Brassica carinata Seed Meal as Soil Amendment and Potential Biofumigant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Seed Meal
2.2. Experimental Set Up
2.3. Soil Organic Carbon Analysis
2.4. Soil Basal Respiration
2.5. Enzymatic Activities
2.6. Volatiles Compounds Analysis
2.7. Bacterial and Fungal Populations Investigation
2.8. Statistical Analysis
3. Results and Discussions
3.1. Soil Structure
3.2. Soil pH
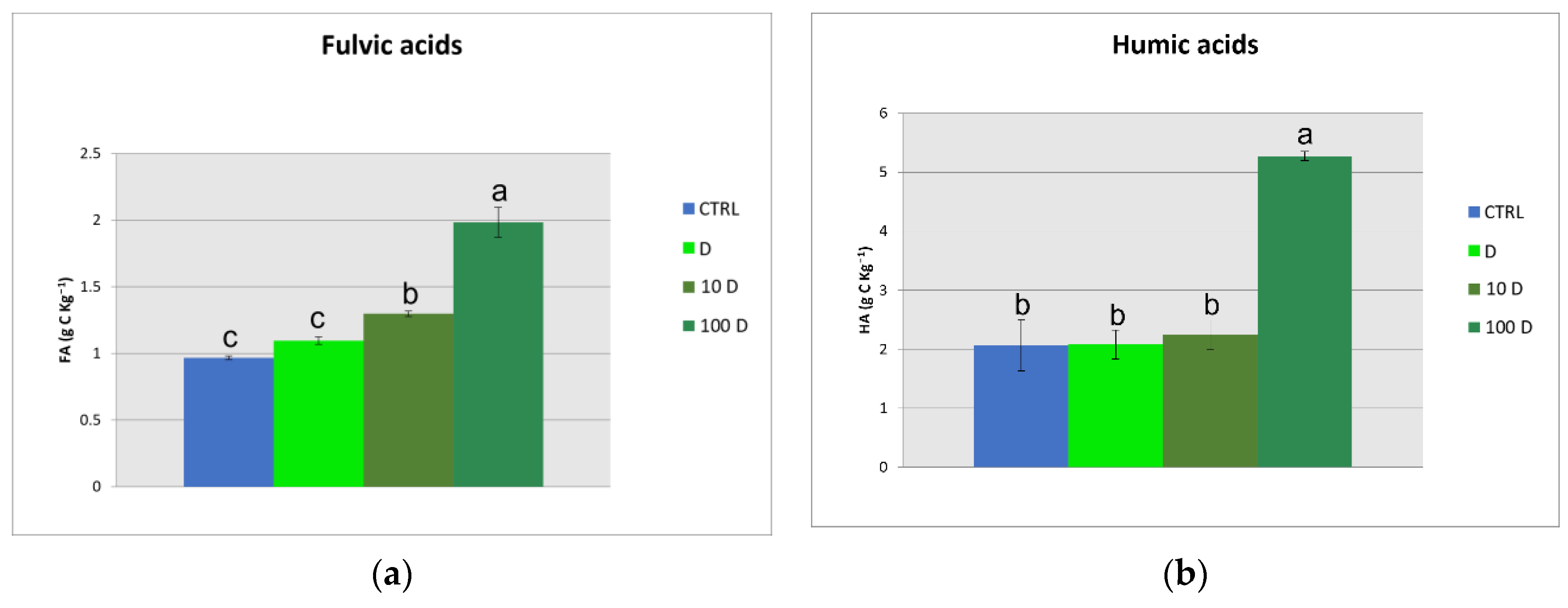
3.3. Soil Organic Carbon
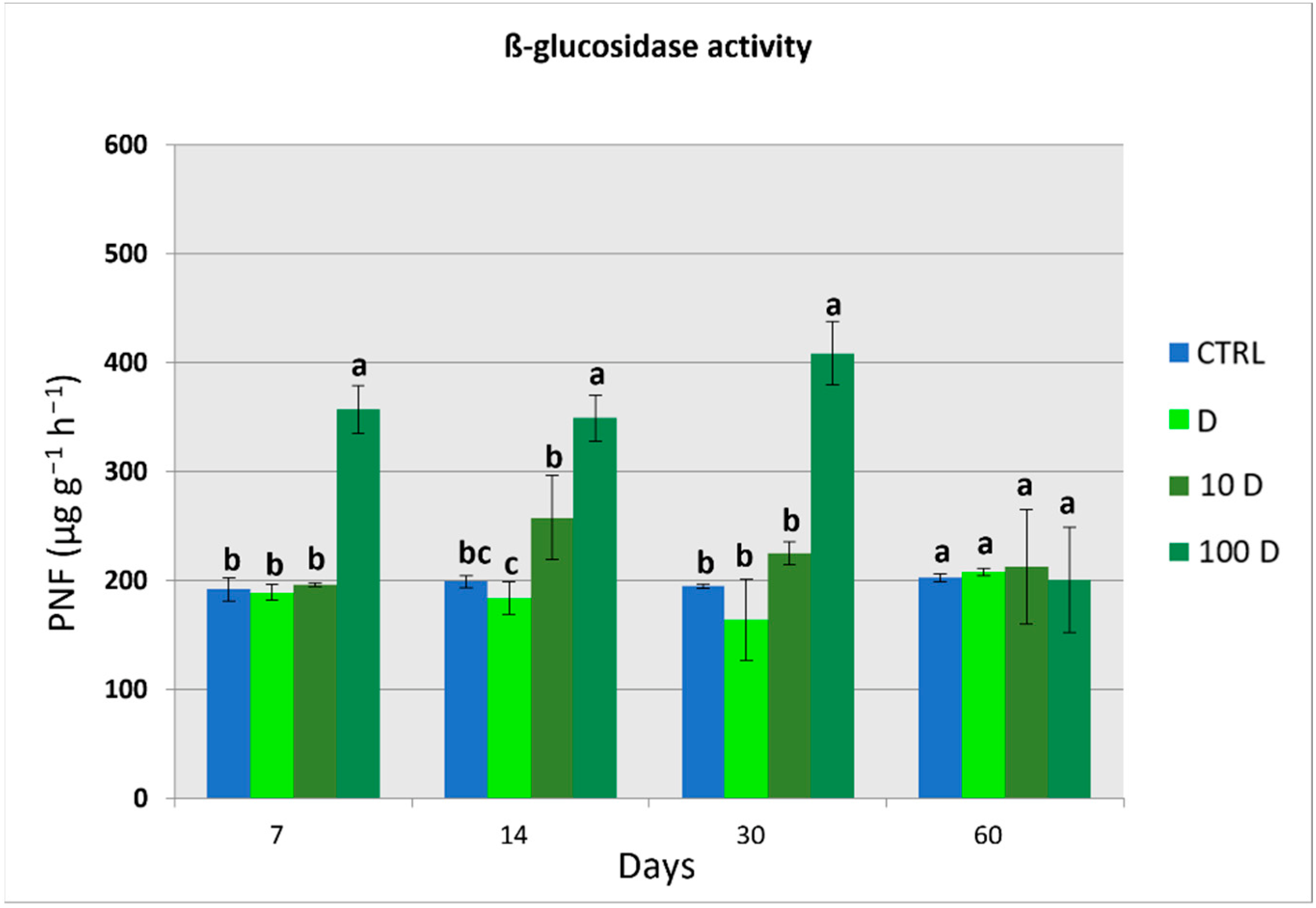
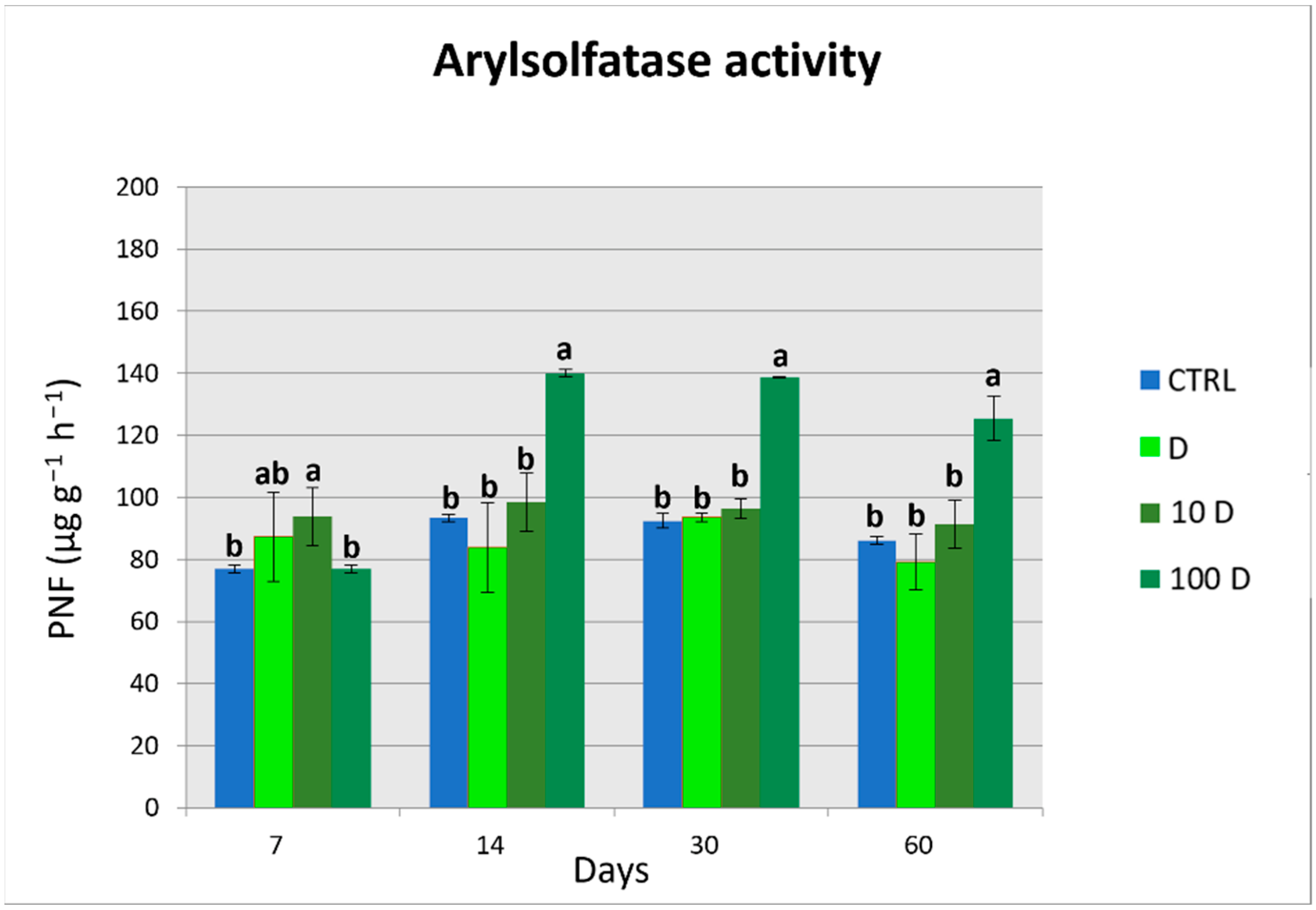
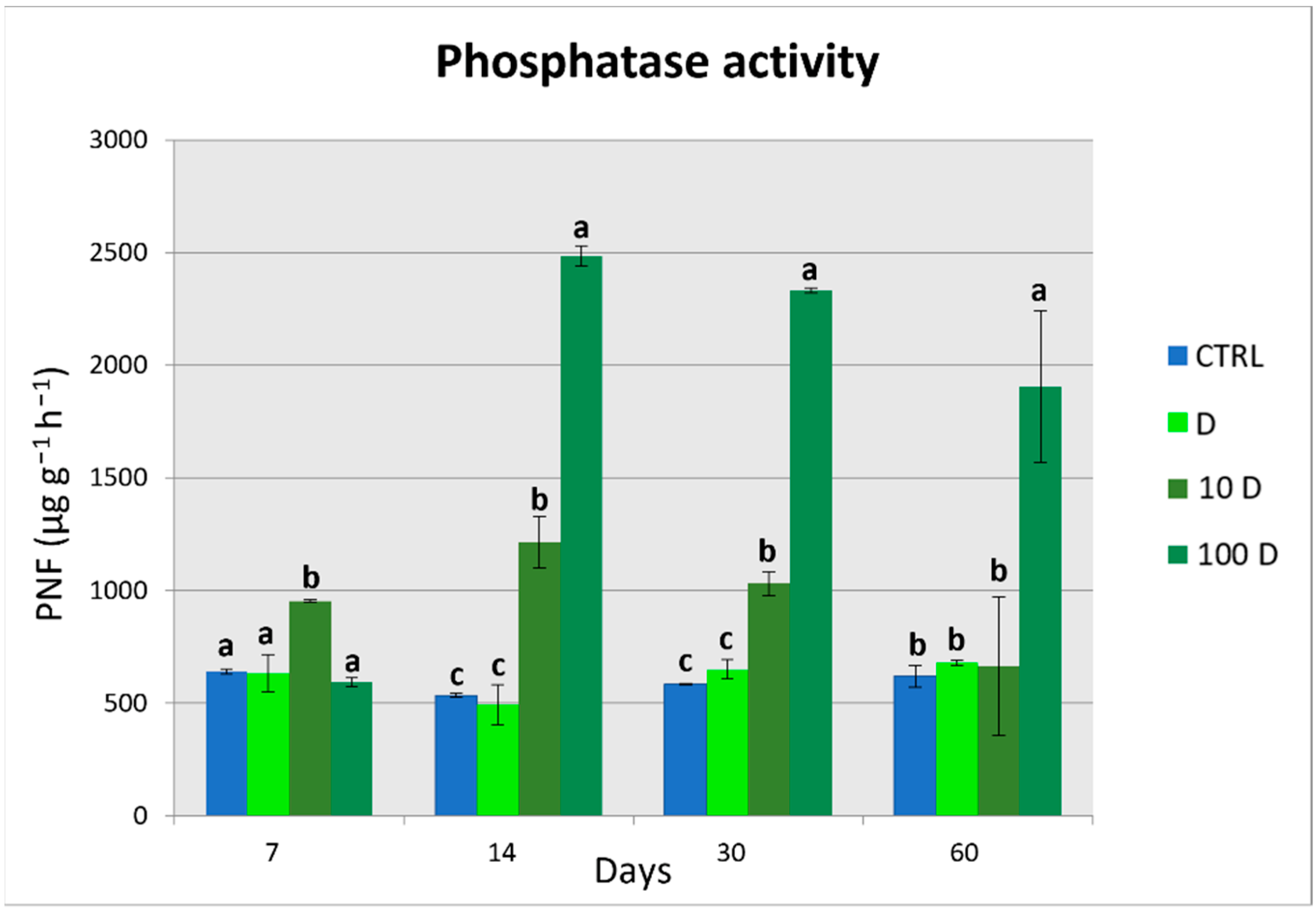
3.4. Soil Enzymatic Activities
3.5. Biofumigant Effect of Brassica Seed Meal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- IFOAM. General Assembly. 2008. Available online: https://www.ifoam.bio/ (accessed on 20 May 2022).
- Popa, M.E.; Mitelut, A.C.; Popa, E.E.; Stan, A.; Popa, V.I. Organic foods contribution to nutritional quality and value. Trends Food Sci. Technol. 2019, 84, 15–18. [Google Scholar] [CrossRef]
- Meemken, E.M.; Qaim, M. Organic Agriculture, Food Security, and the Environment. Annu. Rev. Resour. Econ. 2018, 10, 39–63. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.; Alvarez, R. A review of nitrogen fertilizer and conservation tillage effects on soil organic carbon storage. Soil Use Manag. 2005, 21, 38–52. [Google Scholar] [CrossRef]
- Celestina, C.; Hunt, J.R.; Sale, P.W.G.; Franks, A.E. Attribution of crop yield responses to application of organic amendments: A critical review. Soil Tillage Res. 2019, 186, 135–145. [Google Scholar] [CrossRef]
- Röös, E.; Mie, A.; Wivstad, M.; Salomon, E.; Johansson, B.; Gunnarsson, S.; Wallenbeck, A.; Hoffmann, R.; Nilsson, U.; Sundberg, C.; et al. Risks and opportunities of increasing yields in organic farming. A review. Agron. Sustain. Dev. 2018, 38, 14. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Jan, M.T.; Afzal, M.; Muhammad, I.; Jan, A.; Shah, Z. An integrated approach using organic amendments under a range of tillage practices to improve wheat productivity in a cereal based cropping system. Int. J. Agric. Biol. 2015, 17, 467–474. [Google Scholar] [CrossRef]
- Jahromi, H.; Adhikari, S.; Roy, P.; Hassani, E.; Pope, C.; Oh, T.S.; Karki, Y. Production of green transportation fuels from Brassica carinata oil: A comparative study of noble and transition metal catalysts. Fuel Process. Technol. 2021, 215, 106737. [Google Scholar] [CrossRef]
- Del Gatto, A.; Melilli, M.G.; Raccuia, S.A.; Pieri, S.; Mangoni, L.; Pacifico, D.; Signor, M.; Duca, D.; Foppa Pedretti, E.; Mengarelli, C. A comparative study of oilseed crops (Brassica napus L. subsp. oleifera and Brassica carinata A. Braun) in the biodiesel production chain and their adaptability to different Italian areas. Ind. Crops Prod. 2015, 75, 98–107. [Google Scholar] [CrossRef]
- Duca, D.; Toscano, G.; Riva, G.; Mengarelli, C.; Rossini, G.; Pizzi, A.; Del Gatto, A.; Pedretti, E.F. Quality of residues of the biodiesel chain in the energy field. Ind. Crops Prod. 2015, 75, 91–97. [Google Scholar] [CrossRef]
- Bardi, L.; Rosso, F. Extraction and characterization of brassinosteroids from residues of the biodiesel chain. Ind. Crops Prod. 2015, 75, 24–28. [Google Scholar] [CrossRef]
- Del Gatto, A.; Pieri, S.; Mangoni, L.; Di Candilo, M.; Diozzi, M.; De Mastro, G.; Grassano, N.; Signor, M.; Barbiani, G.; Carboni, M. Scegliere le varietà energetiche di colza. L’Informatore Agrar. 2010, 33, 57–63. [Google Scholar]
- Seepaul, R.; Kumar, S.; Iboyi, J.E.; Bashyal, M.; Stansly, T.L.; Bennett, R.; Boote, K.J.; Mulvaney, M.J.; Small, I.M.; George, S.; et al. Brassica carinata: Biology and agronomy as a biofuel crop. GCB Bioenergy 2021, 13, 582–599. [Google Scholar] [CrossRef]
- Gesch, R.W.; Isbell, T.A.; Oblath, E.A.; Allen, B.L.; Archer, D.W.; Brown, J.; Hatfield, J.L.; Jabro, J.D.; Kiniry, J.R.; Long, D.S.; et al. Comparison of several Brassica species in the north central U.S. for potential jet fuel feedstock. Ind. Crops Prod. 2015, 75, 2–7. [Google Scholar] [CrossRef]
- Vicente, J.G.; Taylor, J.D.; Sharpe, A.G.; Parkin, I.A.P.; Lydiate, D.J.; King, G.J. Inheritance of race-specific resistance to Xanthomonas campestris pv. campestris in Brassica genomes. Phytopathology 2002, 92, 1134–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonguç, M.; Griffiths, P.D. Transfer of powdery mildew resistance from Brassica carinata to Brassica oleracea through embryo rescue. Plant Breed. 2004, 123, 587–589. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G.; Del Gatto, A.; Pieri, S.; Mangoni, L.; Signor, M.; Foppa Pedretti, E.; Toscano, G.; Mengarelli, C.; Duca, D. Morphological, productive and energetic characterization of Brassica carinata in central, north and south areas of Italy. Acta Hortic. 2013, 1005, 419–425. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Vettraino, A.M.; Vannini, A. Efficacy of biofumigation with Brassica carinata commercial pellets (BioFence) to control vegetative and reproductive structures of Phytophthora cinnamomi. Plant Dis. 2016, 100, 324–330. [Google Scholar] [CrossRef] [Green Version]
- DuPont, S.T.; Hewavitharana, S.S.; Mazzola, M. Field scale application of Brassica seed meal and anaerobic soil disinfestation for the control of apple replant disease. Appl. Soil Ecol. 2021, 166, 104076. [Google Scholar] [CrossRef]
- Campanella, V.; Mandalà, C.; Angileri, V.; Miceli, C. Management of common root rot and Fusarium foot rot of wheat using Brassica carinata break crop green manure. Crop Prot. 2020, 130, 105073. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; de Souza Abboud, A.C.; do Carmo, M.G.F. Biofumigation with species of the Brassicaceae family: A review. Ciência Rural 2020, 51. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Villecco, D.; Celano, G.; Scotti, R. Soil amendment with seed meals: Short term effects on soil respiration and biochemical properties. Appl. Soil Ecol. 2013, 72, 225–231. [Google Scholar] [CrossRef]
- Soria, R.; Ortega, R.; Bastida, F.; Miralles, I. Role of organic amendment application on soil quality, functionality and greenhouse emission in a limestone quarry from semiarid ecosystems. Appl. Soil Ecol. 2021, 164, 103925. [Google Scholar] [CrossRef]
- Magdoff, F.; van Es, H. Building Soils for Better Crops: Sustainable Soil Management; Handbook Series Book 10; Sustainable Agriculture Research and Education: Waldorf, MD, USA, 2009. [Google Scholar]
- Larkin, R.P. Soil Health Paradigms and Implications for Disease Management. Annu. Rev. Phytopathol. 2015, 53, 199–221. [Google Scholar] [CrossRef]
- Tagele, S.B.; Kim, R.H.; Shin, J.H. Interactions between Brassica Biofumigants and Soil Microbiota: Causes and Impacts. J. Agric. Food Chem. 2021, 69, 11538–11553. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J. Plant Growth Regul. 2019, 38, 343–356. [Google Scholar] [CrossRef]
- Chhajed, S.; Misra, B.B.; Tello, N.; Chen, S. Chemodiversity of the glucosinolate-myrosinase system at the single cell type resolution. Front. Plant Sci. 2019, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and Subcellular Organization of the Glucosinolate–Myrosinase System against Herbivores and Pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef]
- Mithen, R.F.; Dekker, M.; Verkerk, R.; Rabot, S.; Johnson, I.T. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J. Sci. Food Agric. 2000, 80, 967–984. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Heaney, R.K. Glucosinolates and their breakdown products in cruciferous crops, foods and feedingstuffs. Food Chem. 1983, 11, 249–271. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Giovannini, D.; Brandi, F.; Lanteri, A.P.; Lazzeri, L.; Maltoni, M.L.; Matteo, R.; Minuto, A.; Sbrighi, P.; Stagno, F.; Baruzzi, G. Non-chemical soil fumigation for sustainable strawberry production in southern Italy. Agronomy 2021, 11, 1678. [Google Scholar] [CrossRef]
- Manici, L.M.; Lazzeri, L.; Palmieri, S. In Vitro Fungitoxic Activity of Some Glucosinolates and Their Enzyme-Derived Products toward Plant Pathogenic Fungi. J. Agric. Food Chem. 1997, 45, 2768–2773. [Google Scholar] [CrossRef]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of glucosinolate-derived isothiocyanates on fungi: A comprehensive review on direct effects, mechanisms, structure-activity relationship data and possible agricultural applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Borek, V.; Elberson, L.R.; McCaffrey, J.P.; Morra, M.J. Toxicity of rapeseed meal and methyl isothiocyanate to larvae of the black vine weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 1997, 90, 109–112. [Google Scholar] [CrossRef]
- Lazzeri, L.; Curto, G.; Dallavalle, E.; D’Avino, L.; Malaguti, L.; Santi, R.; Patalano, G. Nematicidal efficacy of biofumigation by defatted Brassicaceae meal for control of Meloidogyne incognita (Kofoid et White) Chitw. on a full field zucchini crop. J. Sustain. Agric. 2009, 33, 349–358. [Google Scholar] [CrossRef]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-hashem, M.; El-domiaty, M. Glucosinolate Biosynthesis and the Glucosinolate–Myrosinase System in Plant Defense Shweta. Agronomy 2020, 10, 12–14. [Google Scholar] [CrossRef]
- Furlan, L.; Bonetto, C.; Finotto, A.; Lazzeri, L.; Malaguti, L.; Patalano, G.; Parker, W. The efficacy of biofumigant meals and plants to control wireworm populations. Ind. Crops Prod. 2010, 31, 245–254. [Google Scholar] [CrossRef]
- Galletti, S.; Sala, E.; Leoni, O.; Burzi, P.L.; Cerato, C. Trichoderma spp. tolerance to Brassica carinata seed meal for a combined use in biofumigation. Biol. Control 2008, 45, 319–327. [Google Scholar] [CrossRef]
- Hollister, E.B.; Hu, P.; Wang, A.S.; Hons, F.M.; Gentry, T.J. Differential impacts of brassicaceous and nonbrassicaceous oilseed meals on soil bacterial and fungal communities. FEMS Microbiol. Ecol. 2013, 83, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.F.; Yamasaki, H.; Mazzola, M. Brassica napus seed meal soil amendment modifies microbial community structure, nitric oxide production and incidence of Rhizoctonia root rot. Soil Biol. Biochem. 2005, 37, 1215–1227. [Google Scholar] [CrossRef]
- Ren, G.; Ma, Y.; Guo, D.; Gentry, T.J.; Hu, P.; Pierson, E.A.; Gu, M. Soil bacterial community was changed after Brassicaceous seed meal application for suppression of Fusarium wilt on pepper. Front. Microbiol. 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.X.; Gao, F.; Muhammad, I.; Huang, J.H.; Zhou, X.B. Effect of water conditions and nitrogen application on maize growth, carbon accumulation and metabolism of maize plant in subtropical regions. Arch. Agron. Soil Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Muhammad, I.; Lv, J.Z.; Yang, L.; Ahmad, S.; Farooq, S.; Zeeshan, M.; Zhou, X.B. Low irrigation water minimizes the nitrate nitrogen losses without compromising the soil fertility, enzymatic activities and maize growth. BMC Plant Biol. 2022, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F. Biochemical properties of soils under crop rotation. Appl. Soil Ecol. 2008, 39, 133–143. [Google Scholar] [CrossRef]
- Lakhdar, A.; Scelza, R.; Scotti, R.; Rao, M.A.; Jedidi, N.; Gianfreda, L.; Abdelly, C. The effect of compost and sewage sludge on soil biologic activities in salt affected soil. Rev. Cienc. Suelo Nutr. Veg. 2010, 10, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Lakhdar, A.; Scelza, R.; ben Achiba, W.; Scotti, R.; Rao, M.A.; Jedidi, N.; Abdelly, C.; Gianfreda, L. Effect of municipal solid waste compost and sewage sludge on enzymatic activities and wheat yield in a clayey-loamy soil. Soil Sci. 2011, 176, 15–21. [Google Scholar] [CrossRef]
- Monaci, E.; Angeletti, C.; Casucci, C.; Vischetti, C. Nitrogen release from pelletized poultry fertilizer in two soils: Influence of soil moisture and microbial biomass. Rev. Bras. Cienc. do Solo 2022, 46, e0210101. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Casucci, C.; Vischetti, C. Effect of recycling wood ash on microbiological and biochemical properties of soils. Agron. Sustain. Dev. 2006, 26, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Saini, R.; Singh, A.; Deb, S.K. Effect of seed meals on weed control and soil physical properties in direct-seeded pumpkin. Sustain. 2020, 12, 5811. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys, 2nd ed.; Natural Resources Conservation Service U.S. Departament Agriculture Handbook; CRC Press: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Angeletti, C.; Monaci, E.; Giannetta, B.; Polverigiani, S.; Vischetti, C. Soil organic matter content and chemical composition under two rotation management systems in a Mediterranean climate. Pedosphere 2021, 31, 903–911. [Google Scholar] [CrossRef]
- Serrano-Pérez, P.; De Santiago, A.; Rodríguez-Molina, M.D.C. Biofumigation with Pellets of Defatted Seed Meal of Brassica carinata: Factors Affecting Performance against Phytophthora nicotianae in Pepper Crops. Front. Sustain. Food Syst. 2021, 5, 4531. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Vischetti, C.; Coppola, L.; Monaci, E.; Mincarelli, L.; Casucci, C.; Taffi, M.; Agnelli, A. Effect of different fractions of organic carbon on adsorption of metalaxyl in waste organic substrates and soils. Fresenius Environ. Bull. 2013, 22, 200–206. [Google Scholar]
- Schnitzer, M. Organic matter characterization. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1982; pp. 581–594. [Google Scholar] [CrossRef]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Karbonate im Boden. Z. Pflanz. Düngung Bodenkd. 1952, 56, 26–38. [Google Scholar] [CrossRef]
- Bloem, J.; Hopkins, D.W.; Benedetti, A. Microbiological Methods for Assessing Soil Quality; CABI: Oxfordshire, UK, 2005; Volume 79, ISBN 0851990983. [Google Scholar]
- Italian Official Gazette. Approvazione dei Metodi Ufficiali di Analisi Chimica del Suolo; Italian Official Gazette: Rome, Italy, 2004. [Google Scholar]
- Tabatabai, M.A.; Soil Enzymes, M. Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; Weaver, R.W., Angel, J.S., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. ISBN 9780891188650. [Google Scholar]
- Juma, N.G.; Tabatabai, M.A. Effects of trace elements on β-glucosaminidase activity in soils. Soil Sci. Soc. Am. J. 1997, 41, 343–346. [Google Scholar] [CrossRef]
- Klose, S.; Tabatabai, M.A. Urease activity of microbial biomass in soils. Soil Biol. Biochem. 1999, 31, 205–211. [Google Scholar] [CrossRef]
- Toscano, G.; Duca, D.; Amato, A.; Pizzi, A. Emission from realistic utilization of wood pellet stove. Energy 2014, 68, 644–650. [Google Scholar] [CrossRef]
- Clarkson, J.; Michel, V.; Neilson, R. Biofumigation for the Control of Soil-Borne Diseases. 2015. Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/9_eip_sbd_mp_biofumigation_final_0.pdf (accessed on 16 July 2021).
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Van Bemmelen, J. Über Die Bestimmung Des Wassers, Des Humus, Des Schwefels, Der in Den Colloïdalen Silikaten Gebundenen Kieselsäure, Des Mangans U. S. W. Im Ackerboden. Die Landwirthschaftlichen Vers.-Stn. 1890, 37, 279–290. [Google Scholar]
- Wang, A.S.; Hu, P.; Hollister, E.B.; Rothlisberger, K.L.; Somenahally, A.; Provin, T.L.; Hons, F.M.; Gentry, T.J. Impact of indian mustard (Brassica juncea) and flax (Linum usitatissimum) seed meal applications on soil carbon, nitrogen, and microbial dynamics. Appl. Environ. Soil Sci. 2012, 2012, 351609. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Feng, J.; Tang, M.; Zhu, B. Soil priming effect and its responses to nutrient addition along a tropical forest elevation gradient. Glob. Chang. Biol. 2021, 27, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Mazzoncini, M.; Antichi, D.; Tavarini, S.; Silvestri, N.; Lazzeri, L.; D’Avino, L. Effect of defatted oilseed meals applied as organic fertilizers on vegetable crop production and environmental impact. Ind. Crops Prod. 2015, 75, 54–64. [Google Scholar] [CrossRef]
- Chen, M.; Wang, C.; Wang, B.; Bai, X.; Gao, H.; Huang, Y. Enzymatic mechanism of organic nitrogen conversion and ammonia formation during vegetable waste composting using two amendments. Waste Manag. 2019, 95, 306–315. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Benedicto, S.; Lee, H.C.; Cook, H.F. Enzyme activity and C and N pools in soil following application of mulches. Can. J. Soil Sci. 2004, 84, 19–30. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Ronchi, V.; Bauer, C. Kinetic parameters of dehydrogenase in the assessment of the response of soil to vermicompost and inorganic fertilisers. Biol. Fertil. Soils 2000, 32, 479–483. [Google Scholar] [CrossRef]
- Albiach, R.; Canet, R.; Pomares, F.; Ingelmo, F. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioresour. Technol. 2000, 75, 43–48. [Google Scholar] [CrossRef]
- Crecchio, C.; Curci, M.; Mininni, R.; Ricciuti, P.; Ruggiero, P. Short-term effects of municipal solid waste compost amendments on soil carbon and nitrogen content, some enzyme activities and genetic diversity. Biol. Fertil. Soils 2001, 34, 311–318. [Google Scholar] [CrossRef]
- Ros, M.; Pascual, J.A.; Garcia, C.; Hernandez, M.T.; Insam, H. Hydrolase activities, microbial biomass and bacterial community in a soil after long-term amendment with different composts. Soil Biol. Biochem. 2006, 38, 3443–3452. [Google Scholar] [CrossRef]
- Fitzgerald, J.W. Sulfate ester formation and hydrolysis: A potentially important yet often ignored aspect of the sulfur cycle of aerobic soils. Bacteriol. Rev. 1976, 40, 698–721. [Google Scholar] [CrossRef] [PubMed]
- Dick, W.A.; Tabatabai, M. Kinetic parameters of phosphatases in soils and organic waste materials. Soil Sci. 1984, 137, 7–15. [Google Scholar] [CrossRef]
- Amador, J.A.; Glucksman, A.M.; Lyons, J.B.; Görres, J.H. Spatial distribution of soil phosphatase activity within a riparian forest1. Soil Sci. 1997, 162, 808–825. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Zofío, M.; Larregla, S.; Garbisu, C. Application of organic amendments followed by soil plastic mulching reduces the incidence of Phytophthora capsici in pepper crops under temperate climate. Crop Prot. 2011, 30, 1563–1572. [Google Scholar] [CrossRef]
- Lazzeri, L.; Leoni, O.; Manici, L.M.; Palmieri, S.; Patalano, G. Use of Seed Flour as Soil. Pesticide. Patent n. US 7.749.549, 6 July 2010. [Google Scholar]
- Zaccardelli, M.; De Nicola, F.; Villecco, D.; Scotti, R. The development and suppressive activity of soil microbial communities under compost amendment. J. Soil Sci. Plant Nutr. 2013, 13, 730–742. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaci, E.; Casucci, C.; De Bernardi, A.; Marini, E.; Landi, L.; Toscano, G.; Romanazzi, G.; Vischetti, C. Brassica carinata Seed Meal as Soil Amendment and Potential Biofumigant. Crops 2022, 2, 233-246. https://doi.org/10.3390/crops2030017
Monaci E, Casucci C, De Bernardi A, Marini E, Landi L, Toscano G, Romanazzi G, Vischetti C. Brassica carinata Seed Meal as Soil Amendment and Potential Biofumigant. Crops. 2022; 2(3):233-246. https://doi.org/10.3390/crops2030017
Chicago/Turabian StyleMonaci, Elga, Cristiano Casucci, Arianna De Bernardi, Enrica Marini, Lucia Landi, Giuseppe Toscano, Gianfranco Romanazzi, and Costantino Vischetti. 2022. "Brassica carinata Seed Meal as Soil Amendment and Potential Biofumigant" Crops 2, no. 3: 233-246. https://doi.org/10.3390/crops2030017
APA StyleMonaci, E., Casucci, C., De Bernardi, A., Marini, E., Landi, L., Toscano, G., Romanazzi, G., & Vischetti, C. (2022). Brassica carinata Seed Meal as Soil Amendment and Potential Biofumigant. Crops, 2(3), 233-246. https://doi.org/10.3390/crops2030017








