Role of Seaweeds for Improving Soil Fertility and Crop Development to Address Global Food Insecurity
Abstract
1. Introduction
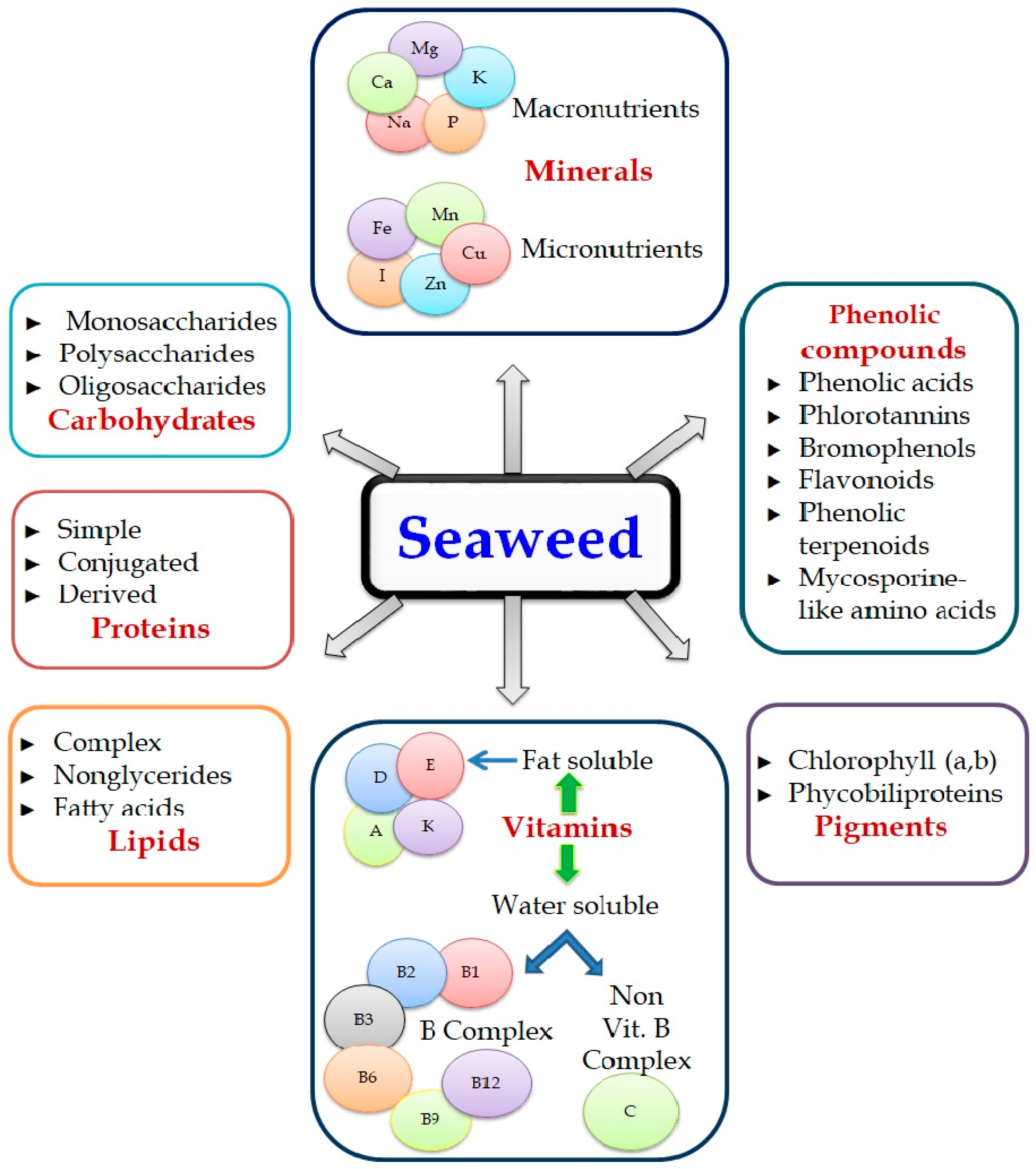
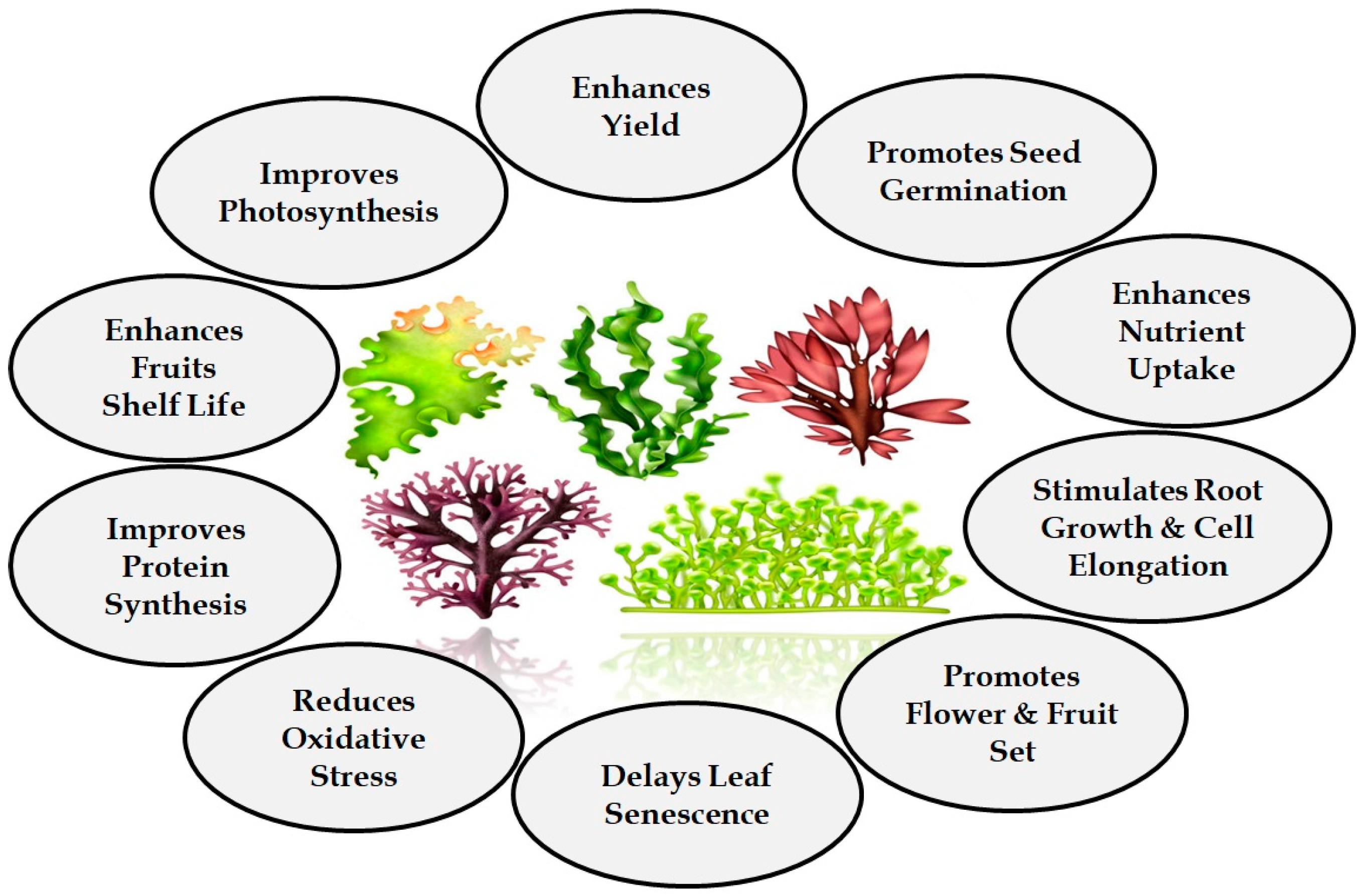
2. Role of Bioactive Compounds Extracted from Seaweeds for Crop Improvement
3. Impact of Seaweeds on Soil Health
4. Effects of Seaweeds on Plant Growth and Stress Tolerance
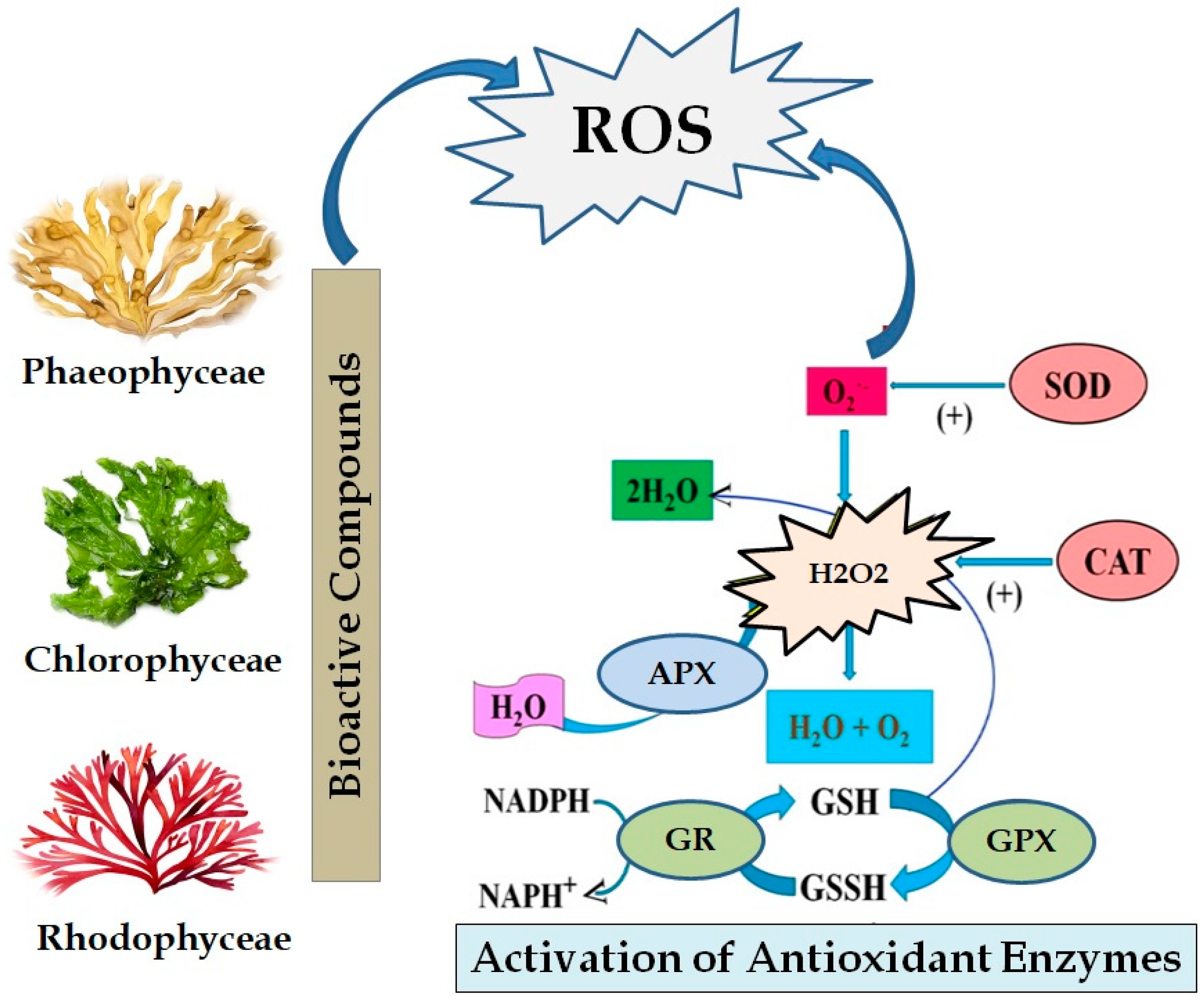
4.1. Antioxidant Properties of Seaweeds
4.2. Role of Seaweeds in Alleviating Plant Abiotic Stresses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| United Nations | UN |
| Food and Agriculture Organization | FAO |
| Compound Annual Growth Rate | CAGR |
| European Union | EU |
| Phycobiostimulants | PBSs |
| Reactive Oxygen Species | ROS |
| Hydrogen Peroxide | H₂O₂ |
| Superoxide Dismutase | SOD |
| Ascorbate Peroxidase | APX |
| Guaiacol Peroxidase | POD |
| Catalase | CAT |
| Malondialdehyde | MDA |
| Cation Exchange Capacity | CEC |
| Heavy Metal | HM |
| Biochemical Oxygen Demand | BOD |
| Dissolved Organic Carbon | DOC |
| Dissolved Oxygen | DO |
| Total Suspended Solids | TSSs |
References
- Brown, K.A.; Venkateshmurthy, N.S.; Law, C.; Harris, F.; Kadiyala, S.; Shankar, B.; Knai, C. Moving towards sustainable food systems: A review of Indian food policy budgets. Glob. Food Secur. 2021, 28, 100462. [Google Scholar] [CrossRef] [PubMed]
- Rathinapriya, P.; Maharajan, T.; Jothi, R.; Prabakaran, M.; Lee, I.B.; Yi, P.H.; Jeong, S.T. Unlocking biochar impacts on abiotic stress dynamics: A systematic review of soil quality and crop improvement. Front. Plant Sci. 2025, 15, 1479925. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, B.L.; Vyas, R.V.; Patel, H.K.; Jhala, Y.K. Perspectives of seaweed as organic fertilizer in agriculture. Soil Fertil. Manag. Sustain. Dev. 2019, 267–289. [Google Scholar] [CrossRef]
- Rathinapriya, P.; Satish, L.; Pandian, S.; Rameshkumar, R.; Balasangeetha, M.; Rakkammal, K.; Ramesh, M. Effects of liquid seaweed extracts in improving the agronomic performance of foxtail millet. J. Plant Nutr. 2020, 43, 2857–2875. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. Food and Agriculture Organization of the United Nations. 2006. Available online: http://faostat.fao.org (accessed on 5 February 2025).
- GlobeNewswire. Seaweed Extracts Biostimulant Market Report 2023–2030. GlobeNewswire. 2023. Available online: https://www.globenewswire.com (accessed on 17 February 2025).
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects 2022; United Nations Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022; Available online: https://population.un.org/wpp/ (accessed on 21 March 2025).
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Waldron, D.S.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. An examination of the potential of seaweed extracts as functional ingredients in milk. Int. J. Dairy Technol. 2014, 67, 182–193. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A.M. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- de Souza Celente, G.; Sui, Y.; Acharya, P. Seaweed as an alternative protein source: Prospective protein extraction technologies. Innov. Food Sci. Emerg. Technol. 2023, 86, 103374. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Akbar, W.A.; Rahim, H.U.; Rutigliano, F.A. Cole-and seaweed-based biopolymers: Sources, extractions and implications for soil quality improvement and environmental sustainability—A review. J. Environ. Manag. 2024, 359, 120964. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xie, C.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Alginates from brown seaweeds as a promising natural source: A review of its properties and health benefits. Food Rev. Int. 2024, 40, 2682–2710. [Google Scholar] [CrossRef]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan from Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, structure, production, and application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef]
- Ali, M.Q.; Azhar, M.A.; Munaim, M.S.A.; Ruslan, N.F.; Alsubhi, L.M.; Ahmad, N.; Noman, A.E. Seaweed organic compounds source of hydrocolloids and sustainable food packaging: Properties, application, and future direction. Discov. Food 2024, 4, 101. [Google Scholar] [CrossRef]
- Vicente, T.F.; Félix, C.; Félix, R.; Valentão, P.; Lemos, M.F. Seaweed as a natural source against phytopathogenic bacteria. Mar. Drugs 2022, 21, 23. [Google Scholar] [CrossRef]
- Behera, D.P.; Vadodariya, V.; Veeragurunathan, V.; Sigamani, S.; Moovendhan, M.; Srinivasan, R.; Ingle, K.N. Seaweeds cultivation methods and their role in climate mitigation and environmental cleanup. Total Environ. Res. Themes 2022, 3, 100016. [Google Scholar] [CrossRef]
- Aina, O.; Bakare, O.O.; Daniel, A.I.; Gokul, A.; Beukes, D.R.; Fadaka, A.O.; Klein, A. Seaweed-derived phenolic compounds in growth promotion and stress alleviation in plants. Life 2022, 12, 1548. [Google Scholar] [CrossRef]
- Mughunth, R.J.; Velmurugan, S.; Mohanalakshmi, M.; Vanitha, K. A review of seaweed extract’s potential as a biostimulant to enhance growth and mitigate stress in horticulture crops. Sci. Hortic. 2024, 334, 113312. [Google Scholar] [CrossRef]
- Thapa, U.; Ansari, Z.G.; Ramesh, S.; Anbalagan, K.; Rabi, A. Plant hormones and growth regulators: Mechanisms, interactions, and agricultural applications. Agric. Arch. Int. J. 2024, 3, 11–20. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Jeon, B.H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Sariñana-Aldaco, O.; Rivera-Solís, L.L.; Benavides-Mendoza, A.; Robledo-Olivo, A.; Rodríguez-Jasso, R.M.; González-Morales, S. Using brown algae in the plant–soil system: A sustainable approach to improving the yield and quality of agricultural crops. Horticulturae 2025, 11, 94. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rameshkumar, S.; Pednekar, M.; Fitzpatrick, S.; Rai, D.K.; Tiwari, B.K. Sequential extraction of fucoidan, laminarin, mannitol, alginate and protein from brown macroalgae Ascophyllum nodosum and Fucus vesiculosus. Int. J. Biol. Macromol. 2024, 256, 128195. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cano, B.; Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and perspectives of the use of alginate as a polymer matrix for microorganisms applied in agro-industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef]
- Dey, S.; Purakayastha, T.J.; Sarkar, B.; Rinklebe, J.; Kumar, S.; Chakraborty, R.; Shivay, Y.S. Enhancing cation and anion exchange capacity of rice straw biochar by chemical modification for increased plant nutrient retention. Sci. Total Environ. 2023, 886, 163681. [Google Scholar] [CrossRef]
- Kergosien, N.; Stiger-Pouvreau, V.; Connan, S.; Hennequart, F.; Brébion, J. Mini-Review: Brown macroalgae as a promising raw material to produce biostimulants for the agriculture sector. Front. Agron. 2023, 5, 1109989. [Google Scholar] [CrossRef]
- Cole, A.J.; Roberts, D.A.; Garside, A.L.; de Nys, R.; Paul, N.A. Seaweed compost for agricultural crop production. J. Appl. Phycol. 2016, 28, 629–642. [Google Scholar] [CrossRef]
- Chanthini, K.M.P.; Senthil-Nathan, S.; Pavithra, G.S.; Malarvizhi, P.; Murugan, P.; Deva-Andrews, A.; Krutmuang, P. Aqueous seaweed extract alleviates salinity-induced toxicities in rice plants (Oryza sativa L.) by modulating their physiology and biochemistry. Agriculture 2022, 12, 2049. [Google Scholar] [CrossRef]
- Mendes, M.; Cotas, J.; Pacheco, D.; Ihle, K.; Hillinger, A.; Cascais, M.; Gonçalves, A.M. Red seaweed (Rhodophyta) phycocolloids: A road from the species to the industry application. Mar. Drugs 2024, 22, 432. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Agrasar, A.M.T.; Mallo, F.; Rúa, M.L.; Fucinos, C. Red seaweed proteins: Valuable marine-origin compounds with encouraging applications. Algal Res. 2023, 75, 103262. [Google Scholar] [CrossRef]
- Michalak, I. The application of seaweeds in environmental biotechnology. In Advances in Botanical Research; Academic Press: New York, NY, USA, 2020; Volume 95, pp. 85–111. [Google Scholar]
- Farghali, M.; Mohamed, I.M.; Osman, A.I.; Rooney, D.W. Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: A review. Environ. Chem. Lett. 2023, 21, 97–152. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, N.V.; Rajkumar, R.; Kumar, N.S.; Arunkumar, P.; Alromaeh, A.I.; AlReshaidan, S.B.; Al-Fatesh, A.S. Seaweed biosorption: A green solution for heavy metal remediation in aquatic and soil environments. Desalination Water Treat. 2025, 321, 101036. [Google Scholar] [CrossRef]
- Gurau, S.; Imran, M.; Ray, R.L. Algae: A cutting-edge solution for enhancing soil health and accelerating carbon sequestration—A review. Environ. Technol. Innov. 2024, 37, 103980. [Google Scholar] [CrossRef]
- Raghu, T. A study on agriculture and foodgrain management in India. Cent. Dev. Econ. Stud. 2022, 9, 30–40. [Google Scholar] [CrossRef]
- Kaur, R.; Shivay, Y.S.; Singh, G.; Virk, H.K.; Sen, S.; Rajni, R. Increasing area under pulses and soil quality enhancement in pulse-based cropping systems-Retrospect and prospects. Indian J. Agric. Sci. 2018, 88, 10–21. [Google Scholar] [CrossRef]
- USDA. India’s Agricultural Exports Climb to Record High; United States Department of Agriculture: Washington, DC, USA, 2014. Available online: http://www.fas.usda.gov/data/india-s-agricultural-exports-climb-record-high (accessed on 25 January 2025).
- Hernandez-Herrera, R.M.; Santacruz-Ruvalcaba, M.A.; Ruiz-Lopez, J.; Norrie, G.; Hernandez-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Pereira, N. Seaweed: Promising plant of the millennium. Sci. Cult. 2005, 71, 60–66. [Google Scholar]
- Nedumaran, T.; Arulbalachandran, D. Seaweeds: A promising source for sustainable development. In Environmental Sustainability; Springer: New Delhi, India, 2015; pp. 65–68. [Google Scholar]
- Satish, L.; Rameshkumar, R.; Rathinapriya, P.; Pandian, S.; Rency, A.S.; Sunitha, T.; Ramesh, M. Effect of seaweed liquid extracts and plant growth regulators on in vitro mass propagation of brinjal (Solanum melongena L.) through hypocotyl and leaf disc explants. J. Appl. Phycol. 2015, 27, 993–1002. [Google Scholar] [CrossRef]
- FAO. The Rediscovered Potential of Seaweed Dietary Additives. Bangkok. 2022. Available online: https://www.fao.org/3/cc0319en/cc0319en.pdf (accessed on 25 January 2025).
- Martínez-Martínez, E.; Slocum, A.H.; Ceballos, M.L.; Aponte, P.; Bisonó-León, A.G. Beyond the Bloom: Invasive Seaweed Sargassum spp. as a Catalyst for Sustainable Agriculture and Blue Economy—A Multifaceted Approach to Biodegradable Films, Biostimulants, and Carbon Mitigation. Sustainability 2025, 17, 3498. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Isolation and identification of cytokinins in a new commercial seaweed product made from Fucus serratus L. J. Appl. Phycol. 1997, 9, 327. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Norrie, J.; Keathley, J.P. Benefits of Ascophyllum nodosum marine-plant extract applications to “Thompson seedless” grape production. Acta Hortic. 2006, 727, 243–247. [Google Scholar] [CrossRef]
- Basavaraja, P.K.; Yogendra, N.D.; Zodape, S.T.; Prakash, R.; Ghosh, A. Effect of seaweed sap as foliar spray on growth and yield of hybrid maize. J. Plant Nutr. 2018, 41, 1851–1861. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Kumar, G.; Sahoo, D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J. Appl. Phycol. 2011, 23, 251–255. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Ghosh, A.; Zodape, S.T. Effect of seaweed sap derived from two marine algae Kappaphycus and Gracilaria on growth and improvement of black gram. Indian J. Mar. Sci. 2016, 45, 789–794. [Google Scholar]
- Pramanick, B.; Brahmachari, K.; Ghosh, A. Effect of seaweed saps on growth and yield improvement of green gram. Afr. J. Agric. Res. 2013, 8, 1180–1186. [Google Scholar]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Kumar, G.; Nanda, S.; Singh, S.K.; Kumar, S.; Singh, D.; Singh, B.N.; Mukherjee, A. Seaweed extracts: Enhancing plant resilience to biotic and abiotic stresses. Front. Mar. Sci. 2024, 11, 1457500. [Google Scholar] [CrossRef]
- Raja, B.; Vidya, R. Application of seaweed extracts to mitigate biotic and abiotic stresses in plants. Physiol. Mol. Biol. Plants 2023, 29, 641–661. [Google Scholar] [CrossRef]
- Jeannin, I.; Lescure, J.C.; Morot-Gaudry, J.F. The effects of aqueous seaweed sprays on the growth of maize. Bot. Mar. 1991, 34, 469–473. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Ghosh, A.; Zodape, S.T. Effect of seaweed saps on growth and yield improvement of transplanted rice in old alluvial soil of West Bengal. Bangladesh J. Bot. 2014, 43, 53–58. [Google Scholar] [CrossRef]
- Shah, M.T.; Zodape, S.T.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Seaweed sap as an alternative liquid fertilizer for yield and quality improvement of wheat. J. Plant Nutr. 2013, 36, 192–200. [Google Scholar] [CrossRef]
- Leindah Devi, N.; Mani, S. Effect of seaweed saps Kappaphycus alvarezii and Gracilaria on growth, yield, and quality of rice. Indian J. Sci. Technol. 2015, 8, 19. [Google Scholar] [CrossRef]
- Pal, A.; Dwivedi, S.K.; Maurya, P.K.; Kanwar, P. Effect of seaweed saps on growth, yield, nutrient uptake, and economic improvement of maize (sweet corn). J. Appl. Nat. Sci. 2015, 7, 970–975. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A commercial extract of brown macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of spinach in vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Kalaivanan, C.; Chandrasekaran, M.; Venkatesalu, V. Effect of seaweed liquid extract of Caulerpa scalpelliformis on growth and biochemical constituents of black gram (Vigna mungo (L.) Hepper). Phykos 2012, 42, 46–53. [Google Scholar]
- Featonby-Smith, B.C.; Van Staden, J. The effect of seaweed concentrate and fertilizer on the growth of Beta vulgaris. Z. Für Pflanzenphysiol. 1983, 112, 155–162. [Google Scholar] [CrossRef]
- Beckett, R.P.; Van Staden, J. The effect of seaweed concentrate on the growth and yield of potassium-stressed wheat. Plant Soil 1989, 116, 29–36. [Google Scholar] [CrossRef]
- Atzmon, N.; Van Staden, J. The effect of seaweed concentrate on the growth of Pinus pinea seedlings. New For. 1994, 8, 279–288. [Google Scholar] [CrossRef]
- Ferreira, M.I.; Lourens, A.F. The efficacy of liquid seaweed extract on the yield of canola plants. S. Afr. J. Plant Soil 2002, 19, 159–161. [Google Scholar] [CrossRef]
- Arthur, G.D.; Stirk, W.A.; Van Staden, J.; Scott, P. Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S. Afr. J. Bot. 2003, 69, 207–211. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological effects of liquid applications of a seaweed extract and a humic acid on creeping bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef]
- Nedumaran, T.; Perumal, P. Effect of seaweed liquid fertilizer on the germination and growth of seedling of mangrove Rhizophora mucronata Boir. J. Phytol. 2009, 1, 142–146. [Google Scholar]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield, and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Latique, S.; Chernane, H.; Mansori, M.; El Kaoua, M. Seaweed liquid fertilizer effect on physiological and biochemical parameters of bean plant (Phaesolus vulgaris variety Paulista) under hydroponic system. Eur. Sci. J. 2013, 9, 174–191. [Google Scholar]
- Zodape, S.T.; Mukhopadhyay, S.; Eswaran, K.; Reddy, M.P.; Chikara, J. Enhanced yield and nutritional quality in green gram (Phaseolus radiata L.) treated with seaweed (Kappaphycus alvarezii) extract. Indian J. Mar. Sci. 2010, 69, 468–471. [Google Scholar]
- Thirumaran, G.; Arumugam, M.; Arumugam, R.; Anantharaman, P. Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus (L.) Medikus. Am.-Eurasian J. Agron. 2009, 2, 57–66. [Google Scholar]
- Ramya, S.S.; Nagaraj, S.; Vijayanand, N. Biofertilizing efficiency of brown and green algae on growth, biochemical and yield parameters of Cyamopsis tetragonolaba (L.) Taub. Recent Res. Sci. Technol. 2010, 2, 45–52. [Google Scholar]
- Sridhar, S.; Rengasamy, R. Significance of seaweed liquid fertilizers for minimizing chemical fertilizers and improving yield of Arachis hypogaea under field trial. Recent Res. Sci. Technol. 2010, 2, 73–80. [Google Scholar]
- Sridhar, S.; Rengasamy, R. Influence of seaweed liquid fertilizer on growth and biochemical characteristics of Arachis hypogaea L. under field trial. J. Ecobiotechnol. 2011, 3, 18–22. [Google Scholar]
- Gireesh, R.; Haridevi, C.K.; Salikutty, J. Effect of Ulva lactuca extract on growth and proximate composition of Vigna unguiculata L. Walp. J. Res. Biol. 2011, 8, 624–630. [Google Scholar]
- Ganapathy Selvam, G.; Balamurugan, M.; Thinakaran, T.; Sivakumar, K. Developmental changes in the germination, growth, and chlorophyllase activity of Vigna mungo L. using seaweed extract of Ulva reticulata Forsskål. Int. Res. J. Pharm. 2013, 4, 252–254. [Google Scholar]
- Jadhao, G.R.; Chaudhary, D.R.; Khadse, V.A.; Zodape, S.T. Utilization of seaweeds in enhancing productivity and quality of black gram [Vigna mungo (L.) Hepper] for sustainable agriculture. Indian J. Nat. Prod. Resour. 2015, 6, 16–22. [Google Scholar]
- Kumar, N.A.; Vanlalzarzova, B.; Sridhar, S.; Baluswami, M. Effect of liquid seaweed fertilizer of Sargassum wightii Grev. on the growth and biochemical content of green gram (Vigna radiata (L.) R. Wilczek). Recent Res. Sci. Technol. 2012, 4, 40–45. [Google Scholar]
- Krajnc, A.U.; Ivanus, A.; Kristl, J.; Susek, A. Seaweed extract elicits the metabolic responses in leaves and enhances growth of Pelargonium cuttings. Eur. J. Hortic. Sci. 2012, 77, 170–181. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Houdusse, F. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Vinoth, S.; Gurusaravanan, P.; Jayabalan, N. Effect of seaweed extracts and plant growth regulators on high-frequency in vitro mass propagation of Lycopersicon esculentum L. (tomato) through double cotyledonary nodal explant. J. Appl. Phycol. 2012, 24, 1329–1337. [Google Scholar] [CrossRef]
- Fan, D.; Kandasamy, S.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. Pre-harvest treatment of spinach with Ascophyllum nodosum extract improves post-harvest storage and quality. Sci. Hortic. 2014, 170, 70–74. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Selvaraj, N.; Ganapathi, A.; Manickavasagam, M. Improved production of withanolides in shoot suspension culture of Withania somnifera (L.) Dunal by seaweed extracts. Plant Cell Tissue Organ Cult. 2014, 119, 221–225. [Google Scholar] [CrossRef]
- Layek, J.; Das, A.; Ramkrushna, G.I.; Trivedi, K.; Yesuraj, D.; Chandramohan, M.; Kubavat, D.; Agarwal, P.K.; Ghosh, A. Seaweed sap: A sustainable way to improve productivity of maize in North-East India. Int. J. Environ. Stud. 2015, 72, 305–315. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.K.; Pal, S.K.; Trivedi, K.; Yesuraj, D.; Singh, C.S.; Zodape, S.T. Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. J. Appl. Phycol. 2016, 28, 2099–2112. [Google Scholar] [CrossRef]
- Satish, L.; Rathinapriya, P.; Rency, A.S.; Ceasar, S.A.; Pandian, S.; Rameshkumar, R.; Ramesh, M. Somatic embryogenesis and regeneration using Gracilaria edulis and Padina boergesenii seaweed liquid extracts and genetic fidelity in finger millet (Eleusine coracana). J. Appl. Phycol. 2016, 28, 2083–2098. [Google Scholar] [CrossRef]
- Singh, S.K.; Thakur, R.; Singh, M.K.; Singh, C.S.; Pal, S.K. Effect of fertilizer level and seaweed sap on productivity and profitability of rice (Oryza sativa). Indian J. Agron. 2015, 60, 420–425. [Google Scholar] [CrossRef]
- Torres, P.; Novaes, P.; Ferreira, L.G.; Santos, J.P.; Mazepa, E.; Duarte, M.E.R.; dos Santos, D.Y. Effects of extracts and isolated molecules of two species of Gracilaria (Gracilariales, Rhodophyta) on early growth of lettuce. Algal Res. 2018, 32, 142–149. [Google Scholar] [CrossRef]
- Lee, H.H.; Lin, C.T.; Yang, L.L. Neuroprotection and free radical scavenging effects of Osmanthus fragrans. J. Biomed. Sci. 2007, 14, 819–827. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, K.L.; Cheung, C.H.; Chang, J.Y. Autophagy induced by cathepsin S inhibition induces early ROS production, oxidative DNA damage, and cell death via xanthine oxidase. Free Radic. Biol. Med. 2013, 65, 1473–1486. [Google Scholar] [CrossRef]
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Wang, F.J.; Pan, C.L. The comparison of anti-oxidative properties of seaweed oligosaccharides fermented by two lactic acid bacteria. J. Mar. Sci. Technol. 2010, 18, 537–545. [Google Scholar] [CrossRef]
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic effects of seaweed polysaccharides in pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–549. [Google Scholar] [CrossRef]
- Kasim, W.A.; Hamada, E.A.; El-Din, N.S.; Eskander, S. Influence of seaweed extracts on the growth, some metabolic activities, and yield of wheat grown under drought stress. Int. J. Agron. Agric. Res. 2015, 7, 173–189. [Google Scholar]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; El Kaoua, M. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.). J. Appl. Phycol. 2015, 27, 1689–1698. [Google Scholar] [CrossRef]
- Shakya, R.; Capilla, E.; Torres-Pagán, N.; Muñoz, M.; Boscaiu, M.; Lupuţ, I.; Verdeguer, M. Effect of two biostimulants, based on Ascophyllum nodosum extracts, on strawberry performance under mild drought stress. Agriculture 2023, 13, 2108. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology, and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improves drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef]
- Bradacova, K.; Weber, N.F.; Morad-Talab, N.; Asim, M.; Imran, M.; Weinmann, M.; Neumann, G. Micronutrients (Zn/Mn), seaweed extracts, and plant growth-promoting bacteria as cold-stress protectants in maize. Chem. Biol. Technol. Agric. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Repke, R.A.; Silva, D.M.R.; dos Santos, J.C.C.; de Almeida Silva, M. Increased soybean tolerance to high-temperature through biostimulant based on Ascophyllum nodosum (L.) seaweed extract. J. Appl. Phycol. 2022, 34, 3205–3218. [Google Scholar] [CrossRef]
- Goyal, V.; Kumari, A.; Avtar, R.; Baliyan, V.; Mehrotra, S. Orthosilicic acid and seaweed extract alleviate the deteriorative effects of high-temperature stress in Brassica juncea (L.) Czern & Coss. Silicon 2023, 15, 4909–4919. [Google Scholar]
- Carrasco-Gil, S.; Hernandez-Apaolaza, L.; Lucena, J.J. Effect of several commercial seaweed extracts in the mitigation of iron chlorosis of tomato plants (Solanum lycopersicum L.). Plant Growth Regul. 2018, 86, 401–411. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology, and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef]
- Ahmed, D.A.E.A.; Gheda, S.F.; Ismail, G.A. Efficacy of two seaweeds dry mass in bioremediation of heavy metal polluted soil and growth of radish (Raphanus sativus L.) plant. Environ. Sci. Pollut. Res. 2021, 28, 12831–12846. [Google Scholar] [CrossRef]
- Sousa, F.; Martins, M.; Sousa, B.; Soares, C.; Azenha, M.; Pereira, R.; Fidalgo, F. The potential of beach wrack as a plant biostimulant to mitigate metal toxicity: Mineral composition, antioxidant properties, and effects against Cu-induced stress. J. Appl. Phycol. 2022, 34, 667–678. [Google Scholar] [CrossRef]
- Trivedi, K.; Gopalakrishnan, V.A.K.; Kumar, R.; Ghosh, A. Transcriptional analysis of maize leaf tissue treated with seaweed extract under drought stress. Front. Sustain. Food Syst. 2021, 5, 774978. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 10537. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress-related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef]
- Stasio, D.E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-priming with seaweed extracts enhances yield of salt-stressed tomato plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Boukhari, M.E.M.; Barakate, M.; Choumani, N.; Bouhia, Y.; Lyamlouli, K. Ulva lactuca extract and fractions as seed priming agents mitigate salinity stress in tomato seedlings. Plants 2021, 10, 1104. [Google Scholar] [CrossRef]
- Bensidhoum, L.; Nabti, E.H. Role of Cystoseira mediterranea extracts (Sauv.) in the alleviation of salt stress adverse effect and enhancement of some Hordeum vulgare L. (barley) growth parameters. SN Appl. Sci. 2021, 3, 116. [Google Scholar] [CrossRef]
- Jafarlou, B.M.; Pilehvar, B.; Modaresi, M.; Mohammadi, M. Seaweed liquid extract as an alternative biostimulant for the amelioration of salt-stress effects in Calotropis procera (Aiton) W.T. J. Plant Growth Regul. 2023, 42, 449–464. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Ali, R.M.; Hemida, K.A.; Sayed, M.A. Role of Ulva lactuca extract in alleviation of salinity stress on wheat seedlings. Sci. World J. 2014, 2014, 847290. [Google Scholar] [CrossRef]
- Shaddad, M.A.K.; Farghl, A.A.M.; Galal, H.R.; Hassan, E.A. Influence of seaweed extracts (Sargassum dentifolium or Padina gymnospora) on the growth and physiological activities of faba bean and wheat plants under salt stress. Egypt. J. Bot. 2014, 54, 121–135. [Google Scholar]
- Ali, A.H.; Said, E.M.; Abdelgawad, Z.A. The role of seaweed extract on improving drought tolerance of wheat revealed by osmoprotectants and DNA (cpDNA) markers. Braz. J. Bot. 2022, 45, 857–867. [Google Scholar] [CrossRef]
- Shahzad, R.; Harlina, P.W.; Gallego, P.P.; Flexas, J.; Ewas, M.; Leiwen, X.; Karuniawan, A. The seaweed Ascophyllum nodosum-based biostimulant enhances salt stress tolerance in rice (Oryza sativa L.) by remodeling physiological, biochemical, and metabolic responses. J. Plant Interact. 2023, 18, 2266514. [Google Scholar] [CrossRef]
- Carmo, L.P.; do Nascimento Moura, C.W.; Lima-Brito, A. Effects of heat stress and seaweed-derived biostimulants on the germination of Comanthera mucugensis, an endemic plant of fire-prone Campos rupestres of Chapada Diamantina (Brazil). S. Afr. J. Bot. 2021, 141, 49–53. [Google Scholar] [CrossRef]
- Goni, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Spann, T.M.; Little, H.A. Applications of a commercial extract of the brown seaweed Ascophyllum nodosum increases drought tolerance in container-grown ‘Hamlin’ sweet orange nursery trees. HortScience 2011, 46, 577–582. [Google Scholar] [CrossRef]
- Abd-Elkader, A.; Salama, M.; Oraby, S. Mitigating negative effects of salt stress on common bean (Phaseolus vulgaris) using seaweed extracts. Egypt. J. Chem. 2023, 66, 49–60. [Google Scholar]
- Papenfus, H.B.; Kulkarni, M.G.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Effect of a commercial seaweed extract (Kelpak®) and polyamines on nutrient-deprived (N, P, and K) okra seedlings. Sci. Hortic. 2013, 151, 142–146. [Google Scholar] [CrossRef]
| Crops | Seaweeds Used | Effects | References |
|---|---|---|---|
| B. vulgaris | E. maxima | Growth improvement | [71] |
| T. aestivum | E. maxima | Growth and yield improvement | [72] |
| S. wightii | Growth and yield improvement | [58] | |
| K. alvarezii and G. edulis | Yield and quality improvement | [66] | |
| P. pinea | E. maxima | Growth improvement | [73] |
| B. napus | E. maxima | Yield improvement | [74] |
| C. annuum | E. maxima | Growth and yield improvement | [75] |
| A. stolonifera | A. nodosum | Improved physiological activity | [76] |
| R. mucronata | P. boergesenii | Improvement in germination and growth | [77] |
| G. max | K. alvarezii | Growth and yield improvement | [78] |
| P. vulgaris | F. spiralis and U. rigida | Physiological and biochemical improvement | [79] |
| P. radiata | K. alvarezii | Growth and nutritional level improvement | [80] |
| A. esculentus | R. intricata | Growth and pigment concentration enhancement | [81] |
| Cyamopsis tetragonoloba | S. wightii and U. lactuca | Growth, yield, and biochemical property improvement | [82] |
| A. hypogaea | S. wightii and U. lactuca | Yield improvement | [83] |
| S. wightii | Growth, yield, and biochemical property improvement | [84] | |
| V. unguiculata | U. lactuca | Growth improvement | [85] |
| V. sinensis | S. wightii and C. chemnitzia | Growth and biochemical constituent improvement | [74] |
| V. mungo | C. scalpelliformis | Growth, yield, and biochemical property improvement | [70] |
| U. reticulata | Enhanced chlorophyllase activity, germination, and growth | [86] | |
| K. alvarezii and G. edulis | Productivity and quality improvement | [87] | |
| V. radiata | S. wightii | Growth and biochemical content improvement | [88] |
| Kappaphycus and Gracilaria | Growth and yield improvement | [60] | |
| P. peltatum | E. maxima | Growth, photosynthetic pigment, and phenolic and protein content improvement | [89] |
| B. napus | A. nodosum | Physiological improvement | [90] |
| L. esculentum | G. edulis and S. wightii | High-frequency mass propagation and growth improvement | [91] |
| S. lycopersicum | U. lactuca, C. sertularioides, P. gymnospora, and S. liebmannii | Growth improvement | [47] |
| S. melongena | G. salicornia, P. gymnospora, P. boergesenii, and G. acerosa | High-frequency mass propagation and growth improvement | [50] |
| S. oleracea | A. nodosum | Improvement in chlorophyll and ascorbate and lipid peroxidation and postharvest enhancement | [92] |
| W. somnifera | G. edulis and S. wightii | Productivity improvement | [93] |
| Z. mays | K. alvarezii and G. edulis | Productivity improvement | [94] |
| G. edulis and K. alvarezii | Yield and quality improvement | [95] | |
| E. coracana | G. edulis and P. boergesenii | Improvement in plant growth | [96] |
| O. sativa | K. alvarezii and G. edulis | Productivity improvement | [97] |
| L. sativa | G. caudata and G. domingensis | Growth improvement | [98] |
| Common Name of the Plant | Botanical Name of the Plant | Various Abiotic Stresses | Seaweeds Used to Mitigate Abiotic Stresses | Effects of Seaweeds Under Abiotic Stress | References |
|---|---|---|---|---|---|
| Chickpea | C. arietinum | NaCl (50 and 150 mM) | S. muticum and Jania rubens | Increased shoot dry weight; shoot length; root dry weight; photosynthetic pigments; soluble sugars; phenols; K+ concentration in shoot and root; activities of SOD, POD, CAT and APX. Reduced Na+ level in root and shoot, H2O2, and MDA content. | [119] |
| Foxtail fern | A. aethiopicus | NaCl (2000 and 4000 ppm) | A. nodosum | Increased branch length, branches per plant, fresh and dry weight per plant, CAT and SOD activities, total chlorophyll content, soluble sugar content, proline content, photosynthetic rate, transpiration rate, and stomatal conductance. | [120] |
| Tomato | S. lycopersicum | NaCl (6.3 dS m−1) | A. nodosum | Increased root-to-shoot ratio, leaf area, shoot fresh weight, root dry weight, fruits fresh weight, harvest index, firmness, and number of fruits. | [121] |
| NaCl (2, 4, 8 dS m−1) | U. lactuca | Increased shoot weight, leaf area, root length, soluble sugars, total proteins, chlorophyll a and b, and total carotenoids. Reduced H2O2 concentration in leaves. | [122] | ||
| Barley | H. vulgare | NaCl (350 mM) | Cystoseira mediterranea | Increased seed germination, plant height, root length, fresh and dry weight of shoot and root, and chlorophyll contents. Reduced membrane integrity, MDA, and H2O2. | [123] |
| Heavy metals (Cu-induced stress) | Fagopyrum esculentum | Increased length and biomass of leaf and root. | [117] | ||
| Giant milkweed | C. procera | NaCl (15 dS m−1) | S. angustifolium | Increased plant height; specific leaf area; root length and volume; root and shoot dry weight; K+ uptake; chlorophyll a and b; and activities of CAT, SOD, POD, and ascorbate. Decreased electrolyte leakage and sodium uptake. | [124] |
| Wheat | T. aestivum | NaCl (50–250 mM) | U. lactuca | Increased seed germination, fresh dry matter, and activities of SOD and CAT. | [125] |
| NaCl (150 and 200 mM) | S. dentifolium and P. gymnospora | Increased fresh and dry weight; chlorophyll a and b; and activities of SOD, CAT, POD, and APX in shoots and root. Reduced MDA content in shoot and root. | [126] | ||
| Drought (40% of field capacity) | S. denticulatum | Increased shoot height, fresh and dry weight of shoot, chlorophyll content, starch germination rate, length of shoot and root, total fresh weight, plant total dry weight, plant length, spike length and weight, number of spikelets, seeds yield per plant, seed weight per spike, and 1000 seeds’ weight. | [127] | ||
| Drought (40% field capacity) | S. latifolium and U. lactuca | Increased root depth, shoot height, leaf area, chlorophyll a and b, carotenoids, chlorophyll a/b ratio, photosynthetic activity, activities of POD and CAT, and ascorbic acid content. | [106] | ||
| Rice | O. sativa | NaCl (200 mM) | A. nodosum | Increased shoot and root length; fresh and dry weight of shoot and root; concentration of K+, Mg2+, and Ca2+; chlorophyll a and b; total chlorophyll; carotenoid; net photosynthetic rate; transpiration rate; intercellular CO2; stomatal conductance; maximum efficiency of photosystem II; water use efficiency; and activities of SOD and CAT. Reduced sodium, MDA, and H2O2. | [128] |
| Maize | Z. mays | Cold (12–14 °C) | A. nodosum, Fucus sp., and Laminaria sp. | Increased root length and calcium, phosphorus, magnesium, potassium, zinc, manganese, iron, and copper concentrations in shoot. Reduced necrotic leaf area and leaf chlorosis, subsequently turning into necrotic spots and anthocyanin formation. | [111] |
| Drought | K. alvarezii | Increased dry matter of root, leaf, and stem; root volume; number of dry leaves per plant; chlorophyll a and b; chlorophyll index; photosynthetic rate; grain weight, length, and diameter; number of seeds, and total yield. Reduced photo inhibition and lipid peroxidation. | [118] | ||
| Radish | R. sativus | Heavy metals (Pb-, Cu-, Zn-, and Ni- induced stress) | U. fasciata and S. lacerifolium | Increased root and shoot length; fresh and dry weight of shoot and root; leaf area; concentrations of nitrogen, phosphorus, potassium, calcium, and magnesium in shoot and root; carbohydrate and protein contents in shoot and root; chlorphyll a and b; and carotenoids. Reduced the contents of cadmium, lead, copper, chromium, and nickel in shoot and root. | [116] |
| - | C. mucugensis | Heat stress (40, 45, 50, and 55 °C) | Agardhiella subulata and Hypnea pseudomusciformis, | Improved seed germination. | [129] |
| Mustard greens | B. juncea | Heat stress (>20 °C) | A. nodosum | Increased plant height, primary and secondary branches per plant, days to maturity, 1000-seed weight, number of siliqua per plant, biological yield, seed yield harvest index, photosynthetic rate, and chlorophyll content. Reduced MDA content and membrane injury. | [113] |
| Tomato | S. lycopersicum | Drought | A. nodosum | Increased plant fresh and dry weight, chlorophyll content, and relative water content. Reduced MDA contents. | [130] |
| Nutrient stress (iron deficiency) | A. nodosum and Durvillea potatorum | Increased dry weight of leaf and root; iron-chelate reductase; activities of SOD, CAT, and MDA contents in leaf and root; concentrations of iron, zinc, manganese, and copper in root and leaf. Reduced chlorosis. | [114] | ||
| Sweet orange | C. sinensis | Drought (50% of evapotranspiration) | A. nodosum | Increased shoot length, leaf and stem dry weight, and total root length. | [131] |
| Common Bean | P. vulgaris | Drought | U. rigida and F. spiralis | Increased chlorophyll a and b and glycine betaine content; plant height; dry weight; total phenolic content; and activities of SOD, CAT, and APX. | [107] |
| NaCl (50 and 100 Mm) | Gelidiumvagum | Increased leaf length and width, leaf area, leaf fresh weight, total phenolic compounds, proline, total carbohydrates, free amino acids, chlorophyll a and b, POD, SOD, APX, CAT, and PAL. Reduced electrolyte leakage and MDA contents. | [132] | ||
| Drought | U. rigida and F. spiralis | Increased shoot length, dry weight, chlorophyll a and b, and glycine betaine content in leaves, polyphenol content in leaves, and activities of SOD and APX in leaves. Reduced MDA content in leaves. | [107] | ||
| Soybean | G. max | Drought | A. nodosum | Increased relative water content, stomatal conductance, and antioxidant activity. | [110] |
| Heat stress (40 °C) | A. nodosum | Increased plant height; number of nodules in root; root dry weight; number of pods per plant; CO2 assimilation rate; stomatal conductance; transpiration rate; carboxylation efficiency; and activities of SOD, CAT, and APX. Reduced leaf temperature, reductase nitrate, and proline concentration. | [112] | ||
| Faba bean | V. faba | NaCl (150 and 200 mM) | S. dentifolium and P. gymnospora | Increased fresh and dry weight; chlorophyll a and b; and activities of SOD, CAT, POD, and APX in shoots and root. Reduced MDA content in shoot and root. | [126] |
| Strawberry | F. × ananassa | Drought (50% field capacity) | A. nodosum | Increased number of leaves, length of the longest leaf, leaf area, number of flowers and fruits, chlorophyll content, and fresh and dry weight of root and leaf. | [108] |
| Spinach | S. oleracea | Drought (50% evapotranspiration) | A. nodosum | Increased leaf relative water content, specific leaf area, and fresh and dry weight of leaf. Reduced ferrous ion-chelating ability. | [109] |
| Lettuce | L. sativa | Nutrients stress (potassium deficiency) | A. nodosum | Increased leaf number, leaf length, biomass fresh weight, biomass dry matter, root fresh weight, root dry matter, root length, relative growth, activities of SOD and CAT, leaf photosynthetic rate, stomatal conductance, internal CO2 concentration, leaf fluorescence, chlorophyll a and b, total chlorophyll and K+ concentration. | [115] |
| Okra | A. esculentus | Nutrient stress (nitrogen, phosphorus, and potassium deficiency) | E. maxima | Enhanced seedling vigor and increased length of shoot and root length, number of leaf and root, stem thickness, fresh and dry weight of shoot, and root and leaf area. | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmeen, A.R.; Maharajan, T.; Rameshkumar, R.; Sindhamani, S.; Banumathi, B.; Prabakaran, M.; Atchaya, S.; Rathinapriya, P. Role of Seaweeds for Improving Soil Fertility and Crop Development to Address Global Food Insecurity. Crops 2025, 5, 29. https://doi.org/10.3390/crops5030029
Yasmeen AR, Maharajan T, Rameshkumar R, Sindhamani S, Banumathi B, Prabakaran M, Atchaya S, Rathinapriya P. Role of Seaweeds for Improving Soil Fertility and Crop Development to Address Global Food Insecurity. Crops. 2025; 5(3):29. https://doi.org/10.3390/crops5030029
Chicago/Turabian StyleYasmeen, Ali Rafi, Theivanayagam Maharajan, Ramakrishnan Rameshkumar, Subbiah Sindhamani, Balan Banumathi, Mayakrishnan Prabakaran, Sundararajan Atchaya, and Periyasamy Rathinapriya. 2025. "Role of Seaweeds for Improving Soil Fertility and Crop Development to Address Global Food Insecurity" Crops 5, no. 3: 29. https://doi.org/10.3390/crops5030029
APA StyleYasmeen, A. R., Maharajan, T., Rameshkumar, R., Sindhamani, S., Banumathi, B., Prabakaran, M., Atchaya, S., & Rathinapriya, P. (2025). Role of Seaweeds for Improving Soil Fertility and Crop Development to Address Global Food Insecurity. Crops, 5(3), 29. https://doi.org/10.3390/crops5030029








