RGB-Derived Indices Accurately Detect Genotypic and Agronomic Differences in Canopy Variation in Durum Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Field Trials
2.2. Phenotyping Measurements
2.2.1. Ground Phenotyping Measurements
2.2.2. Aerial Platform Description and RGB-Derived Vegetation Indexes
2.3. Yield and Protein Content Prediction
2.4. Statistical Analyses
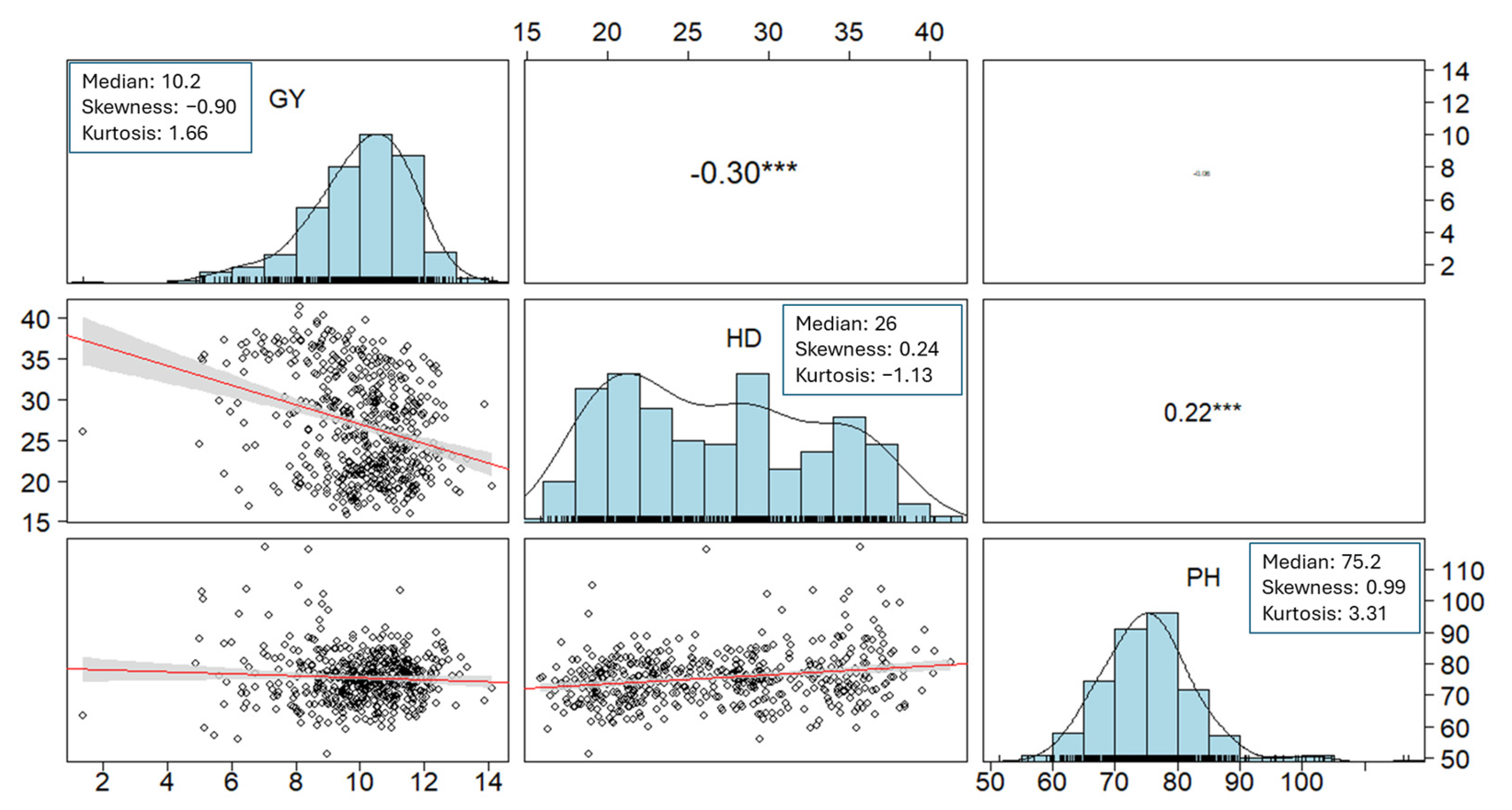
3. Results
3.1. Experiment 1
3.1.1. Germplasm Survey and Genotypic Differences
3.1.2. Temporal Patterns and Discrimination Power of RGB-Derived Vegetation Indices
3.1.3. Multivariate Analysis and Selection of Contrasting Genotypes
3.2. Experiment 2
3.2.1. Effects of Management on VIs: Sowing Rate and N Fertilization
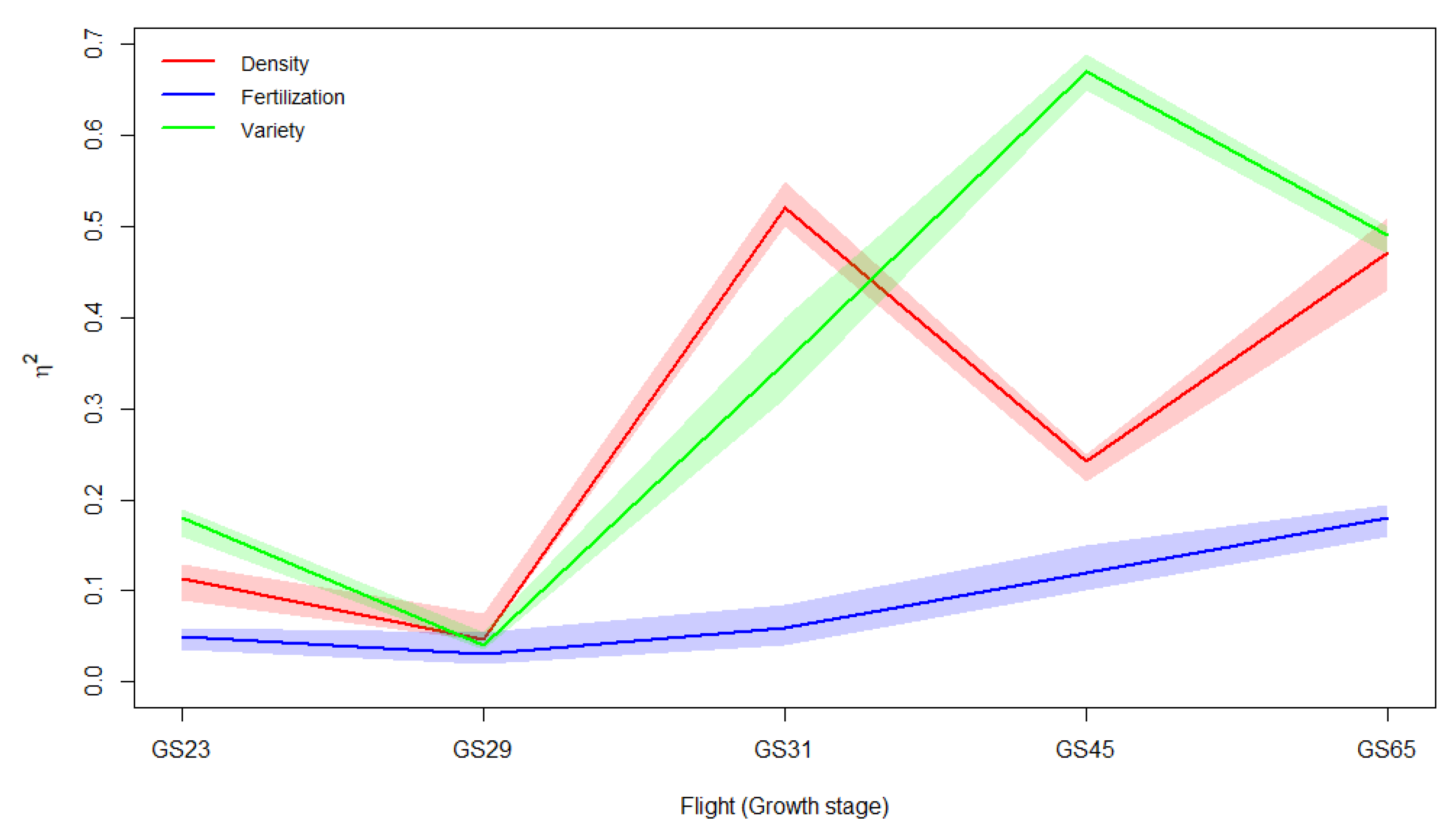
3.2.2. Main Effects of Genotype, Sowing Rate, and Nitrogen Dose
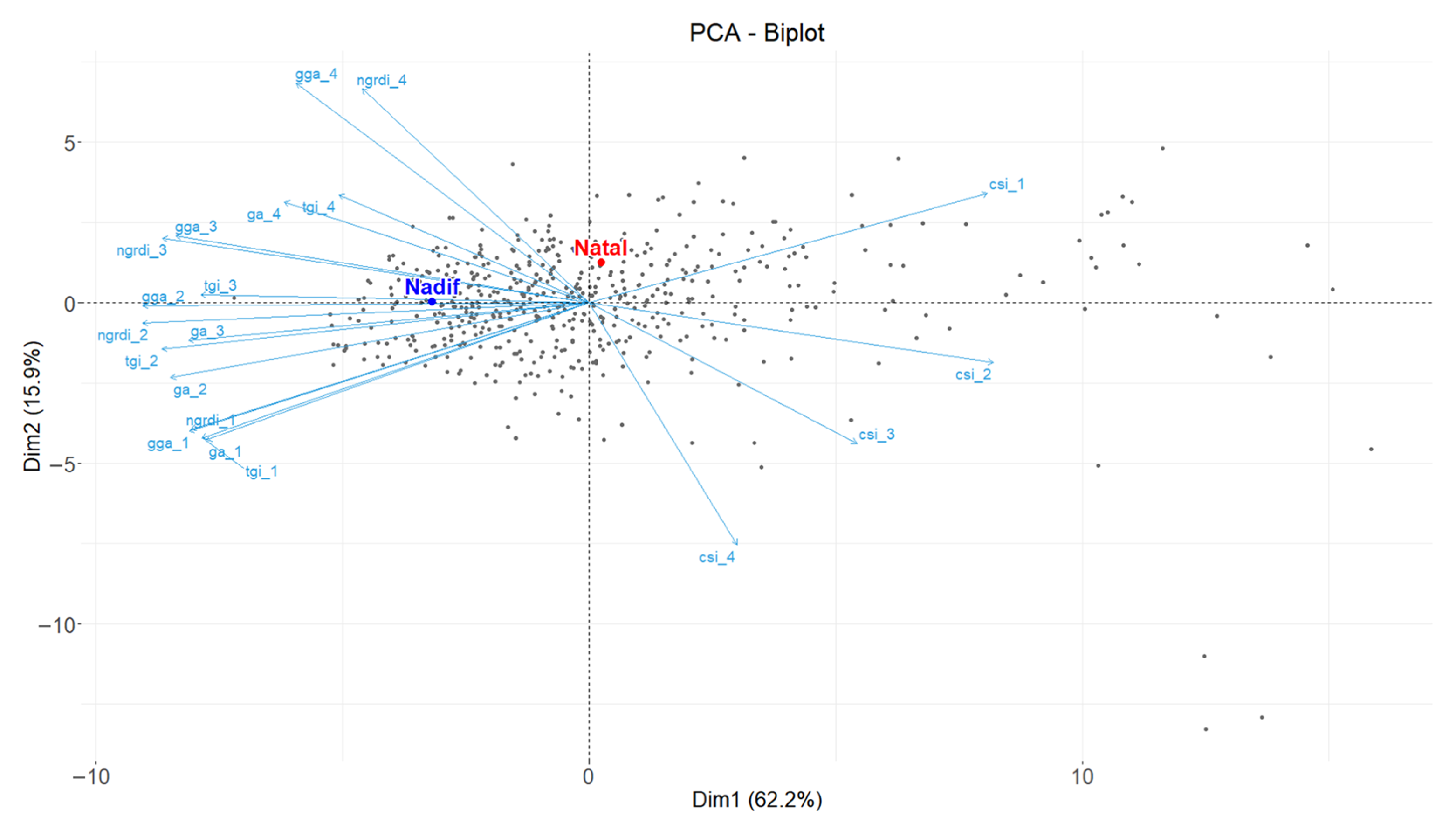
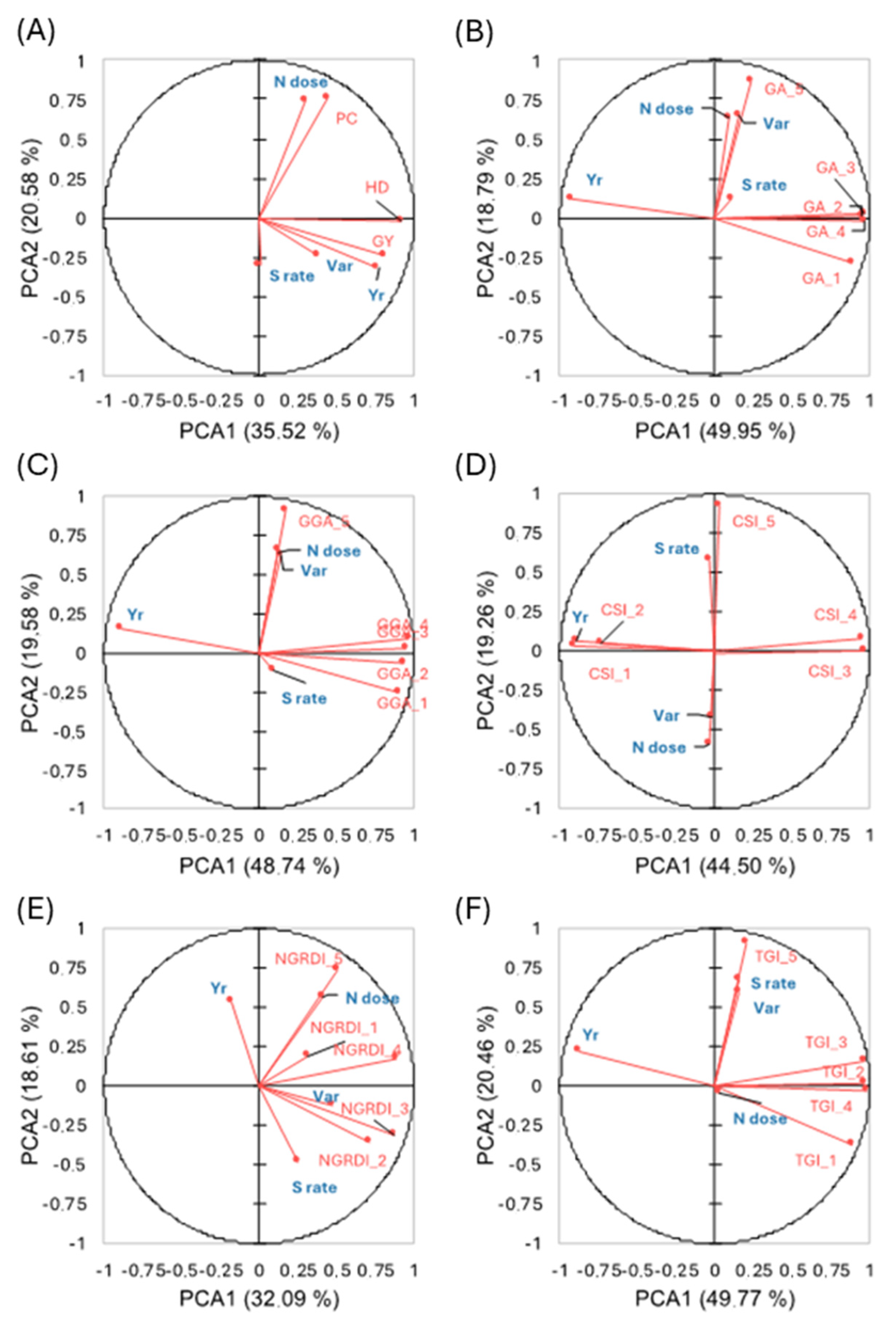
3.2.3. Heritability and Correlation Analyses and PCA of Vegetation Indices
3.2.4. Yield and Protein Prediction
4. Discussion
4.1. Assessment of Soil Coverage Using RGB-Derived Indices
4.2. Influence of Agronomic Management Practices
4.3. Environmental Modulation of VI Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UAV | Unmanned Aerial Vehicle |
| GA | Green Area |
| GGA | Greener Area |
| NGRDII | Normalized Green Red Difference Index |
| TGI | Triangular Greenness Index |
| CSI | Crop Senescence Index |
| VI | Vegetation index |
| QTL | Quantitative Trait Loci |
| HD | Heading Date |
| PH | Plant Height |
| GSD | Ground Sample Distance |
References
- De Vita, P.; Taranto, F. Durum Wheat (Triticum turgidum ssp. durum) Breeding to Meet the Challenge of Climate Change. In Advances in Plant Breeding Strategies: Cereals; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 5, pp. 471–524. ISBN 978-3-030-23108-8. [Google Scholar]
- Ferrise, R.; Moriondo, M.; Bindi, M. Probabilistic assessments of climate change impacts on durum wheat in the Mediterranean region. Nat. Hazards Earth Syst. Sci. 2011, 11, 1293–1302. [Google Scholar] [CrossRef]
- UnNisa, Z.; Govind, A.; Marchetti, M.; Lasserre, B. A review of crop water productivity in the Mediterranean basin under a changing climate: Wheat and barley as test cases. Irrig. Drain. 2022, 71, 51–70. [Google Scholar] [CrossRef]
- Ventrella, D.; Charfeddine, M.; Moriondo, M.; Rinaldi, M.; Bindi, M. Agronomic adaptation strategies under climate change for winter durum wheat and tomato in southern Italy: Irrigation and nitrogen fertilization. Reg. Environ. Change 2012, 12, 407–419. [Google Scholar] [CrossRef]
- Richards, R.A.; Lukacs, Z. Seedling vigour in wheat—Sources of variation for genetic and agronomic improvement. Aust. J. Agric. Res. 2002, 53, 41–50. [Google Scholar] [CrossRef]
- Beres, B.L.; Rahmani, E.; Clarke, J.M.; Grassini, P.; Pozniak, C.J.; Geddes, C.M.; Porker, K.D.; May, W.E.; Ransom, J.K. A Systematic Review of Durum Wheat: Enhancing Production Systems by Exploring Genotype, Environment, and Management (G × E × M) Synergies. Front. Plant Sci. 2020, 11, 568657. [Google Scholar] [CrossRef]
- Hendriks, P.-W.; Gurusinghe, S.; Weston, P.A.; Ryan, P.R.; Delhaize, E.; Weston, L.A.; Rebetzke, G.J. Introgression of early shoot vigour in wheat modifies root systems, increases competitiveness and provides options for integrated weed management. Plant Soil 2024, 504, 717–736. [Google Scholar] [CrossRef]
- Hendriks, P.-W.; Gurusinghe, S.; Ryan, P.R.; Rebetzke, G.J.; Weston, L.A. Competitiveness of Early Vigour Wheat (Triticum aestivum L.) Genotypes Is Established at Early Growth Stages. Agronomy 2022, 12, 377. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum Wheat Breeding in the Mediterranean Region: Current Status and Future Prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Vadez, V.; Grondin, A.; Chenu, K.; Henry, A.; Laplaze, L.; Millet, E.J.; Carminati, A. Crop traits and production under drought. Nat. Rev. Earth Environ. 2024, 5, 211–225. [Google Scholar] [CrossRef]
- Pérez-Méndez, N.; Miguel-Rojas, C.; Jimenez-Berni, J.A.; Gomez-Candon, D.; Pérez-de-Luque, A.; Fereres, E.; Catala-Forner, M.; Villegas, D.; Sillero, J.C. Plant Breeding and Management Strategies to Minimize the Impact of Water Scarcity and Biotic Stress in Cereal Crops under Mediterranean Conditions. Agronomy 2022, 12, 75. [Google Scholar] [CrossRef]
- Nelsen, T.S.; Hegarty, J.; Tamagno, S.; Lundy, M.E. NDVI-based ideotypes as a cost-effective tool to support wheat yield stability selection under heterogeneous environments. Field Crops Res. 2025, 322, 109727. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Richards, R.A. Genetic improvement of early vigour in wheat. Aust. J. Agric. Res. 1999, 50, 291. [Google Scholar] [CrossRef]
- Soltani, A.; Galeshi, S. Importance of rapid canopy closure for wheat production in a temperate sub-humid environment: Experimentation and simulation. Field Crops Res. 2002, 77, 17–30. [Google Scholar] [CrossRef]
- Wilson, P.B.; Rebetzke, G.J.; Condon, A.G. Of growing importance: Combining greater early vigour and transpiration efficiency for wheat in variable rainfed environments. Funct. Plant Biol. 2015, 42, 1107. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Ludwig, F.; Asseng, S. Potential benefits of early vigor and changes in phenology in wheat to adapt to warmer and drier climates. Agric. Syst. 2010, 103, 127–136. [Google Scholar] [CrossRef]
- Mullan, D.J.; Reynolds, M.P. Quantifying genetic effects of ground cover on soil water evaporation using digital imaging. Funct. Plant Biol. 2010, 37, 703–712. [Google Scholar] [CrossRef]
- Zhao, Z.; Rebetzke, G.J.; Zheng, B.; Chapman, S.C.; Wang, E. Modelling impact of early vigour on wheat yield in dryland regions. J. Exp. Bot. 2019, 70, 2535–2548. [Google Scholar] [CrossRef]
- Devi, J.M.; Sinclair, T.R.; Chen, P.; Carter, T.E. Evaluation of Elite Southern Maturity Soybean Breeding Lines for Drought-Tolerant Traits. Agron. J. 2014, 106, 1947–1954. [Google Scholar] [CrossRef]
- Lobell, D.B.; Hammer, G.L.; McLean, G.; Messina, C.; Roberts, M.J.; Schlenker, W. The critical role of extreme heat for maize production in the United States. Nat. Clim. Change 2013, 3, 497–501. [Google Scholar] [CrossRef]
- Messina, C.D.; Sinclair, T.R.; Hammer, G.L.; Curan, D.; Thompson, J.; Oler, Z.; Gho, C.; Cooper, M. Limited-Transpiration Trait May Increase Maize Drought Tolerance in the US Corn Belt. Agron. J. 2015, 107, 1978–1986. [Google Scholar] [CrossRef]
- Schoppach, R.; Sadok, W. Differential sensitivities of transpiration to evaporative demand and soil water deficit among wheat elite cultivars indicate different strategies for drought tolerance. Environ. Exp. Bot. 2012, 84, 1–10. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Hammer, G.L.; van Oosterom, E.J. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Funct. Plant Biol. 2005, 32, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.B.; Rebetzke, G.J.; Condon, A.G. Pyramiding greater early vigour and integrated transpiration efficiency in bread wheat; trade-offs and benefits. Field Crops Res. 2015, 183, 102–110. [Google Scholar] [CrossRef]
- Liao, M.; Fillery, I.R.P.; Palta, J.A. Early vigorous growth is a major factor influencing nitrogen uptake in wheat. Funct. Plant Biol. 2004, 31, 121–129. [Google Scholar] [CrossRef]
- Pang, J.; Palta, J.A.; Rebetzke, G.J.; Milroy, S.P. Wheat genotypes with high early vigour accumulate more nitrogen and have higher photosynthetic nitrogen use efficiency during early growth. Funct. Plant Biol. 2013, 41, 215–222. [Google Scholar] [CrossRef]
- Li, G.; Bai, G.; Carver, B.F.; Elliott, N.C.; Bennett, R.S.; Wu, Y.; Hunger, R.; Bonman, J.M.; Xu, X. Genome-wide association study reveals genetic architecture of coleoptile length in wheat. Theor. Appl. Genet. 2017, 130, 391–401. [Google Scholar] [CrossRef]
- Sharma, D.; Kumari, A.; Sharma, P.; Singh, A.; Sharma, A.; Mir, Z.A.; Kumar, U.; Jan, S.; Parthiban, M.; Mir, R.R.; et al. Meta-QTL analysis in wheat: Progress, challenges and opportunities. Theor. Appl. Genet. 2023, 136, 247. [Google Scholar] [CrossRef]
- Vukasovic, S.; Alahmad, S.; Christopher, J.; Snowdon, R.J.; Stahl, A.; Hickey, L.T. Dissecting the Genetics of Early Vigour to Design Drought-Adapted Wheat. Front. Plant Sci. 2022, 12, 754439. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C. Breeding to adapt agriculture to climate change: Affordable phenotyping solutions. Curr. Opin. Plant Biol. 2018, 45, 237–247. [Google Scholar] [CrossRef]
- Jiang, N.; Zhu, X.-G. Modern phenomics to empower holistic crop science, agronomy, and breeding research. J. Genet. Genom. 2024, 51, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Angidi, S.; Madankar, K.; Tehseen, M.M.; Bhatla, A. Advanced High-Throughput Phenotyping Techniques for Managing Abiotic Stress in Agricultural Crops—A Comprehensive Review. Crops 2025, 5, 8. [Google Scholar] [CrossRef]
- Karim, M.R.; Reza, M.N.; Jin, H.; Haque, M.A.; Lee, K.-H.; Sung, J.; Chung, S.-O. Application of LiDAR Sensors for Crop and Working Environment Recognition in Agriculture: A Review. Remote Sens. 2024, 16, 4623. [Google Scholar] [CrossRef]
- Farhan, S.M.; Yin, J.; Chen, Z.; Memon, M.S. A Comprehensive Review of LiDAR Applications in Crop Management for Precision Agriculture. Sensors 2024, 24, 5409. [Google Scholar] [CrossRef]
- Atefi, A.; Ge, Y.; Pitla, S.; Schnable, J. Robotic Technologies for High-Throughput Plant Phenotyping: Contemporary Reviews and Future Perspectives. Front. Plant Sci. 2021, 12, 611940. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Vergara-Díaz, O.; Gracia-Romero, A.; Rezzouk, F.Z.; Segarra, J.; Buchaillot, M.L.; Chang-Espino, M.; Vatter, T.; Sanchez-Bragado, R.; et al. Crop phenotyping in a context of global change: What to measure and how to do it. J. Integr. Plant Biol. 2022, 64, 592–618. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Kefauver, S.C.; Fernandez-Gallego, J.A.; Vergara-Díaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat. Remote Sens. 2019, 11, 1244. [Google Scholar] [CrossRef]
- White, J.W.; Andrade-Sanchez, P.; Gore, M.A.; Bronson, K.F.; Coffelt, T.A.; Conley, M.M.; Feldmann, K.A.; French, A.N.; Heun, J.T.; Hunsaker, D.J.; et al. Field-based phenomics for plant genetics research. Field Crops Res. 2012, 133, 101–112. [Google Scholar] [CrossRef]
- Liu, J.; Pattey, E. Retrieval of leaf area index from top-of-canopy digital photography over agricultural crops. Agric. For. Meteorol. 2010, 150, 1485–1490. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Kefauver, S.C.; Vergara-Díaz, O.; Zaman-Allah, M.A.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L. Comparative Performance of Ground vs. Aerially Assessed RGB and Multispectral Indices for Early-Growth Evaluation of Maize Performance under Phosphorus Fertilization. Front. Plant Sci. 2017, 8, 2004. [Google Scholar] [CrossRef]
- Abrougui, K.; Khemis, C.; Guebsi, R.; Ouni, A.; Mohammadi, A.; Amami, R.; Kefauver, S.; Mansour, H.B.; Chehaibi, S. Efficient management of potato fields: Integrating ground and UAV vegetation indexes for optimal mechanical planting parameters. Euro-Mediterr. J. Environ. Integr. 2024, 10, 2033–2048. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Gracia-Romero, A.; Vergara-Diaz, O.; Zaman-Allah, M.A.; Tarekegne, A.; Cairns, J.E.; Prasanna, B.M.; Araus, J.L.; Kefauver, S.C. Evaluating Maize Genotype Performance under Low Nitrogen Conditions Using RGB UAV Phenotyping Techniques. Sensors 2019, 19, 1815. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Vatter, T.; Gutiérrez, N.A.; Nieto-Taladriz, M.T.; Araus, J.L. Low-cost assessment of grain yield in durum wheat using RGB images. Eur. J. Agron. 2019, 105, 146–156. [Google Scholar] [CrossRef]
- Hamdane, Y.; Segarra, J.; Buchaillot, M.L.; Rezzouk, F.Z.; Gracia-Romero, A.; Vatter, T.; Benfredj, N.; Hameed, R.A.; Gutiérrez, N.A.; Torró, I.T.; et al. Using Ground and UAV Vegetation Indexes for the Selection of Fungal-Resistant Bread Wheat Varieties. Drones 2023, 7, 454. [Google Scholar] [CrossRef]
- Torres-Sánchez, J.; Peña, J.M.; de Castro, A.I.; López-Granados, F. Multi-temporal mapping of the vegetation fraction in early-season wheat fields using images from UAV. Comput. Electron. Agric. 2014, 103, 104–113. [Google Scholar] [CrossRef]
- Marcial-Pablo, M.d.J.; Gonzalez-Sanchez, A.; Jimenez-Jimenez, S.I.; Ontiveros-Capurata, R.E.; Ojeda-Bustamante, W. Estimation of vegetation fraction using RGB and multispectral images from UAV. Int. J. Remote Sens. 2019, 40, 420–438. [Google Scholar] [CrossRef]
- Prey, L.; Von Bloh, M.; Schmidhalter, U. Evaluating RGB Imaging and Multispectral Active and Hyperspectral Passive Sensing for Assessing Early Plant Vigor in Winter Wheat. Sensors 2018, 18, 2931. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Li, D.; Zhou, X.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of RGB, Color-Infrared and Multispectral Images Acquired from Unmanned Aerial Systems for the Estimation of Nitrogen Accumulation in Rice. Remote Sens. 2018, 10, 824. [Google Scholar] [CrossRef]
- Kipp, S.; Mistele, B.; Baresel, P.; Schmidhalter, U. High-throughput phenotyping early plant vigour of winter wheat. Eur. J. Agron. 2014, 52, 271–278. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, M.; Wang, N.; Qiu, R.; Miao, Y. Plant Phenotyping. In Soil and Crop Sensing for Precision Crop Production; Li, M., Yang, C., Zhang, Q., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 185–250. ISBN 978-3-030-70432-2. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Lukina, E.V.; Stone, M.L.; Raun, W.R. Estimating vegetation coverage in wheat using digital images. J. Plant Nutr. 1999, 22, 341–350. [Google Scholar] [CrossRef]
- Hunt, E.R.; Cavigelli, M.; Daughtry, C.S.T.; Mcmurtrey, J.E.; Walthall, C.L. Evaluation of Digital Photography from Model Aircraft for Remote Sensing of Crop Biomass and Nitrogen Status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Vergara, O.; Araus, J.L.; Tarekegne, A.; Magorokosho, C.; Zarco-Tejada, P.J.; Hornero, A.; Albà, A.H.; Das, B.; Craufurd, P.; et al. Unmanned aerial platform-based multi-spectral imaging for field phenotyping of maize. Plant Methods 2015, 11, 35. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Casadesús, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V.; et al. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Vergara-Díaz, O.; Zaman-Allah, M.A.; Masuka, B.; Hornero, A.; Zarco-Tejada, P.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L. A Novel Remote Sensing Approach for Prediction of Maize Yield Under Different Conditions of Nitrogen Fertilization. Front. Plant Sci. 2016, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.J.; Doraiswamy, P.C.; Hunt, R.E., Jr. Changes of crop rotation in Iowa determined from the United States Department of Agriculture, National Agricultural Statistics Service cropland data layer product. J. Appl. Remote Sens. 2012, 6, 063590. [Google Scholar] [CrossRef]
- Hunt, E.R., Jr.; Daughtry, C.S.T.; Eitel, J.U.H.; Long, D.S. Remote Sensing Leaf Chlorophyll Content Using a Visible Band Index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 October 2025).
- Fox, J.; Weisberg, S.; Price, B. Car: Companion to Applied Regression, Version 3.1-3; Sage: Thousand Oaks, CA, USA, 2001.
- Rodríguez-Álvarez, M.X.; Boer, M.P.; van Eeuwijk, F.A.; Eilers, P.H.C. Correcting for spatial heterogeneity in plant breeding experiments with P-splines. Spat. Stat. 2018, 23, 52–71. [Google Scholar] [CrossRef]
- Cohen, J. (Ed.) Statistical Power Analysis for the Behavioral Sciences. In Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1977; p. iii. ISBN 978-0-12-179060-8. [Google Scholar]
- Kefauver, S.C.; El-Haddad, G.; Vergara-Diaz, O.; Araus, J.L. RGB picture vegetation indexes for High-Throughput Phenotyping Platforms (HTPPs). In Remote Sensing for Agriculture, Ecosystems, and Hydrology XVII, Proceedings of the SPIE Remote Sensing, Toulouse, France, 21–24 September 2015; Neale, C.M.U., Maltese, A., Eds.; SPIE: Bellingham, WA, USA, 2015; p. 96370J. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Feng, L.; Du, Q.; Runge, T. Combining Multi-Source Data and Machine Learning Approaches to Predict Winter Wheat Yield in the Conterminous United States. Remote Sens. 2020, 12, 1232. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.C.; Guo, Y.; Zheng, B. Dynamic Monitoring of NDVI in Wheat Agronomy and Breeding Trials Using an Unmanned Aerial Vehicle. Field Crops Res. 2017, 210, 71–80. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Ul-Allah, S.; Siddique, K.H.M. Physiological and Agronomic Approaches for Improving Water-Use Efficiency in Crop Plants. Agric. Water Manag. 2019, 219, 95–108. [Google Scholar] [CrossRef]
- Jha, P.; Kumar, V.; Godara, R.K.; Chauhan, B.S. Weed Management Using Crop Competition in the United States: A Review. Crop Prot. 2017, 95, 31–37. [Google Scholar] [CrossRef]
- Kristensen, L.; Olsen, J.; Weiner, J. Crop Density, Sowing Pattern, and Nitrogen Fertilization Effects on Weed Suppression and Yield in Spring Wheat. Weed Sci. 2008, 56, 97–102. [Google Scholar] [CrossRef]
- Chalise, D.P.; Snider, J.L.; Virk, G. Assessing the Effects of Cultivar and Nitrogen Application Rate on Seedling Vigor and Early-Season Canopy Growth in Cotton. Agron. J. 2023, 115, 713–725. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Bi, X.; Feng, B.; Wang, Z.; Wang, F.; Si, J.; Shi, J.; Liu, K. Nitrogen Fertilizer Management on Wheat Yield and Nitrogen Utilization. J. Plant Nutr. 2022, 45, 1953–1960. [Google Scholar] [CrossRef]
- Moussavi-Nik, M.; Pearson, J.N.; Hollamby, G.J.; Graham, R.D. Dynamics of Nutrient Remobilization during Germination and Early Seedling Development in Wheat. J. Plant Nutr. 1998, 21, 421–434. [Google Scholar] [CrossRef]
- Maresma, Á.; Ariza, M.; Martínez, E.; Lloveras, J.; Martínez-Casasnovas, J. Analysis of Vegetation Indices to Determine Nitrogen Application and Yield Prediction in Maize (Zea mays L.) from a Standard UAV Service. Remote Sens. 2016, 8, 973. [Google Scholar] [CrossRef]
- Jin, X.; Liu, S.; Baret, F.; Hemerlé, M.; Comar, A. Estimates of Plant Density of Wheat Crops at Emergence from Very Low Altitude UAV Imagery. Remote Sens. Environ. 2017, 198, 105–114. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Jin, X.; Ding, J.; Zhu, X.; Sun, C.; Guo, W. Evaluation of Seed Emergence Uniformity of Mechanically Sown Wheat with UAV RGB Imagery. Remote Sens. 2017, 9, 1241. [Google Scholar] [CrossRef]
- Wilke, N.; Siegmann, B.; Postma, J.A.; Muller, O.; Krieger, V.; Pude, R.; Rascher, U. Assessment of Plant Density for Barley and Wheat Using UAV Multispectral Imagery for High-Throughput Field Phenotyping. Comput. Electron. Agric. 2021, 189, 106380. [Google Scholar] [CrossRef]
- Deery, D.M.; Smith, D.J.; Davy, R.; Jimenez-Berni, J.A.; Rebetzke, G.J.; James, R.A. Impact of Varying Light and Dew on Ground Cover Estimates from Active NDVI, RGB, and LiDAR. Plant Phenomics 2021, 2021, 9842178. [Google Scholar] [CrossRef] [PubMed]
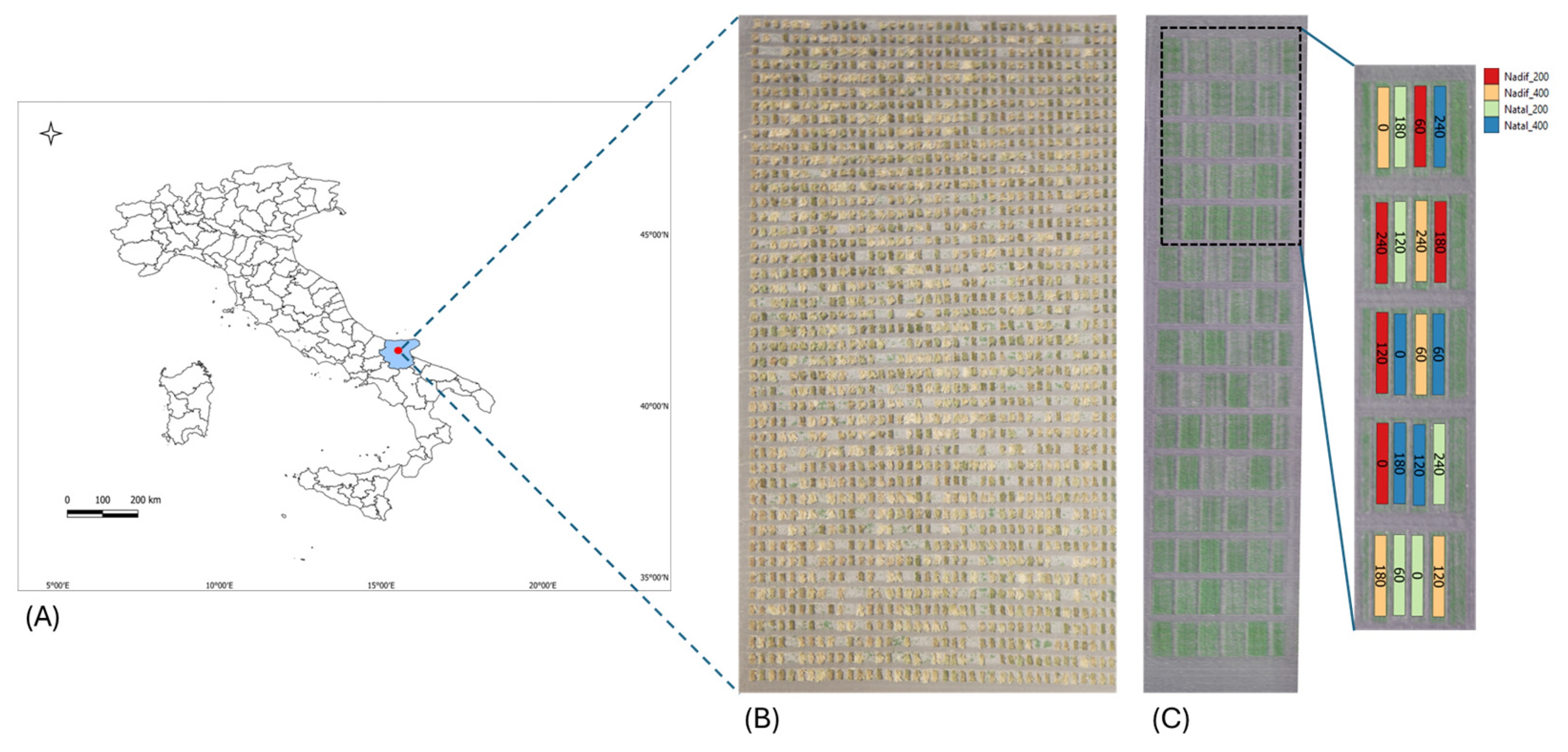

| Trial | Year | Sowing Date | Day of Flight | Phenological Growth Stage | Zadoks Growth Scale (GS) [53] |
|---|---|---|---|---|---|
| Exp. 1 | 2015–2016 | 15 December 2015 | 9 March 2016 | Tillering | 25 |
| 23 March 2016 | Stem Elongation | 31 | |||
| 10 April 2016 | Booting | 45 | |||
| 4 May 2016 | Late Flowering | 69 | |||
| Exp. 2 | 2016–2017 | 13 December 2016 | 15 February 2017 | Early Tillering | 23 |
| 2 March 2017 | Late Tillering | 29 | |||
| 16 March 2017 | Stem Elongation | 31 | |||
| 5 April 2017 | Booting | 45 | |||
| 2 May 2017 | Flowering | 65 | |||
| 2017–2018 | 11 December 2017 | 16 February 2018 | Early Tillering | 23 | |
| 1 March 2018 | Late Tillering | 29 | |||
| 14 March 2018 | Stem Elongation | 31 | |||
| 29 March 2018 | Booting | 45 | |||
| 25 April 2018 | Heading | 55 | |||
| 2018–2019 | 14 December 2018 | 19 February 2019 | Early Tillering | 23 | |
| 28 February 2019 | Late Tillering | 29 | |||
| 13 March 2019 | Stem Elongation 1st node detectable | 31 | |||
| 18 March 2019 | Stem Elongation 2nd node detectable | 32 |
| Spectral Index | Equation | Reference |
|---|---|---|
| Green Area (GA) | 60° < Hue < 180° | [58] |
| Greeness Area (GGA) | 80° < Hue < 180° | [58] |
| Crop Senescence Index (CSI) | 100 × (GA − GGA)/GA | [59] |
| Normalized Green Red Difference Index (NGRDI) | (Green − Red)/(Green + Red) | [60] |
| Triangular Green Index (TGI) | −0.5 [190 (Red − Green) − 120 (Red − Blue)] | [61] |
| VIs | Genotype | Sowing Rate | N Dose | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS23 | GS29 | GS31 | GS45 | GS65 | GS23 | GS29 | GS31 | GS45 | GS65 | GS23 | GS29 | GS31 | GS45 | GS65 | |||
| GA | ** (0.48) | ** (−1.08) | *** (−0.15) | *** (−0.18) | |||||||||||||
| GGA | ** (−0.71) | *** (−0.46) | *** (−0.43) | ||||||||||||||
| CSI | ** (0.21) | *** (0.61) | *** (0.24) | ||||||||||||||
| NGRDI | ** (−0.55) | ** (0.31) | *** (0.45) | *** (0.80) | ** (−0.44) | *** (−0.93) | *** (−0.32) | *** (−0.58) | |||||||||
| TGI | *** (−0.34 | *** (0.25) | *** (0.80) | *** (0.23) | *** (0.10) | ** (−0.59) | *** (−1.06) | *** (−0.30) | *** (−0.29) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fania, F.; Pecorella, I.; Romano, E.; Spadanuda, P.; Pecchioni, N.; Esposito, S.; De Vita, P. RGB-Derived Indices Accurately Detect Genotypic and Agronomic Differences in Canopy Variation in Durum Wheat. Crops 2025, 5, 85. https://doi.org/10.3390/crops5060085
Fania F, Pecorella I, Romano E, Spadanuda P, Pecchioni N, Esposito S, De Vita P. RGB-Derived Indices Accurately Detect Genotypic and Agronomic Differences in Canopy Variation in Durum Wheat. Crops. 2025; 5(6):85. https://doi.org/10.3390/crops5060085
Chicago/Turabian StyleFania, Fabio, Ivano Pecorella, Elio Romano, Patrizio Spadanuda, Nicola Pecchioni, Salvatore Esposito, and Pasquale De Vita. 2025. "RGB-Derived Indices Accurately Detect Genotypic and Agronomic Differences in Canopy Variation in Durum Wheat" Crops 5, no. 6: 85. https://doi.org/10.3390/crops5060085
APA StyleFania, F., Pecorella, I., Romano, E., Spadanuda, P., Pecchioni, N., Esposito, S., & De Vita, P. (2025). RGB-Derived Indices Accurately Detect Genotypic and Agronomic Differences in Canopy Variation in Durum Wheat. Crops, 5(6), 85. https://doi.org/10.3390/crops5060085







