Abstract
Background: Yerba-mate (Ilex paraguariensis) is a perennial native tree from South America. Improper management of yerba-mate crops results in low yield. This work evaluated the recovery and the vegetative capacity of the crop after coppicing. Methods: A 2-year field observation approach was used and the study area was monitored from 2019 to 2021 in a 20-year-old yerba-mate crop with low yield. Drastic coppicing was used as a strategy, aiming to study the effect of the month and the height of pruning. Four groups were defined combining the trunk heights of 10 and 40 cm and the months of pruning June and August. Results: Yerba-mate plants showed a low mortality rate of 2%. In the first year after the intervention, the height of the cutting was the most important factor that influenced the amount of primary and secondary branches, validating response surfaces with r2 values of 0.9942 and 0.9084, respectively. In the second year of the experiment, full recovery in productivity was reached, with a mean rise of 109.7% in the plants of the group coppiced in June 40 cm above the soil. Conclusion: The techniques used in this study are appropriate for vigor recovery in yerba-mate plants inadequately managed and allow a new architecture on plants, enabling mechanical harvesting.
1. Introduction
Yerba-mate (Ilex paraguariensis A. St. Hil) is a perennial tree native to South America, belonging to the Aquifoliaceae family [1]. Its geographic distribution extends to Brazil, Argentina and Paraguay. In Brazil, it occurs in Rio Grande do Sul, Santa Catarina, and Paraná states and a small part of Mato Grosso do Sul [2]. Yerba-mate has great socioeconomic and cultural importance in these states. Its leaves and small branches, when processed, are used in the production of infusion drinks, such as chimarrão. Although the most consumed infusion is “mate” or “chimarrão” (hot beverage), there are different forms of consumption, such as tea, carbonated drinks and “tererê” (cold beverage) [3].
Currently, yerba-mate is cultivated in an agroforestry system or homogeneous under a full sun [4]. In terms of extractive products, it represents one of the most cultivated non-timber species. The Brazilian yield of yerba-mate is around 7000 ton·ha·−1·year−1 and Rio Grande do Sul produces about 8000 ton·ha·−1·year−1 [2].
The implantation of a yerba-mate crop requires an adjusted schedule, as it is a perennial plant, and when this aspect is neglected, the result is a low yield [5]. Inappropriate harvesting practices carried out on the yerba-mate crops over the years cause deformation in the plant architecture, decreasing biomass production [6]. Its stems can be attacked by Hedypathes betulinus beetle (corinthian), one of the most important pests of yerba-mate, causing severe damage to the plants and significant economic losses. The insect’s larvae, during feeding, build longitudinal and ascending galleries in the trunk, branches and roots of the plant, affecting their development and even causing death [7,8].
Yerba-mate has a rhythmic growth characterized by Rauh’s architectural model [9]. The growth rate of yerba-mate plants regularly presents two stops: one in the summer (overall or partial), most likely related to photoperiod; and another in the winter, caused by low minimum temperatures, inducing dormancy [10].
The present study aimed to evaluate the recovery of vigor and vegetative capacity of a low-yield yerba-mate crop, after drastic coppicing. There is a limited amount of data in the literature about the recovery of degraded yerba-mate crops, and the available articles do not explore the measurement of yield as a response and are written in the Portuguese language [11,12]. Thus, the findings of the present work may give new directions for the recovery of yerba-mate crops with low yield.
2. Methods
2.1. Study Area
The research was conducted in a yerba-mate crop in southern Brazil, with the area localization shown in Figure 1a–c. The yellow area in Figure 1c was divided in 16 plots with 22 to 39 plants, totalizing 453 yerba-mate plants. The region’s climate is defined as humid subtropical (Cfa), according to the Köppen classification, with an average annual temperature between 16 °C and 20 °C, and temperatures below 0 °C during the winter in short periods. The average annual precipitation ranges from 1600 mm to 2200 mm [13]. The experiment monitored the 20-year-old yerba-mate crop that was poorly conducted and presented low yield, over 3 years, from 2019 to 2021. The yerba-mate crop before and after the intervention is shown in Figure 2a,b, respectively.
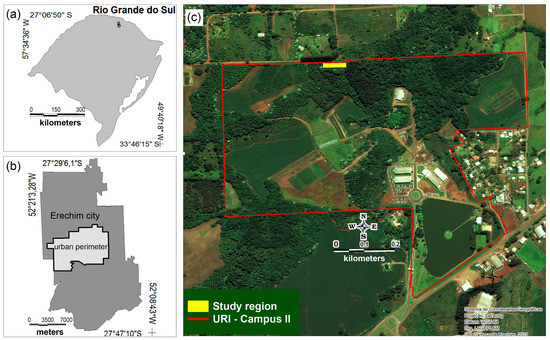
Figure 1.
Geographic localization and design of the experiment. In (a) is the Rio Grande do Sul map (Brazil South); in (b) is the Erechim city map; and in (c) is the study region.

Figure 2.
Yerba-mate plants before (a) and after (b) the experimental intervention.
2.2. Experiment Design
The experimental conditions were carried out using a 2 × 2 factorial design with repetitions, having as variables the height and month of pruning (Table 1). The higher and lower level of each factor were represented by the symbols +1 and −1, respectively. The study protocol was performed in completely randomized plots. The experiment was carried out two times within the vegetative dormancy period (June and August), coppicing at 10 cm and 40 cm above the soil. The coppicing was carried out in a bevel, using a chainsaw with vegetable oil, and fungicide was not applied. During each data collection, the parameters investigated were measured in at least 80 plants uniformly distributed across all plots of the study area.

Table 1.
Treatments adopted for revitalization of yerba-mate crop.
2.3. Follow-Up of the Experiment in 2019
From September to November/2019, the morphogenic development of the plants was monitored by the counting of the number of primary and secondary branches. The experiment follow-up in the first year is described in Figure 3a–c.
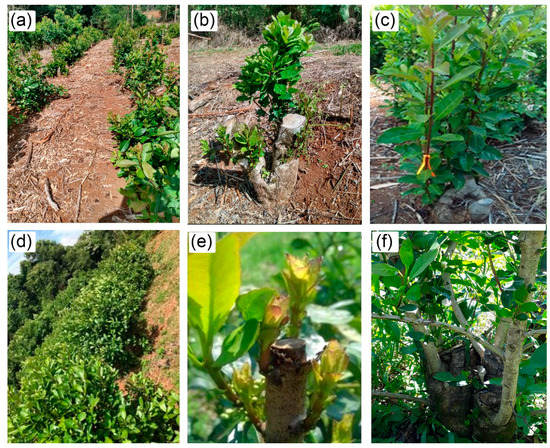
Figure 3.
Monitoring of the experiment during the years 2019 (a–c) and 2020 (d–f). In (a) is growth profile of yerba-mate plants; in (b) is start of primary branches emergence; and in (c) is follow-up of randomly selected branches; in (d) is morphological profile of plants in 2020; in (e) is new branches after pruning in 2020; and in (f) is detail in the plant architecture, originated from the five branches selected in the previously year.
2.4. Follow-Up of the Experiment in 2020
In the yerba-mate plants, during the vegetative dormancy period (2020/August), excess branches were removed and the more developed branches were selected (about five), to conduct a new architecture. This part of the experiment is shown in Figure 3d–f, with Figure 3f showing a plant that originated from the branches that were kept in the previous year.
New branches that emerged (secondary, tertiary and quaternary) were counted. The diameter and height of these secondary branches were measured with a ruler. Morphogenetic monitoring was carried out every two weeks, from September 2020 to November 2020.
The biomass of each plot of the yerba-mate crop that meets the requirements of standard preconizated by the industry was conditioned in bags and the mass was measured using a hook scale.
2.5. Data Analysis
The analysis of normality distribution was performed using the Shapiro–Wilk test and, according to this result, appropriate versions of ANOVA and post-ANOVA tests were applied. The profile of morphological characteristics in the second year was investigated by principal component analysis. The analysis of factorial design was conducted aiming the obtention of Pareto charts and response surfaces. All the analyses were carried out using the software GraphPad Prims 9.0 (San Diego, CA, USA) and Statistic 9.0 (StaSoft. Inc., Tulsa, OK, USA).
3. Results
3.1. Morphological Development during the First Year (2019/September–2019/November)
The morphological development of yerba-mate plants located in different plots under the four different experimental conditions was monitored regarding the primary and secondary branches’ emergence. The measurements were performed in the months of September and November, involving the natural period of vegetative growth of the species. Figure 4a–d shows the evolution of primary branches’ emergence over time. The experimental conditions in June/40 and August/40 (Figure 4a,b) presented a higher number of primary branches, while the condition in June/10 (Figure 4c) and August/10 (Figure 4d) showed low levels of vegetative development. This behavior is in full agreement with the number of secondary branches, as depicted in Figure 4e–h. The secondary branches’ emergence was stimulated in August/10 compared to June/10, as shown in Figure 4g,h.
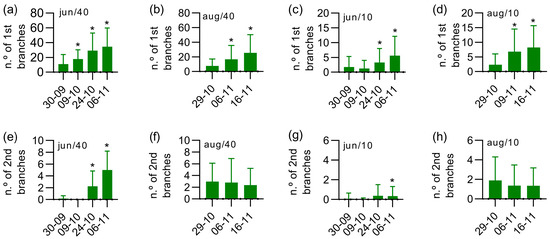
Figure 4.
Behavior of branches’ emergence during the vegetative development of yerba-mate plants from pruning under four experimental conditions. The data are represented as mean ± standard deviation of at least 80 measurements. The plants were coppiced in 2019/June or 2019/August, as specified in the graphs. * p < 0.05 in relation to the first measurement according to Kruskal–Wallis analysis followed by Dunn’s test. During this period, the temperature was 20.29 ± 6.06 °C (36.3–4.3 °C) and the rainfall was 313 mm, according to data from a local meteorological monitoring service. The raw data are available on electronic supporting information (Table S1).
From these results, November was selected for comparisons of the number of branches in the four experimental conditions. Regarding the primary branches’ emergence (Figure 5a), the most promising condition was June/40, which yielded 34.45 ± 25.45 branches/plant, this value was different from that of June/10 (5.57 ± 6.559; p < 0.0001), August/10 (6.81 ± 7.707; p < 0.0001) and August/40 (20.21 ± 21.93; p = 0.0159). The condition August/40 produced the second highest value of primary branches/plant, which was higher than the value produced under the other conditions: August/10 (p < 0.0001) and June/10 (p < 0.0001).
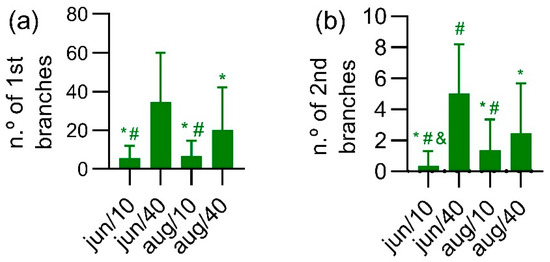
Figure 5.
Effect of experimental conditions on the number of primary branches (a) and secondary branches (b) in the end of the vegetative growth period (2019/November). The data are represented as mean ± standard deviation of at least 80 measurements. * p < 0.05 compared to June/40, # p < 0.05 compared to August/40, and & p < 0.05 compared to Aug/10, according to the Kruskal–Wallis analysis followed by Dunn’s test.
When the same analysis was performed for the secondary branches, a similar profile may be observed (Figure 5b). The condition Jun/40 produced 5.00 ± 3.19 branches/plant, different from June/10 (0.346 ± 0.9647, p < 0.0001), August/10 (1.36 ± 1.98, p < 0.0001) and August/40 (2.46 ± 3.23, p < 0.0001). Similarly to the primary branches behavior, the second highest value of secondary branches/plant was found for the August/40 condition (2.46 ± 3.23 branches/plant), different from those of June/10 (p < 0.001), August/10 (p < 0.001) and June/40 (p = 0.0013). In addition, a significant difference was found between June/10 and August/10 (p = 0.0051).
The analysis of the factorial experiment showed that the height of cutting was the variable with the strongest effect on the number of primary and secondary branches/plant, as can be showed in Figure 6, where the chart includes a vertical line at the critical t-value for an α = 0.05. The interaction between the height and month of cutting produced a smaller effect on these responses, while the month of cutting alone was not able to affect significantly the emergence of primary and secondary branches (Figure 6a,e). For both primary and secondary branches, when the variable height of cutting changed from the smaller to the higher level, an intense increase in the branches’ emergence was observed, as depicted in Figure 6b,f. Although when the same analysis was performed for the month of cutting, very small changes in these responses were found (Figure 6c,g).
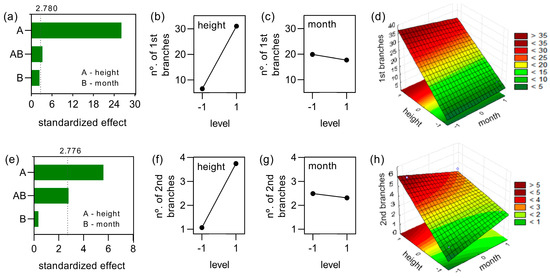
Figure 6.
Factorial analysis of the experiment considering the number/plant of primary (a–d) and secondary (e–h) branches. Pareto chart of the standardized effects at p = 0.05 for number of primary and secondary branches (a,e). The graphs of the main effects on these responses considering variables height and month of cutting are shown in (b,c,f,g). Response surfaces for the number of primary branches/plant (d) and the number of secondary branches/plant (h) having as factors the height and the month of cutting of the yerba-mate plants.
Considering the number/plant of primary and secondary branches as response, two linear models were validated and capable of representing these responses as a function of month and height of cutting. For the number of primary branches, the variance analysis yielded high r2 value (0.9942). This response surface described in Figure 6d confirms that the high number of primary branches was produced by the experimental condition Jun/40, while the primary branch emergence was prejudiced by the low height of cutting. A similar profile was obtained for secondary branches’ emergence (Figure 6h), r2 value of 0.9084. The data of ANOVA validation of these models are reported in Table 2. The two models generated showed that the branches’ emergence was maximized in the experimental condition in June/40.

Table 2.
ANOVA responses for number of primary and secondary branches.
3.2. Morphological Development during the Second Year (2020/September–2020/November)
The five branches selected after the first year of monitoring are depicted in Figure 7, with their classification of primary, secondary, tertiary and quaternary. The branches that originated directly from the trunk were identified as primary branches, and the branches that originated from these, were identified as secondary branches. Tertiary and quaternary branches were similarly defined. In the selected branches (primary) the emergence of the secondary, tertiary and quaternary branches was monitored in the 16 parcels of the yerba-mate crop, according to the four treatments. This strategy is a tool to give directions to plant growth, making suitable the use of mechanical harvesting.
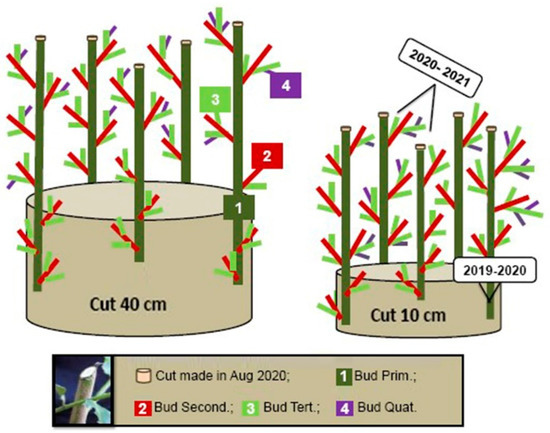
Figure 7.
Schematic profile of the vegetative development of yerba-mate plants after removal of unnecessary branches.
At the end of the experimental monitoring period (2020/November) the variables number of leaves, height and diameter of secondary branches and number of tertiary and quaternary branches were measured (Figure 8). The experimental condition in June/40 produced higher number of leaves/branches when compared to that of June/10 (p = 0.0339), as shown in Figure 8a. In addition, this experimental condition produced the higher number of leaves/branches (75.00 ± 23.67), while the lowest number of leaves/branches was 55.05 ± 22.55 for the June/10 and 58.07 ± 17.87 for the August/10 conditions. The conditions of August/10 yielded secondary branches with the lowest height (48.60 ± 16.30 cm), as may be observed in Figure 8b. This value was statistically different when compared to that of August/40 (p = 0.0158) and June/40 (p = 0.0288) conditions, with 71.20 ± 19.65 cm and 70.55 ± 27.06 cm, respectively. It should be highlighted that the more drastic condition (August/10) hindered the new secondary branches’ emergence, so no plant grew new secondary branches (Figure 8d). The cutting at 10 cm above the soil also avoided the 3rd branches’ emergence, as may be observed in Figure 8f. Regarding the diameter of secondary branches and number of secondary old branches differences across the four treatments (Figure 8c,e) were not found.
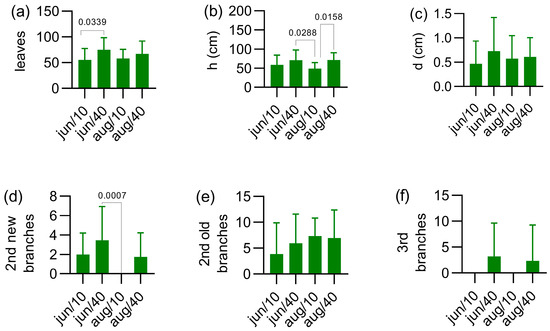
Figure 8.
Effect of experimental conditions on the number of leaves (a), height of secondary branches (b), diameter of secondary branches (c), number of new secondary branches (d), number of old secondary branches (e) and number of tertiary branches (f). The data are represented as mean ± standard deviation of at least 80 measurements. In (a,b) ANOVA + Tukey’s test were used, and in (d) Kruskal–Wallis analysis + Dunn’s test were used. During this period the temperature was 20.44 ± 6.08 °C (37.8–4.2 °C) and the rainfall was 196 mm, according to data from a local meteorological monitoring service.
Aiming to improve the understanding of the role of experimental conditions on the responses, the data were submitted to principal component analysis. The categorical variable that produced better clustering profile of the experimental points was height of cutting (Figure 9). The first two components, PC1 and PC2, explained 67.4% of the total data variability. The plants that were coppiced at 10 cm above the soil, were placed mainly at the left of the plot (green points) as depicted in Figure 9a, with lower PC1 values.
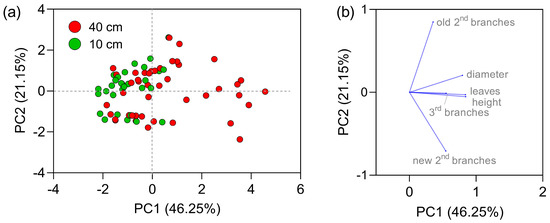
Figure 9.
(a) Distribution in the hyperspace of the scores of the first two components of the data collected in 2020/Nov according to their morphological development. (b) Vector-correlation plot among the variables examined. The variables analyzed were the number of old and new secondary branches, diameter and number of leaves of old branches, and number of tertiary branches.
As may be observed in the vector plot (Figure 9b), PC1 was affected in a large extension mainly by the height and diameter of secondary branches and number of leaves. Most of the plants with higher PC1 values were coppiced at 40 cm above the soil. The variable’s number of old and new secondary branches produced the dispersion of experimental points along the y-axis, with an effect on PC2 values. The vector plot described in Figure 9b also highlights the positive strong correlation in the height of secondary branches, the number of leaves and the emergence of 3rd branches, and the negative correlation between the number of old and new secondary branches.
The correlations among all the variables monitored during all the times of the experiment were investigated by a correlogram construction (Figure 10). The data used for Figure 10 construction involved 1579 experimental measurements in sampled branches from 2020/September to 2020/November, which involved the period of more intense vegetative development in yerba-mate plants. Among all the correlations, the strongest of them are described here. The number of new tertiary branches presented a strong correlation with the diameter of secondary branches (r = 0.50; p < 0.0001), and number of secondary old branches (r = 0.42; p < 0.0001). The emergence of new secondary branches was correlated with the height of secondary branches (r = 0.41; p < 0.0001). An r-value of 0.30 was found between the number of quaternary and new tertiary branches.
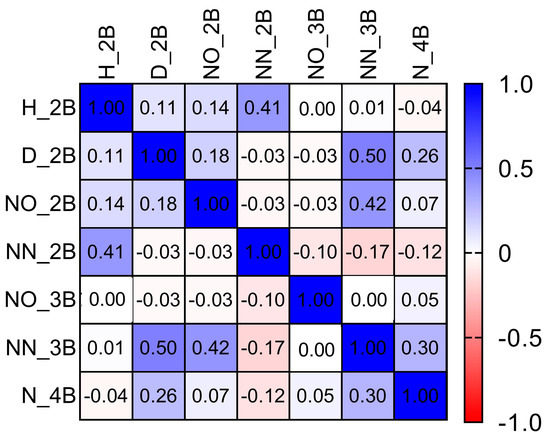
Figure 10.
Pairwise Spearman correlation of the morphological features monitored. The scale. Color key scaled from −1 to + 1 is illustrated as red and blue colors, respectively, with white color representing the absence of correlations. H_2B: height of secondary branches; D_2B: diameter of secondary branches; NO_2B: number of old secondary branches; NN_2B: number of new secondary branches; NO_3B: number of old tertiary branches; NN_3B: number of new tertiary branches; N_4B: number of quaternary branches.
3.3. Characteristics of Yerba-Mate Crop and Yield Assessment
This investigation analyzed 453 yerba-mate plants placed in an unproductive crop. The investigated plant stems’ diameter was 9.74 ± 3.59 cm, ranging from 2 to 26 cm.
The number of yerba-mate plants per plot ranged from 22 to 35 plants. Of the 453 yerba-mate plants, only 9 plants did not develop branches, 4 of them were coppiced at 40 cm above the soil, 2 of them were coppiced in June and 2 in August. For plants that were coppiced at 10 cm above the soil, 5 did not develop branches, 3 of them were coppiced in June and 2 of them in August. These results show a low plant mortality rate and that the plants lost were randomly distributed in the four groups of the experiment design, without the effect of experimental conditions on mortality.
Table 3 shows the yerba-mate biomass produced in each parcel analyzed, in the four experimental conditions. The higher yield values after 2 years (2021) were found when the yerba-mate plants were coppiced in June, 40 cm above the soil, the condition which yielded 4.32 kg/plant, the value 109.7% higher than that of the harvesting performed at the experiment’s start. It should be highlighted here that the first time the plants were submitted to radical harvesting, when they were coppiced. The plants that were coppiced in August at 40 cm above the soil, showed the second highest increase in yield, 4.23 kg/plant, value 75.5% higher than the basal value. Other interesting values of yield was found in the plants that were coppiced in August at 10 cm above the soil. This experimental condition produced 3.01 kg/plant, 54.3% higher than the basal value. The lowest yield values were reached when the plants were coppiced in June at 10 cm above the soil, with 1.94 kg/plant, value lower than the basal yield level. The other aspect to be highlighted is that, in the second evaluation, about 10% of leaves were kept in the plants, aiming to avoid excessive damage to their new architecture.

Table 3.
Yerba-mate biomass production in two different moments: at the start of the experiment and after two years of the intervention.
Aiming to identify the variable with most expressive effect on the yield, the factorial design was analyzed. This analysis highlighted that the most important variable that affected yield was the height of cutting, as can be observed in the Pareto chart of Figure 11a. This variable showed a significant effect when α = 0.1 (standardized effect = 1.860). When the level of height changes from lower to higher, an expressive increase in the yield was observed (Figure 11b). Minor modifications in yield were observed when the variable month of cutting goes from the lower to the higher level, as may be observed in Figure 11c.
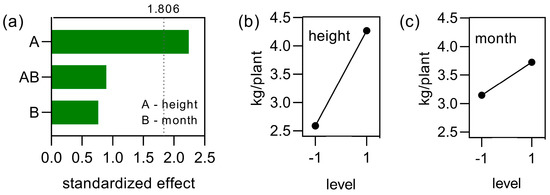
Figure 11.
Factorial analysis of the experiment considering yield as response. Pareto chart of the standardized effects at p = 0.10 for yield of yerba-mate plants is shown in (a). The graphs of the main effects for yield considering variables height and month of cutting are shown in (b) and (c).
4. Discussion
In the study conducted by Rakocevic et al. 2011 [14], it was noted that in the yerba-mate, all the axes are equivalent with regard to the orientation and phylotactic angles. There is a lateral bud in the axilla of each yerba-mate leaf, and the phyllotaxy is in a constant spiral (2/5) between two adjacent leaves. Flowers and fruits are developed in axillary positions along the branches. The lateral branches are grouped before the scars of very short internodes, which separate the annual buds into successive growth units. In this investigation, the branches’ emergence was in agreement with the vegetative growth of the species. Yerba-mate plants naturally show two periods of vegetative growth: the first, more intense, occurs in September, while the second occurs in January and presents lower intensity [14].
Despite this well-defined behavior of the Ilex paraguariensis species, it is susceptible to seasonality and climate changes, which may produce growth stops and its resumption as an adaptive process [15]. Erechim-RS city, where the experiment was carried out, has a warm and temperate climate, with significant rainfall throughout the year, even in the driest month. It is worth highlighting here that, during the experiment period, the climate pattern was not different compared to the annual characteristic climate of the region. According to the study conducted by Nicolini et al. 2012 [16], with regard to the growth and rest periods in Parkia velutina, the phases can be periodic or irregular. In other plants (cocoa), the presence of synchronized or non-synchronized axes was identified [17].
According to Medrado et al., 2002 [18], recovery pruning has been one of the main tools in the management of degraded yerba-mate crops, and should be carried out between the months of July and August, during the plant’s physiological rest. The vigor-recovering tests carried out in this study, as they were conducted during the dormancy period, resulted in 98% of the plants responding with the emergence of new branches, corroborating the study carried out by Stuepp et al. 2016 [11] in a 17-year-old yerba-mate crop and coppicing carried out at 15, 30 and 60 cm above the soil, which obtained about a 95.8% as survival rate. The presence of dormant buds at the base of the trunk of old trees can be explained by the fact that they form in a period closer to seed germination, which enables the induction of juvenile dormant buds [19].
The yerba-mate plants coppiced in August delayed the branches’ emergence; however, statistically, the average number of branches per plant was not different when compared with the other period of coppicing. According to Penteado and Goulart 2017 [6], the ideal time for coppicing is between the month of August and mid-September, the final period of the physiological rest of the plants.
Regarding the number of branches/plant, the highest values were obtained in the coppiced carried out 40 cm above the soil in June. In the study carried out by Stuepp et al. 2016 [11]. the cutting performed at 60 cm from the soil resulted in the highest values for this parameter. However, this height of the trunk would not be advisable for the development of an efficient architecture of the yerba-mate plants. Similarly to our findings, the greater number of branches obtained in the treatment coppiced at 60 cm from the soil is due to the larger lateral surface of the stump, which provided a greater amount of nutrients for the development of new branches, when compared to the plants that were coppiced at 15 and 30 cm above the soil.
Regarding biomass production, a full recovery with an increase in production was achieved in the second year of monitoring. This increase was reached though, in an intermediate period between the two harvests, a large biomass amount was removed, aiming to give the new architecture to yerba-mate plants and make possible mechanical harvesting.
The stochastic behavior of vegetative buds must be highly related to biomass production, which depends on the environment and plant architecture. Pruning promotes traumatic reiterations, a natural process that allows the plant to fully and partially modify its architecture [9], but also increases the growth of the remaining unpruned axes [20]. The asynchronism observed among the yerba-mate individuals may also result from heterogeneous initial stages of the branches, after drastic pruning. Branches that emerge after severe pruning of large branches, also called epicormic, generally develop more than other branches, such as peach and almond trees [10], behavior also observed in this study. The height of the trunk affected the vegetative development, which was observed by Stuepp et al. 2016 [11].
There is an interest in the replacement of trees with strong apical dominance by smaller and more productive trees. Yerba-mate plants show a moderate to strong apical dominance. Another strategy employed aiming to release apical dominance in yerba-mate crops was treating the nursery-grown seedlings with a synthetic cytokinin benzyladenine, which produced an increase in bud swelling and branching [21]. The pruning method is another strategy able to control the apical dominance of the plants, which was explored in this investigation. The pruning can also interfere with the plant architecture, as well as the production of biomass. The pruning of plants with strong apical dominance affects the hormonal-mediated stimulation of dormant buds, which yields an intensive ramification [22].
After the pruning, the tree’s reaction will be to recompose the original foliage, through dormant epicormic buds placed in the trunk bark. This fact is related to the hormonal levels in the plant, since auxin is the hormone that promotes apical growth and its biosynthesis occurs mainly in tissues with rapid cell division, especially in the aerial parts. With radical coppicing, the number of apical buds was reduced and, consequently, the auxin flow may have decreased, producing a reduction in the apical dormancy. This effect may have contributed to the stimulation of lateral branches [19,23].
It should be noticed that the investigation reported here was performed on yerba-mate plants that originated from seedlings and not from clones. This condition contributes to the genetic and morphological variability of the plants and, consequently, to the non-uniformity of the vegetative behavior and the data variability.
Since plant architecture is a result of genes and environmental effects, we explored in this research the role of pruning conditions on plant architecture. In the current time the condition which involved the cutting at 40 cm above the soil, appears to be the most promising regarding the yield and branches’ emergence. However, the morphological behavior of the plants needs further investigation in order to obtain crops in which mechanical harvest can be implemented.
5. Conclusions
The experimental design used in this study allowed the recovery of vigor and vegetative capacity of yerba-mate plants, with a high rate of survival. In the first two years of monitoring, the height of coppicing was the most important variable with an effect on buds’ emergence and vigor. In weak yerba-mate crops, recovery pruning may be a promising alternative for the resumption of vegetative strength and stimulation of branches’ emergence.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/crops3010004/s1; The temperatures and rainfalls of the two vegetative periods investigated are available as an Excel file (Table S1).
Author Contributions
A.T.V.: Conceptualization, Methodology, Data analysis, Investigation, Writing—original draft, Writing review and editing. E.M.: Conceptualization, Methodology, Data analysis, Writing—review and editing—original draft. J.C.: Methodology, Data analysis, Investigation. I.L.G.: Data analysis, Writing—review and editing—original draft. E.N.B.: Methodology, Investigation. R.L.C.: Conceptualization, Methodology, Writing—original draft, Writing review and editing. E.M.Z.: Conceptualization, Methodology, Data analysis, Investigation, Writing—original draft, Writing review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors are thankful to Saulo Madalozzo for the data of climatic monitoring (https://saulo.madalozzo.it/).
Conflicts of Interest
The authors declare to have no conflict of interest.
References
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Delalibera Finzer, J.R. Chemistry, pharmacology and new trends in traditional functional and medicinal beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef] [PubMed]
- IBGE-SIDRA, Produção Agrícola Municipal—PEVS—Produção da Extração Vegetal e da Silvicultura. 2020. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9105-producao-da-extracao-vegetal-e-da-silvicultura.html?=&t=destaques (accessed on 1 July 2022).
- Lewinski, C.S.; Gonçalves, I.L.; Piovezan Borges, A.C.; Dartora, N.; de Souza, L.M.; Valduga, A.T. Effects of UV light on the physic-chemical properties of yerba-mate. Nutr. Food Sci. 2015, 45, 221–228. [Google Scholar] [CrossRef]
- Schuler, H.R.; Alarcon, G.G.; Joner, F.; dos Santos, K.L.; Siminski, A.; Siddique, I. Ecosystem services from ecological agroforestry in Brazil: A systematic map of scientific evidence. Land 2022, 11, 83. [Google Scholar] [CrossRef]
- Schmalko, M.E.; Prat Krikum, S.D.; Kanzi, R.G. La Yerba Mate Tecnología de la Producción y Propiedades; EdUNaM: Missiones, Argentina, 2015. [Google Scholar]
- Penteado Junior, J.; Goulart, I.D.R. Poda em erva-mate plantada. In Embrapa Florestas; Embrapa: Colombo, Brazil, 2017; 28p. [Google Scholar]
- Guedes, J.V.C.; d’Avila, M.; Dornelles, S.H.B. Behavior of Hedypathes betulinus (Klug, 1825) on the Paraguay tea plants. Cienc. Rural 2000, 30, 1059–1061. [Google Scholar] [CrossRef]
- Andrade, S.M.M.; Szczerbowski, D.; Vidal, D.M.; Allison, J.D.; Zarbin, P.H.G. Mate recognition by the green mate borer, Hedypathes betulinus (coleoptera: Cerambycidae): The role of cuticular compounds. J. Insect Behav. 2019, 32, 120–133. [Google Scholar] [CrossRef]
- Hallé, F.; Oldeman, R.A.; Tomlinson, P.B. Tropical Trees and Forests: An Architectural Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Guédon, Y.; Costes, E.; Rakocevic, M. Modulation of the yerba-mate metamer production phenology by the cultivation system and the climatic factors. Ecol. Model. 2018, 384, 188–197. [Google Scholar] [CrossRef]
- Stuepp, C.A.; Bitencourt, J.D.; Wendling, I.; Koehler, H.S.; Zuffellato-Ribas, K.C. Epicormic shoot induction through girdling and coppicing in ‘erva-mate’ trees. Ciência Florest. 2016, 26, 1009–1022. [Google Scholar] [CrossRef]
- Santin, D.; Wendling, I.; Benedetti, E.L.; Brondani, G.E.; Reissmann, C.B.; Morandi, D.; Roveda, L.F. Pruning and bark girdling or erva-mate (Ilex paraguariensis) aiming induction of basal sproutings. Pesqui. Florest. Bras. 2008, 56, 97–104. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Rakocevic, M.; Medrado, M.J.S.; Martim, S.F.; Assad, E.D. Sexual dimorphism and seasonal changes of leaf gas exchange in the dioecious tree Ilex paraguariensis grown in two contrasted cultivation types. Ann. Appl. Biol. 2009, 154, 291–301. [Google Scholar] [CrossRef]
- Rinne, P.L.; Kaikuranta, P.M.; van der Schoot, C. The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. Plant J. 2001, 26, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, E.; Beauchêne, J.; de la Vallée, B.L.; Ruelle, J.; Mangenet, T.; Heuret, P. Dating branch growth units in a tropical tree using morphological and anatomical markers: The case of Parkia velutina Benoist (Mimosoïdeae). Ann. For. Sci. 2012, 69, 543–555. [Google Scholar] [CrossRef]
- Greathouse, D.C.; Laetsch, W.M.; Phinney, B.O. The Shoot-Growth Rhythm of a Tropical Tree, Theobroma cacao. Am. J. Bot. 1971, 58, 281–286. [Google Scholar] [CrossRef]
- Medrado, J.S.M.; Dalzoto, D.N.; Olizeski, A.; Mosele, S.H. Recuperação de ervais degradados. Embrapa Florestas-Comunicado Técnico; Embrapa: Colombo, Brazil, 2002. [Google Scholar]
- Meier, A.R.; Saunders, M.R.; Michler, C.H. Epicormic buds in trees: A review of bud establishment, development and dormancy release. Tree Physiol. 2012, 32, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Fumey, D.; Lauri, P.; Guédon, Y.; Godin, C.; Costes, E. How young trees cope with removal of whole or parts of shoots: An analysis of local and distant responses to pruning in 1-year-old apple (Malus × domestica; Rosaceae) trees. Am. J. Bot. 2011, 98, 1737–1751. [Google Scholar] [CrossRef] [PubMed]
- Sansberro, P.; Mroginski, L.; Bottini, R. Stimulation of lateral branch formation on Ilex paraguariensis (Aquifoliaceae) seedlings. Aust. J. Exp. Agric. 2006, 46, 707–710. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Leyser, O. Regulation of shoot branching by auxin. Trends Plant Sci. 2003, 8, 541–545. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).