Isolation and Characterization of Nitrate Reducing Bacteria for Conversion of Vegetable-Derived Nitrate to ‘Natural Nitrite’
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures and Media
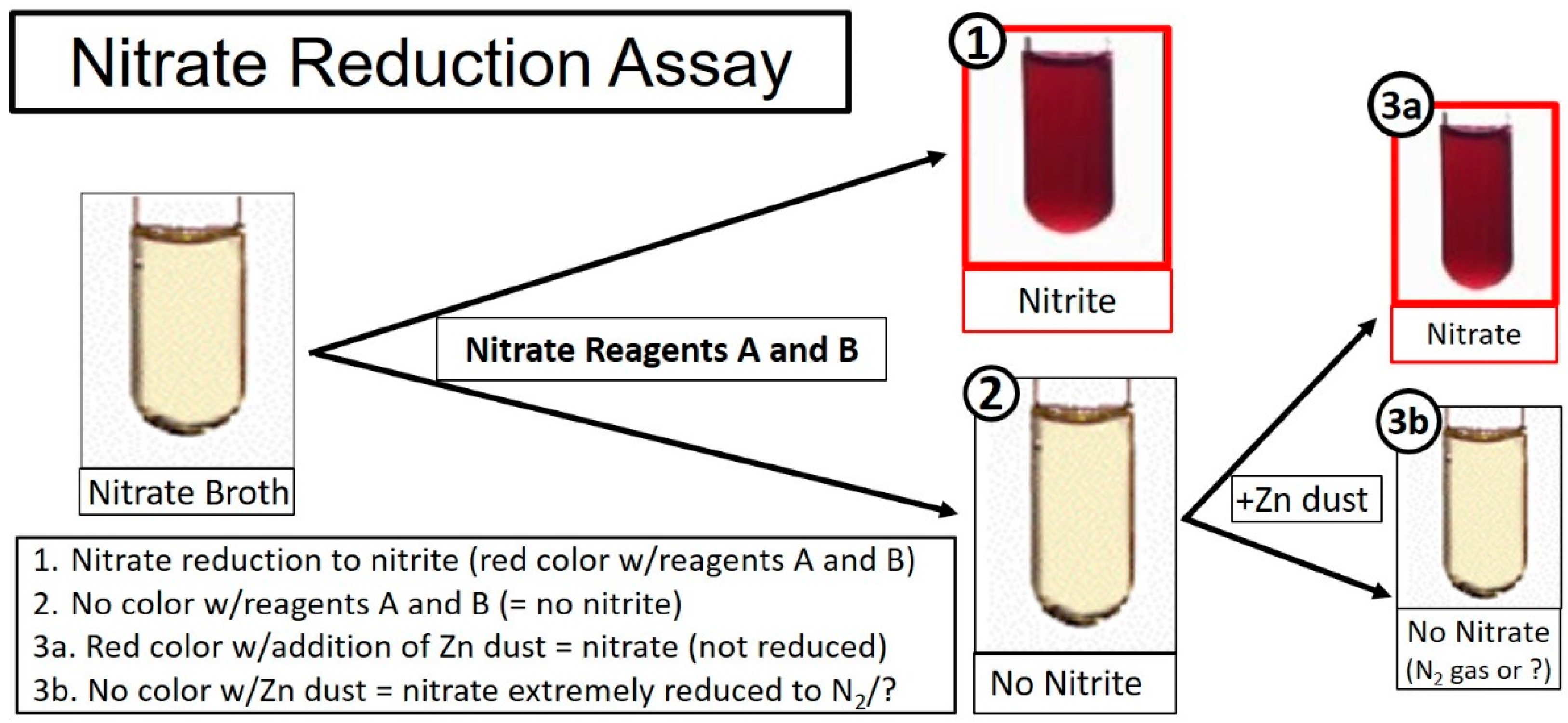
2.2. The Nitrate Reduction Test (in Broth)
- Nitrate reagent A: sulfanilic acid (Fisher); 1 g in 200 mL of 5 N acetic acid,
- Nitrate reagent B: alpha-napthylamine (Fisher); 2 g in 250 mL of 5 N acetic acid,
- Reagent C: Zinc powder (50 mg; Fisher).
2.3. The Nitrate Reduction Assay (on Agar)
2.4. Isolation and Identification of Nitrate Reducing Bacteria
2.5. Vegetable Juice Extraction and Nitrate Detection
2.6. Fermentation Using Nitrate-Reducing Bacterial Isolates
2.7. Quantitation of Nitrate and Nitrite Using High Performance Liquid Chromatography (HPLC)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Nitrate Reduction Assay (Liquid) Using Modified M17-Nitrate Broth
3.2. The Nitrite Assay Modified for On-Agar Use to Screen Bacterial Samples from Foods and Animals
3.3. Identification of Nitrate Reducing Bacteria by 16S rRNA DNA Sequencing
3.4. HPLC Analysis of Nitrate and Nitrite in Vegetable Juices, Nitrate Broth, and after Bacterial Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brkić, D.; Bošnir, J.; Bevardi, M.; Bošković, A.G.; Miloš, S.; Lasić, D.; Krivohlavek, A.; Racz, A.; Ćuić, A.M.; Trstenjak, N.U. Nitrate in leafy green vegetables and estimated intake. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 31–41. [Google Scholar]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. EFSA panel on food additives nutrient sources added to food: Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA Jour. 2017, 15, e04786. [Google Scholar]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. EFSA panel on food additives nutrient sources added to food: Re-evaluation of sodium nitrate (E 251) and potassium nitrate (E 252) as food additives. EFSA Jour. 2017, 15, e04787. [Google Scholar]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products - Invited review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate or nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef]
- Jo, K.; Lee, S.; Yong, H.I.; Choi, Y.-S.; Jung, S. Nitrite sources for cured meat products. LWT 2020, 129, 109583. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Jackson-Davis, A.L.; Myers, K.L.; Lavieri, N.A. Beyond celery and starter culture: Advances in natural/organic curing processes in the United States. Meat Sci. 2012, 92, 267–273. [Google Scholar] [CrossRef]
- Sullivan, G.A.; Jackson-Davis, A.L.; Niebuhr, S.E.; Xi, Y.; Schrader, K.D.; Sebranek, J.G.; Dickson, J.S. Inhibition of Listeria monocytogenes using natural antimicrobials in no-nitrate-or-nitrite-added ham. J. Food Prot. 2012, 75, 1071–1076. [Google Scholar] [CrossRef]
- Djeri, N.; Williams, S.K. Celery juice powder used as nitrite substitute in sliced vacuum-packaged turkey bologna stored at 4C for 10 weeks under retail display light. J. Food Qual. 2014, 37, 361–370. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Azizi, F.; Ghasemi, A.; Hadaegh, F. Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J. Food Compos. Anal. 2016, 51, 93–105. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kim, J.S.; Kim, M.; Hong, M.K.; Lee, J.O.; Kim, C.M.; Song, I.S. Survey of nitrate and nitrite contents of vegetables grown in Korea. Food Additive Contamination 2003, 20, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.M.; Park, J.H.; Yoon, K.S. Nitrite formation from vegetable sources and its use as a preservative in cooked sausage. J. Sci. Food Agric. 2017, 97, 1774–1783. [Google Scholar] [CrossRef]
- Mcdonnell, L.M.; Glass, K.A.; Sindelar, J.J. Identifying ingredients that delay outgrowth of Listeria monocytogenes in natural, organic, and clean-label ready-to-eat meat and poultry products. J. Food Prot. 2013, 76, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.E.; Kim, T.K.; Kim, H.W.; Seo, D.H.; Kim, Y.B.; Jeon, K.H.; Choi, Y.S. Effect of natural pre-converted nitrite sources on color development in raw and cooked pork sausage. Asian-Australas J. Anim. Sci. 2018, 31, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Blaiotta, G.; Mauriello, G.; Pepe, O.; Villani, F. Technological activities of Staphylococcus carnosus and Staphylococcus simulans strains isolated from fermented sausages. Meat Sci. 2005, 71, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R. Nitrate and nitrite reduction test protocols. In Laboratory Protocols; American Society for Microbiology: Washington, DC, USA, 2011; Available online: https://www.asmscience.org/content/education/protocol/protocol.3660 (accessed on 5 March 2021).
- Henning, C.; Vijayakumar, P.; Adhikari, R.; Jagannathan, B.; Gautam, D.; Muriana, P.M. Isolation and taxonomic identity of bacteriocin-producing lactic acid bacteria from retail foods and animal sources. Microorganisms 2015, 3, 80–93. [Google Scholar] [CrossRef]
- Garver, K.I.; Muriana, P.M. Detection, identification and characterization of bacteriocin-producing lactic acid bacteria from retail food products. Int. J. Food Microbiol. 1993, 20, 241–258. [Google Scholar] [CrossRef]
- Kang, D.H.; Fung, D.Y.C. Application of thin agar layer method for recovery of injured Salmonella Typhimurium. Int. J. Food Microbiol. 2000, 54, 127–132. [Google Scholar] [CrossRef]
- Wu, V.C.H.; Fung, D.Y.C.; Kang, D.H.; Thompson, L.K. Evaluation of thin agar layer method for recovery of acid-injured foodborne pathogens. J. Food Prot. 2001, 64, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Pitulle, C.; Citron, D.M.; Bochner, B.; Barbers, R.; Appleman, M.D. Novel bacterium isolated from a lung transplant patient with cystic fibrosis. J. Clin. Microbiol. 1999, 37, 3851–3855. [Google Scholar] [CrossRef]
- Luna, R.A.; Fasciano, L.R.; Jones, S.C.; Boyanton, B.L.; Ton, T.T.; Versalovic, J. DNA pyrosequencing-based bacterial pathogen identification in a pediatric hospital setting. J. Clin. Microbiol. 2007, 45, 2985. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, M.C.; Bertolo, P.L. Simultaneous determination of nitrites, nitrates and amines by ion-interaction reversed-phase high-performance liquid chromatography: Application to lagoon water. J. Chromatogr. A 1990, 509, 147–156. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Silva, S. Quantification of residual nitrite and nitrate in ham by reverse-phase high performance liquid chromatography/diode array detector. Talanta 2008, 74, 1598–1602. [Google Scholar] [CrossRef]
- Chou, S.S.; Chung, J.C.; Hwang, D.F. A high performance liquid chromatography method for determining nitrate and nitrite levels in vegetables. J. Food Drug Anal. 2003, 11, 233–238. [Google Scholar] [CrossRef]
- Cheng, C.F.; Tsang, C.W. Simultaneous determination of nitrite, nitrate and ascorbic acid in canned vegetable juices by reverse-phase ion-interaction HPLC. Food Addit Contam 1998, 15, 753–758. [Google Scholar] [CrossRef]
- Najdenkoska, A. Development of HPLC method for analysis of nitrite and nitrate in vegetable. JAFES 2016, 67, 33–39. [Google Scholar]
- Brown, J.N.; Hewins, M.; Van Der Linden, J.H.M.; Lynch, R.J. Solvent degassing and other factors affecting liquid chromatographic detector stability. J. Chromatogr. A 1981, 204, 115–122. [Google Scholar] [CrossRef]
- Hongsibsong, S.; Poliem, W.; Narksen, W.; Kerdnoi, T.; Prapamontol, T. Determination of nitrate in the edible part of vegetables from markets around Chiang Mai City, northern Thailand by using high performance liquid chromatography. Asian J. Agricult. Res. 2014, 8, 7. [Google Scholar]
- López-Gutiérrez, J.C.; Henry, S.; Hallet, S.; Martin-Laurent, F.; Catroux, G.; Philippot, L. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 2004, 57, 399–407. [Google Scholar] [CrossRef]
- Cook, G.T. A plate test for nitrate reduction. J. Clin. Pathol. 1950, 3, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, B.G.; Rutter, M.; Nedwell, D.B. Selection by temperature of nitrate-reducing bacteria from estuarine sediments: Species composition and competition for nitrate. FEMS Microbiol. Ecol. 1997, 23, 11–22. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, B.; Morrill, P.L.; Husain, T. Isolation of nitrate-reducing bacteria from an offshore reservoir and the associated biosurfactant production. RSC Adv. 2018, 8, 26596–26609. [Google Scholar] [CrossRef]
- Morin, A. Pantoea. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 1028–1032. [Google Scholar]
- Morin, A.; Parveen, Z. Pantoea. In Encyclopedia of Food Microbiology; Robinson, R.K., Ed.; Elsevier: Oxford, UK, 1999; pp. 1623–1630. [Google Scholar]
- Connor, P.M.; Shea, E.F.; Guinane, C.M.; Sullivan, O.; Cotter, P.D.; Ross, R.P.; Hill, C. Nisin H is a new nisin variant produced by the gut-derived strain Streptococcus hyointestinalis DPC6484. Appl. Environ. Microbiol. 2015, 81, 3953. [Google Scholar] [CrossRef]
- Kavitha, S.; Harikrishnan, A.; Jeevaratnam, K. Characterization and evaluation of antibacterial efficacy of a novel antibiotic-type compound from a probiotic strain Lactobacillus plantarum KJB23 against foodborne pathogens. LWT 2020, 118, 108759. [Google Scholar] [CrossRef]
- Kuleaşan, H.; Çakmakçı, M.L. Effect of reuterin, produced by Lactobacillus reuteri on the surface of sausages to inhibit the growth of Listeria monocytogenes and Salmonella spp. Food/Nahrung 2002, 46, 408–410. [Google Scholar] [CrossRef]
- Magrinyà, N.; Bou, R.; Rius, N.; Codony, R.; Guardiola, F. Effect of fermentation time and vegetable concentrate addition on quality parameters of organic botifarra catalana, a cured–cooked sausage. J. Agric. Food Chem. 2012, 60, 6882–6890. [Google Scholar] [CrossRef]
- Neubauer, H.; Götz, F. Physiology and interaction of nitrate and nitrite reduction in Staphylococcus carnosus. J. Bacteriol. 1996, 178, 2005–2009. [Google Scholar] [CrossRef]




| Isolates | Source |
|---|---|
| Clostridium bifermentums P-5 | FAPC culture collection |
| Clostridium bifermentums P-42 | FAPC culture collection |
| Escherichia coli 309-7 | Hog small intestinal sample |
| Escherichia coli 69 | Hog small intestinal sample |
| Escherichia coli NCYU-26-73 | Hog small intestinal sample |
| Escherichia fergusonii Z6 | Hog small intestinal sample |
| Escherichia coli PL-AGW6 | Hog small intestinal sample |
| Escherichia coli F9792 | Hog small intestinal sample |
| Lactobacillus plantarum ML811 | FAPC culture collection |
| Lactobacillus reuteri PIG1-2 | FAPC culture collection |
| Lactobacillus reuteri PIG1-3 | FAPC culture collection |
| Lactobacillus reuteri PIG3-1 | FAPC culture collection |
| Pantoea agglomerans Lett1 | Food sample (iceberg lettuce) |
| Shigella flexneri SFL1520 | Hog small intestinal sample |
| Staphylococcus caprae Cab1 | Food sample (white cabbage) |
| Streptococcus hyointestinalis 1336 | Hog small intestine sample |
| Streptococcus hyointestinalis 1340 | Hog small intestine sample |
| Staphylococcus carnosum | Control strain (FAPC culture collection) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhusal, A.; Muriana, P.M. Isolation and Characterization of Nitrate Reducing Bacteria for Conversion of Vegetable-Derived Nitrate to ‘Natural Nitrite’. Appl. Microbiol. 2021, 1, 11-23. https://doi.org/10.3390/applmicrobiol1010002
Bhusal A, Muriana PM. Isolation and Characterization of Nitrate Reducing Bacteria for Conversion of Vegetable-Derived Nitrate to ‘Natural Nitrite’. Applied Microbiology. 2021; 1(1):11-23. https://doi.org/10.3390/applmicrobiol1010002
Chicago/Turabian StyleBhusal, Arjun, and Peter M. Muriana. 2021. "Isolation and Characterization of Nitrate Reducing Bacteria for Conversion of Vegetable-Derived Nitrate to ‘Natural Nitrite’" Applied Microbiology 1, no. 1: 11-23. https://doi.org/10.3390/applmicrobiol1010002
APA StyleBhusal, A., & Muriana, P. M. (2021). Isolation and Characterization of Nitrate Reducing Bacteria for Conversion of Vegetable-Derived Nitrate to ‘Natural Nitrite’. Applied Microbiology, 1(1), 11-23. https://doi.org/10.3390/applmicrobiol1010002







