Carbon Sequestration for Global-Scale Climate Change Mitigation: Overview of Strategies Plus Enhanced Roles for Perennial Crops
Abstract
1. Introduction
2. Restoring the Carbon Cycle: The Role of Emissions Reduction and Sequestration
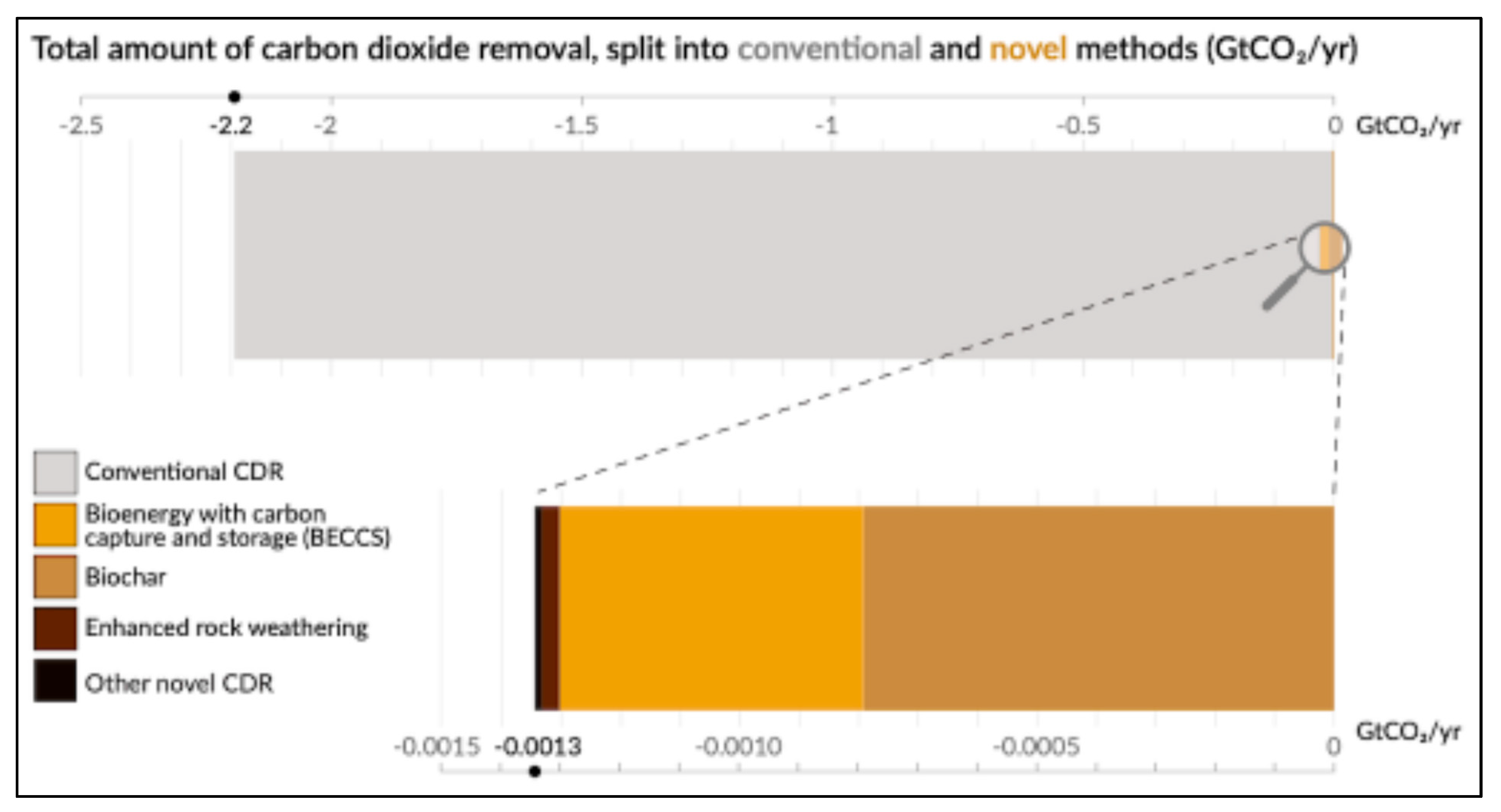
3. Industrial Carbon Sequestration
4. Biological Carbon Sequestration
4.1. Re-Engineering Biological CO2 Fixation
4.2. Natural Vegetation
4.3. Afforestation and Reforestation
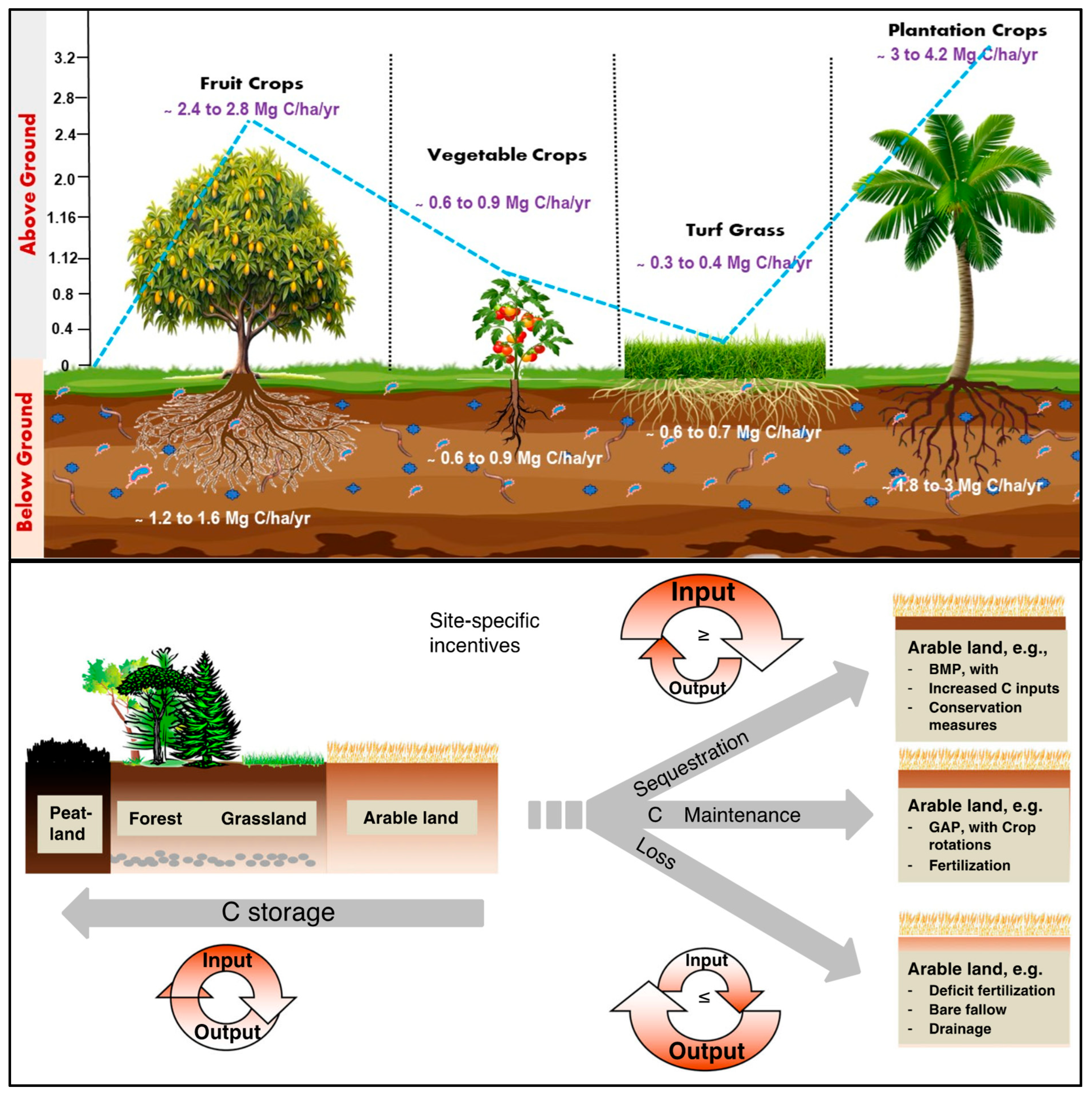
4.4. Soil Carbon Sequestration
4.5. Ocean Fertilization
4.6. Biomass
4.7. Biochar
4.8. Wood Chips/Pellets
5. The Potential of Tropical Perennial Crops
5.1. Cocoa
5.2. Coffee
5.3. Banana
5.4. Coconut
5.5. Rubber
5.6. Oil Palm
6. The Potential of Selected Non-Tropical Perennial Crops
6.1. Olives
6.2. Grapes
6.3. Temperate/Boreal Forestry Crops
7. The Roles of Carbon Trading Schemes
- Utilize carbon offset mechanisms and carbon credits to incentivize sustainable practices across the entire value chain, enhancing environmental responsibility.
- Monetization Opportunities: Emphasize the improved potential for stakeholders, particularly smallholders, to monetize carbon credits, creating an additional income stream and supporting their economic viability.
- Integration with International Markets: Leverage the Voluntary Carbon Exchange to facilitate global trading of carbon credits, expanding Malaysia’s role in the voluntary carbon market and increasing revenue from carbon credits.
- Wetland Agriculture: Promote practices that maintain elevated water tables in peatlands to support sustainable crop cultivation, which can generate carbon credits and physical products to provide income for farmers through sustainable peatland management.
- Forest Preservation Incentives: Incentivize preservation of tropical forests by highlighting the benefits of carbon credits, biodiversity protection, and sustainable tourism to counteract drivers of deforestation.
- Address challenges associated with accurately measuring carbon sequestration and emissions to ensure effective implementation and credibility of the carbon credit system. This links with the need for more accurate baseline emissions data and land-use conversion calculations as these will feed into the value of future carbon credits.
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Murphy, D.J. Biological carbon sequestration: From deep history to the present day. Earth 2024, 5, 195–213. [Google Scholar] [CrossRef]
- NASA. The Carbon Cycle, Earth Observatory. 2011. Available online: https://earthobservatory.nasa.gov/features/CarbonCycle (accessed on 14 May 2025).
- Schlesinger, W.H.; Bernhardt, E.S. The Global Carbon Cycle. In Biogeochemistry, An Analysis of Global Change, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 419–444. [Google Scholar]
- Feulner, G. Formation of most of our coal brought Earth close to global glaciation. Proc. Natl. Acad. Sci. USA 2017, 114, 11333–11337. [Google Scholar] [CrossRef] [PubMed]
- Betts, R.A. Mauna Loa Carbon Dioxide Forecast for 2025; UK Met Office: Exeter, UK, 2025; Available online: https://tiny.cc/vbvh001 (accessed on 14 May 2025).
- Möller, T.; Högner, A.E.; Schleussner, C.F.; Bien, S.; Bien, S.; Kitzman, N.H.; Lamboll, R.D.; Rojelj, J.; Donges, J.F.; Rockstrom, J.; et al. Achieving net zero greenhouse gas emissions critical to limit climate tipping risks. Nat. Commun. 2024, 15, 6192. [Google Scholar] [CrossRef]
- Kiel, J. Data from Earth’s Past Holds a warning for Our Future Under Climate Change. In Yale Climate Connections; Yale University Press: New Haven, CO, USA, 2019; Available online: https://tiny.cc/lbvh001 (accessed on 14 May 2025).
- Kiehl, J.T.; Shields, C.A.; Snyder, M.A.; Zachos, J.C.; Rothstein, M. Greenhouse- and orbital-forced climate extremes during the early Eocene. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 20170085. [Google Scholar] [CrossRef]
- Wray, S. Climate Concerns Persist in 2025 But Faith in Government Action Wanes. Global Government Forum, 1 August 2025. Available online: https://www.globalgovernmentforum.com/climate-concerns-persist-in-2025-but-faith-in-government-action-wanes/ (accessed on 14 May 2025).
- Climate Backtracker. Colombia Law School. 2025. Available online: https://climate.law.columbia.edu/content/climate-backtracker (accessed on 14 May 2025).
- Fursman, L. The Climate Paradox: Why We Need to Reset Action on Climate Change; Tony Blair Institute for Global Change: London, UK, 2025; Available online: https://institute.global/insights/climate-and-energy/the-climate-paradox-why-we-need-to-reset-action-on-climate-change (accessed on 14 May 2025).
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.K.R.; Mcbratney, A.B. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, 145A. [Google Scholar] [CrossRef]
- Nayak, N.; Mehrotra, R.; Mehrotra, S.S. Carbon biosequestration strategies: A review. Carbon Capture Sci. Technol. 2022, 4, 1000065. [Google Scholar] [CrossRef]
- Salk Institute. Harnessing Plants Initiative. 2025. Available online: https://www.salk.edu/harnessing-plants-initiative/research/ (accessed on 14 May 2025).
- Murphy, D.J.; Cardona, T. Photosynthetic Life: Origin, Evolution and Future; Oxford University Press: Oxford, UK, 2022; Available online: https://global.oup.com/ukhe/product/photosynthetic-life-9780198815723?cc=&lang=en& (accessed on 14 May 2025).
- NASA. The Relentless Rise of Carbon Dioxide. 2024. Available online: https://tiny.cc/yavh001 (accessed on 14 May 2025).
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.A.; Koch, A. Restoring natural forests is the best way to remove atmospheric carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef]
- Murphy, D.J. Carbon Sequestration by Tropical Trees and Crops: A Case Study of Oil Palm. Agriculture 2024, 14, 1133. [Google Scholar] [CrossRef]
- Ratnasingam, J.; Ramasany, G.; Toong, W.; Abrudan, I. Carbon Stocking in the Natural Forests—The Case of Malaysia. Not. Bot. Horti Agrobot. 2015, 43, 1842–4309. [Google Scholar]
- Muller, J.D. Decadal Trends in the Oceanic Storage of Anthropogenic Carbon From 1994 to 2014. AGU Adv. 2023, 4, 875. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Li, X.; O’Sullivan, M.; Wigneron, J.P.; Sitch, S.; Ciais, P.; Frankenberg, C.; Fischer, W.W. Recent gains in global terrestrial carbon stocks are mostly stored in non-living pools. Science 2025, 387, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Amelung, W.; Bossio, D.; de Vries, W.; Kogel-Knaber, I.; Lehmann, J.; Amundson, R.; Bol, R. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 11, 5427. [Google Scholar] [CrossRef] [PubMed]
- Angermayr, S.A.; Gorchs Rovira, A.; Hellingwerf, K.J. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015, 33, 352–361. [Google Scholar] [CrossRef]
- Olajire, A.A.; Essien, J.P. Aerobic degradation of petroleum components by microbial consortia. J. Petroleum Environ. Biotechnol. 2014, 5, 195. [Google Scholar] [CrossRef]
- Pant, D.; Shah, K.K.; Sharma, S. Soil and Ocean Carbon Sequestration, Carbon Capture, Utilization, and Storage as Negative Emission Strategies for Global Climate Change. J. Soil Sci. Plant Nutr. 2023, 23, 1421–1437. [Google Scholar] [CrossRef]
- Goren, A.Y.; Erdemir, D.; Dincer, I. Comprehensive review and assessment of carbon capturing methods and technologies: An environmental research. Environ. Res. 2024, 240, 117503. [Google Scholar] [CrossRef]
- Zhao, K.; Jia, C.; Li, Z.; Du, X.; Wang, Y.; Li, J.; Yao, Z.; Yao, J. Recent Advances and Future Perspectives in Carbon Capture, Transportation, Utilization, and Storage (CCTUS) Technologies: A Comprehensive Review. Fuel 2023, 351, 128913. [Google Scholar] [CrossRef]
- Boele, G. Could Carbon Sequestration Technologies Help to Reach Net-Zero? ABN.AMRO Economic Bureau: Amsterdam, The Netherlands, 2024; Available online: https://www.abnamro.com/research/en/our-research/esg-economist-could-carbon-sequestration-technologies-help-to-reach-net-zero (accessed on 14 May 2025).
- Wang, N.; Akimbo, K.; Nemet, G. What went wrong? Learning from three decades of carbon capture, utilization and sequestration (CCUS) pilot and demonstration projects. Energy Policy 2021, 158, 112546. [Google Scholar] [CrossRef]
- Anderson, O.L. CCUS in the United States of America. In Carbon Capture Utilization and Storage; Sustainable Development Goals Series; Pereira, E.G., Fossa, A.J., Muinzer, T.L., Eds.; Palgrave Macmillan: London, UK, 2025. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. Carbon sequestration in an uncertain world. Adv. Environ. Res. 2019, 6, 2–11. [Google Scholar]
- Bisotti, B.; Hoff, K.A.; Mathisen, A.; Hovland, J. Direct Air capture (DAC) deployment: A review of the industrial deployment. Chem. Eng. Sci. 2024, 283, 119416. [Google Scholar] [CrossRef]
- Majid, A.; Almulla, M. 3 Essentials for Carbon Capture and Storage to Really Take Off. World Economic Forum, 26 May 2025. Available online: https://www.weforum.org/stories/2025/03/carbon-capture-storage-essentials-uptake/ (accessed on 14 May 2025).
- Chalmin, A. Fossil Fuel Industry and Investments in CCS & CCUS. Geoengineering Monitor, 15 November 2021. Available online: https://www.geoengineeringmonitor.org/fossil-fuel-industry-and-investments-in-ccs-ccus#:~:text=The%20data%20shows%20that%20the,disclose%20all%20of%20their%20sponsors (accessed on 14 May 2025).
- Feigin, S.V.; Wiebers, D.O.; Lueddeke, G.; Morand, S.; Lee, K.; Knight, A.; Brainin, M.; Feigin, V.L.; Whitfort, A.; Marcum, J.; et al. Proposed solutions to anthropogenic climate change: A systematic literature review and a new way forward. Heliyon 2023, 9, e20544. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.L.; Gao, Q.X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim. Change Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- IPCC. Carbon Dioxide Removal, 2nd ed.; IPCC: Geneva, Switzerland, 2024; Available online: https://static1.squarespace.com/static/633458017a1ae214f3772c76/t/665ed1e2b9d34b2bf8e17c63/1717490167773/The-State-of-Carbon-Dioxide-Removal-2Edition.pdf (accessed on 14 May 2025).
- Bevacqua, E.; Schleussner, C.F.; Zscheischler, J. A year above 1.5 °C signals that Earth is most probably within the 20-year period that will reach the Paris Agreement limit. Nat. Clim. Change 2025, 15, 262–265. [Google Scholar] [CrossRef]
- Wynes, S.; Davis, S.J.; Dickau, M.; Ly, S.; Maibach, E.; Rogelj, J.; Zickfeld, K. Perceptions of carbon dioxide emission reductions and future warming among climate experts. Commun. Earth Environ. 2024, 5, 498. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaerv, K.M. Review of technological progress in carbon dioxide capture, storage, and utilization. Gas Sci. Technol. 2023, 117, 205070. [Google Scholar] [CrossRef]
- Global CCS Institute. Global Status of CCS 2024. 2024. Available online: https://www.globalccsinstitute.com/resources/global-status-report/ (accessed on 14 May 2025).
- BCG. Shifting the Direct Air Capture Paradigm; BCG: Boston, MA, USA, 2023; Available online: https://www.bcg.com/publications/2023/solving-direct-air-carbon-capture-challenge (accessed on 14 May 2025).
- Krishnan, A.; Nighojkar, A.; Kandasubramanian, B. Emerging towards zero carbon footprint via carbon dioxide capturing and sequestration. Carbon Capture Sci. Technol. 2023, 9, 100137. [Google Scholar] [CrossRef]
- McLaren, D.; Corry, O. Carbon Dioxide Removal: What Is Sustainable and Just? Environment 2025, 67, 59–69. [Google Scholar] [CrossRef]
- Elsener, R. Carbon Capture Utilization and Storage is Gaining Traction in the USA Thanks to Ground Breaking Legislation; MAN Energy Solutions USA: Brookshire, TX, USA, 2024; Available online: https://www.man-es.com/discover/inflation-reduction-act-ccus#:~:text=The%20Inflation%20Reduction%20Act%20(IRA,and%20Storage%20(CCUS)%20projects (accessed on 14 May 2025).
- Economist. How Saudi Aramco Plans to Win the Oil Endgame. Economist Business, 2 June 2024. Available online: https://www.economist.com/business/2024/06/02/how-saudi-aramco-plans-to-win-the-oil-endgame (accessed on 14 May 2025).
- Lima, D. Opinion: What To Know About US Carbon Capture in 2025. Carbon Herald, 11 December 2024. Available online: https://carbonherald.com/opinion-what-to-know-about-us-carbon-capture-in-2025/ (accessed on 14 May 2025).
- Neeraj; Yadav, S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Appl. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Licht, G.; Licht, S. Transformation of the greenhouse gas carbon dioxide to graphene. J. CO2 Utilization 2018, 36, 288–294. [Google Scholar] [CrossRef]
- Ozkan, M.; Quiros, K.A.M.; Watkins, J.M.; Nelson, T.M.; Singh, N.D.; Chowdhury, M.; Namboodiri, T.; Talluri, K.R.; Yuan, E. Curbing pollutant CO2 by using two-dimensional MXenes and MBenes. Chem 2024, 10, 443–483. [Google Scholar] [CrossRef]
- Zhu, Q.; Qu, H.; Avci, G.; Hafizi, R.; Zhao, C.; Day, G.M.; Cooper, A.I. Computationally guided synthesis of a hierarchical [4[2+3]+6] porous organic ‘cage of cages’. Nat. Synth. 2024, 3, 825–834. [Google Scholar] [CrossRef]
- Robertson, B.; Mousavian, M. The Carbon Capture Crux, Lessons Learned; Inst. Energy Economics & Financial Analysis: Lakewood, OH, USA, 2020; Available online: https://tiny.cc/n70i001 (accessed on 14 May 2025).
- Lamb, W.; Gasser, T.; Roman Cuesta, R.M.; Grassi, G.; Gidden, M.; Powis, C.; Geden, O. The carbon dioxide removal gap. Nat. Clim. Change 2024, 14, 644–651. [Google Scholar] [CrossRef]
- IEEFA (Institute for Energy Economics and Financial Analysis). Carbon Capture and Storage: Europe’s Climate Gamble; IEEFA: Lakewood, OH, USA, 2024; Available online: https://ieefa.org/resources/carbon-capture-and-storage-europes-climate-gamble (accessed on 14 May 2025).
- Kazlou, T.; Cherp, A.; Jewell, J. Feasible deployment of carbon capture and storage and the requirements of climate targets. Nat. Clim. Change 2024, 14, 1047–1055. [Google Scholar] [CrossRef]
- NOAA. Carbon Dioxide Removal. In NOAA State of the Science Factsheet; 19 September 2024. Available online: https://www.climate.gov/news-features/understanding-climate/carbon-dioxide-removal-noaa-state-science-factsheet#:~:text=The%20State%20of%20Carbon%20Dioxide,primarily%20through%20conventional%20CDR%20methods (accessed on 14 May 2025).
- Andreoni, P.; Emmerling, J.; Tavoni, M. Inequality repercussions of financing negative emissions. Nat. Clim. Change 2024, 14, 1. [Google Scholar] [CrossRef]
- Bartosz, D.; Krzyzynska, R.; Klas, A. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Wood Mackenzie. Global CCUS Investment Requires US$ 196B Through 2034, According to Wood Mackenzie. 2024. Available online: https://www.woodmac.com/press-releases/2024-press-releases/global-ccus-investment-requires-us$-196b-through-2034-according-to-wood-mackenzie/ (accessed on 14 May 2025).
- Zhang, Y.; Jackson, C.; Krevor, S. The feasibility of reaching gigatonne scale CO2 storage by mid-century. Nat. Commun. 2024, 15, 6913. [Google Scholar] [CrossRef]
- Ganti, G.; Pelz, S.; Klönne, U.; Gidden, M.J.; Schleussner, C.F.; Nicholls, Z. Fair carbon removal obligations under climate response uncertainty. Clim. Policy 2025, 2025, 1–13. [Google Scholar] [CrossRef]
- Lebling, K.; Gangotra, A.; Hausker, K.; Byrum, Z. 7 Things to Know About Carbon Capture, Utilization and Sequestration; World Resources Institute: New York, NY, USA, 2025. [Google Scholar]
- Markusson, N.; Lund, J.F.; Buck, H.; Carton, W.; Dooley, K.; Hougaard, I.M. Mitigation Deterrence and Carbon Removal in the Age of Net Zero. In Environmental Science & Policy; 2024; Available online: https://www.sciencedirect.com/special-issue/10Q2VFJNMF0 (accessed on 14 May 2025).
- Brad, A.; Schneider, E. Carbon dioxide removal and mitigation deterrence in EU climate policy: Towards a research approach. Environ. Sci. Policy 2023, 150, 103591. [Google Scholar] [CrossRef]
- Carton, W.; Hougaard, I.-M.; Markusson, N.; Lund, J.F. Is carbon removal delaying emission reductions? WIRES Clim. Change 2023, 14, e826. [Google Scholar] [CrossRef]
- Deprez, A.; Leadley, P.; Dooley, K.; Williamson, P.; Cramer, W.; Gattuso, J.-P.; Rankovic, A.; Carlson, E.L.; Creutzig, F. Sustainability limits needed for CO2 removal. Science 2024, 383, 484–486. [Google Scholar] [CrossRef]
- Stuart-Smith, R.F.; Rajamani, L.; Rogelj, J.; Wetzer, T. Legal limits to the use of CO2 removal. Science 2023, 382, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Schleussner, C.F.; Ganti, G.; Lejeune, Q.; Zhu, B.; Pfleiderer, P.; Prütz, R.; Ciais, P.; Frölicher, T.L.; Fuss, S.; Gasser, T.; et al. Overconfidence in climate overshoot. Nature 2024, 634, 366–373. [Google Scholar] [CrossRef]
- Luck, M.; Wang, F.; De Temmerman, G. Carbon Dioxide Removal (CDR) Evidence Review. An Overview of CDR and Bottlenecks to Overcome; Quadrature Climate Foundation: London, UK, 2024; Available online: https://www.qc.foundation/files//Carbon-dioxide-removal.pdf (accessed on 14 May 2025).
- Bacilieri, A.; Black, R.; Way, R. Assessing the Relative Costs of High-CCS and Low-CCS Pathways to 1.5 Degrees. Oxf. Smith Sch. Enterp. Environ. 2023, 4, 8–23. [Google Scholar]
- Garritano, A.N.; Song, W.; Thomas, T. Carbon fixation pathways across the bacterial and archaeal tree of life. Proc. Natl. Acad. Sci. USA Nexus 2022, 1, pgac226. [Google Scholar] [CrossRef] [PubMed]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef]
- Bailey, R.; King, R. Betting on BECCS? Exploring Land-Based Negative Emissions Technologies; Chatham House: London, UK, 2018; Available online: https://accelerator.chathamhouse.org/article/betting-on-beccs-exploring-land-based-negative-emissions-technologies/ (accessed on 14 May 2025).
- Taylor-Kearney, L.J.; Wang, R.Z.; Shih, P.M. Evolution and origins of rubisco. Curr. Biol. 2024, 34, R764–R767. [Google Scholar] [CrossRef] [PubMed]
- Erb, T.J.; Zarzycki, J. A short history of RubisCO: The rise and fall (?) of Nature’s predominant CO2 fixing enzyme. Curr. Opin. Biotechnol. 2018, 49, 100–107. [Google Scholar] [CrossRef]
- Tcherkez, G. How atmospheric oxygen is captured by RuBisCo. Nat. Rev. Mol. Cell Biol. 2021, 22, 304. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Occhialini, A.; Andralojc, P.; Parry, M.A.; Hanson, M.R. A faster Rubisco with potential to increase photosynthesis in crops. Nature 2014, 513, 547–550. [Google Scholar] [CrossRef]
- Bouvier, J.W.; Emms, D.M.; Kelly, S. Rubisco is evolving for improved catalytic efficiency and CO2 assimilation in plants. Proc. Natl. Acad. Sci. USA 2024, 121, e2321050121. [Google Scholar] [CrossRef]
- Zhao, L.; Cai, Z.; Li, Y.; Zhang, Y. Engineering Rubisco to enhance CO2 utilization. Synth. Syst. Biotechnol. 2024, 9, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Schwander, T.; Schada, V.; Borzyskowski, L.; Burgener, S.; Cortina, N.S.; Erb, T.J. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 2016, 354, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Osmanoglu, Ö.; Dandekar, T. Synthetic Rewiring of Plant CO2 Sequestration Galvanizes Plant Biomass Production. Trends Biotechnol. 2020, 38, 354–359. [Google Scholar] [CrossRef]
- Santos Correa, S.; Schultz, J.; Lauersen, K.J.; Rosado, A.S. Natural carbon fixation and advances in synthetic engineering for redesigning and creating new fixation pathways. J. Adv. Res. 2022, 47, 75–92. [Google Scholar] [CrossRef]
- Luo, S.; Lin, P.P.; Nieh, L.Y.; Liao, G.B.; Tang, P.W.; Chen, C.; Liao, J.C. A cell-free self-replenishing CO2-fixing system. Nat. Catal. 2022, 5, 154–162. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers of IPCC Special Report on Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018. Available online: https://www.ipcc.ch/sr15/ (accessed on 14 May 2025).
- Crockford, P.W.; Bar On, Y.M.; Ward, L.M.; Milo, R.; Halevy, I. The geologic history of primary productivity. Curr. Biol. 2023, 33, 4741–4750.e5. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.A. Carbon cycling: How much life has ever existed on Earth? Curr. Biol. 2023, 33, R1153–R1155. [Google Scholar] [CrossRef]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G.; et al. Mapping tree density at a global scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Joyard, J. Distribution of Biomass on the Planet. In Encyclopedia of the Environment; 2025; Available online: https://www.encyclopedie-environnement.org/en/life/distribution-biomass-planet/ (accessed on 14 May 2025).
- FAO; UNEP. The State of the World’s Forests 2020. In Forests, Biodiversity and People; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- FAO. Land Use; FAOSTAT: Rome, Italy, 2023; Available online: http://www.fao.org/faostat/en/#data/RL (accessed on 14 May 2025).
- Sha, Z.; Bai, Y.; Li, R.; Lan, H.; Zhang, X.; Li, J.; Liu, X.; Xie, Y. The global carbon sink potential of terrestrial vegetation can be increased substantially by optimal land management. Commun. Earth Environ. 2022, 3, 8. [Google Scholar] [CrossRef]
- Fletcher, M.S.; Hamilton, R.; Dressler, W.; Palmer, L. Indigenous knowledge and the shackles of wilderness. Proc. Natl. Acad. Sci. USA 2021, 118, e2022218118. [Google Scholar] [CrossRef]
- Lewis, S.; Lopez-Gonzalez, G.; Sonké, B. Increasing Carbon Storage in Intact African Tropical Forests. Nature 2009, 457, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.C. Decay of Wood; CBD-111: Kolkata, India, 1969; Available online: https://web.mit.edu/parmstr/Public/NRCan/CanBldgDigests/cbd111_e.html (accessed on 14 May 2025).
- Manici, L.M.; De Meo, I.; Ludovica Saccà, M.; Ceotto, E.; Caputo, F.; Paletto, A. The relationship between tree species and wood colonising fungi and fungal interactions influences wood degradation. Ecol. Indicators. 2023, 151, 110312. [Google Scholar] [CrossRef]
- NRC. Does Harvesting in Canada’s Forests Contribute to Climate Change? In Canadian Forest Service Notes; 2007; Available online: https://web.archive.org/web/20151001012436/ (accessed on 14 May 2025).
- Hua, F.; Wang, X.; Zheng, X.; Fisher, B.; Wang, L.; Zhu, J.; Tang, Y.; Yu, D.W.; Wilcove, D.S. Opportunities for biodiversity gains under the world’s largest reforestation programme. Nat. Commun. 2016, 7, 12717. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, Q.P.M.; Hollingworth, P.; Beckschafer, H.; Chen, R.J.; Zomer, L.; Zhang, M.; Wang, J.; Xu, J. China’s fight to halt tree cover loss. Proc. R. Soc. Biol. Sci. 2017, 284, 1854. [Google Scholar] [CrossRef]
- Cook-Patton, S.C.; Leavitt, S.M.; Gibbs, D.; Harris, N.L.; Lister, K.; Anderson-Teixeira, K.J.; Briggs, R.D.; Chazdon, R.L.; Crowther, T.W.; Ellis, P.W.; et al. Mapping carbon accumulation potential from global natural forest regrowth. Nature 2020, 585, 545–550. [Google Scholar] [CrossRef]
- Harris, N.; Gibbs, D. Forests Absorb Twice as Much Carbon as They Emit Each Year; World Resources Institute: Washington, DC, USA, 2021; Available online: https://www.wri.org/insights/forests-absorb-twice-much-carbon-they-emit-each-year (accessed on 14 May 2025).
- Harris, N.L.; Gibbs, D.A.; Baccini, A.R.A.; Birdsey, S.; de Bruin, W. Global maps of twenty-first century forest carbon fluxes. Nat. Clim. Change 2021, 11, 234–240. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Houghton, R.A.; Fang, J.; Kauppi, P.E.; Keith, H. The enduring world forest carbon sink. Nature 2024, 631, 563–569. [Google Scholar] [CrossRef]
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Walker, W. The global potential for increased storage of carbon on land. Proc. Natl. Acad. Sci. USA 2022, 119, e2111312119. [Google Scholar] [CrossRef]
- European Space Agency. Mission Overview. In Biomass Infographic; 2025; Available online: https://earth.esa.int/eogateway/missions/biomass/description (accessed on 14 May 2025).
- National Academies of Sciences. Developing a Research Agenda for Carbon Dioxide Removal and Reliable Sequestration; National Academies Press (US): Cambridge, MA, USA, 2019; Available online: https://www.nationalacademies.org/our-work/developing-a-research-agenda-for-carbon-dioxide-removal-and-reliable-sequestration (accessed on 14 May 2025).
- Trenberth, K.E.; Smith, L. The Mass of the Atmosphere: A Constraint on Global Analyses. J. Clim. 2005, 18, 864–875. [Google Scholar] [CrossRef]
- Button, E.S. Deep-C storage: Biological, chemical and physical strategies to enhance carbon stocks in agricultural subsoils. Soil Biol. Biochem. 2022, 170, 108697. [Google Scholar] [CrossRef]
- Zomer, R.J.; Trabucco, A.; Coe, R.; Place, F. Trees on Farm: Analysis of Global Extent and Geographical Patterns of Agroforestry; ICRAF. 2009. Working Paper 89; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Front. Climate 2019, 1, 8. [Google Scholar] [CrossRef]
- FAO. Global Soil Sequestration Potential (GSOCseq) Map. FAO Soils Portal. 2025. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-soil-organic-carbon-sequestration-potential-map-gsocseq/en/ (accessed on 14 May 2025).
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Kabato, W.; Getnet, G.T.; Sinore, T.; Nemeth, A.; Molnár, Z. Towards Climate-Smart Agriculture: Strategies for Sustainable Agricultural Production, Food Security, and Greenhouse Gas Reduction. Agronomy 2025, 15, 565. [Google Scholar] [CrossRef]
- Melillo, J.; Gribkoff, E. Soil-Based Carbon Sequestration. MIT Climate Portal. 2021. Available online: https://climate.mit.edu/explainers/soil-based-carbon-sequestration (accessed on 14 May 2025).
- Melillo, J.M.; Frey, S.D.; DeAngelis, K.M.; Werner, W.; Bernard, M.J.; Bowles, F.P.; Pold, G.; Knorr, M.A.; Grandy, A.S. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 2017, 358, 101–105. [Google Scholar] [CrossRef]
- Padarian, J.; Minasny, B.; McBratney, A.; Smith, P. Soil carbon sequestration potential in global croplands. Peer J. 2022, 10, e13740. [Google Scholar] [CrossRef]
- Leifeld, J.; Wüst-Galley, C.; Page, S. Intact and managed peatland soils as a source and sink of GHGs from 1850 to 2100. Nat. Climate Change 2019, 9, 945–947. [Google Scholar] [CrossRef]
- Prananto, J.P.; Minasny, B.; Comeau, L.P.; Grace, P. Drainage increases CO2 and N2O emissions from tropical peat soils. Glob. Change Biol. 2020, 26, 4583–4600. [Google Scholar] [CrossRef]
- Girkin, N.T.; Cooper, H.V.; Ledger, M.J. Tropical peatlands in the Anthropocene: The present and the future. Anthropocene 2022, 40, 100354. [Google Scholar] [CrossRef]
- Armentano, T.V.; Menges, E.S. Patterns of change in the carbon balance of organic soil-wetlands of the temperate zone. J. Ecol. 1986, 74, 755–774. [Google Scholar] [CrossRef]
- Wilson, D. Greenhouse gas emission factors associated with rewetting of organic soils. Mires Peat 2016, 17, 1–28. [Google Scholar]
- Knox, S.H.; Sturtevant, C.; Matthes, J.H.; Koteen, L.; Verfaillie, J.; Baldocchi, D. Agricultural peatland restoration: Effects of land-use change on greenhouse gas (CO2 and CH4) fluxes in the Sacramento-San Joaquin Delta. Glob. Change Biol. 2015, 21, 750–765. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.A.; Smith, P.; Verhagen, J. Carbon sequestration in the agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Lessmann, M.; Ros, G.H.; Young, M.D.; de Vries, W. Global variation in soil carbon sequestration potential through improved cropland management. Glob. Change Biol. 2022, 28, 1162–1177. [Google Scholar] [CrossRef]
- Goodrick, I.; Nelson, P.N.; Banabas, M.; Wursster, C.M.; Bird, M.I. Soil carbon balance following conversion of grassland to oil palm. GCB Bioenergy 2015, 7, 263–272. [Google Scholar] [CrossRef]
- Borchard, N.; Bulusu, M.; Meyer, N.; Rodionov, A.; Herawati, H.; Blagodatsky, S.; Cadisch, G. Deep soil carbon storage in tree- dominated land use systems in tropical lowlands of Kalimantan. Geoderma 2019, 354, 113864. [Google Scholar] [CrossRef]
- Brindis-Santos, A.I.; Lopez, D.J.; Mata-Zayas, E.E.; Palma, D.J. Impacts of oil palm cultivation on soil organic carbon stocks in Mexico: Evidence from plantations in Tabasco State. Cah. Agric. 2021, 30, 47. [Google Scholar] [CrossRef]
- Sprunger, C.D.; Martin, T.; Mann, M. Systems with greater perenniality and crop diversity enhance soil biological health. Agric. Environ. Lett. 2020, 5, e20030. [Google Scholar] [CrossRef]
- Fakhraee, M.; Tarhan, L.G.; Planavsky, N.J.; Reinhard, C.T. A largely invariant marine dissolved organic carbon reservoir across Earth’s history. Proc. Natl. Acad. Sci. USA 2021, 118, e2103511118. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Milo, R. The Biomass Composition of the Oceans: A Blueprint of Our Blue Planet. Cell 2019, 179, 1451–1454. [Google Scholar] [CrossRef]
- Lauderdale, J.M.; Braakman, R.; Forget, G.; Dutkiewicz, S.; Follows, M.J. Microbial feedbacks optimize ocean iron availability. Proc. Natl. Acad. Sci. USA 2020, 117, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Hance, J. Is Ocean Iron Fertilization Back from the Dead as a CO2 Removal Tool? Mongabay. 2023. Available online: https://news.mongabay.com/2023/11/is-ocean-iron-fertilization-back-from-the-dead-as-a-co₂-removal-tool/ (accessed on 14 May 2025).
- Silverman-Roati, K.; Webb, R.M.; Gerrard, M. Removing Carbon Dioxide Through Ocean Fertilization: Legal Challenges and Opportunities, Sabin Center for Climate Change Law, Columbia Law School. 2022. Available online: https://scholarship.law.columbia.edu/faculty_scholarship/3637 (accessed on 14 May 2025).
- Petit, J.; Jouzel, J.; Raynaud, D.; Barkov, N.I.; Barnola, J.M.; Basile, I.; Bender, M.; Chappellaz, J.; Davis, M.; Delaygue, G.; et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 1999, 399, 429–436. [Google Scholar] [CrossRef]
- Strong, A.; Chisholm, S.; Miller, C.; Cullen, J. Ocean fertilization: Time to move on. Nature 2009, 461, 347–348. [Google Scholar] [CrossRef]
- Martin, P.; Van der Loeff, M.R.; Cassar, N.; Vandromme, P.; D’Ovidio, F.; Stemmann, L.; Rengarajan, R.; Soares, M.; Gonzalez, H.E.; Ebersbach, F.; et al. Iron fertilization enhanced net community production but not downward particle flux during the Southern Ocean iron fertilization experiment LOHAFEX. Glob. Biogeochem. Cycles 2013, 27, 871–881. [Google Scholar] [CrossRef]
- Bach, L.T.; Gill, S.J.; Rickaby, R.E.M.; Gpre, S.; Renforth, P. CO2 removal with enhanced weathering and ocean alkalinity enhancement: Potential risks and co-benefits for marine pelagic ecosystems. Front. Clim. 2019, 1, 7. [Google Scholar] [CrossRef]
- Goldenberg, S.U.; Spisla, C.; Sánchez, N.; Taucher, J.; Spillimg, K.; Swat, M.; Fiesinger, A.S.; Mendez, M.F.; Krock, B.; Hauss, H.; et al. Diatom-mediated food web functioning under ocean artificial upwelling. Sci. Rep. 2024, 14, 3955. [Google Scholar] [CrossRef] [PubMed]
- Ocean Nets. Artificial Upwelling. 2020. Available online: https://www.oceannets.eu/artifical-upwelling/#:~:text=Enhancing%20the%20upward%20transport%20of%20nutrient-rich%20deep%20waters,increases%20the%20carbon%20uptake%20of%20the%20upper%20ocean (accessed on 14 May 2025).
- National Academies of Sciences. A Research Strategy for Ocean-Based Carbon Dioxide Removal and Sequestration; National Academies Press (US): Cambridge, MA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK580046/ (accessed on 14 May 2025).
- Jurcott, M.; Oschlies, A.; Koeve, W. Artificial Upwelling—A Refined Narrative. Geophys. Res. Lett. 2023, 50, e2022GL101870. [Google Scholar] [CrossRef]
- Takahashi, T.; Feely, R.A.; Weiss, R.F.; Wanninkhof, R.H.; Chipman, D.W.; Sutherland, S.C.; Takahashi, T.T. Global air-sea flux of CO2: An estimate based on measurements of sea-air pCO2 difference. Proc. Natl. Acad. Sci. USA 1997, 94, 8292–8299. [Google Scholar] [CrossRef]
- Castañón, L. An Ocean of Opportunity. Oceanus, 7 December 2021. Available online: https://www.whoi.edu/oceanus/feature/an-ocean-of-opportunity/ (accessed on 14 May 2025).
- Doney, S.C.; Buck, H.; Buesseler, K.; Iglesias-Rodriguez, M.D.; Moran, K.; Oschlies, A.; Renforth, P.; Roman, J.; Sant, G.N.; Siegel, D.; et al. A research strategy for ocean-based carbon dioxide removal and sequestration. In Consensus Study Report Highlights; 2021; Available online: https://www.nap.edu/resource/26278/Ocean_CDR_2021.pdf (accessed on 14 May 2025).
- Lubofsky, E. The Ocean Has a Serious Case of Heartburn. Is Relief on the Way. Oceanus, 1 July 2021. Available online: https://www.whoi.edu/oceanus/feature/ocean-alkalinity/ (accessed on 14 May 2025).
- Maribus. The Ocean—A Climate Champion? How to Boost Marine Carbon Dioxide Uptake. World Ocean. Rev. 2024, 8, 54–67. [Google Scholar]
- Denvir, A.; Leslie-Bole, H. Biomass Can Fight Climate Change, But Only If You Do It Right; World Resources Institute: New York, NY, USA, 2025; Available online: https://www.wri.org/insights/sustainable-biomass-carbon-removal#:~:text=Sequestering%20the%20carbon%20in%20biomass,benefits%2C%20like%20mitigating%20wildfire%20risk (accessed on 14 May 2025).
- Lamlom, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Bioenergy 2003, 4, 381–388. [Google Scholar] [CrossRef]
- Rajakal, J.P.; Ng, F.Y.; Zulkifli, A.; How, B.S.; Sunarso, J.; Ng, D.K.S.; Andiappan, V. Analysis of current state, gaps, and opportunities for technologies in the Malaysian oil palm estates and palm oil mills towards net-zero emissions. Heliyon 2024, 10, e30768. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochem. Cosmochima Acta. 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management, 2nd ed.; Routledge: London, UK, 2015. [Google Scholar] [CrossRef]
- De Gryze, S.; Cullen, M.; Durschinger, L. Evaluation of the Opportunities for Generating Carbon Offsets from Soil Sequestration of Biochar; Climate Action Reserve: Los Angeles, CA, USA, 2010; Available online: https://climateactionreserve.org/wp-content/uploads/2009/03/Soil_Sequestration_Biochar_Issue_Paper1.pdf (accessed on 14 May 2025).
- Varkolu, M.; Gundekari, S.; Omvesh; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Zhang, Y.; Maierdan, Y.; Guo, T.; Chen, B.; Fang, S.; Zhao, L. Biochar as carbon sequestration material combines with sewage sludge incineration ash to prepare lightweight concrete. Constr. Build. Mater. 2022, 343, 128116. [Google Scholar] [CrossRef]
- Saxena, S. Pyrolysis and beyond: Sustainable valorization of plastic waste. Appl. Energy Combust. Sci. 2025, 21, 100311. [Google Scholar] [CrossRef]
- Smith, S.M.; Geden, O.; Gidden, M.J.; Lamb, W.F.; Nemet, G.F.; Minx, J.C. (Eds.) The State of Carbon Dioxide Removal, 2nd ed.; Oxford University: Oxford, UK, 2024; Available online: https://osf.io/f85qj/ (accessed on 14 May 2025).
- Paper Advance. Quebec Biochar: Carbonity Begins Production. In Biomass; 2025; Available online: https://www.paperadvance.com/bioeconomy/biomass/quebec-biochar-carbonity-begins-production.html (accessed on 14 May 2025).
- Camia, A.; Giuntoli, J.; Jonsson, R.; Robert, N.; Cazzaniga, N.E.; Jasinevičius, G.; Avitabile, V.; Grassi, G.; Barredo, J.I.; Mubareka, S. The Use of Woody Biomass for Energy Purposes in the EU; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Sterman, J.D.; Moomaw, W.; Rooney-Varga, J.; Siegel, L. Does wood bioenergy help or harm the climate? Bull. At. Sci. 2022, 78, 128–138. [Google Scholar] [CrossRef]
- Booth, M.S. The Great Biomass Boondoggle; New York Review of Books: New York, NY, USA, 2019; Available online: https://www.nybooks.com/online/2019/10/14/the-great-biomass-boondoggle/ (accessed on 14 May 2025).
- Sterman, J.D.; Siegel, L.; Rooney-Varga, J. Does replacing coal with wood lower CO2 emissions? Dynamic lifecycle analysis of wood bioenergy. Environ. Res. Lett. 2018, 13, 01500. [Google Scholar]
- Snowdon, C. Trees for Burning: The Biomass Controversy; Working Paper; Institute of Economic Affairs: London, UK, 2024; Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4716864 (accessed on 14 May 2025).
- Tran, H.; Jino, E.; Arunachalam, S. Emissions of wood pelletization and bioenergy use in the United States. Renew. Energy 2023, 219, 119536. [Google Scholar] [CrossRef]
- Buchholz, T.; Gunn, J.S.; Sharma, B. When Biomass Electricity Demand Prompts Thinnings in Southern US Pine Plantations: A Forest Sector Greenhouse Gas Emissions Case Study. Front. For. Glob. Change 2021, 4, 642569. [Google Scholar] [CrossRef]
- NRDC. A Bad Biomass Bet. In Issue Brief; NRDC: New York, NY, USA, 2021; Available online: https://www.nrdc.org/sites/default/files/bad-biomass-bet-beccs-ib.pdf (accessed on 14 May 2025).
- National Audit Office. The Government’s Support for Biomass. 2024. Available online: www.nao.org.uk (accessed on 14 May 2025).
- Lawson, D. Time’s Up for Drax’s Tree Burning Racket. Sunday Times, 4 June 2023. Available online: https://www.thetimes.co.uk/article/times-up-for-draxs-tree-burning-racket-drw2gd23x (accessed on 14 May 2025).
- Mavrokefalidis, D. NAO: Government Fails to Ensure Biomass Sustainability in £20bn Support. Energy Live News, 24 January 2024. Available online: https://www.energylivenews.com/2024/01/24/nao-government-fails-to-ensure-biomass-sustainability-in-20bn-support/ (accessed on 14 May 2025).
- Millard, R. UK Cannot Prove Sustainability of Biomass Power Plants, Warns Watchdog. Financial Times, 2024. Available online: https://www.ft.com/content/adee9de4-c36f-435c-8551-f28b77737246 (accessed on 14 May 2025).
- Crowley, J. Key Power Station Didn’t Properly Disclose Burning Forest Wood. BBC News, 9 February 2025. Available online: https://www.bbc.co.uk/news/articles/cdxnpzzjed1o (accessed on 14 May 2025).
- Muirhead, C. Ed Miliband Embroiled in New Drax Greenwashing Row… over a Town in Mississippi. This Is Money, 27 April 2025. Available online: https://www.thisismoney.co.uk/money/markets/article-14650335/Energy-Secretary-embroiled-new-Drax-greenwashing-row.html (accessed on 14 May 2025).
- Williams, M. Drax Makes a Mockery of International Day of Forests. NRDC, 2024. Expert Blog. 2024. Available online: https://www.nrdc.org/bio/matt-williams/drax-makes-mockery-international-day-forests (accessed on 14 May 2025).
- Mayo, F. The Largest Emitters in the UK: Annual Review; Ember: London, UK, 2024; Available online: https://ember-energy.org/app/uploads/2024/08/The-largest-emitters-in-the-UK_-annual-review-1-1.pdf (accessed on 14 May 2025).
- Anderson, K. Letter to Review UK CCUS Policy; Campaign Against Climate Change: London, UK, 2024; Available online: https://www.campaigncc.org/sites/data/files/sites/data/files/Docs/letter_to_sos_-_blue_hydrogen_and_ccus.pdf (accessed on 14 May 2025).
- UK Parliament. Carbon Capture, Usage and Storage, Eighth Report of Session 2024–2025. 2025. Available online: https://publications.parliament.uk/pa/cm5901/cmselect/cmpubacc/351/report.html (accessed on 14 May 2025).
- Pratley, N. MPs Question Value of Billions in Subsidies Granted to Drax Power Plant. Spending Watchdog Warns £6.5bn in Funding May Not Offer Value for Public Money Amid Sustainability Concerns. Guardian, 25 April 2025. Available online: https://www.theguardian.com/business/2025/apr/25/mps-question-value-of-billions-in-subsidies-granted-to-drax-power-plant (accessed on 14 May 2025).
- Partnership for Policy Integrity. The Drax Whistleblower Case: Its Significance for UK Biomass Policy; Partnership for Policy Integrity: Amherst, MA, USA, 2025; Available online: https://www.pfpi.net/2025/03/the-drax-whistleblower-case-its-significance-for-uk-biomass-policy/ (accessed on 14 May 2025).
- Ilakiya, T.; Parameswari, E.; Swarnapriya, R.; Yazhini, G.; Kalaiselvi, P.; Davamani, V.; Singh, S.; Vinothini, N.; Dharani, C.; Garnepudi, S.L.; et al. Unlocking the Carbon Sequestration Potential of Horticultural Crops. Crops 2024, 10, 65. [Google Scholar] [CrossRef]
- Phalan, B.; Bertzky, M.; Butchart, S.H.M.; Donald, P.F.; Scharlemann, J.P.W.; Stattersfield, A.J.; Balmford, A. Crop expansion and conservation priorities in tropical countries. PLoS ONE 2018, 8, e51759. [Google Scholar] [CrossRef]
- Mahli, Y.; Grace, J. Tropical forests and atmospheric carbon dioxide. Trends Ecol. Evol. 2000, 15, 332–337. [Google Scholar]
- Patthanaissaranukool, W.; Polprasert, C. Carbon Mobilization in Oil Palm Plantation and Milling Based on a Carbon-Balanced. Environ. Asia 2011, 24, 17–26. [Google Scholar]
- Agricultural Policy Monitoring and Evaluation. 2022: Reforming Agricultural Policies for Climate Change Mitigation; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Martinez-Nuñez, C.; Velado-Alonso, E.; Avelino, J.; Rey, P.J.; Hoopen, G.M.; Peer, G.; Zou, Y.; Liu, Y.; Agyei, P.A.; Rusch, A.; et al. Tailored policies for perennial woody crops are crucial to advance sustainable development. Nat. Sustain. 2024, 8, 133–141. [Google Scholar] [CrossRef]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Sommer, R.; Verchot, L.V. Global Sequestration Potential of Increased Organic Carbon in Cropland Soils. Sci. Rep. 2017, 7, 15554. [Google Scholar] [CrossRef] [PubMed]
- Koussihouèdé, H.; Aholoukpè, H.; Adjibodou, J.; Hinkati, H.; Dunos, B.; Chapius-Lardy, L.; Barthes, B.G. Comparative analysis of nutritional status and growth of immature oil palm in various intercropping systems in southern Benin. Exp. Agric. 2020, 56, 371–386. [Google Scholar] [CrossRef]
- Leonel, S.; Leonel, M.; Jesus, P.R.R.d.; Tecchio, M.A.; Silva, M.d.S.; Cândido, H.T.; Molha, N.Z.; Ouros, L.F.d. Achievements of Banana (Musa sp.)-Based Intercropping Systems in Improving Crop Sustainability. Horticulturae 2024, 10, 956. [Google Scholar] [CrossRef]
- Maheswarappa, H.P.; Palaniswami, C.; Dhanapal, R.; Subramanian, P. Coconut based intercropping and mixed cropping systems. J. Plant. Crops. 2010, 37, 14–16. [Google Scholar]
- Tilden, G.M.; Aranka, J.N.; Curry, G.N. Ecosystem services in coffee agroforestry: Their potential to improve labour efficiency amongst smallholder coffee producers. Agroforest Syst. 2024, 98, 383–400. [Google Scholar] [CrossRef]
- Fleiss, S.; Waddell, E.; Ola Bernadus, B.; Banin, L.F.; Benedick, S.; Sailim, A.B. Conservation set-asides improve carbon storage and support associated plant diversity in certified sustainable oil palm plantations. Biol. Conserv. 2020, 248, 108631. [Google Scholar] [CrossRef]
- Mohd Hanafiah, K.; Abd Mutalib, A.H.; Miard, P.; Goh, C.S.; Sah, S.A.M.; Ruppert, N. Impact of Malaysian palm oil on sustainable development goals: Co-benefits and trade-offs across mitigation strategies. Sustain. Sci. 2022, 17, 1639–1661. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, J.E.; O’Hanley, J.R.; Armsworth, P.R.; Slade, E.M.; Deere, N.J.; Mitchell, S.L.; Hemproch-Bennett, D. Enhancing the ecological value of oil palm agriculture through set-asides. Nat. Sustain. 2023, 6, 513–525. [Google Scholar] [CrossRef]
- Liu, W.; Hughes, A.C.; Bai, Y.; Li, Z.; Mei, C.; Ma, Y. Using Landscape Connectivity Tools to Identify Conservation Priorities in Forested Areas and Potential Restoration Priorities in Rubber Plantation in Xishuangbanna, Southwest China. Landsc. Ecol. 2020, 35, 389–402. [Google Scholar] [CrossRef]
- Tiko, J.M.; Ndjadi, S.S.; Obandza-Ayessa, J.L.; Mweru, J.P.M.; Michel, B.; Beeckman, H.; Rakotondrasoa, O.L.; Hulu, J.P.M.T. Carbon Sequestration Potential in Rubber Plantations: A Complementary Approach to Tropical Forest Conservation Strategies, a Review. Earth 2025, 6, 21. [Google Scholar] [CrossRef]
- Jackson, E. Natural Rubber Market Shows Strong Growth Amid Global Trade Developments and Sustainability Initiatives. Chemical Analyst, 20 December 2024. Available online: https://www.chemanalyst.com/NewsAndDeals/NewsDetails/natural-rubber-market-shows-strong-growth-amid-global-trade-developments-32324 (accessed on 14 May 2025).
- The Nation. Green light given to Rubber Plantations to Sell Carbon Credits. 2024. Available online: https://www.nationthailand.com/blogs/special-edition/sustainability/40036741 (accessed on 14 May 2025).
- Miharza, T.; Wijayanto, N.; Roshetko, J.M.; Siregar, I.Z. Carbon stocks and footprints of smallholder cacao systems in Polewali Mandar, West Sulawesi. Front. Environ. Sci. 2023, 11, 680984. [Google Scholar] [CrossRef]
- Michel, I.; Blanco, J.; Essouma, F.M.; Carrière, S.M. Complex cocoa agroforestry systems shaped within specific socioeconomic and historical contexts in Africa: Lessons from Cameroonian farmers. Agric. Syst. 2024, 221, 2610. [Google Scholar] [CrossRef]
- Robinson, N. Cocoa prices shock 2025 financial outlook for big food. Food Navigator, 13 February 2025. Available online: https://www.foodnavigator.com/Article/2025/02/13/how-cocoa-prices-impact-big-food-companies-like-unilever/ (accessed on 14 May 2025).
- Atangana, A.; Khasa, D.; Chang, S.; Degrande, A. Tropical Agroforestry; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Somarriba, E.; Deheuvels, O.; Cerda, R. Carbon stocks and cocoa yields in agroforestry systems of Central America. Agric. Ecosyst. Environ. 2013, 173, 46–57. [Google Scholar] [CrossRef]
- Nguyen-Duy, N.; Talsma, T.; Nguyen, K.T.; Nguyen, T.Q.; Laderach, P. Carbon Assessment for Cocoa Cropping Systems in Lampung, Indonesia; International Center for Tropical Agriculture: Cali, Colombia, 2018; p. 32. Available online: https://ccafs.cgiar.org/resources/publications/carbon-assessment-cocoa-cropping-systems-lampung-indonesia (accessed on 14 May 2025).
- Thomson, A.; Konig, S.; Bakhtary, H.; Young, K.J. Developing Cocoa Agroforestry Systems in Ghana and Cote d’Ivoire. In Climate Focus; 2020; Available online: https://climatefocus.com/wp-content/uploads/2022/06/Developing-Cocoa-Agroforesty-Systems-in-Ghana-and-Cote-dIvoire.pdf (accessed on 14 May 2025).
- Schroth, G.; Laderach, P.; Martinez-Valle, A.I.; Bunn, C.; Jassogne, L. Vulnerability to climate change of cocoa in West Africa: Patterns, opportunities and limits to adaptation. Sci. Total Environ. 2016, 556, 231–241. [Google Scholar] [CrossRef]
- Grüter, R.; Trachsel, T.; Laube, P.; Jaisli, I. Expected global suitability of coffee, cashew and avocado due to climate change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef]
- Murphy, D.J. Coffee May Become More Scarce and Expensive Thanks to Climate Change—New Research. The Conversation, 27 January 2022. Available online: https://theconversation.com/coffee-may-become-more-scarce-and-expensive-thanks-to-climate-change-new-research-175766 (accessed on 14 May 2025).
- Toledo, V.M.; Moguel, P. Coffee and sustainability: The multiple values of traditional shaded coffee. Sustain. Agric. 2012, 36, 353–377. [Google Scholar] [CrossRef]
- Haggar, J.; Casanoves, F.; Cerda, R.; Cerretelli, S.; Gonzalez-Mollinedo, S.; Lanza, G. Shade and agronomic intensification in coffee agroforestry systems: Trade-off or synergy? Front. Sustain. Food Syst. 2021, 5, 645948. [Google Scholar] [CrossRef]
- Vallejos-Torres, G.; Gaona-Jimenez, N.; Pichis-Garcıa, R.; Ordoñez, L.; Garcıa-Gonzales, P.; Quinteros, A.; Lozano, A.; Saavedra-Ramırez, J. Carbon reserves in coffee agroforestry in the Peruvian Amazon. Front. Plant Sci. 2024, 15, 1410418. [Google Scholar] [CrossRef] [PubMed]
- Buechley, E.R.; Şekercioglu, C.H.; Atickem, A.; Gebremichael, G.; Ndungu, J.K.; Mahamued, B.A. Importance of Ethiopian shade coffee farms for forest bird conservation. Biol. Conserv. 2015, 188, 50–60. [Google Scholar] [CrossRef]
- Hylander, K.; Nemomissa, S.; Delrue, J.; Enkosa, W. Effects of coffee management on deforestation rates and forest integrity. Conserv. Biol. 2013, 27, 1031–1040. [Google Scholar] [CrossRef]
- Koutouleas, A.; Sarzynski, T.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N. A Shaded-Coffee: A Nature-Based Strategy for Coffee Production Under Climate Change? A Review. Front. Sustain. Food Syst. 2022, 6, 877476. [Google Scholar] [CrossRef]
- Tesfay, F.; Moges, Y.; Asfaw, Z. Woody species composition, structure, and carbon stock of coffee-based agroforestry system along an elevation gradient in the moist mid-highlands of Southern Ethiopia. Int. J. For. Res. 2022, 1, 4729336. [Google Scholar] [CrossRef]
- Lara-Estrada, L.; Rasche, L.; Schneider, U.A. Land in Central America will become less suitable for coffee cultivation under climate change. Reg. Environ. Change 2021, 21, 88. [Google Scholar] [CrossRef]
- Birhanu, A.; Terefe, D. The Role of Shade Trees in Coffee Production Systems: The Case of 37 Yayo District, Ilubabora Zone, Oromiya Region, Southwest Ethiopia. South Asian Res. J. Bio Appl. Biosci. 2022, 4, 37–50. [Google Scholar]
- Niguse, G.; Iticha, B.; Kebede, G.; Chimdi, A. Contribution of coffee plants to carbon sequestration in agroforestry systems of Southwestern Ethiopia. J. Agric. Sci. 2022, 160, 440–447. [Google Scholar] [CrossRef]
- Gelaye, Y.; Getahun, S. A review of the carbon sequestration potential of fruit trees and their implications for climate change mitigation: The case of Ethiopia. Cogent Food Agric. 2024, 10, 2294544. [Google Scholar] [CrossRef]
- Lugo-Pérez, J.; Hajian-Forooshani, Z.; Perfecto, I.; Vandermeer, J. The importance of shade trees in promoting carbon storage in the coffee agroforest systems. Agric. Ecosyst. Environ. 2023, 355, 108594. [Google Scholar] [CrossRef]
- Harmand, J.M.; Hergoualc’h, K.; De Miguel, S.; Dzib, B.; Siles, P.; Vaast, P. Carbon sequestration in coffee agroforestry plantations of Central America. In Proceedings of the 21st International Conference on Coffee Science, Montpellier, France, 11–15 September 2021; Available online: https://agritrop.cirad.fr/540109/ (accessed on 14 May 2025).
- Nab, C.; Maslin, M. Life cycle assessment synthesis of the carbon footprint of Arabica coffee: Case study of Brazil and Vietnam conventional and sustainable coffee production and export to the United Kingdom. Geo 2020, 7, e00096. [Google Scholar] [CrossRef]
- Henderson, E. 11 Best Independent Coffee Brands to Brighten Your Morning. Independent, 10 February 2025. Available online: https://www.independent.co.uk/extras/indybest/food-drink/best-independent-coffee-brands-ground-bean-ethiopian-colombian-brazilian-a9606086.html (accessed on 14 May 2025).
- Technavio. Specialty Coffee Shops Market to Grow by USD 50.8 Billion (2025–2029); PR Newswire: New York, NY, USA, 2025; Available online: https://www.prnewswire.com/news-releases/specialty-coffee-shops-market-to-grow-by-usd-50-8-billion-2025-2029-driven-by-rising-coffee-consumption-report-on-how-ai-redefines-market-landscape---technavio-302359596.html (accessed on 14 May 2025).
- Solidaridad. Overview of the Carbon Balance in Coffee Production. 2024. Available online: https://www.solidaridadnetwork.org/publications/overview-of-the-carbon-balance-in-coffee-production/ (accessed on 14 May 2025).
- FAOa. Banana. Market Review Preliminary Results. 2025. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/560972a9-e34d-44f5-9079-fc1551e0fd69/content (accessed on 14 May 2025).
- FAOb. Crop Information—Banana. 2025. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/banana/en/ (accessed on 14 May 2025).
- Turrell, C. Saving Cavendish. Nat. Biotechnol. 2024, 42, 545. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Mostert, D.; Yu, H.; Zhou, M.; Li, G.; Zuo, C.; Haridas, S.; Webster, K.; Li, M.; et al. Virulence of banana wilt-causing fungal pathogen Fusarium oxysporum tropical race 4 is mediated by nitric oxide biosynthesis and accessory genes. Nat. Microbiol. 2024, 9, 2232–2243. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Li, M.; Shi, Y.F. Carbon Storage and Carbon Dioxide Sequestration of Banana Plants at Different Growth Stages. Adv. Mater. Res. 2014, 1010–1012, 662–665. [Google Scholar] [CrossRef]
- Varma, V.; Mosedale, J.R.; Alvarez, J.A.G.; Bebber, D. Socio-economic factors constrain climate change adaptation in a tropical export crop. Nat. Food 2025, 6, 343–352. [Google Scholar] [CrossRef]
- Descals, A.; Wich, S.; Szantoi, Z.; Struebig, M.J.; Dennis, R.; Hatton, Z.; Ariffin, T.; Unus, N.; Gaveau, D.L.A.; Meijaard, E. High-resolution global map of closed-canopy coconut palm. Earth Syst. Sci. Data 2023, 15, 3991–4010. [Google Scholar] [CrossRef]
- FAO. Banana Market Review 2022; FAO: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/cd3e1df8-6e70-461a-9963-9827ad69389f/content (accessed on 14 May 2025).
- Murphy, D.J.; Goggin, K.A.; Patterson, R. Oil palm crops in the 2020s and beyond: Challenges and solutions. CABI J. Agric. Biosci. 2021, 2, 39. [Google Scholar]
- Statista. Harvested Area of Coconuts Worldwide from 2010 to 2023 (in Million Hectares). 2025. Available online: https://tiny.cc/bcvh001 (accessed on 14 May 2025).
- Ranasinghe, C.S.; Silva, L.R.S. Photosynthetic assimilation, carbohydrates in vegetative organs and carbon removal in nut-producing and sap-producing coconut palms. Cocos 2007, 8, 45–57. [Google Scholar] [CrossRef][Green Version]
- Atapattu, A.J.; Udumann, S.S. Leveraging Agroforestry Principles for Nature-Based Climate-Smart Solutions for Coconut Cultivation. In Handbook of Nature-Based Solutions to Mitigation and Adaptation to Climate Change; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Namitha, V.V.; Raj, S.K.; Prathapan, K. Carbon Sequestration Potential in Coconut based Cropping System: A Review. Agric. Rev. 2025, 46, 143–146. [Google Scholar] [CrossRef]
- Bhagya, H.P.; Maheswarappa, H.P.; Surekha, P.; Bhat, R. Carbon sequestration potential in coconut-based cropping systems. Indian J. Hort. 2017, 74, 1–5. [Google Scholar] [CrossRef]
- Rani, S.R.; Subbulakshmi, S.; Kavitha, K.; Hassan, S.N.; Latha, R.; Suresh, S. A review on coconut based intercropping. Ind. J. Res. Agron. 2024, 9, 243–247. [Google Scholar] [CrossRef]
- Rubber Authority of Thailand. Thailand’s natural rubber producers are preparing for new market requirements. In Briefing; 2024; Available online: https://efi.int/sites/default/files/files/publication-bank/2024/Briefing%20-%20Thailand’s%20natural%20rubber%20producers%20are%20preparing%20for%20new%20market%20requirements.pdf (accessed on 14 May 2025).
- Moss, J. Will Rubber Prices Continue Their Slide from Seven-Year Highs This Year? International Banker, 15 January 2025. Available online: https://internationalbanker.com/brokerage/will-rubber-prices-continue-their-slide-from-seven-year-highs-this-year/ (accessed on 14 May 2025).
- Statista. Consumption of Natural and Synthetic Rubber Worldwide from 1990 to H1. 2024. Available online: https://www.statista.com/statistics/275399/world-consumption-of-natural-and-synthetic-caoutchouc/ (accessed on 14 May 2025).
- Blagodatsky, S.; Cadisch, G.; Xu, J.C. Carbon balance of rubber (Hevea brasiliensis) plantations: A review of uncertainties at plot, landscape and production level. Agric. Ecosyst. Environ. 2016, 221, 8–19. [Google Scholar] [CrossRef]
- Menoh, A. Carbon Storage of Some Rubber Trees (Hevea brasiliensis) Clones in HEVECAM’s Plantations in South Cameroon. In Biodiversity of Ecosystems; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Adingra, O.M.M.A.; Kassi, N.J. Dynamique de la végétation de Bamo et stocks de carbone dans la mosaïque de végétation. Eur. Sci. J. 2016, 12, 359–374. [Google Scholar] [CrossRef]
- Ziegler, A.D.; Phelps, J.; Yuen, J.Q.; Webb, E.L.; Lawrence, D.; Fox, J.M.; Bruun, T.B.; Leisz, S.J.; Ryan, C.M.; Dressler, W.; et al. GCB Carbon outcomes of major land cover transitions in SE Asia: Great uncertainties and REDD policy implications. Glob. Change Biol. 2012, 18, 3087–3099. [Google Scholar] [CrossRef]
- Satakhun, D.; Chayawat, C.; Sathornkich, J.; Phattaralerphong, J.; Chantuma, P.; Thaler, P.; Gay, F. Carbon sequestration potential of rubber-tree plantation in Thailand. IOP Conf. Ser. Mater. Sci. Eng. 2019, 526, 012036. [Google Scholar] [CrossRef]
- Fox, J.M.; Castella, J.C.; Ziegler, A.D.; Westley, S.B. Rubber Plantations Expand in Mountainous Southeast Asia: What Are the Consequences for the Environment? East-West Center: Honolulu, HI, USA, 2014; No. 114; Available online: https://www.files.ethz.ch/isn/179885/api114.pdf (accessed on 14 May 2025).
- Pinizzotto, S.; Kadir, A.; Gitz, V.; Sainte-Beuve, J.; Nair, L.; Gohet, E.; Meybeck, A. Natural Rubber and Climate Change. In Policy Paper No. 6; CIFOR: Bogor, Indonesia, 2021. [Google Scholar] [CrossRef]
- Kadir, A.B.S.A.; Gitz, V.; Gohet, E.; Jacob, J.; Nair, L.; Pinizzotto, S.; Nguyen, A.N.; Blagodatsky, S.; Brady, M.; Cerutti, P.O.; et al. Natural Rubber Contributions to Mitigation of Climate Change. In Proceedings of the World Forestry Congress, Seoul, Republic of Korea, 2–6 May 2022; pp. 1–9. Available online: https://www.cifor.org/publications/pdf_files/Papers/WFC2022-Kadir.pdf (accessed on 14 May 2025).
- Zou, R.; Sultan, H.; Muse Muhamed, S.; Khan, M.N.; Pan, J.; Liao, W.; Li, Q.; Cheng, S.; Tian, J.; Cao, Z.; et al. Sustainable Integration of Rubber Plantations Within Agroforestry Systems in China: Current Research and Future Directions. Plant Sci. Today 2024, 11, 421–431. [Google Scholar] [CrossRef]
- Solidaridad. Palm Oil Barometer 2025—Procurement for Prosperity. 2025. Available online: https://www.solidaridadnetwork.org/publications/palm-oil-barometer-2025/ (accessed on 14 May 2025).
- Southey, F. From Cocaine to Palm Oil: The Highs and Lows of Transforming a Narcotic Landscape. Food Navigator, 24 February 2022. Available online: https://www.foodnavigator.com/Article/2022/02/24/from-cocaine-to-palm-oil-the-highs-and-lows-of-transforming-a-narcotic-landscape/ (accessed on 14 May 2025).
- Abdul Rahim, K.S.B.; Samsuri, A.B.; Jamal, S.H.B.; Mohd Nor, S.A.B.; Rusly, S.N.A.B.; Ariff, H.B.; Abdul Latif, N.S.B. Redefining biofuels: Investigating oil palm biomass as a promising cellulose feedstock for nitrocellulose-based propellant production. Def. Technol. 2024, 37, 111–132. [Google Scholar] [CrossRef]
- Ariesca, R.; Sau, A.A.W.T.; Adinugroho, W.C.; Setiawan, A.A.R.; Ahamed, T.; Noguchi, R. Land Swap Option for Sustainable Production of Oil Palm Plantations in Kalimantan, Indonesia. Sustainability 2023, 15, 2394. [Google Scholar] [CrossRef]
- Henson, I.E. Notes on oil palm productivity. IV. Carbon dioxide gradients and fluxes and evapotranspiration, above and below the canopy. J. Oil Palm Res. 1999, 11, 33–40. [Google Scholar]
- Henson, I.; Ruiz, R.R.; Romero, H.M. The greenhouse gas balance of the oil palm industry in Colombia: A preliminary analysis. I. Carbon sequestration and carbon. Agron. Colomb. 2012, 30, 359–369. [Google Scholar]
- Henson, I.; Ruiz, R.R.; Romero, H.M. The greenhouse gas balance of the oil palm industry in Colombia: A preliminary analysis. II. Greenhouse gas emissions and the carbon budget. Agron. Colomb. 2012, 30, 370–378. [Google Scholar]
- Pulhin, F.B.; Urquiola, J.P.; Lasco, R.D. Carbon Sequestration Potential of Oil Palm in Bohol, Philippines. Ecosyst. Devel. J. 2014, 4, 14–19. [Google Scholar]
- Daud, N.N.; Chinenyenwa, A.S.; Rhys, T.H.; Ken, L.; Lee, H. Carbon Sequestration in Malaysian Oil Palm Plantations—An Overview: Towards a Sustainable Geoenvironment. In Proceedings of the 8th International Congress on Environmental Geotechnics, Hangzhou, China, 28 October–1 November 2018; Springer: Singapore, 2019; Volume 3, pp. 49–56. [Google Scholar] [CrossRef]
- Uning, R.; Latif, M.T.; Othman, M.; Juneng, L.; Mohd Hanif, N.; Nadzir, M.S.M.; Abdul Maulud, K.N.; Jaafar, W.S.W.M.; Said, N.F.S.; Ahamad, F. A Review of Southeast Asian Oil Palm and Its CO2 Fluxes. Sustainability 2020, 12, 5077. [Google Scholar] [CrossRef]
- Alcock, T.D.; Salt, D.E.; Wilson, P.; Ramsden, S.J. More sustainable vegetable oil: Balancing productivity with carbon storage opportunities. Sci. Total Environ. 2022, 829, 154539. [Google Scholar] [CrossRef]
- Cheah, L.W.; Gan, H.H.; Goh, K.J. Production, Stock and Management of Carbon in Oil Palm Plantations on Mineral Soils; AAR Newsletter: Washington, DC, USA, 2015; Available online: https://aarsb.com.my/wp-content/Publication/Newsletter/PDF/2015-Oct.pdf (accessed on 14 May 2025).
- Murphy, D.J.; Soon, C.P.; Onn, H.W.; Evers, S.; Hai, T.C.; Jantan, N.M.; Girkin, N. Sustainable oil palm production. Balancing carbon sequestration and greenhouse gas emissions: A scientific review. Malays. Oil Sci. Technol. 2025, 33, 3–22. [Google Scholar]
- Rahmani, T.A.; Nurrochmat, D.R.; Hero, Y.; Park, M.S.; Boer, R.; Satria, A. Evaluating the feasibility of oil palm agroforestry in Harapan Rainforest, Jambi, Indonesia. For. Soc. 2021, 5, 458–477. [Google Scholar] [CrossRef]
- Rival, A.M.; Ancrenaz, I.; Lackman, S.; Burhan, C.; Zemp, M.; Firdaus, M.; Djama, M. Innovative planting designs for oil palm-based agroforestry. Agroforest. Syst. 2025, 99, 27. [Google Scholar] [CrossRef]
- Rafflegeau, S.; Allinne, C.; Barkaoui, K.; Deheuvels, O.; Jagoret, J.; Garcia, L. Ecosystem services functional motif: A new concept to analyse and design agroforestry systems. In Proceedings of the 4th World Congress on Agroforestry, Montpellier, France, 19–22 May 2019; Dupraz, C., Ed.; Book of Abstracts. p. 733. Available online: https://agroforestry2019.cirad.fr/news-press (accessed on 14 May 2025).
- Masure, A.; Martin, P.; Lacan, X.; Rafflegeau, S. Promoting oil palm-based agroforestry systems: An asset for the sustainability of the sector. Cahiers Agric. 2023, 32, 16. [Google Scholar] [CrossRef]
- Khasanah, N.; van Noordwijk, M.; Slingerland, M.; Sofiyudin, M.; Stomph, D.; Migeon, A.F.; Hairiah, K. Oil Palm Agroforestry Can Achieve Economic and Environmental Gains as Indicated by Multifunctional Land Equivalent Ratios. Front. Sustain. Food Syst. 2020, 3, 122. [Google Scholar] [CrossRef]
- Ahirwal, J.; Sahoo, U.K.; Thangjam, U.; Thong, P. Oil Palm Agroforestry Enhances Crop Yield and Ecosystem Carbon Stock in Northeast India: Implications For UN Sustainable Development Goals. Sustain. Prod. Consum. 2022, 30, 478–487. [Google Scholar] [CrossRef]
- Messier, C.; Bauhus, J.; Sousa-Silva, R.; Auge, H.; Baeten, L.; Barsoum, N.; Zemp, D.C. For the sake of resilience and multifunctionality, let’s diversify planted forests! Conserv. Lett. 2022, 15, e12829. [Google Scholar] [CrossRef]
- Zemp, D.C.; Guerrero-Ramirez, N.; Brambach, F.; Darras, K.; Grass, I.; Potapov, A.; Kreft, H. Tree islands enhance biodiversity and functioning in oil palm landscapes. Nature 2023, 618, 316–321. [Google Scholar] [CrossRef]
- Deines, C. The Global Environmental Consequences of Palm Oil Production: The Role of Industrial Polyculture in Sustainable Solutions. Bachelor’s Thesis, Western Carolina University, Cullowhee, NC, USA, 2024. Available online: https://affiliate.wcu.edu/rasc/wp-content/uploads/sites/298/2025/03/Deines.pdf (accessed on 14 May 2025).
- Frianto, D.; Sutrisno, E.; Wahyudi, A.; Novriyanti, E.; Adinugroho, W.C.; Yunianto, A.S.; Kurniawan, H.; Khotimah, H.; Windyoningrum, A.; Dharmawan, I.W.S.; et al. Carbon stock dynamics of forest to oil palm plantation conversion for ecosystem rehabilitation planning. Glob. J. Environ. Sci. Manag. 2024, 10, 1593–1614. [Google Scholar] [CrossRef]
- Woittiez, L.S.; van Wijk, M.T.; Slingerland, M.; van Noordwijk, M.; Giller, K.E. Yield gaps in oil palm: A quantitative review of contributing factors. Eur. J. Agron. 2017, 83, 57–77. [Google Scholar] [CrossRef]
- Monzon, J.P.; Lim, Y.L.; Tenorio, F.A.; Farrasati, R.; Pradiko, I.; Sugianto, H. Agronomy explains large yield gaps in smallholder oil palm fields. Agric. Syst. 2023, 210, 103689. [Google Scholar] [CrossRef]
- Salim, S. United Plantations Sees Palm Oil Prices Ranging Between RM3,850 and RM4,250 in 2024. The Edge, 5 March 2024. Available online: https://theedgemalaysia.com/node/703551#:~:text=Its%20Malaysian%20estates%20reached%20an,from%205.1%20tonnes%20per%20hectare (accessed on 14 May 2025).
- Guthrie, S.D. Sime Darby Plantation Launches Super-Charged Seeds. Press Release, 8 November 2023. Available online: http://www.sdguthrie.com/press-releases/sime-darby-plantation-launches-super-charged-seeds (accessed on 14 May 2025).
- Escallón-Barrios, M.; Castillo-Gomez, D.; Leal, J.; Montenegro, C.; Medaglia, A.L. Improving harvesting operations in an oil palm plantation. Ann. Oper. Res. 2020, 314, 411–449. [Google Scholar] [CrossRef]
- Lee, J.; Ghazoul, J.; Obidzinski, K.; Koh, P. Oil palm smallholder yields and incomes constrained by harvesting practices and type of smallholder management in Indonesia. Agron. Sustain. Dev. 2014, 34, 501–513. [Google Scholar] [CrossRef]
- Mohanaraj, S.; Donough, C.R. Harvesting practices for maximum yield in oil palm: Results from a re-assessment at IJM plantations. Sabah. Oil Palm Bull. 2016, 72, 32–37. [Google Scholar]
- Isaac, J. Industrial revolution 4.0 for smart oil palm mills. Malays. Oil Sci. Technol. 2019, 28, 28–36. [Google Scholar]
- Keong, N.W. Modernising sales and widening markets. Malays. Oil Sci. Technol. 2019, 28, 32–36. [Google Scholar]
- Descals, A.; Sheil, D.; Wich, S.; Ozigis, M.; Meijaard, E. Extensive Unreported Non-Plantation Oil Palm in Africa. Preprints 2025, 2025021589. [Google Scholar] [CrossRef]
- Leijten, F.; Lantz, C.; Baldos, U.; Johnson, J.A.; Sim, S.; Verburg, P.H. Projecting global oil palm expansion under zero-deforestation commitments: Direct and indirect land use change impacts. iScience 2023, 26, 106971. [Google Scholar] [CrossRef]
- Galán-Martín, Á.; Contreras, M.; Romero, I.; Ruiz, E.; Bueno-Rodríguez, S.; Eliche-Quesada, D.; Castro-Galiano, E. The potential role of olive groves to deliver carbon dioxide removal in a carbon-neutral Europe: Opportunities and challenges. Renew. Sustain. Energy Rev. 2022, 165, 112609. [Google Scholar] [CrossRef]
- Galán-Martín, A.; Contreras, M.; Castro, E. Carbon-negative products to engage society in climate action: The life cycle of olive oil. Sust. Prod. Consumpt. 2024, 37, 516–527. [Google Scholar] [CrossRef]
- Dawson, D. Global Olive Oil Production Will Reach 4.4M Tons by 2050, Expert Projects. Olive Oil Times, 6 December 2021. Available online: https://www.oliveoiltimes.com/world/global-olive-oil-production-reach-4-million-tons-by-2050/101131 (accessed on 14 May 2025).
- Rosati, A.; Paoletti, A.; Lodolini, E.M.; Famiani, F. Cultivar ideotype for intensive olive orchards: Plant vigor, biomass partitioning, tree architecture and fruiting characteristics. Front. Plant Sci. 2024, 15, 1345182. [Google Scholar] [CrossRef]
- OIV. Annual Assessment of the World Vine and Wine Sector in 2022. 2022. Available online: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment-2023.pdf (accessed on 14 May 2025).
- Vendrame, N.; Tezza, L.; Pitacco, A. Study of the carbon budget of a temperate-climate vineyard: Inter-annual variability of CO2 flux. Am. J. Enol. Vitic. 2019, 70, 34–41. [Google Scholar] [CrossRef]
- Chiriaco, M.V.; Belli, C.; Chiti, T.; Trotta, C.; Sabbatini, S. The potential carbon neutrality of sustainable viticulture showed through a comprehensive assessment of the greenhouse gas (GHG) budget of wine production. J. Clean. Prod. 2019, 225, 435–450. [Google Scholar] [CrossRef]
- Callesen, T.O.; Gonzalez, C.V.; Campos, F.B.; Zanotelli, D.; Massimo Tagliavini, M.; Montagnani, L. Understanding carbon sequestration, allocation, and ecosystem storage in a grassed vineyard. Geoderma Reg. 2023, 34, e00674. [Google Scholar] [CrossRef]
- Xue, T.; Zhang, L.; Yang, F.; Li, Y.; Cheng, C.; Hsin, C.; Cheng, L.; Wang, J.; Sang, Q.; Yang, S.; et al. Carbon sink and soil organic carbon sequestration mechanisms in vineyards. J. Clean. Prod. 2024, 469, 143217. [Google Scholar] [CrossRef]
- Mo, L.; Zohner, C.M.; Reich, P.B.; Liang, J.; de Miguel, S.; Nabuurs, G.-J.; Renner, S.S.; Hoogen, J.; Araz, A.; Herold, M.; et al. Integrated global assessment of the natural forest carbon potential. Nature 2023, 624, 92–101. [Google Scholar] [CrossRef]
- Graham, R.T.; Jain, T.B. Silviculture’s role in managing boreal forests. Conserv. Ecol. 1998, 2, 8. [Google Scholar] [CrossRef]
- Lal, R.; Lorenz, K. Carbon Sequestration in Temperate Forests. In Recarbonization of the Biosphere; Lal, R., Lorenz, K., Hüttl, R., Schneider, B., von Braun, J., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Nair, P.K.R. Climate Change Mitigation: A Low-Hanging Fruit of Agroforestry. In Agroforestry—The Future of Global Land Use; Nair, P., Garrity, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 9. [Google Scholar] [CrossRef]
- MET. What is the Carbon Dioxide (CO2) Quota? METGroup: Zug, Switzerland, 2023; Available online: https://group.met.com/en/mind-the-fyouture/mindthefyouture/carbon-dioxide-co2-quota (accessed on 14 May 2025).
- International Carbon Action Partnership (ICAP). Emissions Trading Worldwide: 2025 ICAP Status Report; ICAP: Berlin, Germany, 2025; Available online: https://icapcarbonaction.com/en/publications/emissions-trading-worldwide-icap-status-report-2025 (accessed on 14 May 2025).
- Jones, J.P.G.; Lewis, S.L. Forest carbon offsets are failing. Analysis reveals emission reductions from forest conservation have been overestimated. Science 2023, 381, 830–831. [Google Scholar] [CrossRef] [PubMed]
- West, T.A.P.; Wunder, S.; Sills, E.O.; Börner, J.; Rifai, S.W.; Neidermeier, A.N.; Frey, G.P.; Kontoleon, A. Action needed to make carbon offsets from forest conservation work for climate change mitigation. Science 2023, 381, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Probst, B.S.; Toetzke, M.; Kontoleon, A.; Anadon, L.D.; Minx, J.M.; Haya, B.K.; Schneider, L.; Trotter, P.A.; West, P.A.; Gell-Wiehl, A.; et al. Systematic assessment of the achieved emission reductions of carbon crediting projects. Nat. Commun. 2024, 15, 9562. [Google Scholar] [CrossRef]
- Greenfield, P. Cop29: What Are Carbon Credits and Why Are They So Controversial? Guardian, 10 November 2024. Available online: https://tiny.cc/li0j001 (accessed on 14 May 2025).
- Greenfield, P. Cop29’s New Carbon Market Rules Offer Hope After Scandal and Deadlock. Guardian, 24 November 2024. Available online: https://www.theguardian.com/environment/2024/nov/24/cop29s-new-carbon-market-rules-offer-hope-after-scandal-and-deadlock (accessed on 14 May 2025).
- UNFCCC. COP29 UN Climate Conference Agrees to Triple Finance to Developing Countries, Protecting Lives and Livelihoods. 2024. Available online: https://unfccc.int/news/cop29-un-climate-conference-agrees-to-triple-finance-to-developing-countries-protecting-lives-and (accessed on 14 May 2025).
- Gagnon-Lebrun, F.; Casaer-Diaz, K. With the COP29 UN Climate Negotiations Behind Us, Frederic Gagnon-Lebrun, Senior Director, Policy and Strategy and Karolien Casaer-Diez, Senior Director, Article 6 Help Us to Unpack the Outcomes. South Pole, 3 December 2024. Available online: https://www.southpole.com/blog/cop29-highlights-shaping-the-future-of-carbon-markets (accessed on 14 May 2025).
- Zheng, Y. How Carbon Markets can Unlock Green Finance for Global South Countries; Green Central Banking, 2025; Available online: https://greencentralbanking.com/2025/04/16/carbon-markets-unlocking-green-finance/ (accessed on 14 May 2025).
- Economist. Can the Voluntary Carbon Market Save the Amazon? Entrepreneurs in Brazil Are Betting Big on Planting Trees; Economist: New York, NY, USA, 2024; Available online: https://www.economist.com/the-americas/2024/09/19/can-the-voluntary-carbon-market-save-the-amazon (accessed on 14 May 2025).
- Antonelli, A.; Rueda, X.; Calcagno, R.; Nantongo Kalunda, P. How biodiversity credits could help to conserve and restore nature. Nature 2024, 634, 1045–1104. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, D.J. Carbon Sequestration for Global-Scale Climate Change Mitigation: Overview of Strategies Plus Enhanced Roles for Perennial Crops. Crops 2025, 5, 39. https://doi.org/10.3390/crops5030039
Murphy DJ. Carbon Sequestration for Global-Scale Climate Change Mitigation: Overview of Strategies Plus Enhanced Roles for Perennial Crops. Crops. 2025; 5(3):39. https://doi.org/10.3390/crops5030039
Chicago/Turabian StyleMurphy, Denis J. 2025. "Carbon Sequestration for Global-Scale Climate Change Mitigation: Overview of Strategies Plus Enhanced Roles for Perennial Crops" Crops 5, no. 3: 39. https://doi.org/10.3390/crops5030039
APA StyleMurphy, D. J. (2025). Carbon Sequestration for Global-Scale Climate Change Mitigation: Overview of Strategies Plus Enhanced Roles for Perennial Crops. Crops, 5(3), 39. https://doi.org/10.3390/crops5030039





