Implications of Fertilisation on Soil Nematode Community Structure and Nematode-Mediated Nutrient Cycling
Abstract
1. Introduction
2. Mechanisms Through Which Nematodes Contribute to Nutrient Cycling
2.1. Direct Contributions of Nematodes to Nutrient Cycling
2.2. Indirect Effects of Nematodes to Nutrient Cycling
3. Impacts of Organic Fertilisation on Free-Living Nematodes
3.1. Heterogeneity Within Organic Amendments Shapes Nematode Communities
| References | Organic Fertilisers | Management Practice and Crop | BF | FF | OM/PR | PPN | Diversity | Nematode Indices |
|---|---|---|---|---|---|---|---|---|
| [52] | Organic manure applied at various rates, along with chemical fertilisers at different rates | Greenhouse (jackfruit) | ↑ high levels of manure than synthetic fertiliser | ↑ high levels of manure than synthetic fertiliser | ↑ high levels of manure: synthetic fertiliser | ↓ with high levels of manure than synthetic fertiliser | ↑ high levels of manure than synthetic fertiliser | ↑ MI, EI, SI, high levels of manure than synthetic fertiliser |
| [58] | Manure (M), manure + urea (M + U), straw (S), straw + urea (S + U) | Monoculture (sorghum) | ↑ straws than manures | ↑ straws than manures | No effect | ↑ manure than straw | not reported | No effect on CI, EI, SI though SI varied with development stage |
| [73] | Cow manure plus rice straw compost, Sugarcane filter cake compost plus urea | Different cropping patterns (rice, sesame, soybean) | No effect | No effect | No effect | No effect | No effect | No effect |
| [64] | Poplar leaf, maize straw, cow manure + NPK to all | Monoculture (soybean) | ↑ cow manure than straw and poplar leaf | ↑ cow manure than straw and poplar leaf | ↑ straw and poplar leaf than manure | ↑ poplar leaf and maize than straw | ↑ Poplar leaf and straw than manure | No effect on MI; ↑ PPI in poplar leaf than straw and manure ↑ SI in straw compared to manure and poplar leaf |
| [70] | Sugarcane bagasse (SCB), sewage sludge (SES), plant residues (PLR), Sugarcane Refinery Sludge (SCS) | Greenhouse (banana) | ↑ SCB, SCS, and SES than PLR | ↑ SCB at planting ↓ at the final stage. ↑ in PLR at final | No effect; ↑ in SCB at final stage | ↓ in all except vs. control no effect in SES; ↓ at the final stage in SCB | Not reported | ↑ in MI in all except SES; ↑ CI in SCS and PLR |
| [74] | Beef manure (BM), Horse manure (HM), swine manure (SM), poultry manure (PM) | Monoculture (corn) | ↑ horse and beef manure, than swine, while poultry manure had the lowest | ↑ swine and poultry manure than beef, while horse manure had the lowest | ↑ beef and swine manure were the highest, followed by poultry and then horse manure | ↑ in all manures | No effect | No effect in PPI, EI, CI; ↑ MI in swine and poultry manure compared to beef, while horse manure had the least MI; beef and swine manure had higher SI than poultry, while horse had the lowest |
| [75] | Plant based compost, plant-based compost + urea at different ratios (3:1, 1:1, 1:3) | Monoculture (carrot) | No effect | No effect | No effect | No effect | No effect | |
| [69] | Conventional Farmyard Manure (CFM), Compost (COM, Improved Farmyard Manure (IFM), Kraal Manure (KM), Vermicompost (VCT) applied at different rates and in various combinations (16 treatments) with mineral fertilisers in some combinations | Monoculture (rice) | No effect | No effect | ↑ organic treatments, especially those incorporating bat guano and high rates of organic materials | ↑ organic treatments, especially those incorporating bat guano and high rates of organic materials; ↑ compost only than manure only treatments | No differences between all organic treatments | No effect on MI, PPI, CI, EI; ↑ high rates of organic inputs, kraal manure, low input conventional farmyard manure with NPK |
| [72] | Fermented manure (FM) and sawdust (SAW), both individually and in combination with inorganic nitrogen | controlled pot experiment (sugar beet) | ↑ in both FM treatments than SAW treatments; ↑ high levels of FM | ↑ in both FM treatments than SAW treatments; ↑ high levels of FM | ↓ in both FM and SAW | ↓ in FM; no effect SAW | Not reported | Not reported |
| [59] | Cattle Manure Compost + NPK (NPKM), Maize Straw + NPK (NPKS) | Crop rotation (wheat and maize) | ↑ BF biomass carbon in both manure and straw | ↑ FF biomass carbon in straw than manure in wheat | ↓ OM-PR biomass carbon manure than straw | ↑ PPN biomass carbon; however lower biomass carbon in both manure and straw in maize season | Not reported | ↑ in enrichment footprint in straw than manure under wheat. SI footprint lower in maize |
| [24] | Raw Manure, Composted Manure | Monoculture (tomato) | ↑ in both raw and composited manure | ↑ in both raw and composited; more in compost | ↑ in both raw and composited; more in compost | ↓ Raw manure than compost | ↑ in compost than raw manure | ↓ in ∑MI in both; lower in raw manure |
| [57] | Naturally fertilised (NF)-plant residues, Poultry Litter (PL), Cow Manure (CM) | Agroforestry (coffee, banana, royal palm, avocado, peanut) | ↑ in PL and cow manure (CM) lower in NF | ↑ in NF than PL lowest in cow manure | No effect | ↑ NF less in PL and lowest in CM. | ↑ in NF than PL; lowest in cow manure | ↑ in MI in NF than CM; lowest in PL; ↑ in EI in CM than PL, lowest in NF; ↑ in SI in NF than PL, lowest in CM |
| [62] | Traditional compost (C), compost with effective microorganisms (EMC), and chicken dung compost with effective microorganisms (EMCDC). Each was applied at two rates of 7.5 t/ha and 15 t/ha | Crop rotation (wheat and maize) | ↑ in both EMC and EMCDC compared to traditional compost at both rates | No effect | ↓ at high rates. Effective microorganisms have no effect | ↓ in both EMC and EMCDC vs. traditional compost at high rates | affected by the amount of compost, not effective microbes | Low MI; PPI; ∑MI not affected by effective microbes; EI, SI affected by the amount of compost. |
| [56] | Traditional Compost (C), Traditional Compost with Effective Microorganisms (EMC) | Crop rotation (wheat and maize) | ↑ in both composts, but higher in the compost with microbes | ↓ in compost with microbes than traditional compost | ↑ in compost with microbes than in traditional compost | ↑ in both composts, higher in the compost with microbes | Not reported | Not reported |
3.2. Factors Driving Variation in Free-Living Nematode Communities Under Organic Fertilisation
4. Impacts of Inorganic Fertilisation on Free-Living Nematodes
4.1. Changes in Soil Chemistry Alter Nematodes Under Inorganic Fertilisation
4.2. The Role of Disturbance and Ecological Interactions Under Inorganic Fertilisation
5. Impacts of Fertilisation on Plant-Parasitic Nematodes
5.1. Interacting Factors Shaping Plant Parasitic Nematodes in Fertilised Soils
5.2. Plant Parasitic Nematodes Suppression by Organic Fertilisers
6. Impacts of Recycling Derived Fertilisers on Nematode Communities
7. Impacts of Fertilisation on Nematode Diversity
8. Impacts of Fertilisation on Nematode Functional Indices
9. Impacts of Fertilisation on Nematode-Mediated Nutrient Cycling
10. Perspectives, Challenges, and Opportunities
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RDF | Recycled-derived fertiliser |
| PPN | Plant parasitic nematodes |
| C-P | Coloniser-Persister |
| MI | Maturity index |
| CI | Channel index |
| SI | Structural index |
| EI | Enrichment index |
| C | Carbon |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| NPK | Nitrogen, Phosphorus, and Potassium |
References
- Worldometer. Current World Population. Worldometer. 2025. Available online: https://www.worldometers.info/world-population/ (accessed on 29 March 2025).
- FAO. The Future of Food and Agriculture–Alternative Pathways to 2050; Food and Agriculture Organization of the United Nations Rome: Rome, Italy, 2018. [Google Scholar]
- European Union. Regulation of the European Parliament and of the Council laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 2019, 114. [Google Scholar]
- FAO. The use of phosphate rock for sustainable agriculture. In FAO Fertiliser and Plant Nutrition Bulletin; FAO & IAEA: Rome, Italy, 2004; pp. 1–148. [Google Scholar]
- Ryan, D.; Karpinska, A.; Forrestal, P.J.; Ashekuzzaman, S.M.; Kakouli-Duarte, T.; Dowling, D.N.; Germaine, K.J. The Impact of Bio-Based Fertiliser Integration into Conventional Grassland Fertilisation Programmes on Soil Bacterial, Fungal, and Nematode Communities. Front. Sustain. Food Syst. 2022, 6, 832841. [Google Scholar] [CrossRef]
- ReNu2Cycle Consortium. ReNu2Cycle: Recycling of Nutrients to Close the Fertiliser Cycle. 2023. Available online: https://renu2cycle.nweurope.eu/ (accessed on 29 March 2025).
- Rey-Martínez, N.; Torres-Sallan, G.; Morales, N.; Serra, E.; Bisschops, I.; van Eekert, M.H.A.; Borrà, E.; Sanchis, S. Combination of technologies for nutrient recovery from wastewater: A review. Clean. Waste Syst. 2024, 7, 100139. [Google Scholar] [CrossRef]
- Karpinska, A.; Kakouli-Duarte, T.; Ashekuzzaman, S.M.; Byrne, J.; Schmalenberger, A.; Forrestal, P.J. Plant and Soil Effects of Alternative Sources of Phosphorus over Three Years of Application. Agronomy 2024, 14, 1591. [Google Scholar] [CrossRef]
- Ivona, S.; Amrita, S.; Achim, S.; Lagrange, H.; Forrestal, P.; Postma, R.; van Scholl, L.; Verleden, I.; Ryan, D.; Krapinska, A.; et al. ReNu2Farm–Product information sheets. In Product Information Sheets (WPT1_D3.4); ReNu2Farm: Saarbrücken, Germany, 2021. [Google Scholar]
- Bongers, T.; Ferris, H. Nematode community structure as a biomonitor in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S. Feeding Habits in Soil Nematode Families and Genera—An Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T. Indices developed specifically for analysis of nematode assemblages. In Nematodes as Environmental Indicators; Wilson, M.J., Kakouli-Duarte, T., Eds.; CAB International: Oxford, UK, 2009; pp. 124–145. [Google Scholar]
- Neher, D.A.; Darby, B.J. General community indices that can be used for analysis of nematode assemblages. In Nematodes as Environmental Indicators; Wilson, M.J., Kakouli-Duarte, T., Eds.; CaBi Publishing: Oxford, UK, 2009; pp. 107–123. [Google Scholar]
- Wilson, M.J.; Kakouli-Duarte, T. Nematodes as Environmental Indicators; CABI Publishing: Oxford, UK, 2009; Available online: https://ebookcentral.proquest.com/lib/itcarlow/detail.action?docID=455765 (accessed on 10 March 2024).
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; De Ruiter, P.C. The identification and evaluation of food webs in soil: Temporal and spatial heterogeneity of trophic interactions within below-ground food webs. Pedobiologia 1991, 35, 63–69. [Google Scholar]
- Bongers, T. The Maturity Index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil 1999, 212, 13–22. [Google Scholar] [CrossRef]
- Suyadi Sila, S.; Samuel, J. Nematode diversity indices application to determine the soil health status of lembo agroecosystem in west kutai, east kalimantan province, indonesia. Biodiversitas 2021, 22, 2861–2869. [Google Scholar] [CrossRef]
- Krashevska, V.; Kudrin, A.A.; Widyastuti, R.; Scheu, S. Changes in Nematode Communities and Functional Diversity With the Conversion of Rainforest Into Rubber and Oil Palm Plantations. Front. Ecol. Evol. 2019, 7, 487. [Google Scholar] [CrossRef]
- Forge, T.; Ehret, D.; Messiga, A.; Dorais, M. Influences of nitrogen inputs on nematode populations under highbush blueberry. J. Nematol. 2021, 52, e2020–e2056. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, F.; Chen, X.; Li, J.; Zhou, X.; Guo, H. Long-term fertilisation effects on soil biotic communities are mediated by plant diversity in a Tibetan alpine meadow. Plant Soil 2022, 474, 525–540. [Google Scholar] [CrossRef]
- Nahar, M.S.; Grewal, P.S.; Miller, S.A.; Stinner, D.; Stinner, B.R.; Kleinhenz, M.D.; Wszelaki, A.; Doohan, D. Differential effects of raw and composted manure on nematode community, and its indicative value for soil microbial, physical and chemical properties. Appl. Soil Ecol. 2006, 34, 140–151. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; Van Der Meulen, H.R.; Lau, S.S. Nitrogen mineralization by bacterial-feeding nematodes: Verification and measurement. Plant Soil 1998, 203, 159–171. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Bert, W.; Maenhout, P.; Sleutel, S.; De Neve, S. Quantifying the contribution of entire free-living nematode communities to carbon mineralization under contrasting C and N availability. PLoS ONE 2015, 10, e0136244. [Google Scholar] [CrossRef]
- Bouwman, L.A.; Bloem, J.; Hoenderboom, G.H.J.; De Ruiter, P.C. Short-term and long-term effects of bacterivorous nematodes and nematophagous fungi on carbon and nitrogen mineralization in microcosms. Biol. Fertil. Soils 1994, 17, 249–256. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Buchan, D.; De Neve, S. Quantifying the influences of free-living nematodes on soil nitrogen and microbial biomass dynamics in bare and planted microcosms. Soil Biol. Biochem. 2014, 70, 131–141. [Google Scholar] [CrossRef]
- Holajjer, P.; Kamra, A.; Singh, P. Influence of nematode-bacterial interactions on N and P mineralisation in soil and on decomposition of crop residues during aerobic composting. Appl. Ecol. Environ. Res. 2016, 14, 283–299. [Google Scholar] [CrossRef]
- Irshad, U.; Villenave, C.; Brauman, A.; Plassard, C. Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol. Biochem. 2011, 43, 2121–2126. [Google Scholar] [CrossRef]
- Irshad, U.; Yergeau, E. Bacterial subspecies variation and nematode grazing change P dynamics in the wheat rhizosphere. Front. Microbiol. 2018, 9, 1990. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Liu, Y.; Han, Y.; Wang, Y.; Wang, Q.; Liu, T. Nematodes and their bacterial prey improve phosphorus acquisition by wheat. New Phytol. 2023, 237, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, M.; Zhang, J.; Chen, Y.; Chen, X.; Chen, L.; Li, L.; Zhang, X.-X.; Sun, B. Nematode grazing promotes bacterial community dynamics in soil at the aggregate level. ISME J. 2017, 11, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Milkereit, J.; Geisseler, D.; Lazicki, P.; Settles, M.L.; Durbin-Johnson, B.P.; Hodson, A. Interactions between nitrogen availability, bacterial communities, and nematode indicators of soil food web function in response to organic amendments. Appl. Soil Ecol. 2021, 157, 103767. [Google Scholar] [CrossRef]
- Xu, L.; Xu, W.; Jiang, Y.; Hu, F.; Li, H. Effects of interactions of auxin-producing bacteria and bacterial-feeding nematodes on regulation of peanut growths. PLoS ONE 2015, 10, e0124361. [Google Scholar] [CrossRef]
- Ranoarisoa, M.P.; Morel, C.; Andriamananjara, A.; Jourdan, C.; Bernard, L.; Becquer, T.; Rabeharisoa, L.; Rahajaharilaza, K.; Plassard, C.; Blanchart, E.; et al. Effects of a bacterivorous nematode on rice 32P uptake and root architecture in a high P-sorbing ferrallitic soil. Soil Biol. Biochem. 2018, 122, 39–49. [Google Scholar] [CrossRef]
- Liang, S.; Kou, X.; Li, Y.; Lü, X.; Wang, J.; Li, Q. Soil nematode community composition and stability under different nitrogen additions in a semiarid grassland. Glob. Ecol. Conserv. 2020, 22, e00965. [Google Scholar] [CrossRef]
- Sarathchandra, S.U.; Ghani, A.; Yeates, G.W.; Burch, G.; Cox, N.R. Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biol. Biochem. 2001, 33, 953–964. [Google Scholar] [CrossRef]
- Wei, C.; Zheng, H.; Li, Q.; Lü, X.; Yu, Q.; Zhang, H.; Chen, Q.; He, N.; Kardol, P.; Liang, W.; et al. Nitrogen Addition Regulates Soil Nematode Community Composition through Ammonium Suppression. PLoS ONE 2012, 7, e43384. [Google Scholar] [CrossRef]
- Pan, F.; Han, X.; Mclaughlin, N.B.; Li, C.; Zhao, D.; Zhan, L.; Xu, Y. Effect of long-term fertilisation on free-living nematode community structure in Mollisols. J. Soil Sci. Plant Nutr. 2015, 15, 129–141. [Google Scholar]
- Herren, G.L.; Habraken, J.; Waeyenberge, L.; Haegeman, A.; Viaene, N.; Cougnon, M.; Reheul, D.; Steel, H.; Bert, W. Effects of synthetic fertiliser and farm compost on soil nematode community in long-term crop rotation plots: A morphological and metabarcoding approach. PLoS ONE 2020, 15, e0230153. [Google Scholar] [CrossRef]
- Bonkowski, M.; Cheng, W.; Griffiths, B.S.; Alphei, J.; Scheu, S. Microbial-faunal interactions in the rhizosphere and effects on plant growth. Eur. J. Soil Biol. 2000, 36, 135–147. [Google Scholar] [CrossRef]
- Kane, J.L.; Kotcon, J.B.; Freedman, Z.B.; Morrissey, E.M. Fungivorous nematodes drive microbial diversity and carbon cycling in soil. Ecology 2023, 104, e3844. [Google Scholar] [CrossRef] [PubMed]
- Rehman, P.; Nazir, R.; Naqvi, A.; Pervez, A.; Irshad, U. Bacterial feeder nematodes: Facilitator or competitor for plant phosphorus in soil. J. Soil Sci. Plant Nutr. 2018, 18, 1173–1186. [Google Scholar] [CrossRef]
- Xiao, H.; Griffiths, B.; Chen, X.; Liu, M.; Jiao, J.; Hu, F.; Li, H. Influence of bacterial-feeding nematodes on nitrification and the ammonia-oxidizing bacteria (AOB) community composition. Appl. Soil Ecol. 2010, 45, 131–137. [Google Scholar] [CrossRef]
- Sun, F.; Yan, G.; Lin, W.; He, W.; Cheng, X.; Li, Y.; Tariq, A.; Sardans, J.; Penuelas, J.; Wang, J.; et al. Warming enhanced the interaction effects of fungi and fungivores and soil potassium mineralization in tropical forest. Catena 2024, 243, 108229. [Google Scholar] [CrossRef]
- Wardle, D.A.; Yeates, G.W. The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: Evidence from decomposer food-webs. Oecologia 1993, 93, 303–306. [Google Scholar] [CrossRef]
- Wei, K.; Wang, J.; Yuan, C.; Tang, J.; Zhu, B. Relationships of bacterial-feeding nematodes, phosphatase-producing bacteria, phosphatase activity and their effects on soil organic phosphorus mineralization under straw return. Appl. Soil Ecol. 2024, 96, 105280. [Google Scholar] [CrossRef]
- Ingham, R.E.; Trofymow, J.A.; Ingham, E.R.; Coleman, D.C. Interactions of Bacteria, Fungi, and their Nematode Grazers: Effects on Nutrient Cycling and Plant Growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- Tu, C.; Koenning, S.R.; Hu, S. Root-parasitic nematodes enhance soil microbial activities and nitrogen mineralization. Microb. Ecol. 2003, 46, 134–144. [Google Scholar] [CrossRef]
- Su, L.; Bai, T.; Qin, X.; Yu, H.; Wu, G.; Zhao, Q.; Tan, L. Organic manure induced soil food web of microbes and nematodes drive soil organic matter under jackfruit planting. Appl. Soil Ecol. 2021, 166, 103994. [Google Scholar] [CrossRef]
- Edouard Rambaut, L.A.; Vayssières, J.; Versini, A.; Salgado, P.; Lecomte, P.; Tillard, E. 15-year fertilisation increased soil organic carbon stock even in systems reputed to be saturated like permanent grassland on andosols. Geoderma 2022, 425, 116025. [Google Scholar] [CrossRef]
- Okada, H.; Harada, H. Effects of tillage and fertiliser on nematode communities in a Japanese soybean field. Appl. Soil Ecol. 2007, 35, 582–598. [Google Scholar] [CrossRef]
- Sedlář, O.; Balík, J.; Černý, J.; Kulhánek, M.; Smatanová, M. Long-Term Application of Organic Fertilisers in Relation to Soil Organic Matter Quality. Agronomy 2023, 13, 175. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Effective microorganisms and compost favor nematodes in wheat crops. Agron Sustain Dev 2013, 33, 573–579. [Google Scholar] [CrossRef]
- Vieira Júnior, J.O.L.; Pereira, R.C.; Soto, R.L.; Cardoso, I.M.; Mondino, E.A.; Berbara, R.L.L.; Mendonça, E.S. Organic fertilisation influences nematode diversity and maturity index in coffee tree plantations using an agroforestry system. J. Nematol. 2021, 53, e2021–e2054. [Google Scholar] [CrossRef]
- Villenave, C.; Saj, S.; Pablo, A.L.; Sall, S.; Djigal, D.; Chotte, J.L.; Bonzi, M. Influence of long-term organic and mineral fertilisation on soil nematofauna when growing Sorghum bicolor in Burkina Faso. Biol. Fertil. Soils 2010, 46, 659–670. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Xu, M.; Zhang, S.; Huang, S.; Liang, W. Responses of soil micro-food web to long-term fertilisation in a wheat-maize rotation system. Appl. Soil Ecol. 2016, 98, 56–64. [Google Scholar] [CrossRef]
- Coll, P.; Le Cadre, E.; Villenave, C. How are nematode communities affected during a conversion from conventional to organic farming in southern French vineyards? Nematology 2012, 16, 665–676. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y.C. Abundance and diversity of soil nematodes as influenced by different types of organic manure. Helminthologia 2010, 47, 58–66. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Effect of compost and chemical fertiliser on soil nematode community in a Chinese maize field. Eur. J. Soil Biol. 2010, 46, 230–236. [Google Scholar] [CrossRef]
- Ikoyi, I.; Egeter, B.; Chaves, C.; Ahmed, M.; Fowler, A.; Schmalenberger, A. Responses of soil microbiota and nematodes to application of organic and inorganic fertilisers in grassland columns. Biol. Fertil. Soils 2020, 56, 647–662. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Fan, W.; He, R.; Yao, Y.; Sun, L.; Zhao, X.; Wu, J. Comparative effects of different organic materials on nematode community in continuous soybean monoculture soil. Appl. Soil Ecol. 2018, 125, 12–17. [Google Scholar] [CrossRef]
- Mahran, A.; Tenuta, M.; Lumactud, R.A.; Daayf, F. Response of a soil nematode community to liquid hog manure and its acidification. Appl. Soil Ecol. 2009, 43, 75–82. [Google Scholar] [CrossRef]
- Neher, D.A. Nematode Communities in Organically and Conventionally Managed Agricultural Soils 1. J. Nematol. 1999, 31, 142. [Google Scholar]
- Treonis, A.M.; Austin, E.E.; Buyer, J.S.; Maul, J.E.; Spicer, L.; Zasada, I.A. Effects of organic amendment and tillage on soil microorganisms and microfauna. Appl. Soil Ecol. 2010, 46, 103–110. [Google Scholar] [CrossRef]
- Wang, K.H.; McSorley, R.; Marshall, A.; Gallaher, R.N. Influence of organic Crotalaria juncea hay and ammonium nitrate fertilisers on soil nematode communities. Appl. Soil Ecol. 2006, 31, 186–198. [Google Scholar] [CrossRef]
- Raharijaona, S.; Blanchart, E.; Razafindrakoto, M.; Rafolisy, T.; Salgado, P.; Razafimbelo, T.; Autfray, P.; Ratsiatosika, O.; Bernard, L.; Trap, J. Responses of Soil Nematodes to Combined Bio-Organo-Mineral Fertilisers on Upland Rice Cropping in the Highlands of Madagascar. Proc. Zool. Soc. 2023, 76, 224–240. [Google Scholar] [CrossRef]
- Tabarant, P.; Villenave, C.; Risede, J.M.; Roger-Estrade, J.; Thuries, L.; Dorel, M. Effects of four organic amendments on banana parasitic nematodes and soil nematode communities. Appl. Soil Ecol. 2011, 49, 59–67. [Google Scholar] [CrossRef]
- Steel, H.; Vandecasteele, B.; Willekens, K.; Sabbe, K.; Moens, T.; Bert, W. Nematode communities and macronutrients in composts and compost-amended soils as affected by feedstock composition. Appl. Soil Ecol. 2012, 61, 100–112. [Google Scholar] [CrossRef]
- Renč, O.M.; Kova’č, P.; Kova’č Ik, K. Response of Plant Parasitic and Free Living Soil Nematodes to Composted Animal Manure Soil Amendments. J. Nematol. 2012, 44, 329–336. [Google Scholar]
- Nguyen SVan Nguyen, P.T.K.; Araki, M.; Perry, R.N.; Tran, L.B.; Chau, K.M.; Min, Y.Y.; Toyota, K. Effects of cropping systems and soil amendments on nematode community and its relationship with soil physicochemical properties in a paddy rice field in the Vietnamese Mekong Delta. Appl. Soil Ecol. 2020, 156, 103683. [Google Scholar] [CrossRef]
- Benkovic-Lacic, T.; BrMez, M.; Ivezic, M.; Raspudic, E.; Pribetić, D.; Loncaric, Z.; Grubisic, D. Influence of organic and inorganic fertilisers on nematode communities in cornfield240 Agricultural Academy. Bulg. J. Agric. Sci. 2013, 19, 235. [Google Scholar]
- Habteweld, A.; Brainard, D.; Kravchencko, A.; Grewal, P.S.; Melakeberhan, H. Effects of integrated application of plant-based compost and urea on soil food web, soil properties, and yield and quality of a processing carrot cultivar. J. Nematol. 2020, 52, e2020–e2111. [Google Scholar] [CrossRef] [PubMed]
- Atandi, J.G.; Adamtey, N.; Kiriga, A.W.; Karanja, E.N.; Musyoka, M.W.; Matheri, F.M.; Tanga, C.M.; Coyne, D.L.; Fiaboe, K.K.M.; Bautze, D.; et al. Organic maize and bean farming enhances free-living nematode dynamics in sub-Saharan Africa. Agric. Ecosyst. Environ. 2022, 327, 107846. [Google Scholar] [CrossRef]
- Briar, S.S.; Grewal, P.S.; Somasekhar, N.; Stinner, D.; Miller, S.A. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Appl. Soil Ecol. 2007, 37, 256–266. [Google Scholar] [CrossRef]
- Briar, S.S.; Miller, S.A.; Stinner, D.; Kleinhenz, M.D.; Grewal, P.S. Effects of organic transition strategies for peri-urban vegetable production on soil properties, nematode community, and tomato yield. Appl. Soil Ecol. 2011, 47, 84–91. [Google Scholar] [CrossRef]
- Elnasikh, M.; Osman, A.G.; Sherif, A.M. Impact of neem seed cake on soil micro flora and some soil properties. J. Sci. Technol. 2011, 12, 144–150. [Google Scholar]
- Pothula, S.K.; Phillips, G.; Bernard, E.C. Increasing Levels of Physical Disturbance Affect Soil Nematode Community Composition in a Previously Undisturbed Ecosystem. J. Nematol. 2022, 54, 20220022. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hodda, M.; Ash, G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. EPPO Bull. 2013, 43, 334–374. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Wu, L.; Hu, J.; Chen, H.; Wang, B.; Wu, Y.; Bai, Y.; Chen, D. Stronger effects of long-term P enrichment on soil biota than plants in grasslands. Soil Tillage Res. 2023, 229, 105668. [Google Scholar] [CrossRef]
- Olatunji, O.A.; Gong, S.; Tariq, A.; Pan, K.; Sun, X.; Chen, W.; Zhang, L.; Dakhil, M.A.; Huang, D.; Tan, X. The effect of phosphorus addition, soil moisture, and plant type on soil nematode abundance and community composition. J. Soils Sediments 2019, 19, 1139–1150. [Google Scholar] [CrossRef]
- Gruzdeva, L.I.; Matveeva, E.M.; Kovalenko, T.E. Changes in soil nematode communities under the impact of fertilisers. Eurasian Soil Sci. 2007, 40, 681–693. [Google Scholar] [CrossRef]
- Siebert, J.; Sünnemann, M.; Auge, H.; Berger, S.; Cesarz, S.; Ciobanu, M.; Guerrero-Ramírez, N.R.; Eisenhauer, N. The effects of drought and nutrient addition on soil organisms vary across taxonomic groups, but are constant across seasons. Sci. Rep. 2019, 9, 639. [Google Scholar] [CrossRef]
- Chen, X.; Daniell, T.J.; Neilson, R.; O’Flaherty, V.; Griffiths, B.S. Microbial and microfaunal communities in phosphorus limited, grazed grassland change composition but maintain homeostatic nutrient stoichiometry. Soil Biol. Biochem. 2014, 75, 94–101. [Google Scholar] [CrossRef]
- Qi, Y.; Sun, X.; Peng, S.; Tan, X.; Zhou, S. Effects of fertilisation on soil nematode communities in an alpine meadow of Qinghai-Tibet plateau. Front. Ecol. Evol. 2023, 11, 1122505. [Google Scholar] [CrossRef]
- Vestergård, M. Nematode assemblages in the rhizosphere of spring barley (Hordeum vulgare L.) depended on fertilisation and plant growth phase. Pedobiologia 2004, 48, 257–265. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Bai, X.; Grace, J.B.; Bai, Y. Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 2013, 101, 1322–1334. [Google Scholar] [CrossRef]
- Ni, X.; Zhu, X.; Feng, Q.; Zhao, D.; Huang, W.; Pan, F. Effect of Application Rates of N and P Fertilisers on Soil Nematode Community Structure in Mollisols. Agronomy 2024, 14, 507. [Google Scholar] [CrossRef]
- Azpilicueta, C.V.; Cristina Aruani, M.; Chaves, E.; Reeb, P.D. Soil nematode responses to fertilisation with ammonium nitrate after six years of unfertilized apple orchard. Span. J. Agric. Res. 2014, 12, 353–363. [Google Scholar] [CrossRef]
- Nikolaidou, C.; Mola, M.; Papakostas, S.; Aschonitis, V.G.; Monokrousos, N.; Kougias, P.G. The effect of anaerobic digestate as an organic soil fertiliser on the diversity and structure of the indigenous soil microbial and nematode communities. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ikoyi, I.; Fowler, A.; Schmalenberger, A. One-time phosphate fertiliser application to grassland columns modifies the soil microbiota and limits its role in ecosystem services. Sci. Total Environ. 2018, 630, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Cheng, Y.; Feng, X.; Sun, F.; Gu, Y. Effects of fertiliser and weed species richness on soil nematode community in a microcosm field experiment. Soil Ecol. Lett. 2023, 5, 151–168. [Google Scholar] [CrossRef]
- Pulavarty, A.; Egan, A.; Karpinska, A.; Horgan, K.; Kakouli-Duarte, T. Plant parasitic nematodes: A review on their behaviour, host interaction, management approaches and their occurrence in two sites in the republic of ireland. Plants 2021, 10, 2352. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef]
- Kimpinski, J.; Gallant, C.E.; Henry, R.; Macleod, J.A.; Sanderson, J.B.; Sturz, A.V. Effect of compost and manure soil amendments on nematodes and on yields of potato and barley: A 7-year study. J. Nematol. 2003, 35, 289–293. [Google Scholar]
- Oka, Y.; Shapira, N.; Fine, P. Control of root-knot nematodes in organic farming systems by organic amendments and soil solarization. Crop Prot. 2007, 26, 1556–1565. [Google Scholar] [CrossRef]
- Rodriguez-Kabana, R. Organic and Inorganic Nitrogen Amendments to Soil as Nematode Suppressants. J. Nematol. 1986, 18, 129–135. [Google Scholar] [PubMed]
- Wang, K.-H.; Sipes, B.S.; Schmitt, D.P. Suppression of Rotylenchulus reniformis by Crotalaria juncea, Brassica napus, and Tagetes erecta. Nematropica 2001, 31, 235–250. [Google Scholar]
- Nair, A.; Ngouajio, M. Soil microbial biomass, functional microbial diversity, and nematode community structure as affected by cover crops and compost in an organic vegetable production system. Appl. Soil Ecol. 2012, 58, 45–55. [Google Scholar] [CrossRef]
- Natalio, A.I.M.; Ahmed, M.; Back, M.A.; Richards, A.; Jeffery, S. Temporal monitoring of free-living nematode communities for evaluation of soil health in an arable crop rotation. Pedobiologia 2024, 104, 150959. [Google Scholar] [CrossRef]
- Karpinska, A.; Ryan, D.; Germaine, K.; Dowling, D.; Forrestal, P.; Kakouli-Duarte, T. Soil microbial and nematode community response to the field application of recycled bio-based fertilisers in irish grassland. Sustainability 2021, 13, 12342. [Google Scholar] [CrossRef]
- Saju, A.; Van De Sande, T.; Ryan, D.; Karpinska, A.; Sigurnjak, I.; Dowling, D.N.; Germaine, K.; Kakouli-Duarte, T.; Meers, M. Exploring the short-term in-field performance of Recovered Nitrogen from Manure (RENURE) materials to substitute synthetic nitrogen fertilisers. Clean. Circ. Bioecon. 2023, 5, 100043. [Google Scholar] [CrossRef]
- Costa Nde, J.F.; Pedrosa, E.M.R.; da Silva Vicente, T.F.; Machado, A.C.Z.; Guimarães, L.M.P. Nematode community structure in sugarcane fields under continuous vinasse fertigation. Pedobiologia 2024, 104, 150963. [Google Scholar] [CrossRef]
- Korthals, G.W.; Bongers, M.; Fokkema, A.; Dueck, T.A.; Lexmond, T.M. Joint toxicity of copper and zinc to a terrestrial nematode community in an acid sandy soil. Ecotoxicology 2000, 9, 219–228. [Google Scholar] [CrossRef]
- Yeates, G.W. Effects of plants on nematode community structure. Annu. Rev. Phytopathol. 1999, 37, 127–149. [Google Scholar] [CrossRef]
- Dietrich, P.; Cesarz, S.; Liu, T.; Roscher, C.; Eisenhauer, N. Effects of plant species diversity on nematode community composition and diversity in a long-term biodiversity experiment. Oecologia 2021, 197, 297–311. [Google Scholar] [CrossRef]
- AbdelRazek, G.M.; Balah, M.A. Associate plant parasitic nematodes to weed species in some newly reclaimed lands. Sci. Rep. 2023, 13, 21923. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; De Vos, O.J.; Korthals, G.W.; van Bruggen, A.H. Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Grosso, F.; Bååth, E.; De Nicola, F. Bacterial and fungal growth on different plant litter in Mediterranean soils: Effects of C/N ratio and soil pH. Appl. Soil Ecol. 2016, 108, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, S.X. Nitrate N loss by leaching and surface runoff in agricultural land: A global issue (a review). Adv. Agron. 2019, 156, 159–217. [Google Scholar]
- Li, Q.; Jiang, Y.; Liang, W.; Lou, Y.; Zhang, E.; Liang, C. Long-term effect of fertility management on the soil nematode community in vegetable production under greenhouse conditions. Appl. Soil Ecol. 2010, 46, 111–118. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, F.; Zou, B.; Chen, Y.; Zhao, J.; Mo, Q.; Li, Y.; Li, X.; Xia, H. Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol. Fertil. Soils 2015, 51, 207–215. [Google Scholar] [CrossRef]
- Devi, T.S.; Behera, H.S.; Madhu, A.; Samreen, S.; Chaudhary, S.; Koushal, S.; Priya, P. A comprehensive review on integrated pest management in nematode. Int. J. Res. Agron. 2024, 7, 760–765. [Google Scholar] [CrossRef]
- Liu, T.; Guo, R.; Ran, W.; Whalen, J.K.; Li, H. Body size is a sensitive trait-based indicator of soil nematode community response to fertilisation in rice and wheat agroecosystems. Soil Biol. Biochem. 2015, 88, 275–281. [Google Scholar] [CrossRef]
- Geissler, B.; Hermann, L.; Mew, M.C.; Steiner, G. Striving toward a circular economy for phosphorus: The role of phosphate rock mining. Minerals 2018, 8, 395. [Google Scholar] [CrossRef]
- Liu, H. Ammonia Synthesis Catalysts: Innovation and Practice; World Scientific Publishing: Singapore, 2013. [Google Scholar]
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilisers as a route to controlled release of nutrients. In Controlled Release Fertilisers for Sustainable Agriculture; Academic Press: Cambridge, UK, 2021; pp. 231–245. [Google Scholar]
- Huygens, D.; Orveillon, G.; Lugato, E.; Tavazzi, S.; Comero, S.; Jones, A.; Gawlik, B.; Saveyn, H.G.M. Technical Proposals for the Safe Use of Processed Manure Above the Threshold Established for Nitrate Vulnerable Zones by the Nitrates Directive (91/676/EEC); Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
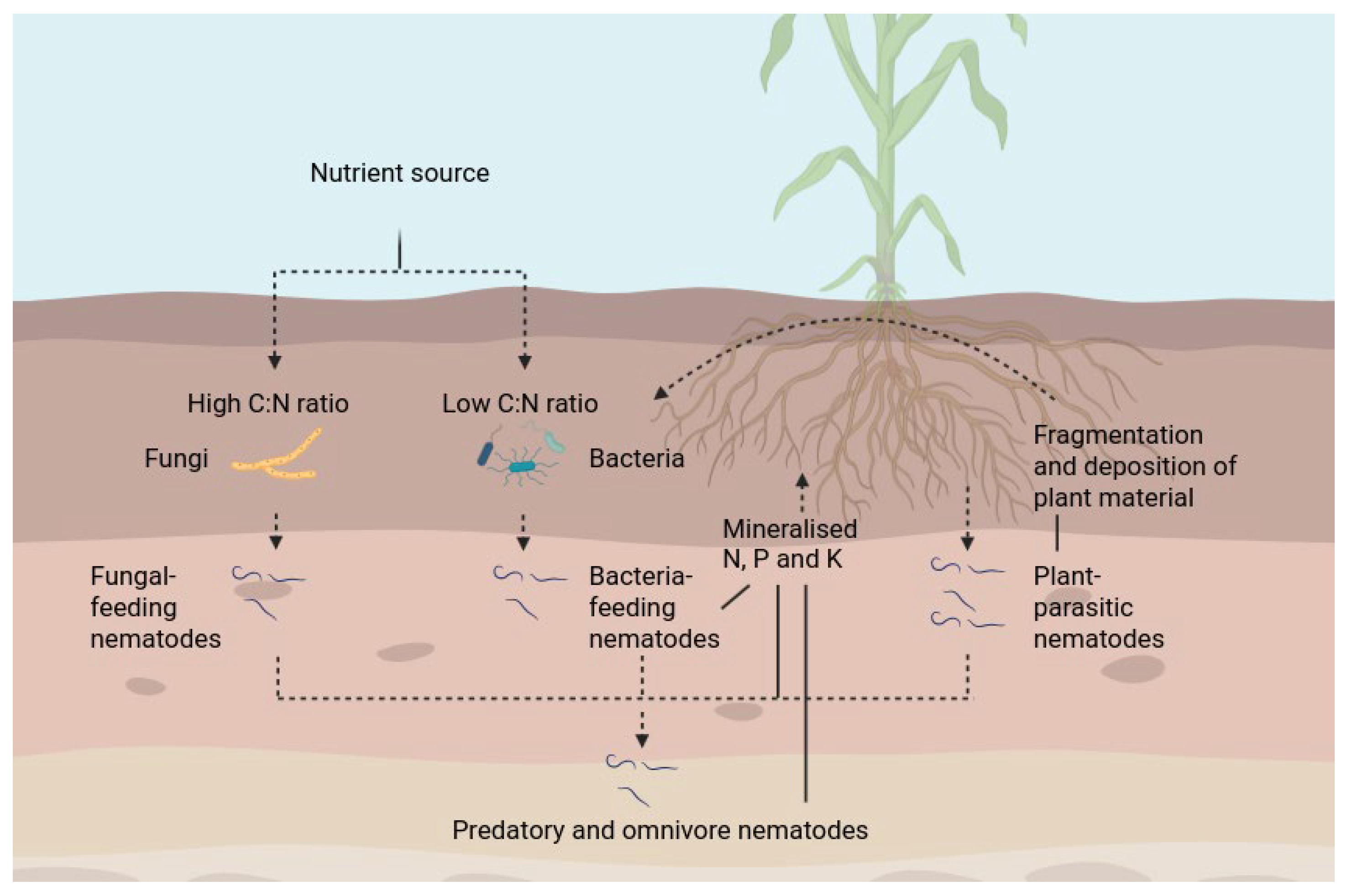
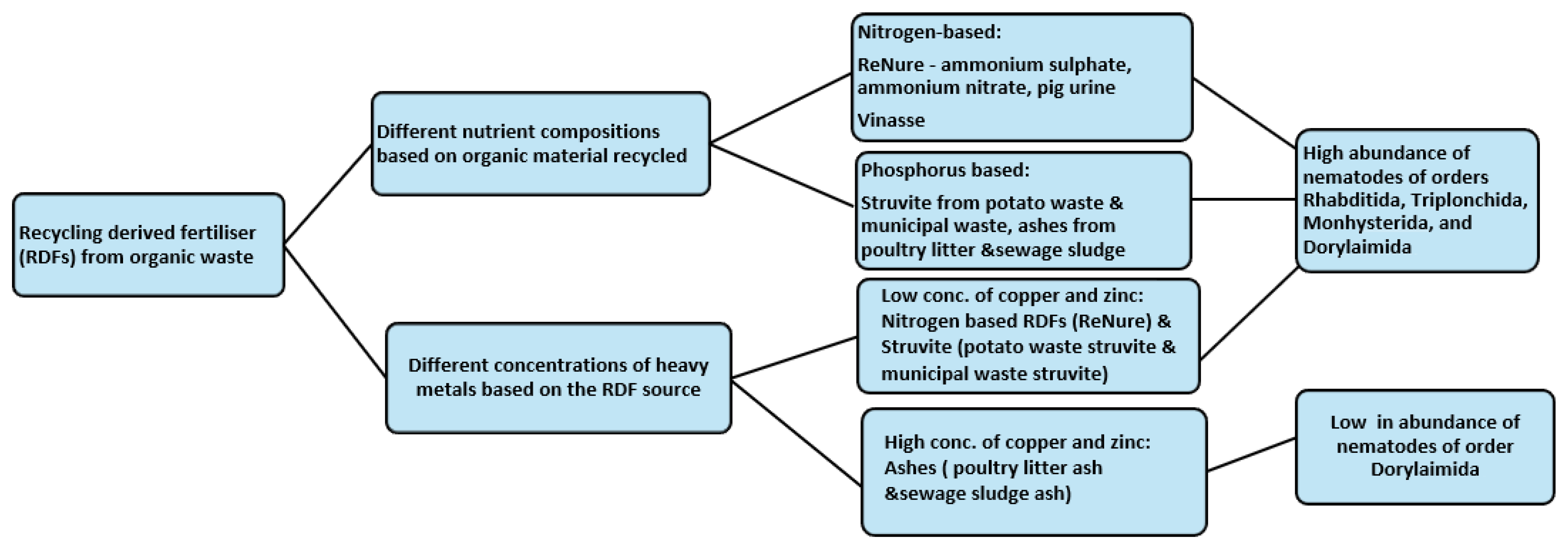
| Factor | BF | FF | O-P | Nematode Indices |
|---|---|---|---|---|
| Organic Fertilisation | ||||
| Type of organic matter based on (C:N) | ↑ at low C:N in organic | slow ↑ at high C:N in organic | ↑ at high C:N in organic | ↑ CI at high C:N; MI varies; ↑ in EI; SI varies; no impact on PPI |
| Application rate | ↑ at high-rate organic | ↑ at high-rate organic | ↑ at high-rate organic | MI varies across studies |
| Quality (compost maturity) | No impact at low maturity | No impact at low maturity | No impact at low maturity | |
| Experimental factors | varies based on conditions | varies based on conditions | varies based on conditions | |
| Tillage | ↑ under tillage | ↓ under both organic and inorganic under tillage | ↓ under both organic and inorganic under tillage | |
| Crop type | May favour some taxa in some crops | May favour some taxa in some crops | May favour some taxa in some crops | |
| Inorganic fertilisation | BF | FF | O-P | Nematode indices |
| Changes in soil chemistry (soil pH) | ↓ under inorganic at low pH | ↓ under inorganic at low pH | ||
| Application rate | ↑ at low application rates | ↓ under inorganic at a high application | ↓ under inorganic at a high application | ↓ in MI |
| Ammonium suppression | ↓ at high rates of N | ↓ at high rates of N | ↓ at high rates of N | |
| Predator–prey abundance | ↑ in the presence of more bacteria | ↑ in the presence of more fungi | ↑ in the presence of prey | ↑ in SI with more omnivore-predator nematodes |
| Tillage | ↑ under tillage and even higher under no tillage | ↓ under tillage lower than under organic | ↓ under no tillage | ↑ SI under no tillage; ↓ in EI under tillage |
| Method of nematode analysis | No effect | No effect | Sensitive to metabarcoding than morphological identification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atira, L.S.; Kakouli-Duarte, T. Implications of Fertilisation on Soil Nematode Community Structure and Nematode-Mediated Nutrient Cycling. Crops 2025, 5, 50. https://doi.org/10.3390/crops5040050
Atira LS, Kakouli-Duarte T. Implications of Fertilisation on Soil Nematode Community Structure and Nematode-Mediated Nutrient Cycling. Crops. 2025; 5(4):50. https://doi.org/10.3390/crops5040050
Chicago/Turabian StyleAtira, Lilian Salisi, and Thomais Kakouli-Duarte. 2025. "Implications of Fertilisation on Soil Nematode Community Structure and Nematode-Mediated Nutrient Cycling" Crops 5, no. 4: 50. https://doi.org/10.3390/crops5040050
APA StyleAtira, L. S., & Kakouli-Duarte, T. (2025). Implications of Fertilisation on Soil Nematode Community Structure and Nematode-Mediated Nutrient Cycling. Crops, 5(4), 50. https://doi.org/10.3390/crops5040050







