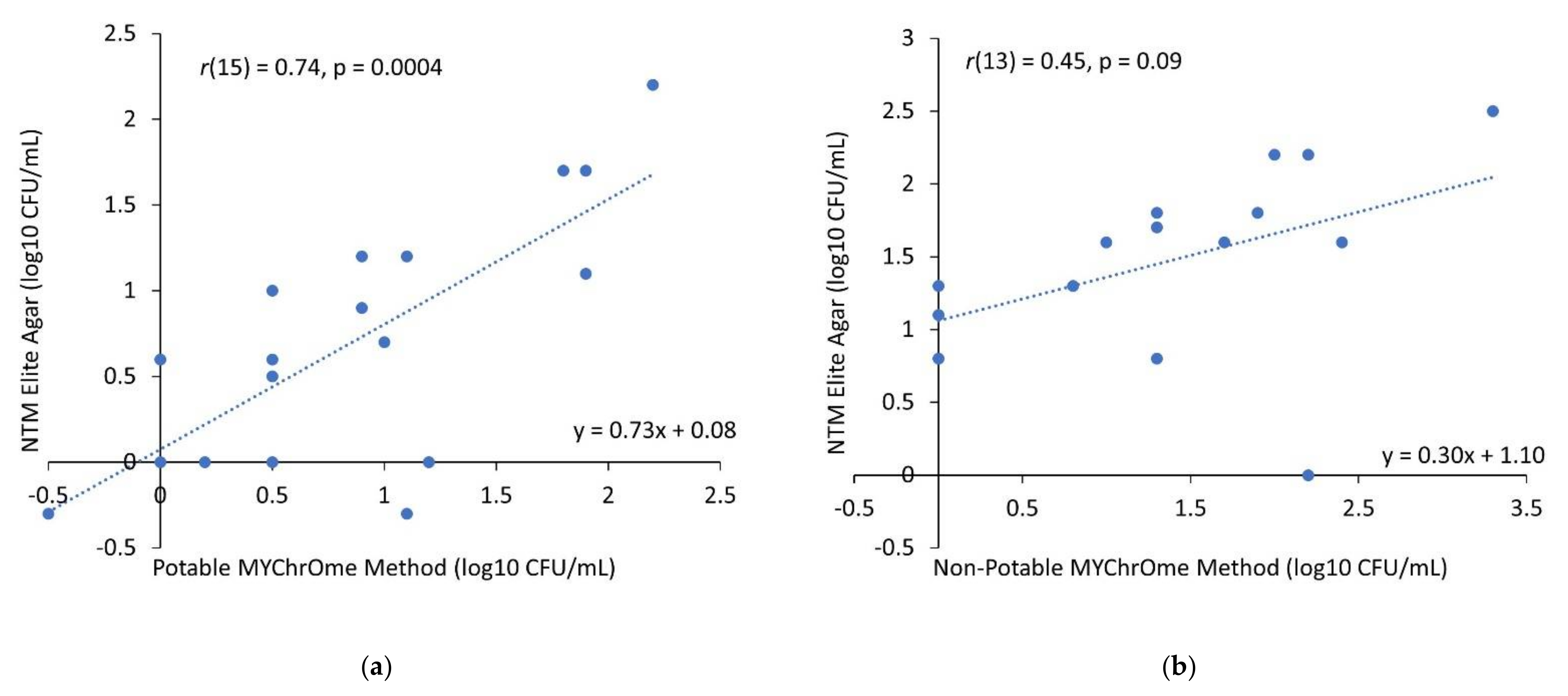
3.2. Potable Water
3.3. Non-Potable Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winthrop, K.; Marras, T.K.; Adjemian, J.; Zhang, H.; Wang, P.; Zhang, Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large, U.S. Managed Care Health Plan, 2008–2015. Ann. Am. Thorac. Soc. 2020, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Deng, L.; Adam, E.A.; Benedict, K.M.; Beshearse, E.M.; Blackstock, A.J.; Bruce, B.B.; Derado, G.; Edens, C.; Fullerton, K.E.; et al. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 2021, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, T.; Chimara, E.; do Prado, G.V.B.; Ferrazoli, L.; Carvalho, N.G.F.; dos Santos Simeão, F.C.; de Souza, A.R.; Costa, C.A.R.; Viana Niero, C.; Brianesi, U.A.; et al. Pseudooutbreak of rapidly growing mycobacteria due to Mycobacterium abscessus subsp bolletii in a digestive and respiratory endoscopy unit caused by the same clone as that of a countrywide outbreak. Am. J. Infect. Control 2016, 44, e221–e226. [Google Scholar] [CrossRef] [PubMed]
- Sommerstein, R.; Ruegg, C.; Kohler, P.; Bloemberg, G.; Kuster, S.P.; Sax, H. Transmission of Mycobacterium chimaera from Heater-Cooler Units during Cardiac Surgery despite an Ultraclean Air Ventilation System. Emerg. Infect. Dis. 2016, 22, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- El Sahly, H.M.; Septimus, E.; Soini, H.; Septimus, J.; Wallace, R.J.; Pan, X.; Williams-Bouyer, N.; Musser, J.M.; Graviss, E.A. Mycobacterium simiae pseudo-outbreak resulting from a contaminated hospital water supply in Houston, Texas. Clin. Infect. Dis. 2002, 35, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Conger, N.G.; O’Connell, R.J.; Laurel, V.L.; Olivier, K.N.; Graviss, E.A.; Williams-Bouyer, N.; Zhang, Y.; Brown-Elliott, B.A.; Wallace, R.J. Mycobacterium simiae pseudo-outbreak associated with a hospital water supply. Infect. Control Hosp. Epidemiol. 2004, 25, 1050–1055. [Google Scholar] [CrossRef]
- Scanlon, M.M.; Gordon, J.L.; McCoy, W.F.; Cain, M.F. Water Management for Construction: Evidence for Risk Characterization in Community and Healthcare Settings: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2168. [Google Scholar] [CrossRef] [Green Version]
- Falkinham, J.O. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg. Infect. Dis. 2011, 17, 419–424. [Google Scholar] [CrossRef]
- Tzou, C.L.; Dirac, M.A.; Becker, A.L.; Beck, N.K.; Weigel, K.M.; Meschke, J.S.; Cangelosi, G.A. Association between mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil a case-control study. Ann. Am. Thorac. Soc. 2020, 17, 57–62. [Google Scholar] [CrossRef]
- Pfaller, S.; King, D.; Mistry, J.H.; Alexander, M.; Abulikemu, G.; Pressman, J.G.; Wahman, D.G.; Donohue, M.J. Chloramine Concentrations within Distribution Systems and Their Effect on Heterotrophic Bacteria, Mycobacterial Species, and Disinfection Byproducts. Water Res. 2021, 205, 117689. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Wallace, R.J. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 2002, 15, 716–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, M.J. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect. Dis. 2018, 18, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strollo, S.E.; Adjemian, J.; Adjemian, M.K.; Prevots, D.R. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann. Am. Thorac. Soc. 2015, 12, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Bradner, L.; Robbe-Austerman, S.; Beitz, D.C.; Stabel, J.R. Chemical decontamination with N-acetyl-L-cysteine-sodium hydroxide improves recovery of viable Mycobacterium avium subsp. paratuberculosis organisms from cultured milk. J. Clin. Microbiol. 2013, 51, 2139–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradner, L.; Robbe-Austerman, S.; Beitz, D.C.; Stabel, J.R. Optimization of hexadecylpyridinium chloride decontamination for culture of mycobacterium avium subsp. paratuberculosis from milk. J. Clin. Microbiol. 2013, 51, 1575–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.D.; Falkinham, J.O. Effect of cetylpyridinium chloride (CPC) on colony formation of common nontuberculous mycobacteria. Pathogens 2018, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Preece, C.L.; Perry, A.; Gray, B.; Kenna, D.T.; Jones, A.L.; Cummings, S.P.; Robb, A.; Thomas, M.F.; Brodlie, M.; O’Brien, C.J.; et al. A novel culture medium for isolation of rapidly-growing mycobacteria from the sputum of patients with cystic fibrosis. J. Cyst. Fibros. 2015, 15, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K.E.; Chhabra, A.K.; Wickenberg, L.P.; McCoy, W.F. Validation of the MYChrOmeTM Culture Plate for Detection and Differentiation of Rapid-Growing Nontuberculous Mycobacteria in potable and non-potable water: AOAC Performance Tested MethodSM 062101. J. Aoac Int. 2021, 5, 549–557. [Google Scholar] [CrossRef]
- Preece, C.L.; Wichelhaus, T.A.; Perry, A.; Jones, A.L.; Cummings, S.P.; Perry, J.D.; Hogardt, M. Evaluation of various culture media for detection of rapidly growing mycobacteria from patients with cystic fibrosis. J. Clin. Microbiol. 2016, 54, 1797–1803. [Google Scholar] [CrossRef] [Green Version]
- Alexander, K.J.; Furlong, J.L.; Id, J.L.B.; Rihs, J.D.; Stephenson, D.; Id, J.D.P.; Stout, J.E. Evaluation of a new culture medium for isolation of nontuberculous mycobacteria from environmental water samples. PLoS ONE 2021, 16, e0247166. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Garlasco, J.; Curtoni, A.; Iannaccone, M.; Zotti, C.M. Chemical susceptibility testing of non-tuberculous mycobacterium strains and other aquatic bacteria: Results of a study for the development of a more sensitive and simple method for the detection of NTM in environmental samples. J. Microbiol. Methods 2022, 193, 106405. [Google Scholar] [CrossRef] [PubMed]
- Radomski, N.; Roguet, A.; Lucas, F.S.; Veyrier, F.J.; Cambau, E.; Accrombessi, H.; Moilleron, R.; Behr, M.A.; Moulin, L. atpE gene as a new useful specific molecular target to quantify Mycobacterium in environmental samples. BMC Microbiol. 2013, 13, 1471–2180. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O. Surrounded by mycobacteria: Nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 2009, 107, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Biomerieux: NTM Elite Agar. Available online: https://www.biomerieux-diagnostics.com/ntm-elite-agar (accessed on 1 March 2022).
- Jones, J.J.; Falkinham, J.O. Decolorization of malachite green and crystal violet by waterborne pathogenic mycobacteria. Antimicrob. Agents Chemother. 2003, 47, 2323–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedley, S.; Bartram, J.; Rees, G.; Dufour, A.; Cotruvo, J.A. Pathogenic Mycobacteria in Water: A Guide to Public Health Consequences, Monitoring and Management; IWA Publishing: London, UK, 2004; pp. 174–175. ISBN 1843390590. [Google Scholar]
- Halstrom, S.; Price, P.; Thomson, R. Review: Environmental mycobacteria as a cause of human infection. Int. J. Mycobacteriol. 2015, 4, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Avg CFU a/mL Potable | Avg CFU/mL Non-Potable | |||||
|---|---|---|---|---|---|---|
| Sample Number | MYChrOme Method | NTM Elite | 7H11S | MYChrOme Method | NTM Elite | 7H11S |
| 1 | ND | ND | ND | ND | ND | ND |
| 2 | 8.2 | 8.5 | 0.2 | 16.7 | 6.7 | 7.2 |
| 3 | 13.5 | 0.5 | 0.5 | 166.7 | 146.7 | 88.8 |
| 4 | 500.0 | 500.0 | 500.0 | 96.7 | 170.0 | 181.0 |
| 5 | 143.3 | 157.3 | 250.5 | ND | 6.7 | 0.7 |
| 6 | ND | ND | ND | 3.3 | 20.0 | ND |
| 7 | 7.3 | 16.5 | 25.2 | 2093.3 | 320.0 | 500.0 |
| 8 | ND | ND | ND | 10.0 | 40.0 | 7.2 |
| 9 | ND | ND | ND | ND | 3.3 | 6.8 |
| 10 | 80.3 | 54.8 | 24.8 | ND | ND | 0.2 |
| 11 | 3.5 | 3.7 | ND | ND | ND | 0.3 |
| 12 | 1.2 | 4.0 | ND | 80.0 | 63.3 | 26.7 |
| 13 | 3.3 | 10.3 | 5.5 | 46.7 | 36.7 | 50.5 |
| 14 | 14.5 | 0.2 | 0.5 | 163.3 | ND | 5.0 |
| 15 | ND | ND | 0.2 | ND | 3.3 | 1.0 |
| 16 | 3.5 | ND | ND | 236.7 | 43.3 | 22.3 |
| 17 | ND | ND | ND | 6.7 | 20.0 | 9.7 |
| 18 | 1.7 | 0.8 | ND | 16.7 | 53.3 | 26.5 |
| 19 | 13.7 | 15.8 | 15.2 | 16.7 | 66.7 | 3.8 |
| 20 | 10.5 | 5.0 | ND | ND | ND | ND |
| 21 | 3.2 | 3.8 | ND | ND | ND | 0.5 |
| 22 | 56.7 | 49.5 | 3.3 | ND | ND | 0.2 |
| 23 | ND | ND | ND | ND | 13.3 | 18.5 |
| 24 | 78.0 | 11.7 | 0.5 | ND | ND | 0.3 |
| 25 | 0.3 | 0.5 | ND | ND | ND | 2.3 |
| Positives | 18 | 17 | 12 | 13 | 16 | 22 |
| Mean b | 52.4 | 49.6 | 68.9 | 227.2 | 63.3 | 43.6 |
| SD c | 118.3 | 122.3 | 153.0 | 565.7 | 84.0 | 110.0 |
| SEM d | 27.9 | 29.7 | 44.2 | 156.9 | 21.0 | 23.4 |
| MYChrOme Method | NTM Elite Agar | |||||
|---|---|---|---|---|---|---|
| Pos a | ND b | Pos | ND | PA c | K d | |
| Potable | 18 | 7 | 17 | 8 | 96% | 0.905 |
| Non-Potable | 13 | 12 | 16 | 9 | 80% | 0.595 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, K.E.; Chhabra, A.K.; Wickenberg, L.P.; McCoy, W.F. A Comparison of Three Culture Media for the Detection of Rapid-Growing Nontuberculous Mycobacteria in Environmental Samples. Appl. Microbiol. 2022, 2, 347-356. https://doi.org/10.3390/applmicrobiol2020026
Fisher KE, Chhabra AK, Wickenberg LP, McCoy WF. A Comparison of Three Culture Media for the Detection of Rapid-Growing Nontuberculous Mycobacteria in Environmental Samples. Applied Microbiology. 2022; 2(2):347-356. https://doi.org/10.3390/applmicrobiol2020026
Chicago/Turabian StyleFisher, Katherine E., Avneet K. Chhabra, Leah P. Wickenberg, and William F. McCoy. 2022. "A Comparison of Three Culture Media for the Detection of Rapid-Growing Nontuberculous Mycobacteria in Environmental Samples" Applied Microbiology 2, no. 2: 347-356. https://doi.org/10.3390/applmicrobiol2020026
APA StyleFisher, K. E., Chhabra, A. K., Wickenberg, L. P., & McCoy, W. F. (2022). A Comparison of Three Culture Media for the Detection of Rapid-Growing Nontuberculous Mycobacteria in Environmental Samples. Applied Microbiology, 2(2), 347-356. https://doi.org/10.3390/applmicrobiol2020026