Effect of Plant Biostimulants on Beetroot Seed Productivity, Germination, and Microgreen Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. The Growing Conditions and Experimental Protocol
2.2. Preparation and Characterization of Selenium Colloidal Solution
2.3. SGE and SGC Determination
2.4. Microgreen Preparation
2.5. Selenium
2.6. Chlorophyll and Carotene Determination
2.7. Betalain Pigment Determination
2.8. Total Polyphenols (TP)
2.9. Antioxidant Activity (AOA)
2.10. Proline
2.11. Statistical Analysis
3. Results and Discussion
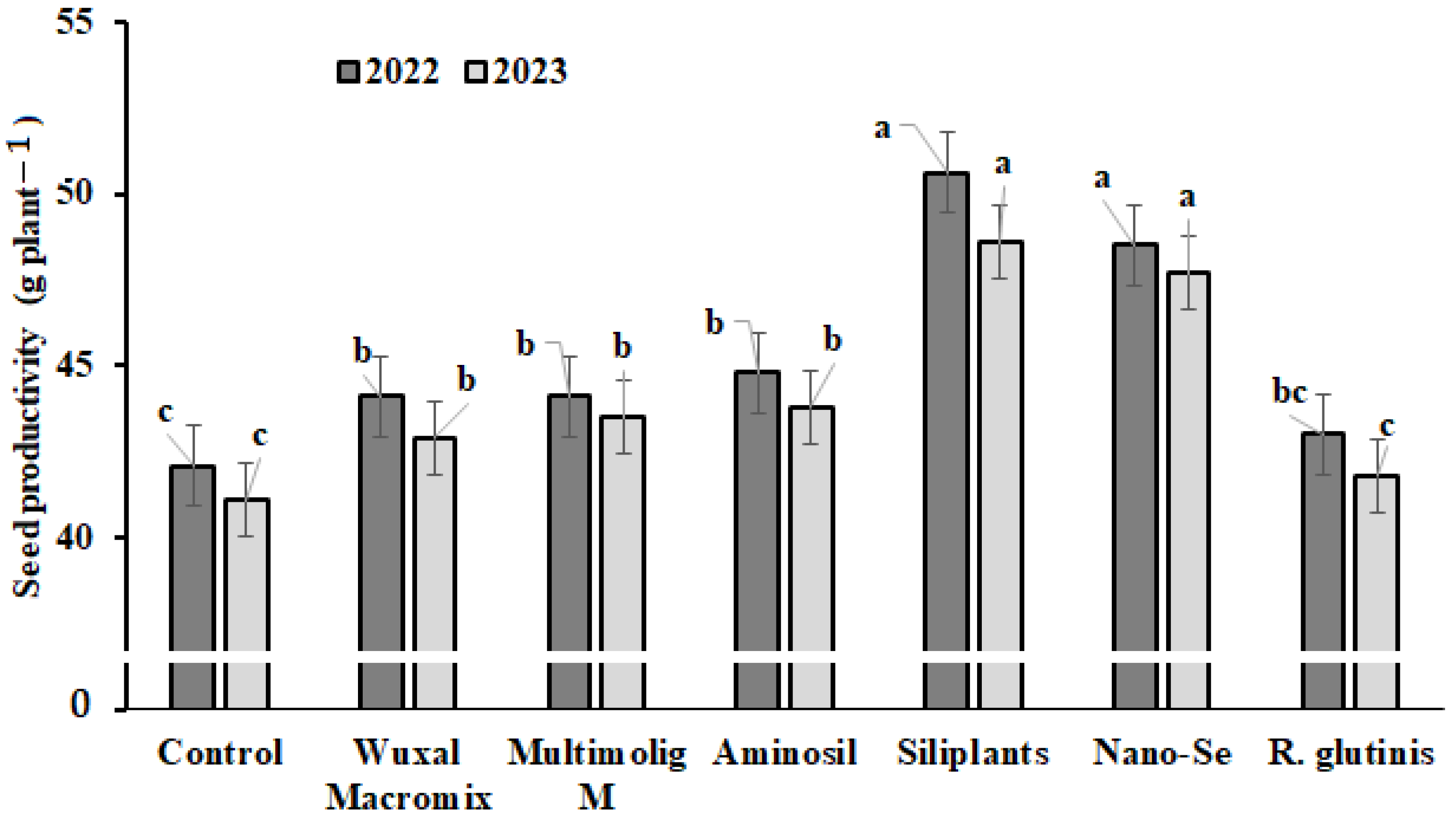
3.1. Seed Yield
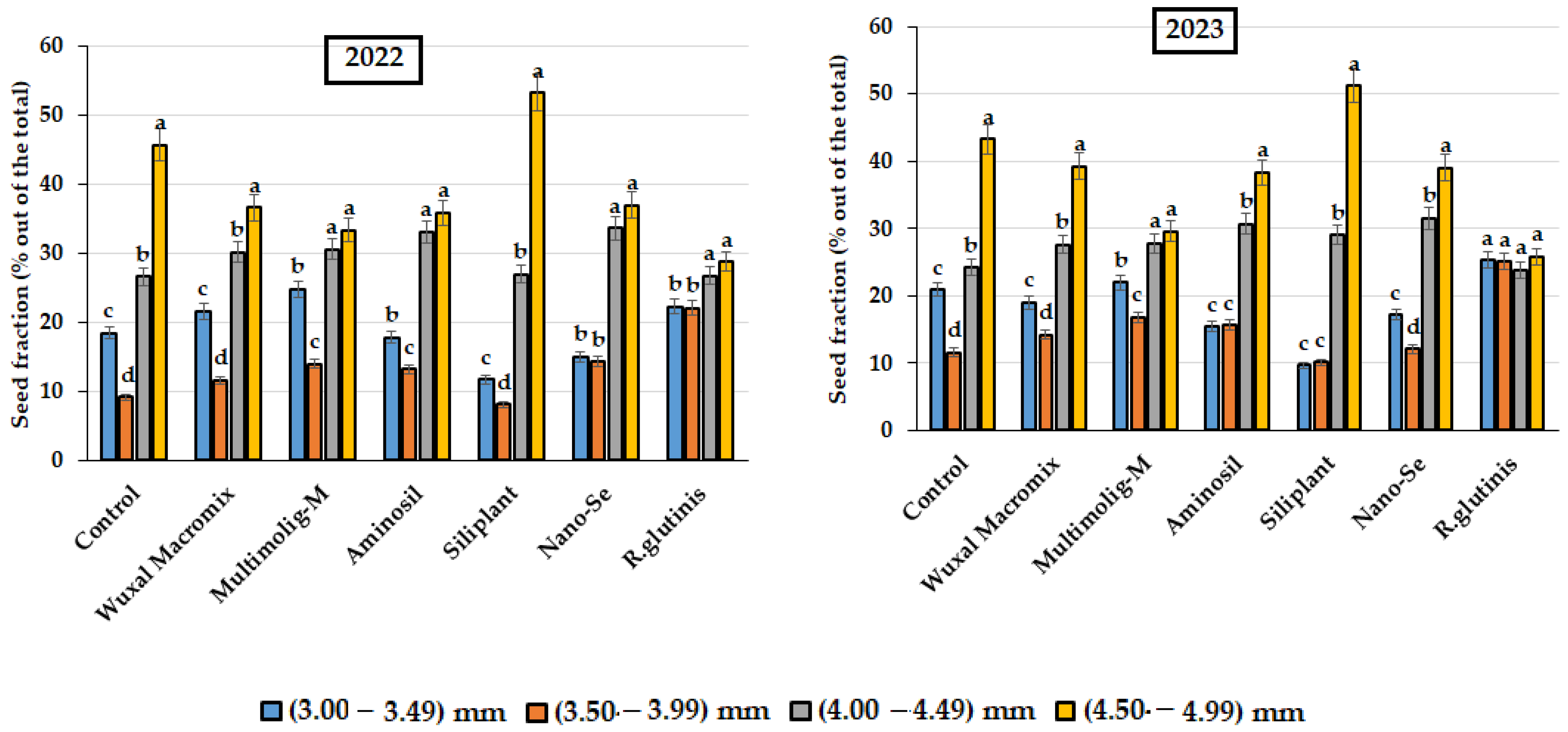
3.2. Seed Size Class Distribution
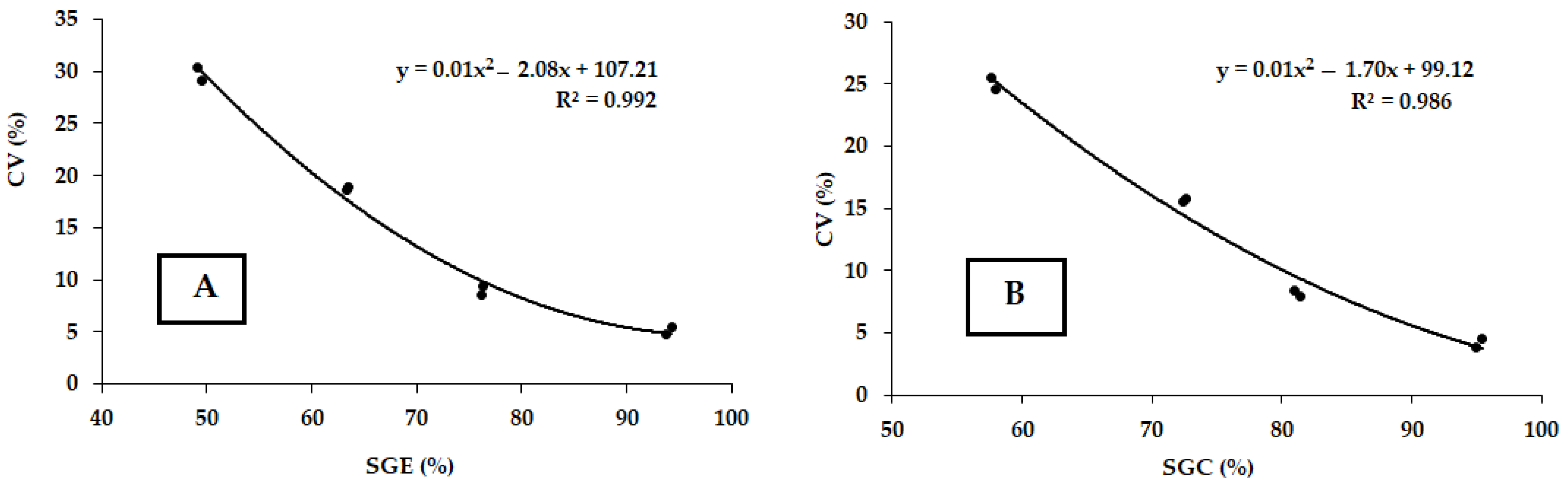
3.3. Seed Germination Energy and Capacity
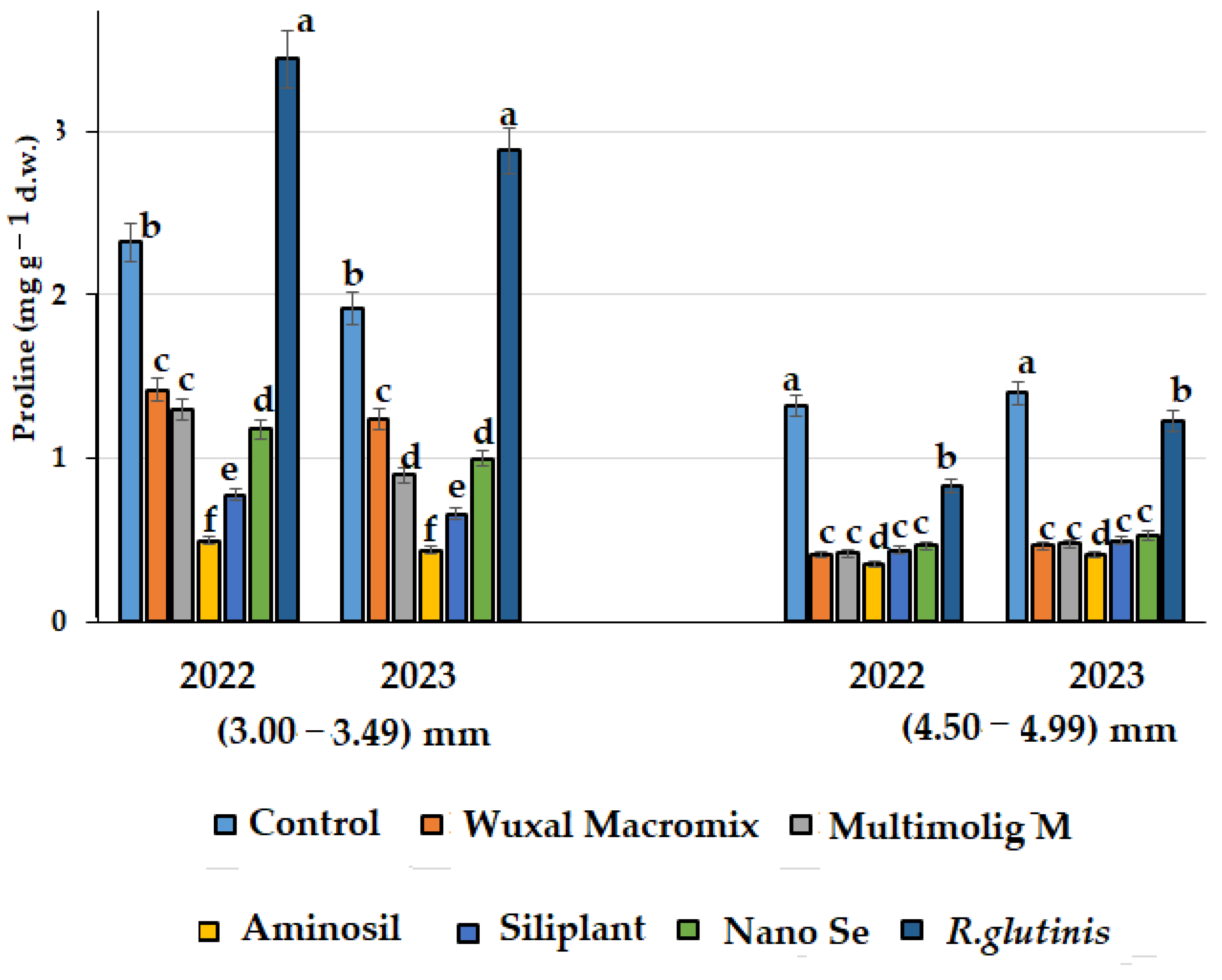
3.4. Antioxidant Status and Proline Content
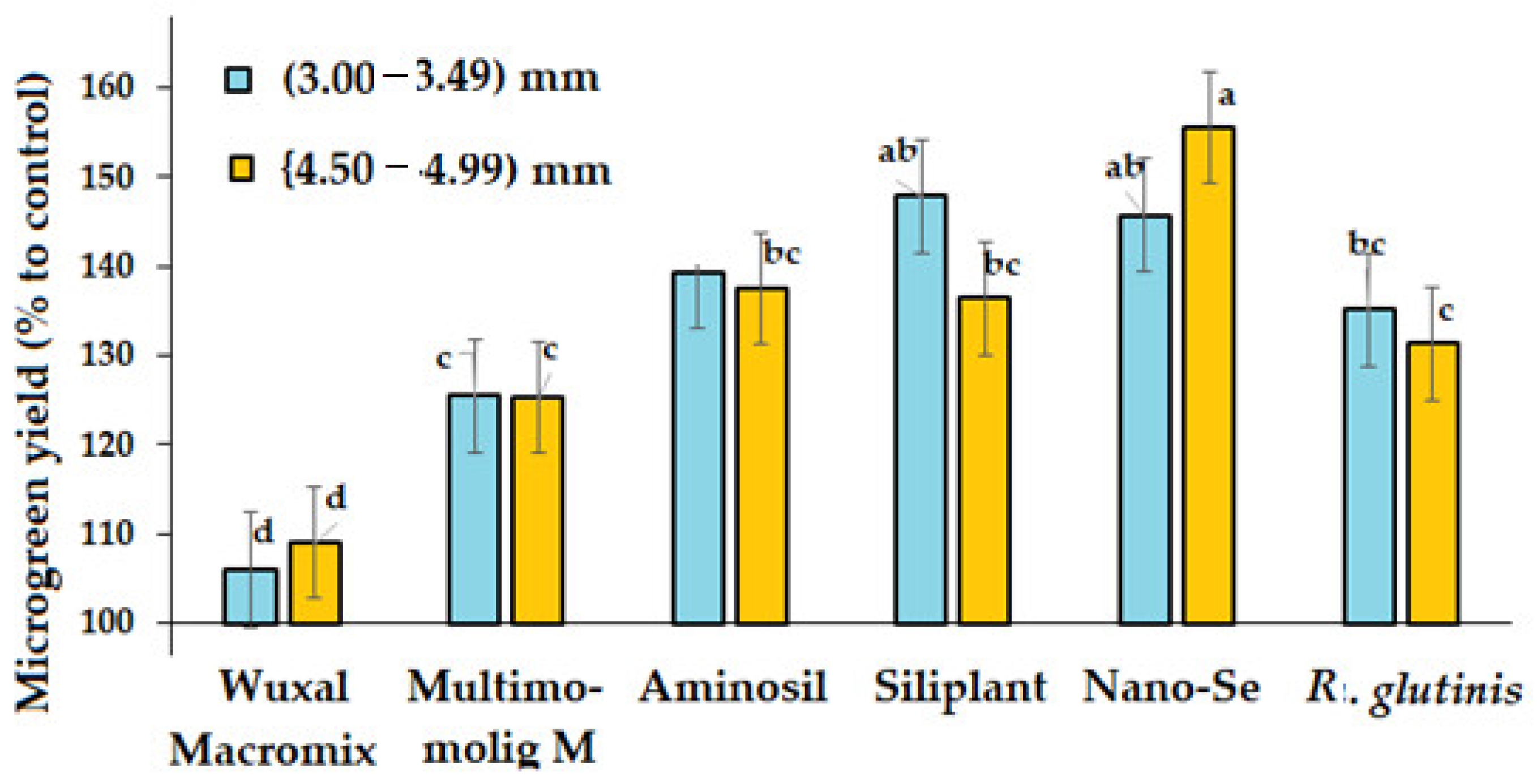
3.5. Microgreen Yield
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aarti, D.; Sarkar, K.; Gupta, S.; Singh, A. A Review on potential health benefits of beetroot. Indian Journal of Health Care. Med. Pharm. Pract. 2024, 5, 96–102. [Google Scholar]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a functional food with huge health benefits: Antioxidant, antitumor, physical function, and chronic metabolomics activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef]
- Freitas, G.M.; Cicotti, L.; Constant, P.B.L.; Zotarelli, M.F.; de Sá, J.P.N.; Mazzitti, S.; de Oliveira, F.A.; Durigon, A. Beetroot microgreens (Beta vulgaris) powder obtained by cast-tape drying and freeze-drying. J. Food Process Eng. 2024, 47, 74. [Google Scholar] [CrossRef]
- Acharya, J.; Gautam, S.; Neupane, P.; Niroula, A. Pigments, ascorbic acid, and total polyphenols content and antioxidant capacities of beet (Beta vulgaris) microgreens during growth. Int. J. Food Prop. 2021, 24, 1175–1186. [Google Scholar] [CrossRef]
- Dubey, S.; Harbourne, N.; Harty, M.; Hurley, D.; Elliott-Kingston, C. Microgreens production: Exploiting environmental and cultural factors for enhanced agronomical benefits. Plants 2024, 13, 2631. [Google Scholar] [CrossRef]
- Yadav, L.P.; Koley, T.K.; Tripathi, A.; Singh, S. Antioxidant potentiality and mineral content of summer season leafy greens: Comparison at mature and microgreen stages using chemometric. Agric. Res. 2019, 8, 165–175. [Google Scholar] [CrossRef]
- Golubkina, N.; Kharchenko, V.; Moldovan, A.; Antoshkina, M.; Ushakova, O.; Sękara, A.; Stoleru, V.; Murariu, O.C.; Tallarita, A.V.; Sannino, M. Effect of selenium and garlic extract treatments of seed-addressed lettuce plants on biofortification level, seed productivity and mature plant yield and quality. Plants 2024, 13, 1190. [Google Scholar] [CrossRef]
- Shrestha, S.; Dhungana, M.; Sahani, S.; Bhattarai, B. Seed quality improvement to approach sustainable yield of field crops by various preparation techniques: Seed priming, treatment and inoculation. A review. Plant Physiol. Soil Chem. 2021, 1, 20–28. [Google Scholar] [CrossRef]
- Weissmann, E.A.; Raja, K.; Gupta, A.; Patel, M.; Buehler, A. Seed quality enhancement. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023. [Google Scholar]
- Baqir, H.A.; Zeboon, N.H.; Al-behadili, A.A.J. The Role and Importance of Amino acids within Plant: A Review. Plant Arch. 2019, 19 (Suppl. 2), 1402–1410. [Google Scholar]
- Márquez, V.G.; Moreno, Á.M.; Mendoza, A.B.; Macías, J.M. Ionic selenium and nanoselenium as biofortifiers and stimulators of plant metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- Khan, A.L. Silicon: A valuable soil element for improving plant growth and CO2 sequestration. J. Adv. Res. 2024, 26. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Miao, X. Effects of exogenous silicon on maize seed germination and seedling growth. Sci. Rep. 2021, 11, 1014. [Google Scholar] [CrossRef]
- Amir, R.; Galili, G.; Cohen, H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Soares, J.N.; Reichardt, K.; Neto, D.D. Seed and foliar application of amino acids improve variables of nitrogen metabolism and productivity in soybean crop. Front. Plant Sci. 2018, 9, 396. [Google Scholar] [CrossRef]
- Kordrostami, M.; Ghasemi-Soloklui, A.A.; Hossain, M.A.; Mostofa, M.G. Breaking barriers: Selenium and silicon-mediated strategies for mitigating abiotic stress in plants. Phyton 2023, 92, 2713–2736. [Google Scholar] [CrossRef]
- Huang, S.; Qin, H.; Jiang, D.; Lu, J.; Zhu, Z.; Huang, X. Bio-nano selenium fertilizer improves the yield, quality, and organic selenium content in rice. J. Food Comp. Anal. 2024, 132, 106348. [Google Scholar] [CrossRef]
- Shanmugaiah, V.; Gauba, A.; Hari, S.K. Effect of silicon micronutrient on plant’s cellular signaling cascades in stimulating plant growth by mitigating the environmental stressors. Plant Growth Regul. 2023, 100, 391–408. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional Role of Silicon to Activate Resilient Plant Growth and to Mitigate Abiotic Stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef]
- Artyszak, A. Effect of Silicon Fertilization on Crop Yield Quantity and Quality-A Literature Review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Artyszak, A.; Kondracka, M.; Gozdowski, D.; Siuda, A.; Litwińczuk-Bis, M. Impact of foliar application of various forms of silicon on the chemical composition of sugar beet plants. Sugar Tech 2021, 23, 546–559. [Google Scholar] [CrossRef]
- Kowalska, J.; Krzymi’nska, J.; Tyburski, J. Yeasts as a potential biological agent in plant disease protection and yield improvement—A short review. Agriculture 2022, 12, 1404. [Google Scholar] [CrossRef]
- Fu, S.F.; Sun, P.F.; Lu, H.Y.; Wei, J.Y.; Xiao, H.S.; Fang, W.T.; Cheng, B.Y.; Chou, J.Y. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata Lab. Fungal Biol. 2016, 120, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, R.A.; Athab, M.A.; Matny, O.N. Management of potato virus Y (PVY) in potato by some biocontrol agents under field conditions. Adv. Environ. Biol. 2013, 7, 441–444. [Google Scholar]
- Chen, Y.-R.; Kuo, C.-Y.; Fu, S.-F.; Chou, J.-Y. Plant growth-promoting properties of the phosphate-solubilizing red yeast Rhodosporidium Paludigenum. World J. Microbiol. Biotechnol. 2023, 39, 54. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S. Plant growth promoting activity and biocontrol potential of soil yeast. Int. J. Environ. Biotechnol. 2022, 15, 75–80. [Google Scholar]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Evaluation of the production of exopolysaccharide by plant growth promoting yeast Rhodotorula sp. strain CAH2 under abiotic stress. Int. J. Biol. Macromol. 2019, 121, 55–62. [Google Scholar] [CrossRef]
- Aly, A.; Eliwa, N.; Abd El Megid, M.H. Improvement of growth, productivity, and some chemical properties of hot pepper by foliar application of amino acids and yeast extract. Potravin. Slovak J. Food Sci. 2019, 13, 831–839. [Google Scholar] [CrossRef]
- Sowmya, R.S.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Effect of amino acids on growth, elemental content, functional groups, and essential oils composition on hydroponically cultivated coriander under different conditions. Ind. Crops Prod. 2023, 197, 116577. [Google Scholar]
- Sinichenko, N.A.; Vanyushkin, I.A.; Kozar, E.G.; Markarova, M.Y. Influence of biological preparations of various nature on the development of alternariosis and the yield of tomato plants in the conditions of Primorsky Krai. News FSVC 2023, 1, 25–31. (In Russian) [Google Scholar]
- Bandehagh, A.; Dehghanian, Z.; Gougerdchi, V.; Hossain, M.A. Selenium: A game changer in plant development, growth, and stress tolerance, via the modulation in gene expression and secondary metabolite biosynthesis. Phyton 2023, 92, 2301–2324. [Google Scholar] [CrossRef]
- FAO; ISTA. Guidelines for the establishment and management of seed testing laboratories. In Joint FAO and ISTA Handbook; FAO: Rome, Italy; ISTA: Vienna, Austria, 2023. [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Anthoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plant Antioxidants and Methods of Their Determination; INFRA-M: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance. Methods in Molecular Biology (Methods and Protocols); Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 639, Chapter 20; pp. 317–331. [Google Scholar]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon in Agriculture. In Effect of Silicon on Crop Growth, Yield and Quality; Springer: Berlin/Heidelberg, Germany, 2015; Chapter 11; pp. 209–223. [Google Scholar]
- Reyes-Perez, J.J.; Tipan-Torres, H.C.; Llerena-Ramos, L.T.; Hernandez-Montiel, L.G.; Rivas-Garcia, T. Silicon increased the growth, productivity, and nutraceutical quality of tomato (Solanum lycopersicum L.). Not. Bot. Horti Agrobot. 2023, 51, 13155. [Google Scholar] [CrossRef]
- Ning, D.; Qin, A.; Liu, Z.; Duan, A.; Xiao, J.; Zhang, J.; Liu, Z.; Zhao, B.; Liu, Z. Silicon-mediated physiological and agronomic responses of maize to drought stress imposed at the vegetative and reproductive stages. Agronomy 2020, 10, 1136. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino acid biostimulants and protein hydrolysates in agricultural sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Puchkov, M.; Shcherbakova, N.; Pakina, E.; Zargar, M.; Lyashko, M. Seed priming with exogenous amino acids improves germination rates and enhances photosynthetic pigments of onion seedlings (Allium cepa L.). Horticulturae 2023, 9, 80. [Google Scholar] [CrossRef]
- Sadak, S.H.M.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar]
- Griazeva, V.I. Effect of fractional seed composition on varietal characteristics of uterine table beet roots. Niva Povolzhya 2018, 4, 14–18. (In Russian) [Google Scholar]
- Hamidi, A.; Chegini, M.A. Effect of seed size of sugar beet varieties on some germination characters and seedling vigor. J. Struct. Biol. 2016, 31, 157–166. [Google Scholar]
- Khan, I.; Awan, S.A.; Rizwan, M.; Brestic, M.; Xie, W. Silicon: An essential element for plant nutrition and phytohormones signaling mechanism under stressful conditions. Plant Growth Regul. 2022, 100, 301–319. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Wang, N.; Jiang, X.; Mao, H.; Zhu, C.; Wen, F.; Wang, X.; Lu, Z.; Yue, G.; et al. Manipulation of auxin response factor affects seed size in the woody perennial Jatropha curcas. Sci. Rep. 2017, 7, 40844. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N. Selenium biorhythms and hormonal regulation. In Selenium. Sources, Functions and Health Effects; Aomori, C., Hokkaido, M., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2012; Chapter 2; pp. 33–74. [Google Scholar]
- Mussagy, C.U.; Ribeiro, H.F.; Pereira, J.F.B. Chapter 4—Rhodotorula sp. as a cell factory for production of valuable biomolecules, Eds: Gregory, S. Makowski, Sima Sariaslani. Adv. Appl. Microbiol. 2023, 123, 133–156. [Google Scholar] [PubMed]
- Chomontowski, C.; Wzorek, H.; Podlaski, S. Impact of sugar beet seed priming on seed quality and performance under diversified environmental conditions of germination, emergence and growth. J. Plant Growth Regul. 2020, 39, 183–189. [Google Scholar] [CrossRef]
- García-Locascio, E.; Valenzuela, E.I.; Cervantes-Avilés, P. Selenium nanoparticles and maize: Understanding the impact on seed germination, growth, and nutrient interactions. Plant Nano Biol. 2025, 11, 100144. [Google Scholar] [CrossRef]
- Huong, N.T.; Tung, D.K.; Ky, V.H.; Nam, P.H.; Anh, N.T.N. Synthesis of nano-selenium and its effects on germination and early seedling growth of four crop plants. AIP Adv. 2024, 14, 25–46. [Google Scholar] [CrossRef]
- Simić, A.; Sredojević, S.; Todorović, M.; Đukanović, L.; Radenović, C. Studies on the relationship between the content of total phenolics in exudates and germination ability of maize seed during accelerated aging. Seed Sci. Technol. 2004, 32, 213–218. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- de Freitas, I.S.; da Costa Mello, S.; Nemali, K. Supplemental light quality affects optimal seeding density of microgreens. Urban Agric. Reg. Food Syst. 2024, 9, e20064. [Google Scholar] [CrossRef]
- Stajčić, S.; Cetković, G.; Tumbas Šaponjac, V.; Travićić, V.; Ilić, P.; Brunet, S.; Tomić, A. Bioactive compounds and the antioxidant activity of selected vegetable microgreens: A correlation study. Processes 2024, 12, 1743. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A comprehensive review of bioactive molecules and health benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef]
- Murphy, C.J.; Llort, K.F.; Pill, W.G. Factors affecting the growth of microgreen table beet. Int. J. Veg. Sci. 2010, 16, 253–266. [Google Scholar] [CrossRef]
- Golubkina, N.; Zayachkovsky, V.; Poluboyarinov, P.; Amagova, Z.; Sękara, A.; Murariu, O.C.; Tallarita, A.V.; Caruso, G. Beneficial effect of organic and inorganic forms of selenium on yield and nutritional characteristics of beetroot. Acta Agric. Slov. 2025, 121, 1–10. [Google Scholar] [CrossRef]
- Kippa, A.P.; Strohm, D.; Brigelius-Floh, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Hesekerd, H.; German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- El-Ramady, H.; Faizy, S.E.-D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.-D.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium biofortification for human health: Opportunities and challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]

| Month | Temperature (°C) | Precipitation (mm) | ||
|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | |
| May | 10.0 | 12.2 | 55.5 | 33.9 |
| June | 18.6 | 16.5 | 24.6 | 66.9 |
| July | 20.2 | 18.1 | 66.1 | 83.9 |
| August | 22.3 | 19.9 | 13.7 | 48.8 |
| September | 9.6 | 14.9 | 125.7 | 10.2 |
| Formulation | Dose | Composition | Company |
|---|---|---|---|
| Wuxal Macromix | 2 mL L−1 | A suspension fertilizer containing N: 240 g L−1; P: 240 g L−1; K: 180 g L−1; B: 0.3 g L−1; Cu: 0.75 g L−1; Zn: 0.75 g L−1; Fe: 1.5 g L−1; Mn: 0.75 g L−1; Mo: 0.015 g L−1. | Aglukon GmbH & Co. KG, Düsseldorf, Germany |
| Multimolig M | 4 mL L−1 | Amino acids; humic acids: 4%; N total: 4%; N-NO3: 0.5%; P2O5: 0.5%; K2O: 1%; dry matter: 25%; organic matter: 30%; Ca: 0.2%; S: 1.5%; Na: 0.5%; Mg: 0.3%; Fe: 10%; Mn: 1.3%; Zn: 1%; Cu: 1.5%; Co: 0.4%; Mo: 1%; B: 0.06%. | Multimolig, St. Petersburg, Russia |
| Aminosil | 20 mL L−1 | Proteinogenic and non-proteinogenic amino acids; N: 1.8%; P: 1.8%; K: 1.8%; Mg: 0.21%; Fe: 0.02%; Ca: 0.55%; Mg: 0.21%; Zn: 0.001%; Mn: 0.002%. | Aminosil, Belgorod, Russia |
| Siliplant | 3 mL L−1 | Si: 7.5–7.8%; K: 1.0%; Fe: 0.30 g L−1; Mg: 0.10 g L−1; Cu: 0.07 g L−1; Zn: 0.08 g L−1; Mn: 0.30 g L−1; Mo: 0.06 g L−1; Co: 0.02 g L−1; B: 0.094 g L−1. | NEST M Inc., Moscow, Russia |
| Nano-Se | 1.27 mM; 100 mg L−1 | 100 nm particles obtained by laser ablation. | Institute of Metallurgy and Material Science, Moscow, Russia |
| Rhodotorula glutinis | 1 mL L−1 | Soil yeast Rhodotorula glutinis formulation with cell titer of 1.5–2.5 MM KOE mL−1 (a source of amino acids, proteins, and phytohormones). | Federal Scientific Vegetable Center, Moscow, Russia |
| Parameter | Seed Diameter Class (mm) | |||
|---|---|---|---|---|
| 3.00–3.49 | 3.50–3.99 | 4.00–4.49 | 4.50–4.99 | |
| Untreated Control | 7.55 | 10.24 | 14.5 | 21.71 |
| Wuxal Macromix | 6.96 | 9.88 | 14.8 | 19.69 |
| Multimolig M | 7.56 | 10.35 | 15.0 | 20.44 |
| Aminosil | 6.88 | 10.13 | 14.9 | 20.76 |
| Siliplant | 6.97 | 9.87 | 15.5 | 21.10 |
| Nano-Se | 7.41 | 9.85 | 15.2 | 21.50 |
| R. glutinis | 7.52 | 10.47 | 15.4 | 20.92 |
| Significance | n.s. | n.s. | n.s. | n.s. |
| M ± SD | 7.26 ± 0.31 d | 10.11 ± 0.25 c | 15.00 ± 0.35 b | 20.87 ± 0.67 a |
| CV (%) | 4.29 | 2.47 | 2.53 | 3.24 |
| Treatment | Seed Diameter Class (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 3.00–3.49 | 3.50–3.99 | 4.00–4.49 | 4.50–4.99 | |||||
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Control | 36 c | 35 c | 53 b | 54 b | 70 ab | 69 b | 94 a | 95 a |
| Wuxal Macromix | 47 b | 48 b | 70 a | 71 a | 80 ab | 79 ab | 97 a | 97 a |
| Multimolig M | 35 c | 34 c | 65 a | 64 ab | 77 ab | 78 ab | 96 a | 97 a |
| Aminosil | 72 a | 73 a | 75 a | 74 a | 85 a | 86 a | 97 a | 98 a |
| Siliplant | 46 b | 45 b | 66 a | 67 ab | 75 ab | 76 ab | 94 a | 95 a |
| Nano-Se | 67 a | 66 a | 72 a | 73 a | 80 ab | 81 a | 94 a | 95 a |
| R. glutinis | 44 b | 43 b | 42 c | 41 c | 66 b | 65 b | 84 a | 83 a |
| Treatment | Seed Diameter Class (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 3.00−3.49 | 3.50−3.99 | 4.00−4.49 | 4.50−4.99 | |||||
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Control | 44 c | 43 c | 60 bc | 61 bc | 76 ab | 75 ab | 95 a | 95 a |
| Wuxal Macromix | 55 b | 54 b | 77 a | 76 a | 80 ab | 81 a | 97 a | 97 a |
| Multimolig M | 46 bc | 45 | 80 a | 79 a | 87 a | 88 a | 97 a | 98 a |
| Aminosil | 80 a | 81 a | 82 a | 83 a | 87 a | 86 a | 97 a | 98 a |
| Siliplant | 52 bc | 53 b | 72 ab | 73 a | 80 ab | 81 a | 96 a | 97 a |
| Nano-Se | 76 a | 75 a | 82 a | 83 a | 87 a | 88 a | 96 a | 97 a |
| R. glutinis | 53 bc | 52 b | 54 c | 53 c | 70 b | 71 b | 87 a | 86 a |
| Treatment | AOA (mg GAE g−1 d.w.) | TP (mg GAE g−1 d.w.) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3.00–3.49 mm | 4.50–4.99 mm | 3.00–3.49 mm | 4.50–4.99 mm | |||||
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Control | 22.3 ab | 20.9 a | 14.5 c | 13.7 bc | 9.5 a | 8.3 a | 7.5 a | 6.7 a |
| Wuxal Macromix | 19.2 b | 16.8 b | 13.9 c | 12.9 c | 8.0 a | 6.8 b | 5.7 b | 4.9 b |
| Multimolig M | 21.7 b | 19.9 a | 14.0 c | 13.3 bc | 8.1 a | 7.5 ab | 6.9 a | 6.1 a |
| Aminosil | 19.6 b | 18.4 a | 16.7 ab | 15.6 ab | 7.9 a | 7.3 ab | 6.9 a | 5.7 ab |
| Siliplant | 22.0 ab | 21.2 a | 17.8 ab | 16.8 a | 8.9 a | 8.1 a | 7.5 a | 6.5 a |
| Nano-Se | 23.5 ab | 22.3 a | 18.6 a | 16.8 a | 8.5 a | 7.7 ab | 6.8 ab | 6.0 a |
| R. glutinis | 26.0 a | 22.4 a | 15.8 bc | 15.0 ab | 8.8 a | 7.4 ab | 7.9 a | 6.5 a |
| Treatment | Microgreen Length (cm) | Microgreen Yield (g per 100 Microgreens) | ||
|---|---|---|---|---|
| 3.00–3.49 mm | 4.50–4.99 mm | 3.00–3.49 mm | 4.50–4.99 mm | |
| Control | 11.5 d | 11.9 d | 94 c | 99 c |
| Wuxal Macromix | 11.6 d | 12.5 cd | 100 c | 108 bc |
| Multimolig M | 11.9 d | 14.3 bcd | 118 b | 124 b |
| Aminosil | 14.5 bc | 15.2 ab | 131 ab | 136 ab |
| Siliplant | 14.8 abc | 15.7 ab | 135 ab | 139 ab |
| Nano-Se | 15.3 ab | 17.8 a | 137 ab | 154 a |
| R. glutinis | 12.8 cd | 14.1 bcd | 127 ab | 130 ab |
| M ± SD | 13.2 ± 1.6 | 14.5 ± 2.0 | 120.3 ± 17.1 | 127.1 ± 18.8 |
| CV (%) | 12.1 | 11.8 | 14.2 | 14.8 |
| Treatment | Betalain Pigments | Total chl | Carotene | AOA | TP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg 100 g−1 d.w. | mg GAE g−1 d.w. | |||||||||
| 3.00– 3.49 mm | 4.50– 4.99 mm | 3.00– 3.49 mm | 4.50– 4.99 mm | 3.09– 3.49 mm | 4.50– 4.99 mm | 3.00– 3.49 mm | 4.50– 4.99 mm | 3.00– 3.49 mm | 4.50– 4.99 mm | |
| Control | 5.8 b | 6.3 b | 88 c | 89 e | 8 d | 9 c | 42.1 | 41.1 | 16.1 | 14.9 |
| Wuxal Macromix | 7.1 a | 7.6 a | 90 c | 112 d | 9 cd | 10 c | 42.1 | 40.8 | 17.0 | 15.0 |
| Multimolig M | 7.0 a | 7.6 a | 91 c | 121 c | 10 c | 9 c | 45.6 | 43.9 | 19.0 | 18.0 |
| Aminosil | 7.3 a | 7.8 a | 123 a | 134 b | 13 a | 13 b | 43.4 | 42.8 | 18.1 | 16.6 |
| Siliplant | 7.7 a | 7.7 a | 126 a | 157 a | 14 a | 17 a | 46.7 | 45.9 | 19.2 | 16.4 |
| Nano-Se | 7.6 a | 7.8 a | 127 a | 151 a | 13 a | 18 a | 50.0 | 47.4 | 18.4 | 17.7 |
| R. glutinis | 6.9 a | 7.6 a | 108 b | 130 b | 12 b | 13 b | 46.0 | 40.5 | 17.7 | 15.9 |
| Significance | n.s. | n.s. | n.s. | n.s. | ||||||
| M | 7.1 a | 7.5 a | 107.6 b | 127.7 a | 11.3 a | 12.7 a | 45.1 a | 43.2 a | 17.9 a | 16.4 a |
| SD | 0.6 | 0.5 | 17.9 | 23.2 | 2.3 | 3.7 | 2.8 | 2.7 | 1.1 | 1.2 |
| CV (%) | 8.5 | 6.7 | 16.6 | 18.2 | 20.4 | 29.1 | 6.2 | 6.3 | 6.1 | 7.3 |
| MG Carotene | MG Yield | MG Length | MG BP | Seed Pro | Seed AOA | SGC | |
|---|---|---|---|---|---|---|---|
| MG Chl | 0.899 a | 0.892 a | 0.937 a | 0.797 a | −0.550 d | −0.133 | 0.587 c |
| MG Carotene | 0.899 a | 0.889 a | 0.656 e | −0.361 | 0.124 | 0.347 | |
| MG yield | 0.934 a | 0.780 a | −0.396 | 0.146 | 0.352 | ||
| MG length | 0.746 a | −0.537 f | −0.036 | 0.524 f | |||
| MG BP | −0.677 e | −0.241 | 0.797 a | ||||
| Seed Pro | 0.598 c | −0.637 b | |||||
| Seed AOA | −0.781 a | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Zayachkovsky, V.; Markarova, M.; Fedotov, M.; Alpatov, A.; Skrypnik, L.; Nadezhkin, S.; Murariu, O.C.; Tallarita, A.V.; Caruso, G. Effect of Plant Biostimulants on Beetroot Seed Productivity, Germination, and Microgreen Quality. Crops 2025, 5, 23. https://doi.org/10.3390/crops5030023
Golubkina N, Zayachkovsky V, Markarova M, Fedotov M, Alpatov A, Skrypnik L, Nadezhkin S, Murariu OC, Tallarita AV, Caruso G. Effect of Plant Biostimulants on Beetroot Seed Productivity, Germination, and Microgreen Quality. Crops. 2025; 5(3):23. https://doi.org/10.3390/crops5030023
Chicago/Turabian StyleGolubkina, Nadezhda, Vladimir Zayachkovsky, Maria Markarova, Mikhail Fedotov, Andrey Alpatov, Lyubov Skrypnik, Sergei Nadezhkin, Otilia Cristina Murariu, Alessio Vincenzo Tallarita, and Gianluca Caruso. 2025. "Effect of Plant Biostimulants on Beetroot Seed Productivity, Germination, and Microgreen Quality" Crops 5, no. 3: 23. https://doi.org/10.3390/crops5030023
APA StyleGolubkina, N., Zayachkovsky, V., Markarova, M., Fedotov, M., Alpatov, A., Skrypnik, L., Nadezhkin, S., Murariu, O. C., Tallarita, A. V., & Caruso, G. (2025). Effect of Plant Biostimulants on Beetroot Seed Productivity, Germination, and Microgreen Quality. Crops, 5(3), 23. https://doi.org/10.3390/crops5030023










