Abstract
Entomopathogenic fungi are among the most promising non-chemical alternatives for the control of many serious phytophagous insect pests, such as moth species. The present research investigates the use of the little-studied entomopathogenic fungus Beauveria varroae as a biocontrol agent against the notorious pests Helicoverpa armigera and Sesamia nonagrioides in laboratory conditions. Conidial suspensions of B. varroae were prepared at 103-104-105-106-107-108 conidia/mL to assess their insecticidal potential. In this study, we used 100 3rd-instar larvae for each concentration. During the lab bioassays, almost complete mortality of 35–96.6% was recorded for H. armigera larvae and 40–96.6% for S. nonagrioides larvae 10 days after exposure. The lethal effect of the entomopathogen was related to both dose and exposure time of the entomopathogen, with higher concentrations resulting in increased mortality. The survival effect of S. nonagrioides and H. armigera larvae was dependent on the hazard effect of the used dose and the exposure time. These findings indicate that B. varroae has potential as a biocontrol agent. Further research will elucidate this new isolate and optimize application methods in field conditions.
1. Introduction
Chewing damage from larvae of stage moth pests (Lepidoptera) has been an issue in yield reduction for a plethora of plants, deteriorating qualitative and quantitative production. Helicoverpa armigera Hübner (Lepidoptera: Noctuidae), commonly known as the cotton bollworm, is a common pest of cotton and one of the most destructive agricultural pests worldwide [1]. It is widely distributed across the world [2,3], including Greece [4]. It typically completes 4–6 generations per year [5] in most cropping areas. Temperatures around 25 °C and optimal climates for the development and reproduction of this pest [6] make Mediterranean and tropical regions ideal habitats [7]. H. armigera larvae have a yellowish-white body with a dark brown or black head capsule and undergo six instars before finishing development [8]. The larvae are known for their aggressive feeding habits on numerous crops such as cotton Gossypium hirsutum L. (Malvales: Malvaceae) [9], chickpea Cicer arietinum L. (Fabales: Fabaceae) [10], and other legumes [2,11,12]. It has been reported that H. armigera has caused significant economic losses of around $3 billion per year [13,14], with infestation rates ranging from 16% to 45% for tomatoes in India [15]. While chemical control with synthetic pesticides such as organophosphates has generally been effective [16,17], resistance to insecticides has been widely documented [18,19,20,21], emphasizing the urgent need for alternative control strategies, including biological control.
Sesamia nonagrioides Lefebvre (Lepidoptera: Noctuidae) is a serious pest of corn with a wide host range among many plant species of the Poaceae family [22,23] and a strong preference for Zea mays L. (Poales: Poaceae) [22,24]. It is considered a major pest in Mediterranean areas [25], including Greece [26], as well as parts of Africa and Asia [26,27]. It has four growth stages (egg, larvae, pupae, and adult), and its larvae overwinter in maize stalks and roots [28,29]. The young larvae feed on the leaf tissue, where they remain in groups, and after two days, they bore galleries into the stems for up to a month [30]. These larval entry points serve as sites of infection for pathogenic fungi [30], leading to a decline in crop quality. In Greece, 3–4 generations per year have been recorded [31]. Chemical control is common for S. nonagrioides but is usually ineffective due to the prolonged flight of the adults, the larvae’s rapid plant penetration, and their cryptic behavior [32]. Also, its control with organophosphates and organochlorides can be very challenging because of the narrow window for insecticide implementation between egg hatching and larval entry to the plants [33].
The overuse of chemical insecticides poses significant risks to human health, contributes to the development of pest resistance, and causes environmental damage due to their long-lasting presence and impact on nontarget species. Therefore, adopting environmentally friendly pest control strategies is essential. Numerous studies highlight the value of natural products as a safer alternative to synthetic pesticides for managing major agricultural pests. Biocontrol of H. armigera and S. nonagrioides with entomopathogenic fungi (EPF) has shown promise in several studies [34,35], can be implemented within the framework of integrated pest management (IPM) [36], generally does not pose environmental concerns, and is recognized as an eco-friendly alternative to broad-spectrum synthetic insecticides [37,38]. Species such as Beauveria bassiana (Hypocreales: Cordycipitaceae) and Metarhizium robertsii (Hypocreales: Clavicipitaceae) have been successfully tested against S. nonagrioides larvae [39]. Isaria fumosorosea (Hypocreales: Cordycipitaceae) and M. robertsii have also been reported as endophytes [40] capable of reducing Lepidoptera feeding damage [41], and their action has proven effective against S. nonagrioides larvae in Sorghum bicolor L. (Poales: Poaceae) [39,42]. Members of the Beauveria genus have been reported as effective for H. armigera in Z. mays [43], broad bean (Vicia faba L.) (Fabales: Fabaceae) [44], and tomato (Solanum lycopersicum L.) (Solanales: Solanaceae) [45]. B. bassiana has been shown to significantly inhibit H. armigera larvae [8,46]. Additionally, B. bassiana and M. robertsii have been effectively used as biocontrol agents against the reproductive phases of H. armigera [47]. B. bassiana and Purpureocillium lilacinum (Hypocreales: Ophiocordycipitaceae) can be an effective tool for H. armigera control while also enhancing cotton growth [48]. Although EPF offer considerable promise for Lepidoptera pest management, further research is needed to explore their full potential. While B. bassiana has been widely studied, B. varroae has only recently been identified as a potential biocontrol agent [49], with very limited studies conducted so far [49,50].
This research aims to evaluate the lethal effects of the EPF B. varroae on the larvae of H. armigera and S. nonagrioides, offering a potential alternative to chemical control strategies for managing these pests in Greece.
2. Materials and Methods
2.1. Insect Laboratory Rearing
Both insects’ larvae were collected from the crop fields of the University of Ioannina, Arta Campus, and reared on artificial substrates under laboratory conditions. For the successive stages of insect development, a room with constant temperature (25 ± 1 °C), humidity of 60–70%, and a photoperiod of 16:8 h light:dark was used.
The artificial diets were composed of 1 g of potassium sorbate, 2 g of nipagin, 3 g of ascorbic acid, 25 g of soy flour, 4 g of vitamin mixture Vanderzant for insects, 25 g maize germ, 400 g cellulose, 50 g of brewer yeast, 40 mL HCL (2N), and 700 mL water for S. nonagrioides, and of 12 g ascorbic acid, 7.5 g methyl 4-hydroxybenzoate, 4.5 g sorbic acid, 0.6 g cholesterol, 0.6 mL cottonseed oil, and 0.6 g vitamin mixture Vanderzant for insects for H. armigera. Adult insects were kept in growth chambers (PHC Europe/Sanyo/Panasonic Biomedical MLR-352-PE) with controlled environmental conditions (25 ± 10C, 65 ± 5% relative humidity, complete darkness).
2.2. B. varroae Laboratory Isolate
The fungal isolate utilized in this study was originally collected from an urban area dominated by Pinus halepensis (Pinales: Pinaceae) in the Dasylio region of Patras [50]. The isolate was incubated in complete darkness on Sabouraud Dextrose Agar at 25 ± 1 °C for a period of 15 days. Fresh conidia were harvested from the cultures following this incubation period for use in bioassays. To prepare the conidial suspensions, the surface of the Petri dishes was gently scraped with a sterile loop, and the collected conidia were transferred into a 500 mL sterile glass beaker containing 50 mL of distilled water supplemented with 0.05% Tween 80. The suspension was filtered through multiple layers of sterile cloth and homogenized using a magnetic stirrer for 5 min [51]. The concentration of conidia was determined using a Neubauer hemocytometer under a phase-contrast microscope (Axioplan; Zeiss, Oberkochen, Germany), based on two methodological approaches, with one being selected for final use. Viability of conidia was assessed following the protocol described by Goettel and Inglis (1997) [52]. Three drops of a diluted suspension (~1 × 106 conidia/mL) were placed on MEPA medium and incubated at 25 ± 1 °C and 65 ± 5% relative humidity in the dark for 24 h. Following staining with lactophenol cotton blue, germination was examined under a microscope (Zeiss Axioplan, ×400). Conidia were considered germinated if the germ tube was at least equal in length to the conidium’s width. In all strains tested, over 90% of the conidia successfully germinated.
2.3. Effect of B. varroae Isolate on the Mortality of the S. nonagrioides and H. armigera Larvae
Conidial suspensions of B. varroae were prepared at concentrations ranging from 103 to 108 conidia/mL to evaluate their insecticidal efficacy. For each concentration, 100 3rd-instar larvae were used. Groups of ten larvae were treated with 2 mL of the respective fungal suspension and transferred into sterile 9 cm Petri dishes. The suspensions were applied using a Potter spray tower (Burkard Manufacturing Co. Ltd., Rickmansworth, Hertfordshire, UK) at a pressure of 1 kgf/cm2. Following treatment, larvae were kept under standard rearing conditions and provided with the artificial diet throughout the experimental period. Mortality was recorded daily over a 10-day observation period. Control groups were treated with 2 mL of sterile distilled water containing 0.05% Tween 80. Dead larvae were collected daily, briefly surface-sterilized using a 2% sodium hypochlorite solution to prevent contamination by opportunistic saprophytic fungi, and then placed on sterile paper towels in Petri dishes. These dishes were incubated in the dark at 25 °C for 5 to 7 days. Larvae exhibiting fungal growth were recorded as infected. The presence of Beauveria was verified microscopically by examining conidial structures and hyphal development.
2.4. Statistical Analysis
In all experimental replicates, mortality in the control group remained consistently low (<3%), and therefore, no correction of mortality rates was deemed necessary. Percentage mortality data were subjected to arcsine square root transformation prior to analysis. A two-way analysis of variance (ANOVA) was conducted using the General Linear Model (GLM) procedure in SPSS software version 29.0. Where significant F-values were detected, pairwise comparisons of means were performed using the Bonferroni post hoc test.
To assess the impact of the fungal isolates on S. nonagrioides and H. armigera, the Cox proportional hazards regression model was employed. This survival analysis model examines how covariates influence the hazard function, which represents the risk of an event (e.g., death) occurring at a specific time, given survival up to that point. Differences in survival curves among treatments were assessed using the Breslow (Generalized Wilcoxon) method [53].
The Cox model is mathematically represented as
h(t) = h0(t) × exp{b1 × 1 + b2 × 2 + ⋯ + bp × p}
The hazard function, typically denoted as h(t) or λ(t), reflects the instantaneous risk that an event will occur at time t for an individual who has survived until that time. While the survivor function estimates the probability of continued survival, the hazard function quantifies the immediate likelihood of the event occurring.
In this equation, h(t) is the hazard at time t, influenced by a set of p covariates (x1, x2, …, xₚ) with corresponding coefficients (b1, b2, …, bₚ). The baseline hazard h0(t) represents the hazard when all covariates are set to zero (since exp(0) = 1), and it may vary with time, as indicated by the t notation.
3. Results
According to the laboratory bioassay results, the tested EPF isolate caused various levels of H. armigera and S. nonagrioides mortality. Larval mortality varied as a function of the applied dose of B. varroae. The cumulative mortality percentages of S. nonagrioides larvae, on day 10 after exposure, were 40 (103) to 96.6% (108), and the mortality of H. armigera larvae was 35 (103) to 96.6% (108) (Figure 1). In the control treatments, which consisted of adults that had been treated only with ddH2O, mortality percentage was 3.3% for the S. nonagrioides and H. armigera larvae (Figure 1).
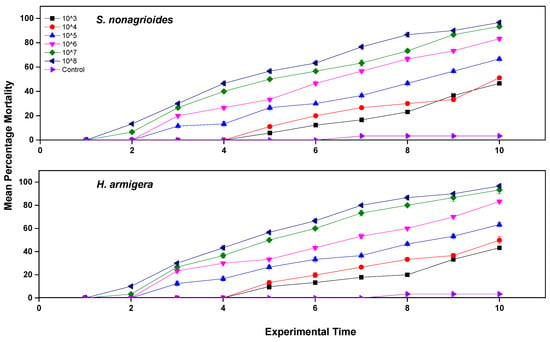
Figure 1.
Mean mortality (% ± sd) of S. nonagrioides and H. armigera 3rd larvae, which were exposed to a range of 103 to 108 conidia mL−1 B. varroae.
The survival effect of S. nonagrioides and H. armigera larvae was dependent on the hazard effect of the used dose and the exposure time (Table 1). The dose with both the highest hazard effect and the lowest survival rate for both of the insects was 108 conidia per mL. The hazard effect was higher than the survival rate, especially at doses over 106 conidia per mL.

Table 1.
Survival and hazard effect of EPF on S. nonagrioides and H. armigera (Cox regression method).
The main effects and interactions of the three factors, such as insect, the dose, and finally the experimental time, proved to be significant. This indicates that B. varroae affected the survival time of the insect in diverse ways (Table 2). The effect of the three factors, Insect * Experimental Time * Dose, was significant for the survival of the insect.

Table 2.
Three-way ANOVA with post hoc Bonferroni test on mortality as a variable.
Experimental duration and fungal dose had a statistically significant effect, with both presenting p-values < 0.001 (Table 3). Among these, dose exerted a pronounced influence on the lethal time for both insect species. The Exp(B) value for the upper bound of the 95.0% confidence interval indicated an increased hazard ratio for both S. nonagrioides and H. armigera. For the insect species factor, the Exp(B) was 0.032, suggesting that it was associated with a lower hazard rate and consequently prolonged survival. This indicates that the species variable had a limited impact on reducing the time to death in treated larvae. Conversely, the coefficient (B) for dose was positive, with an Exp(B) greater than 1, indicating that higher doses were strongly associated with increased hazard and, therefore, reduced survival time in both insect species. Dose consistently exhibited a positive B coefficient and Exp(B) > 1, confirming its strong role in accelerating larval mortality. By contrast, the insect species factor showed a negative B value with an Exp(B) < 1, reinforcing its association with lower mortality risk and extended survival time.

Table 3.
Variables in the equation from Cox regression (Chi–square: 412.114, df = 3, p < 0.001).
4. Discussion
Our findings revealed that B. varroae induced a dose-dependent lethal effect on both S. nonagrioides and H. armigera, confirming its potential as a promising biocontrol agent. The infection process of entomopathogenic fungi (EPF) is both intricate and highly specialized, involving several components that remain only partially elucidated, such as host–pathogen interactions, strain-specific virulence, and variability in host susceptibility [54]. Beyond the genetic diversity inherent to entomopathogenic fungi, differences in susceptibility between insect hosts also play a critical role [39]. Fungal infection typically begins with the attachment of conidia to the insect cuticle, followed by germination and active penetration of the integument. During this process, the fungus must overcome both physical and biochemical defenses of the host [55]. To breach these barriers, EPF secrete cuticle-degrading enzymes, including proteases and chitinases, which facilitate entry by breaking down structural components of the cuticle [56]. The rate at which fungal propagules succeed in penetrating the host cuticle can vary, largely depending on its thickness and structural composition [57].
Amer et al. (2008) [58] observed higher larval mortality rates of S. littoralis (Lepidoptera: Noctuidae) when treated with Beauveria bassiana concentrations of 1 × 105 and 1 × 106 conidia/mL, while treatments with higher concentrations (107–109 conidia/mL) resulted in comparatively lower mortality rates than those reported in the present study. Asi et al. (2013) [59] reported greater efficacy against S. litura larvae at similar dosage levels than those noted in the earlier studies. Another investigation demonstrated that a B. bassiana strain induced high mortality at concentrations of 1 × 106, 1 × 107, and 1 × 108 conidia/mL [60]. Similarly, research on M. anisopliae indicated mortality rates of approximately 88–90% at dosages of 2 × 106 and 1 × 107 conidia/mL against S. littoralis larvae. However, the same fungus showed less than 5% mortality against S. litura larvae even at the highest tested concentration [61]. Furthermore, Fite et al. (2020) [62] reported a broad range of mortality (20–70%) in H. armigera larvae following exposure to different isolates of M. anisopliae.
This is the first report of the use of B. varroae against these very important insects. Overall, an increase in concentrations was found to be more potent for both EPF used against S. nonagrioides and H. armigera. The effectiveness of EPF as insecticides is greatly affected by various factors, including the behavior, age, and population density of the insect species, diet, and genetic traits, as well as its physiology and morphology [63]. Consequently, differences in insect susceptibility to EPF cannot be explained solely by the amount of conidia used. The findings of [64] align with our results, demonstrating that a concentration of 1 × 108 spores/mL produced the highest mortality in Maruca vitrata (Lepidoptera: Crambidae). Additionally, [65] reported that elevated EPF doses caused significantly greater mortality in the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) compared to lower concentrations. However, for S. nonagrioides and H. armigera, mortality rates at low concentrations of B. varroae were moderate. The virulence of fungal strains is largely influenced by the number of spores and their proliferation within the host organism [66]. Moreover, sporulation has been shown to correlate directly with host mortality, as indicated by Pauli et al. (2018) [67].
In the evaluation of B. varroae virulence, significantly high mortality was observed in 3rd-instar larvae of S. nonagrioides and H. armigera. Generally, older larval instars tend to exhibit increased resistance to pathogens, likely due to the development of thicker cuticles; however, this trend varies depending on the specific insect–pathogen interaction. For instance, 1st-instar larvae of Ostrinia nubilalis (Lepidoptera: Crambidae) have been shown to be more susceptible to B. bassiana compared to 4th-instar larvae [68]. Conversely, in the case of the diamondback moth (DBM), 3rd- and 4th-instar larvae demonstrate greater susceptibility to fungal infections than 2nd-instar larvae [69]. Some researchers attribute this variability in susceptibility to the moulting process, as shedding the old cuticle can remove attached fungal spores [70,71]. Nonetheless, additional factors such as alterations in epicuticular wax composition and hemolymph constituents also likely influence susceptibility, as documented in other insect species. For example, Hafez et al. (1997) [72] mentioned that B. bassiana was more virulent to young larvae of the potato tuber moth compared to older instars. Similarly, Qayyum et al. (2015) [45] found that 2nd-instar larvae were more susceptible than older stages, potentially due to cuticle hardening in later instars, as suggested by Wraight et al. (2010) [73]. In our study, mortality rates at the concentration of 1 × 108 conidia/mL of B. varroae were notably high, whereas lower concentrations resulted in moderate mortality.
These differences could be attributed to variations in the timing of application or the repeated subculturing of fungal isolates for purification and propagation purposes. Butt and Goettel (2000) [74] emphasized that to enhance the virulence of an isolate intended for bioassays, it is advisable to passage the fungus through an insect host prior to culturing it on artificial media.
5. Conclusions
Beyond confirming the pathogenicity of B. varroae against insect pests, our study reinforces that the effectiveness of any fungal species is isolate-dependent, with virulence varying according to the host. Successful infection and germination are influenced by a combination of biotic and abiotic factors, including host susceptibility, developmental stage, incubation duration, temperature, and humidity. Both the literature and our findings suggest that an EPF species may exhibit strong virulence against certain hosts while being less effective or ineffective against others. Therefore, comprehensive screening is essential to identify isolates with suitable virulence for further development in fungal-based biocontrol formulations. The application of B. varroae for managing significant crop pests such as S. nonagrioides and H. armigera appears promising within integrated pest management (IPM) frameworks. Nonetheless, additional studies are needed to isolate novel strains and evaluate their virulence and host specificity in depth. Moreover, improvements are necessary to enhance fungal performance under challenging environmental conditions, develop longer-lasting and easier-to-apply formulations, and maintain pathogen virulence. This work represents a preliminary step in exploring new isolates, and further research is critical to establish whether B. varroae can become a reliable component of insect pest control strategies.
Author Contributions
Conceptualization, S.M.; methodology, S.M.; software, S.M.; validation, S.M., P.A.E. and I.L.; formal analysis, V.P.; investigation, S.M., V.P. and P.A.E.; resources, V.P. and S.M.; data curation, S.M. and P.A.E.; writing—original draft preparation, S.M. and V.P.; writing—review and editing, S.M., P.A.E., V.P. and I.L.; visualization, S.M.; supervision, G.P. and S.M.; project administration, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author S.M.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berenbaum, M.R.; Bush, D.S.; Liao, L.H. Cytochrome P450-Mediated Mycotoxin Metabolism by Plant-Feeding Insects. Curr. Opin. Insect Sci. 2021, 43, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Maqsood, A.; Ahmed, M.; Tariq, M.; Ali, M.; Qureshi, R. Toxicity, Antifeedant and Sub-Lethal Effects of Citrullus Colocynthis Extracts on Cotton Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Pak. J. Zool. 2017, 49, 2019–2026. [Google Scholar] [CrossRef]
- Tay, W.T.; Soria, M.F.; Walsh, T.; Thomazoni, D.; Silvie, P.; Behere, G.T.; Anderson, C.; Downes, S. A Brave New World for an Old World Pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE 2013, 8, e80134. [Google Scholar] [CrossRef]
- Milonas, P.; Gogou, C.; Papadopoulou, A.; Fountas, S.; Liakos, V.; Papadopoulos, N.T. Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) and Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) in a Cotton Production Area. Neotrop. Entomol. 2016, 45, 240–251. [Google Scholar] [CrossRef]
- Fitt, G.P. The Ecology of Heliothis Species in Relation to Agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–53. [Google Scholar] [CrossRef]
- Mironidis, G.K.; Savopoulou-Soultani, M. Effects of Constant and Changing Temperature Conditions on Diapause Induction in Helicoverpa armigera (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2012, 102, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Burgio, G.; Ravaglia, F.; Maini, S.; Bazzocchi, G.G.; Masetti, A.; Lanzoni, A. Mating Disruption of Helicoverpa armigera (Lepidoptera: Noctuidae) on Processing Tomato: First Applications in Northern Italy. Insects 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A Review on Biological Interactions and Management of the Cotton Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Dias, P.M.; De Souza Loureiro, E.; Pessoa, L.G.A.; De Oliveira Neto, F.M.; De Souza Tosta, R.A.; Teodoro, P.E. Interactions between Fungal-Infected Helicoverpa armigera and the Predator Chrysoperla externa. Insects 2019, 10, 309. [Google Scholar] [CrossRef]
- Ahmed, K.; Awan, M.S. Integrated Management of Insect Pests of Chickpea Cicer arietinum (L. Walp) in South Asian Countries: Present Status and Future Strategies—A Review. Pak. J. Zool. 2013, 45, 1125–1145. [Google Scholar]
- Cunningham, J.P.; Zalucki, M.P.; West, S.A. Learning in Helicoverpa armigera (Lepidoptera: Noctuidae): A New Look at the Behaviour and Control of a Polyphagous Pest. Bull. Entomol. Res. 1999, 89, 201–207. [Google Scholar] [CrossRef]
- Patankar, A.G.; Giri, A.P.; Harsulkar, A.M.; Sainani, M.N.; Deshpande, V.V.; Ranjekar, P.K.; Gupta, V.S. Complexity in Specificities and Expression of Helicoverpa armigera Gut Proteinases Explains Polyphagous Nature of the Insect Pest. Insect Biochem. Mol. Biol. 2001, 31, 453–464. [Google Scholar] [CrossRef]
- Haile, F.; Nowatzki, T.; Storer, N. Issues Overview of Pest Status, Potential Risk, and Management Considerations of Helicoverpa armigera (Lepidoptera: Noctuidae) for U.S. Soybean Production. J. Integr. Pest. Manag. 2021, 12, 3–4. [Google Scholar] [CrossRef]
- Sharma, H. Heliothis/Helicoverpa Management: The Emerging Trends and Need for Future Research, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429082764. [Google Scholar]
- Thakur, P.; Rana, R.; Sharma, A.; Priyanka Thakur, C.; Kumar, A. Biophysical Characters of Tomato Varieties in Relation to Resistance against Tomato Fruit Borer, Helicoverpa armigera (Hubner). J. Entomol. Zool. Stud. 2017, 5, 108–112. [Google Scholar]
- Saba, S.; Khan, M.M.; Akhtar, I.; Hussain Shah, S.W.; Maan, N.A.; Anum, W.; Manzoor, N.; Rehman, M.; Kanwal, N. Evaluation of Toxicological Responses of Helicoverpa armigera (Hübner) against Some Insecticides by Using Probit Analysis in Laboratory. Plant Prot. 2019, 3, 15–20. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Jaffar, S. Efficacy of Some Selected Chemical Insecticides and Bio-Pesticides against Tomato Fruit Worm, (Helicoverpa armigera) under the Agro Climatic Condition of Gilgit Baltistan, Pakistan. J. Entomol. Zool. Stud. 2014, 3, 50–52. [Google Scholar]
- Buès, R.; Bouvier, J.C.; Boudinhon, L. Insecticide Resistance and Mechanisms of Resistance to Selected Strains of Helicoverpa armigera (Lepidoptera: Noctuidae) in the South of France. Crop Prot. 2005, 24, 814–820. [Google Scholar] [CrossRef]
- Pietrantonio, P.V.; Junek, T.A.; Parker, R.; Mott, D.; Siders, K.; Troxclair, N.; Vargas-Camplis, J.; Westbrook, J.K.; Vassiliou, V.A. Detection and Evolution of Resistance to the Pyrethroid Cypermethrin in Helicoverpa zea (Lepidoptera: Noctuidae) Populations in Texas. Environ. Entomol. 2007, 36, 1174–1188. [Google Scholar] [CrossRef]
- Ugurlu, S.; Gurkan, M.O. Note: Insecticide Resistance in Helicoverpa armigera from Cotton-Growing Areas in Turkey. Phytoparasitica 2007, 35, 376–379. [Google Scholar] [CrossRef]
- Avilla, C.; González-Zamora, J.E. Monitoring Resistance of Helicoverpa armigera to Different Insecticides Used in Cotton in Spain. Crop Prot. 2010, 29, 100–103. [Google Scholar] [CrossRef]
- Cruz, D.; Eizaguirre, M. Host Location Behaviour of Gravid Females in the Mediterranean Corn Borer Sesamia nonagrioides: External Morphology of Antennae and Ovipositor Sensilla. Bull. Insectology 2016, 69, 181–192. [Google Scholar]
- M Camargo, A.; Arias-Martín, M.; Castañera, P.; P Farinós, G. Performance of Sesamia nonagrioides on Cultivated and Wild Host Plants: Implications for Bt Maize Resistance Management. Pest. Manag. Sci. 2020, 76, 3657–3666. [Google Scholar] [CrossRef]
- Pedigo, L.; Rice, M.; Krell, R. Entomology and Pest Management, 7th ed.; Waveland Press: Long Grove, IL, USA, 2021; ISBN 978-1-4786-3992-3. [Google Scholar]
- Malvar, R.A.; Revilla, P.; Velasco, P.; Cartea, M.E.; Ordás, A. Insect Damage to Sweet Corn Hybrids in the South Atlantic European Coast. J. Am. Soc. Hortic. Sci. 2002, 127, 693–696. [Google Scholar] [CrossRef]
- Eizaguirre, M.; Fantinou, A.A. Abundance of Sesamia nonagrioides (Lef.) (Lepidoptera: Noctuidae) on the Edges of the Mediterranean Basin. Psyche A J. Entomol. 2012, 2012, 854045. [Google Scholar] [CrossRef]
- Jika, A.K.N.; Le Ru, B.; Capdevielle-Dulac, C.; Chardonnet, F.; Silvain, J.F.; Kaiser, L.; Dupas, S. Population Genetics of the Mediterranean Corn Borer (Sesamia nonagrioides) Differs between Wild and Cultivated Plants. PLoS ONE 2020, 15, e0230434. [Google Scholar] [CrossRef]
- Gillyboeuf, N.; Anglade, P.; Lavenseau, L.; Peypelut, L. Cold Hardiness and Overwintering Strategy of the Pink Maize Stalk Borer, Sesamia nonagrioides Lef (Lepidoptera, Noctuidae). Oecologia 1994, 99, 366–373. [Google Scholar] [CrossRef]
- Maiorano, A.; Cerrani, I.; Fumagalli, D.; Donatelli, M. New Biological Model to Manage the Impact of Climate Warming on Maize Corn Borers. Agron. Sustain. Dev. 2014, 34, 609–621. [Google Scholar] [CrossRef]
- Kaçar, G.; Butrón, A.; Kontogiannatos, D.; Han, P.; Peñaflor, M.F.G.V.; Farinós, G.P.; Huang, F.; Hutchison, W.D.; de Souza, B.H.S.; Malvar, R.A.; et al. Recent Trends in Management Strategies for Two Major Maize Borers: Ostrinia nubilalis and Sesamia nonagrioides. J. Pest Sci. 2023, 96, 879–901. [Google Scholar] [CrossRef]
- Tsitsipis, J.; Gliatis, A.; Mazomenos, B. Seasonal Appearance of the Corn Stalk Borer, Sesamia nonagrioides, in Central Greece. Meded. Fac. Landbouwwet. Rijksuniv. Gent 1984, 49, 667–674. [Google Scholar]
- Blandino, M.; Saladini, M.A.; Alma, A.; Reyneri, A. Pyrethroid Application Timing to Control European Corn Borer (Lepidoptera: Crambidae) and Minimize Fumonisin Contamination in Maize Kernels. Cereal Res. Commun. 2010, 38, 75–82. [Google Scholar] [CrossRef]
- Shelton, A.M.; Zhao, J.Z.; Roush, R.T. Economic, Ecological, Food Safety, and Social Consequences of the Deployment of Bt Transgenic Plants. Annu. Rev. Entomol. 2002, 47, 845–881. [Google Scholar] [CrossRef]
- Cherry, A.J.; Banito, A.; Djegui, D.; Lomer, C. Suppression of the Stem-Borer Sesamia calamistis (Lepidoptera; Noctuidae) in Maize Following Seed Dressing, Topical Application and Stem Injection with African Isolates of Beauveria bassiana. Int. J. Pest. Manag. 2004, 50, 67–73. [Google Scholar] [CrossRef]
- Tang, L.C.; Hou, R.F. Potential Application of the Entomopathogenic Fungus, Nomuraea Rileyi, for Control of the Corn Earworm, Helicoverpa armigera. Entomol. Exp. Appl. 1998, 88, 25–30. [Google Scholar] [CrossRef]
- Skinner, M.; Parker, B.L.; Kim, J.S. Role of Entomopathogenic Fungi in Integrated Pest Management. Integr. Pest. Manag. Curr. Concepts Ecol. Perspect. 2014, 169–191. [Google Scholar] [CrossRef]
- Kambrekar, D.N.; Aruna, J. Screening for Endophytic Beauveria bassiana from Different Plants and Its Pathogenecity against Chickpea Pod Borer, Helicoverpa armigera (Hubner). J. Exp. Zool. 2018, 21, 727–731. [Google Scholar]
- Priyashantha, A.K.H.; Galappaththi, M.C.A.; Karunarathna, S.C.; Lumyong, S. Entomopathogenic Fungi: Bioweapons against Insect Pests. In The Role of Entomopathogenic Fungi in Agriculture; CRC Press: Boca Raton, FL, USA, 2024; pp. 91–116. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Chondrogiannis, C.; Grammatikopoulos, G. Effects of Three Endophytic Entomopathogens on Sweet Sorghum and on the Larvae of the Stalk Borer Sesamia nonagrioides. Entomol. Exp. Appl. 2015, 154, 78–87. [Google Scholar] [CrossRef]
- Canassa, F.; Tall, S.; Moral, R.A.; de Lara, I.A.R.; Delalibera, I.; Meyling, N.V. Effects of Bean Seed Treatment by the Entomopathogenic Fungi Metarhizium robertsii and Beauveria bassiana on Plant Growth, Spider Mite Populations and Behavior of Predatory Mites. Biol. Control 2019, 132, 199–208. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Bojke, A.; Tkaczuk, C. Effects of the Entomopathogenic Fungi Metarhizium robertsii, Metarhizium flavoviride, and Isaria fumosorosea on the Lipid Composition of Galleria Mellonella Larvae. Mycologia 2021, 113, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Grammatikopoulos, G. The Effect of Three Entomopathogenic Endophytes of the Sweet Sorghum on the Growth and Feeding Performance of Its Pest, Sesamia nonagrioides Larvae, and Their Efficacy under Field Conditions. Crop Prot. 2020, 127, 104952. [Google Scholar] [CrossRef]
- Pelizza, S.A.; Mariottini, Y.; Russo, L.M.; Vianna, M.F.; Scorsetti, A.C.; Lange, C.E. Beauveria bassiana (Ascomycota: Hypocreales) Introduced as an Endophyte in Corn Plants and Its Effects on Consumption, Reproductive Capacity, and Food Preference of Dichroplus maculipennis (Orthoptera: Acrididae: Melanoplinae). J. Insect Sci. 2017, 17, 53. [Google Scholar] [CrossRef]
- Jaber, L.R.; Vidal, S. Fungal Endophyte Negative Effects on Herbivory Are Enhanced on Intact Plants and Maintained in a Subsequent Generation. Ecol. Entomol. 2010, 35, 25–36. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Wakil, W.; Arif, M.J.; Sahi, S.T.; Dunlap, C.A. Infection of Helicoverpa armigera by Endophytic Beauveria bassiana Colonizing Tomato Plants. Biol. Control 2015, 90, 200–207. [Google Scholar] [CrossRef]
- Kalvnadi, E.; Mirmoayedi, A.; Alizadeh, M.; Pourian, H.R. Sub-Lethal Concentrations of the Entomopathogenic Fungus, Beauveria bassiana Increase Fitness Costs of Helicoverpa armigera (Lepidoptera: Noctuidae) Offspring. J. Invertebr. Pathol. 2018, 158, 32–42. [Google Scholar] [CrossRef]
- de Souza Loureiro, E.; Tosta, R.A.d.S.; Dias, P.M.; Pessoa, L.G.A.; Neto, F.M.d.O.; Devoz, G.L.R.; Muchalak, F. Performance of Metarhizium Rileyi Applied on Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Rev. Agric. Neotrop. 2020, 7, 60–65. [Google Scholar] [CrossRef]
- Lopez, D.C.; Sword, G.A. The Endophytic Fungal Entomopathogens Beauveria bassiana and Purpureocillium lilacinum Enhance the Growth of Cultivated Cotton (Gossypium hirsutum) and Negatively Affect Survival of the Cotton Bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Darsouei, R.; Karimi, J.; Stelinski, L.L. Endophytic Colonization of Sugar Beet by Beauveria varroae and Beauveria bassiana Reduces Performance and Host Preference in Army Worm, Spodoptera littoralis. Crop Prot. 2024, 175, 106441. [Google Scholar] [CrossRef]
- Lagogiannis, I.; Mantzoukas, S.; Eliopoulos, P.A.; Poulas, K. First Record of Beauveria Varroae, Cordyceps blackwelliae, and Purpureocillium lavendulum from Greece and Their Pathogenicity against Thaumetopoea pityocampa. Diversity 2023, 15, 312. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors Affecting the Occurrence and Distribution of Entomopathogenic Fungi in Natural and Cultivated Soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Goettel, M.S.; Douglas Inglis, G. Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Academic Press: Cambridge, MA, USA, 1997; pp. 213–249. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Seyedtalebi, F.S.; Safavi, S.A.; Talaei-Hassanloui, R.; Bandani, A.R. Quantitative Comparison for Some Immune Responses among Eurygaster Integriceps, Ephestia Kuehniella and Zophobas Morio against the Entomopathogenic Fungus Beuveria Bassiana. Invertebr. Surviv. J. 2017, 14, 174–181. [Google Scholar] [CrossRef]
- Vianna, M.F.; Pelizza, S.; Russo, M.L.; Toledo, A.; Mourelos, C.; Scorsetti, A.C. ISSR Markers to Explore Entomopathogenic Fungi Genetic Diversity: Implications for Biological Control of Tobacco Pests. J. Biosci. 2020, 45, 136. [Google Scholar] [CrossRef]
- Wang, C.; Feng, M.G. Advances in Fundamental and Applied Studies in China of Fungal Biocontrol Agents for Use against Arthropod Pests. Biol. Control 2014, 68, 129–135. [Google Scholar] [CrossRef]
- Kumar, A.; Sharfuddin, C. Evaluation of Beauveria bassiana and Metarhizium anisopliae, for the Management of Helicoverpa armigera (Hubner). J. Mycopathol. Res. 2022, 60, 91–97. [Google Scholar] [CrossRef]
- Amer, M.M.; El-Sayed, T.I.; Bakheit, H.K.; Moustafa, S.A.; El-Sayed, Y.A. Pathogenicity and Genetic Variability of Five Entomopathogenic Fungi Against Spodoptera littoralis. Res. J. Agric. Biol. Sci. 2008, 4, 354–367. [Google Scholar]
- Asi, M.R.; Bashir, M.H.; Afzal, M.; Zia, K.; Akram, M. Potential of Entomopathogenic Fungi For Biocontrol of Spodoptera litura Fabricius (Lepidoptera: Noctuidae). J. Anim. Plant Sci. 2013, 23, 913–918. [Google Scholar]
- El-Katatny, M.H. Virulence Potential of Some Fungal Isolates and Their Control-Promise against the Egyptian Cotton Leaf Worm, Spodoptera littoralis. Arch. Phytopathol. Plant Prot. 2010, 43, 332–356. [Google Scholar] [CrossRef]
- El Husseini, M.M.M. Effect of the Fungus, Beauveria bassiana (Balsamo) Vuillemin, on the Beet Armyworm, Spodoptera exigua (Hübner) Larvae (Lepidoptera: Noctuidae), under Laboratory and Open Field Conditions. Egypt. J. Biol. Pest. Control 2019, 29, 52. [Google Scholar] [CrossRef]
- Fite, T.; Tefera, T.; Negeri, M.; Damte, T.; Sori, W. Evaluation of Beauveria bassiana, Metarhizium anisopliae, and Bacillus thuringiensis for the Management of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) under Laboratory and Field Conditions. Biocontrol Sci. Technol. 2020, 30, 278–295. [Google Scholar] [CrossRef]
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Vidal, C.; Lacey, L.A.; Lomer, C.J.; Rougier, M. Variability in Susceptibility to Simulated Sunlight of Conidia among Isolates of Entomopathogenic Hyphomycetes. Mycopathologia 1996, 135, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.D.; Wakefield, M.E.; Price, N.; Wildey, K.B.; Chambers, J.; Moore, D.; Aquino De Muro, M.; Bell, B.A. The Potential Use of Insect-Specific Fungi to Control Grain Storage Pests in Empty Grain Stores. HGCA Proj. Rep. 2004, 1–49. [Google Scholar]
- Mehinto, J.T.; Atachi, P.; Douro-Kpindou, O.K.; Dannou, E.A.; Tamò, M. Mortality of Maruca vitrata (Lepidoptera: Crambidae) Larval Stages Induced by Different Doses of the Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana. Int. J. 2014, 2, 273–285. [Google Scholar]
- Freed, S.; Feng-Liang, J.; Naeem, M.; Shun-Xiang, R.; Hussian, M. Toxicity of Proteins Secreted by Entomopathogenic Fungi against Plutella xylostella (Lepidoptera: Plutellidae). Int. J. Agric. Biol. 2012, 14, 291–295. [Google Scholar]
- Ali, S.; Huang, Z.; Ren, S. Production of Cuticle Degrading Enzymes by Isaria fumosorosea and Their Evaluation as a Biocontrol Agent against Diamondback Moth. J. Pest Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Pauli, G.; Mascarin, G.M.; Eilenberg, J.; Delalibera Júnior, I. Within-Host Competition between Two Entomopathogenic Fungi and a Granulovirus in Diatraea saccharalis (Lepidoptera: Crambidae). Insects 2018, 9, 64. [Google Scholar] [CrossRef]
- Feng, Z.; Carruthers, R.I.; Roberts, D.W.; Robson, D.S. Age-Specific Dose-Mortality Effects of Beauveria bassiana (Deuteromycotina: Hyphomycetes) on the European Corn Borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). J. Invertebr. Pathol. 1985, 46, 259–264. [Google Scholar] [CrossRef]
- Vandenberg, J.D.; Ramos, M.; Altre, U.-A.J. Dose-Response and Age-and Temperature-Related Susceptibility Dose-Response and Age-and Temperature-Related Susceptibility of the Diamondback Moth (Lepidoptera: Plutellidae) to Two of the Diamondback Moth (Lepidoptera: Plutellidae) to Two Isolates of Isolates of Beauveria bassiana (Hyphomycetes: Moniliaceae). Entomol. Collect. 1998, 27, 1017–1021. [Google Scholar]
- Ferron, P. Étude En Laboratoire Des Conditions Écologiques Favorisant Le Développement de La Mycose a Beauveria tenella Du Ver Blanc. Entomophaga 1967, 12, 257–293. [Google Scholar] [CrossRef]
- Hafez, M.; Zaki, F.N.; Moursy, A.; Sabbour, M. Biological Effects of the Entomopathogenic Fungus, Beauveria bassiana on the Potato Tuber Moth Phthorimaea operculella (Seller). Anz. Schadlingskunde Pflanzenschutz Umweltschutz 1997, 70, 158–159. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ramos, M.E.; Avery, P.B.; Jaronski, S.T.; Vandenberg, J.D. Comparative Virulence of Beauveria bassiana Isolates against Lepidopteran Pests of Vegetable Crops. J. Invertebr. Pathol. 2010, 103, 186–199. [Google Scholar] [CrossRef]
- Butt, T.M.; Goettel, M.S. Bioassays of Entomogenous Fungi. Bioassays Entomopathog. Microbes Nematodes 2000, 141–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).