Noninvasive Screening of Basal Cell Carcinomas: A Comparison of the Structure and Physical Properties of Large and Small Nodular Lesions Using Vibrational OCT and Histopathology
Simple Summary
Abstract
1. Introduction
2. Methods
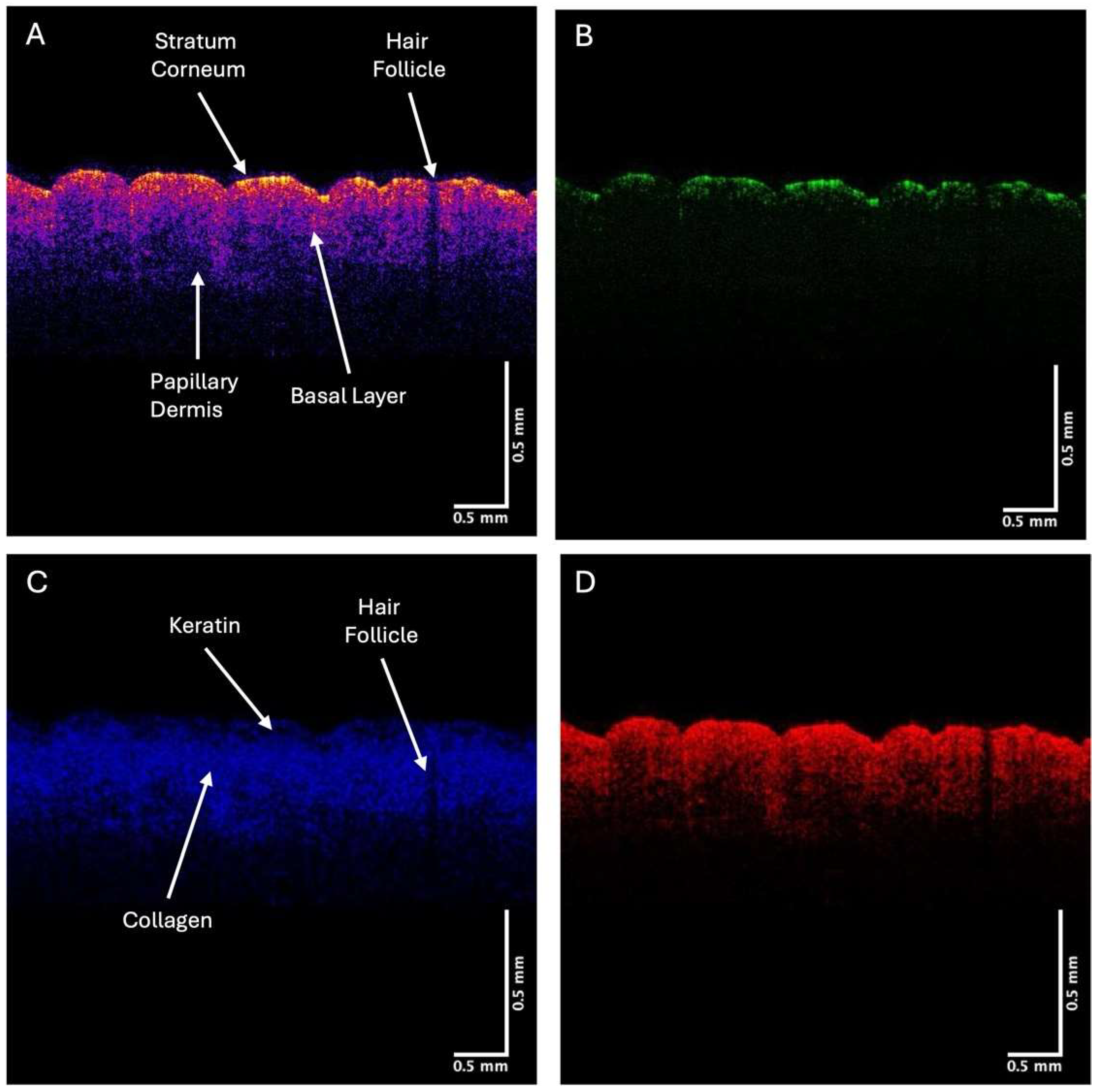
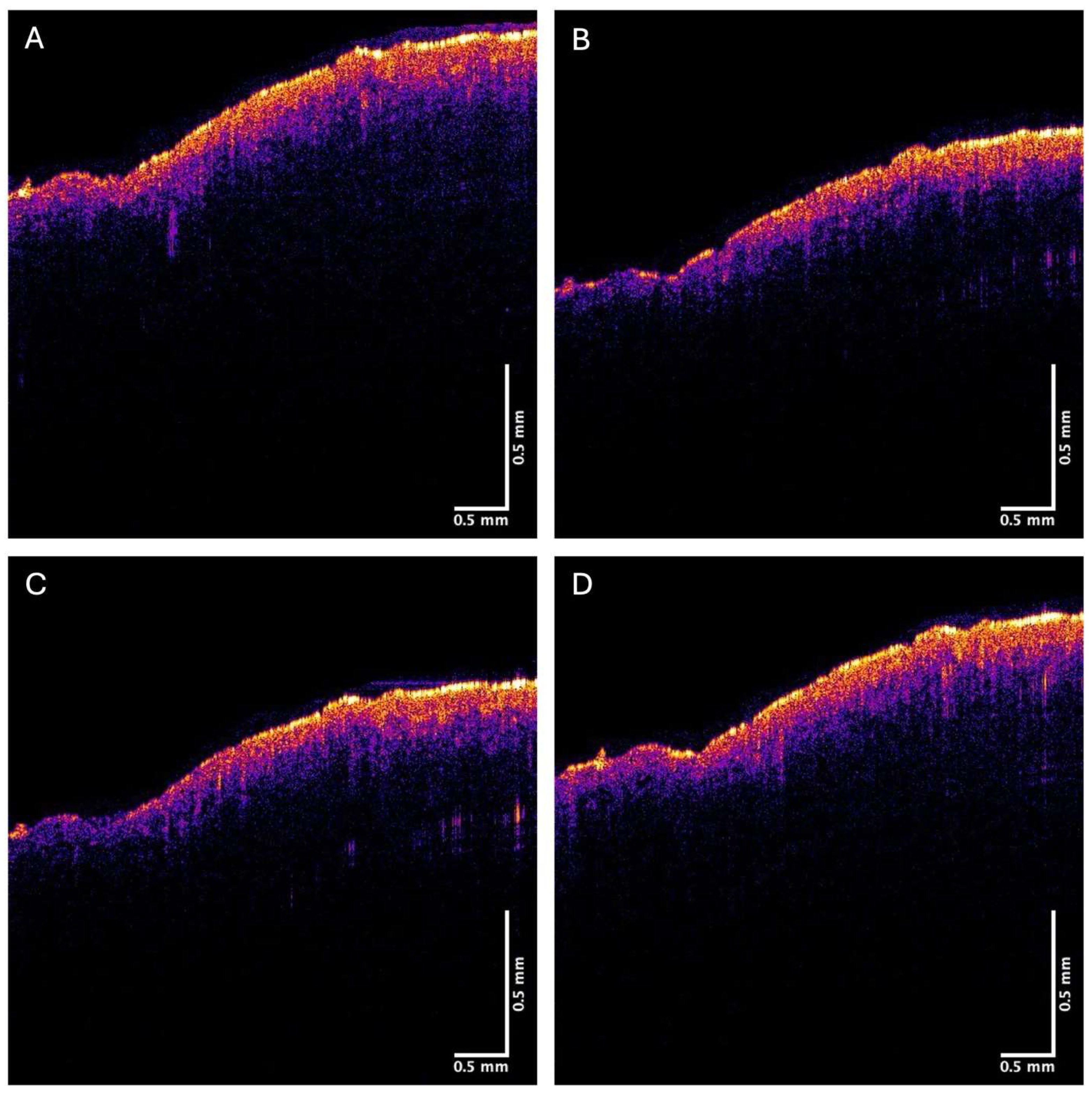
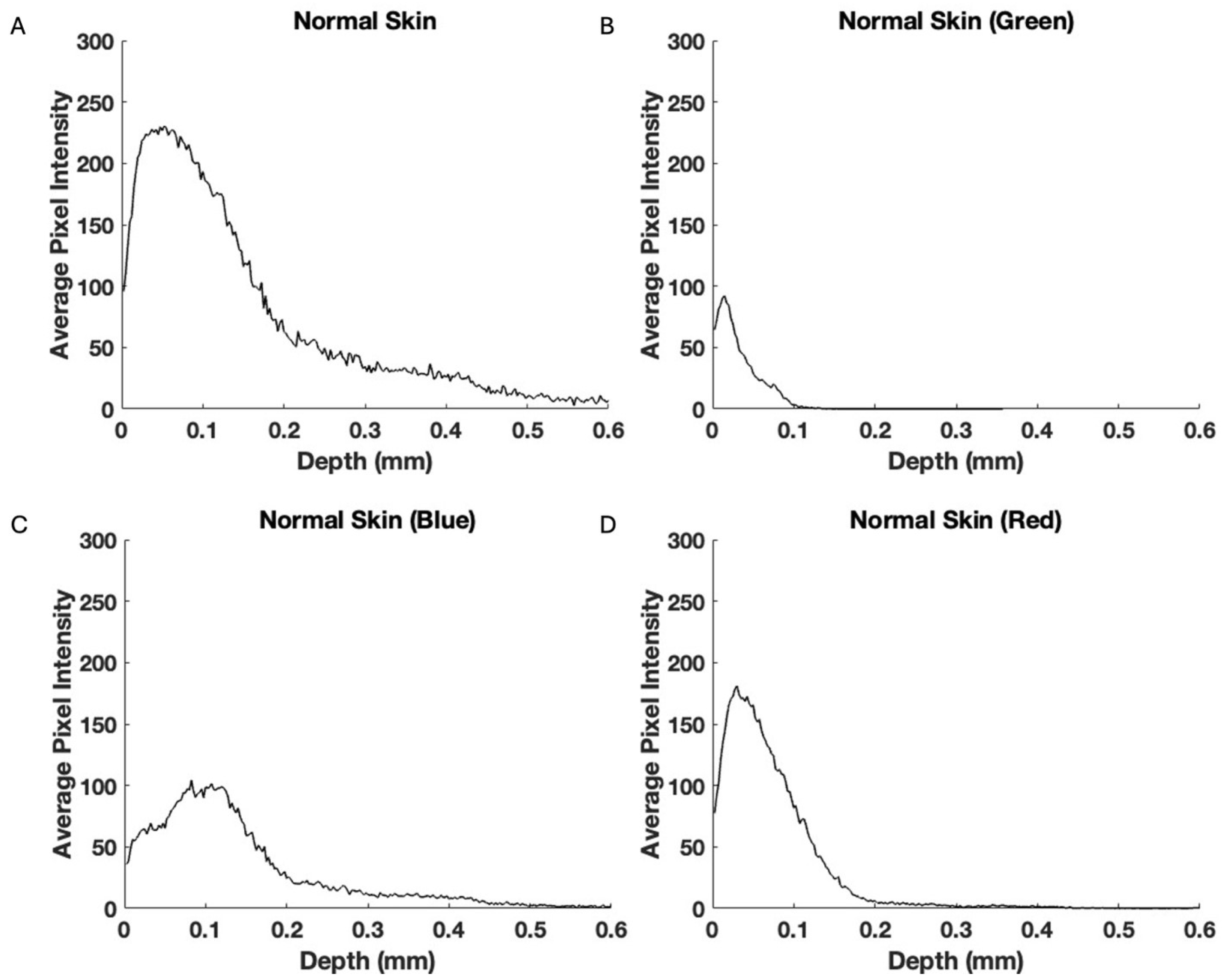
2.1. OCT Image Collection
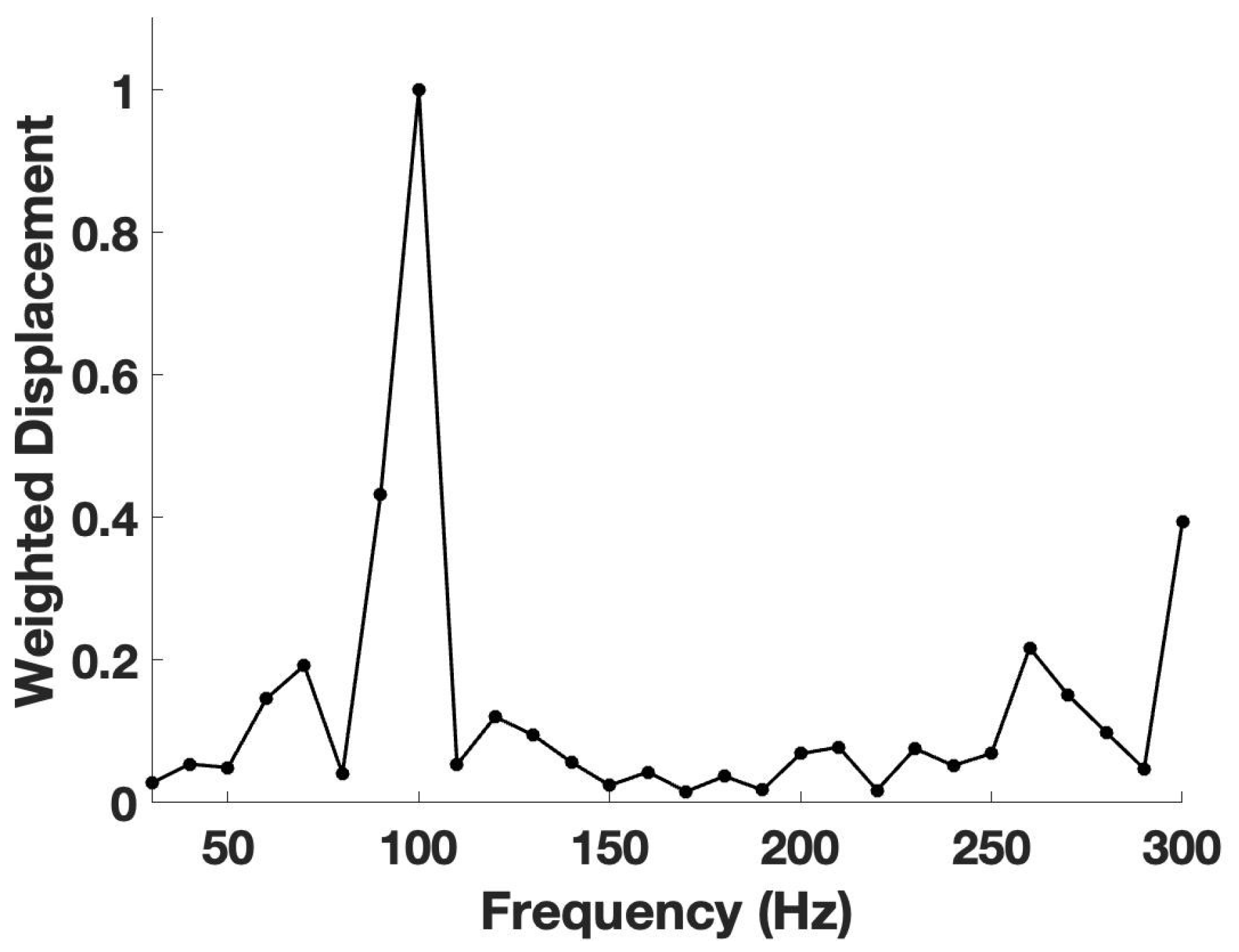
2.2. VOCT Measurements
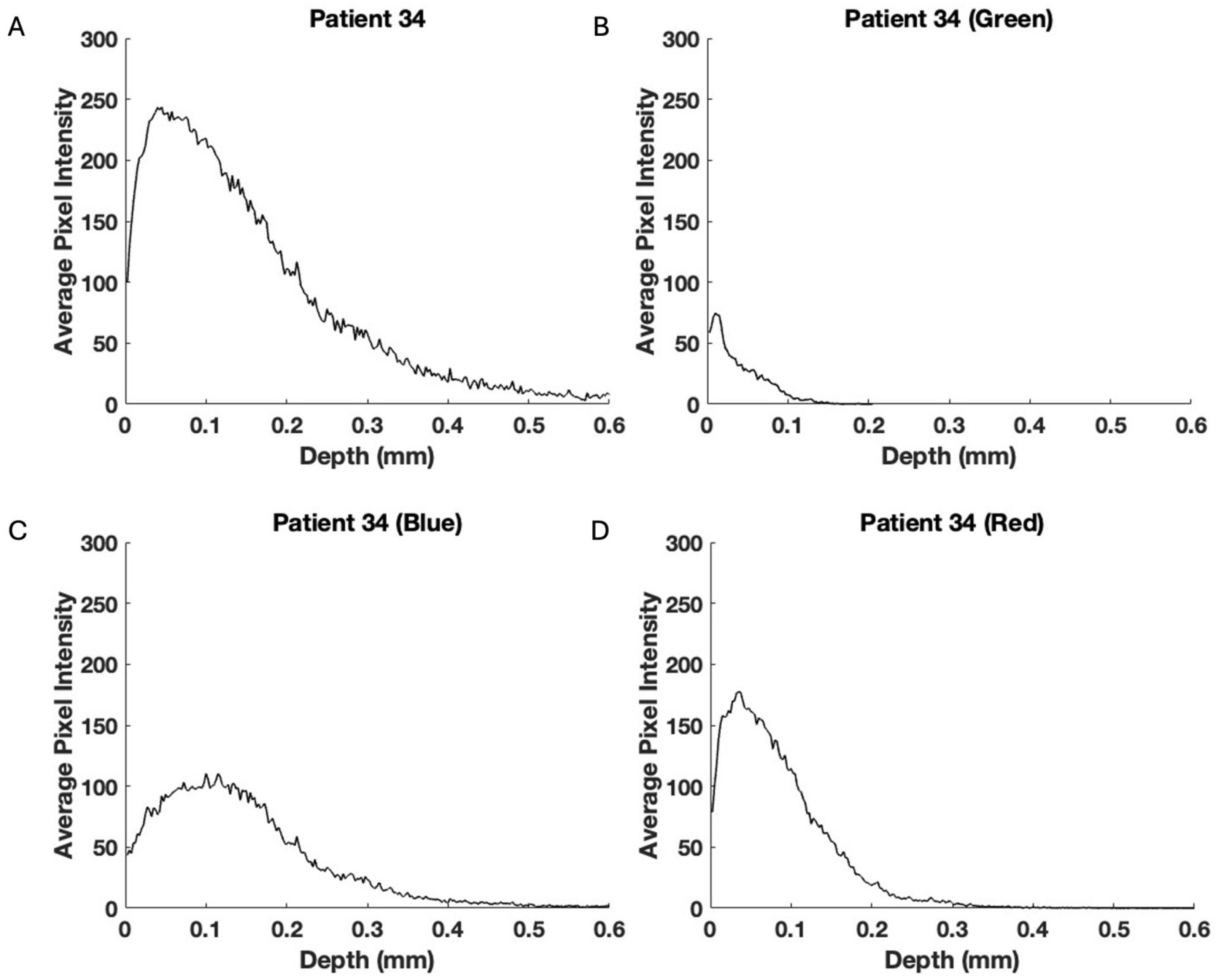
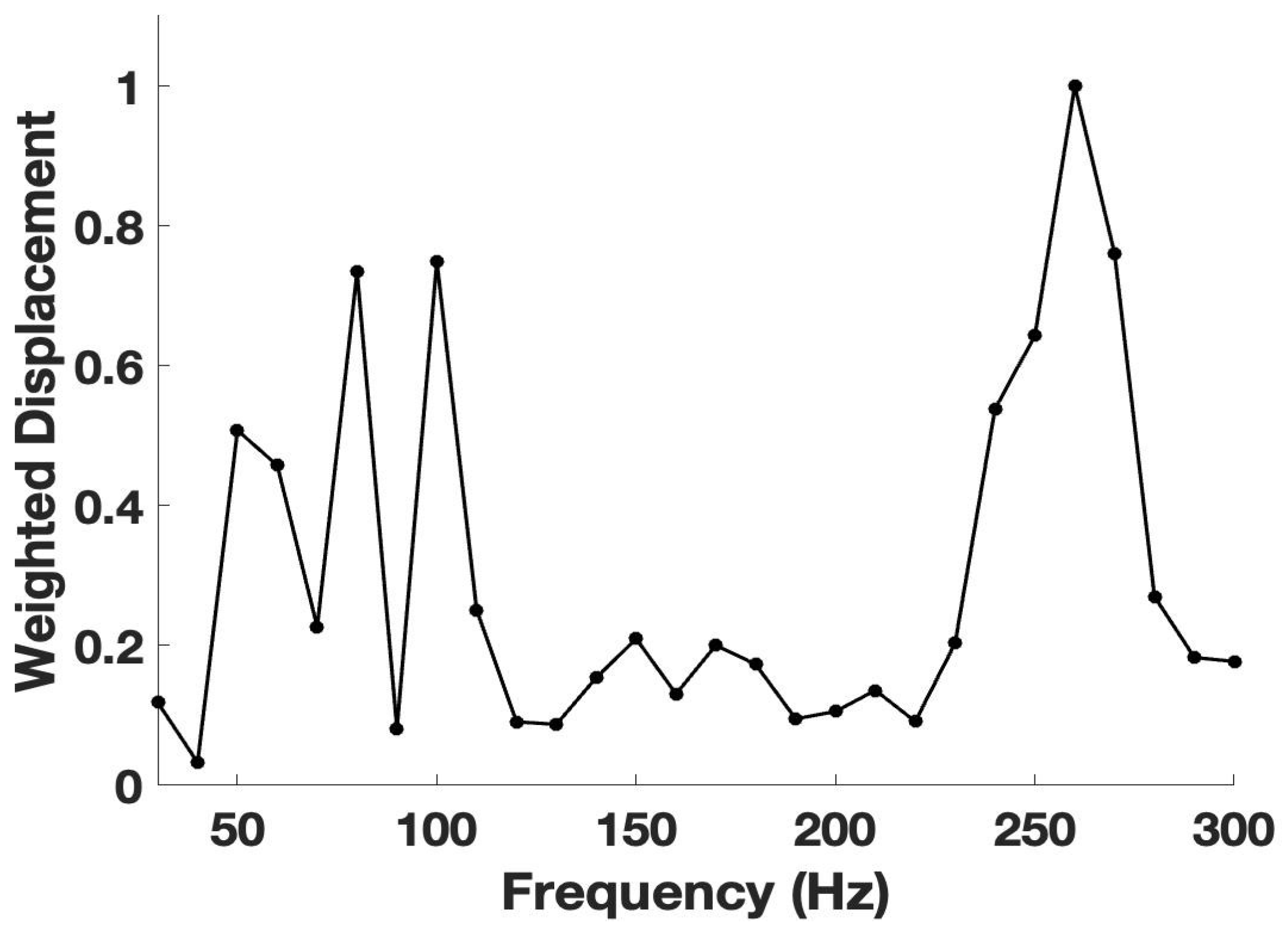
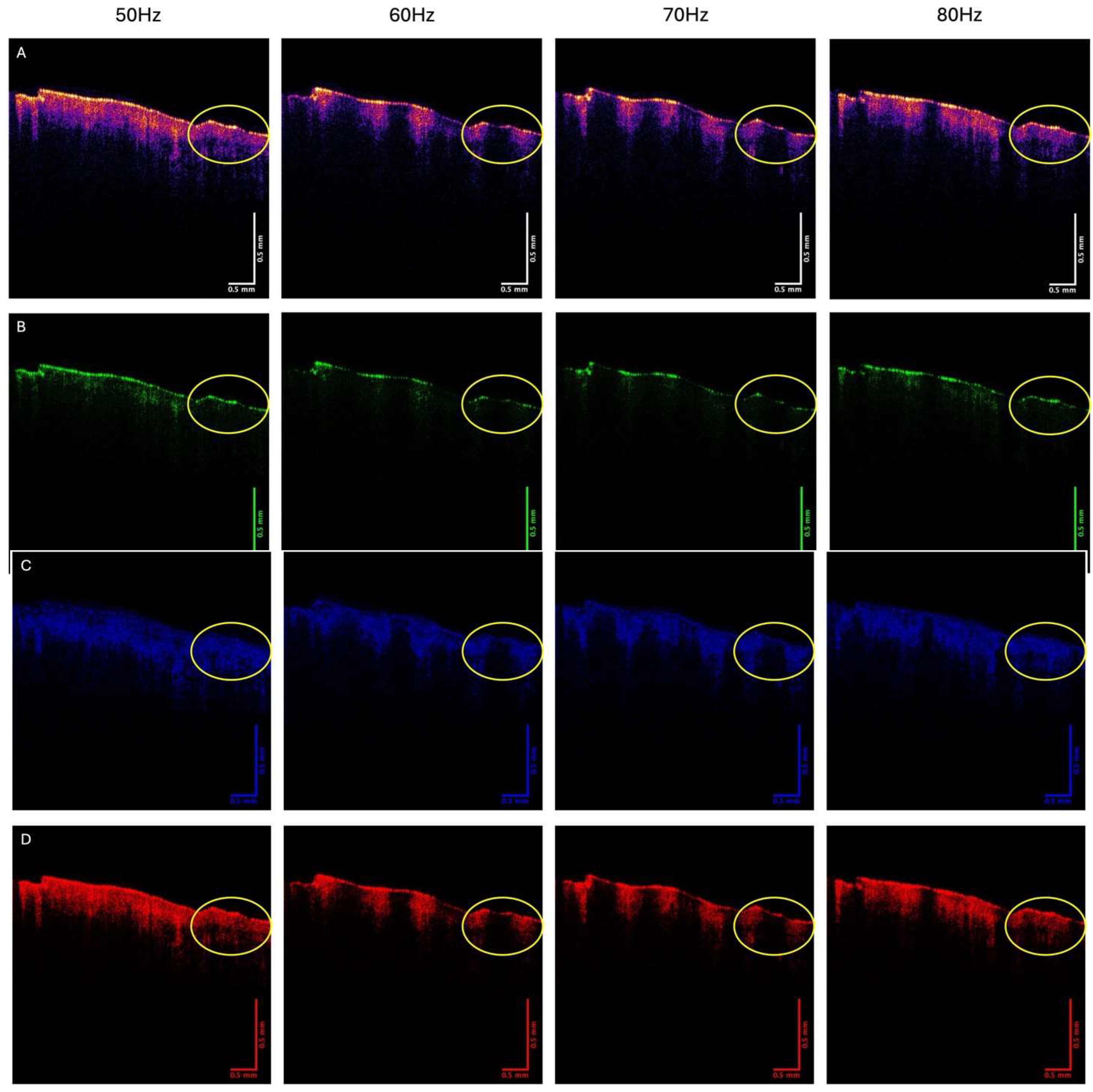
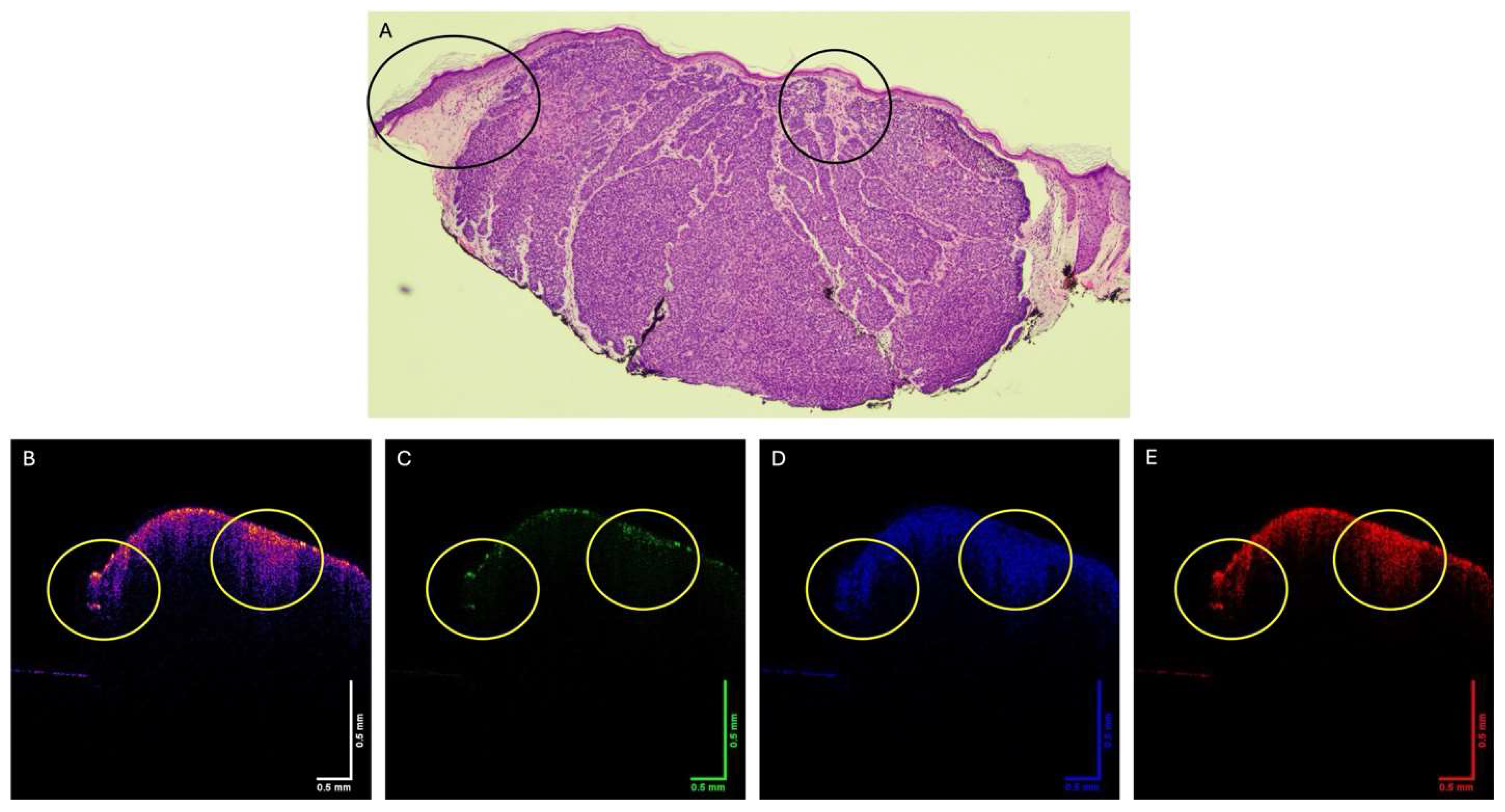
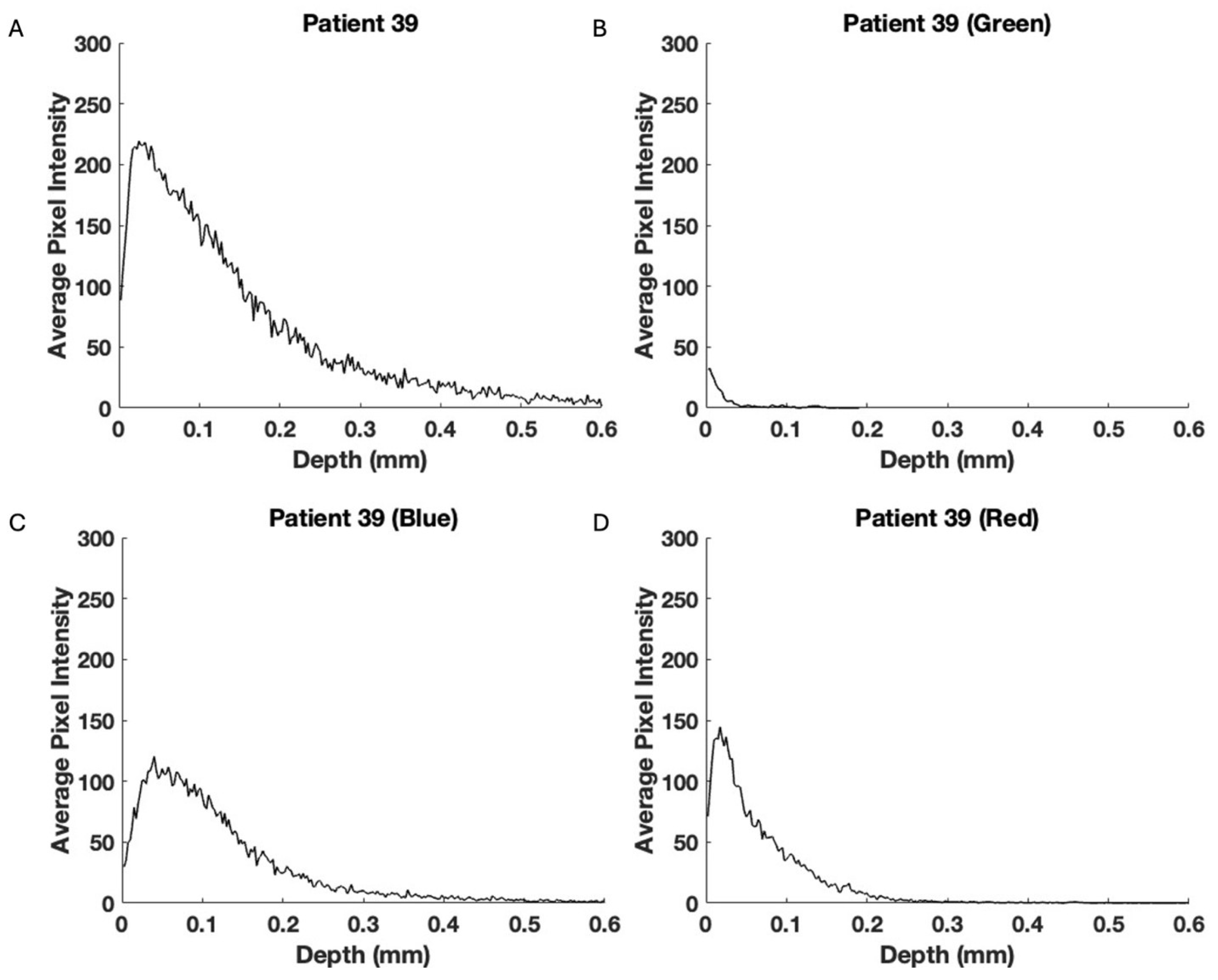
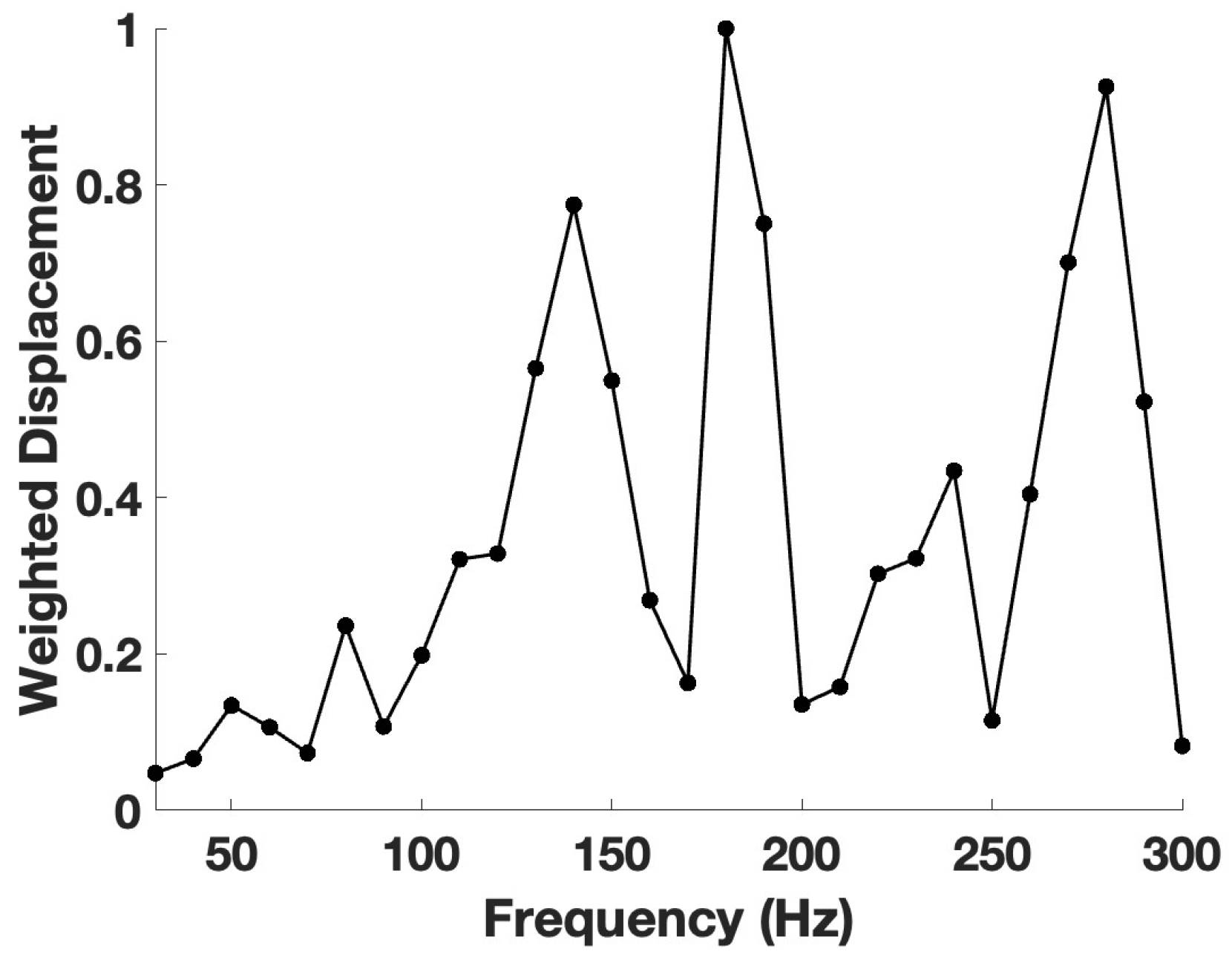
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: www.cancer.org/cancer/types/basal-and-squamous-cell-skin-cancer/about/key-statistics.html (accessed on 6 March 2025).
- Negrutiu, M.; Danescu, S.; Popa, T.; Focsan, M.; Vesa, S.C.; Baican, A. Advancements in Basal Cell Carcinoma Diagnosis: Non-Invasive Imaging and Multimodal Approach. J. Clin. Med. 2024, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Tabanlıoğlu Onan, D.; Şahin, S.; Gokoz, O.; Erkin, G.; Cakır, B.; Elcin, G.; Kayıkcıoğlu, A. Correlation between the dermatoscopic and histopathological features of pigmented basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Ferrante di Ruffano, L.; Dinnes, J.; Deeks, J.J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, CD013189. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Patel, A.; Dhillon, J.; Bobra, A.; Nadiminti, H. Rapid Noninvasive Skin Screening for Basal Cell Carcinomas using Vibrational Optical Coherence Tomography. Br. J. Cancer Res. 2025, 8, 747–755. [Google Scholar] [CrossRef]
- Coleman, A.J.; Richardson, T.J.; Orchard, G.; Uddin, A.; Choi, M.J.; Lacy, K.E. Histological correlates of optical coherence tomography in non-melanoma skin cancer. Ski. Res. Technol. 2013, 19, 10–19. [Google Scholar] [CrossRef]
- Bertheim, U.; Hofer, P.A.; Engström-Laurent, A.; Hellström, S. The stromal reaction in basal cell carcinomas. A prerequisite for tumour progression and treatment strategy. Br. J. Plast. Surg. 2004, 57, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Tzellos, T.G.; Kyrgidis, A.; Vahtsevanos, K.; Triaridis, S.; Printza, A.; Klagas, I.; Zvintzou, E.; Kritis, A.; Karakiulakis, G.; Papakonstantinou, E. Nodular basal cell carcinoma is associated with increased hyaluronan homeostasis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Role of Hyaluronic Acid in Selected Malignant Neoplasms in Women. Biomedicines 2023, 11, 304. [Google Scholar] [CrossRef]
- Jermain, P.R.; Iorizzo, T.W.; Maloney, M.; Mahmoud, B.; Yaroslavsky, A.N. Design and Validation of a Handheld Optical Polarization Imager for Preoperative Delineation of Basal Cell Carcinoma. Cancers 2022, 14, 4049. [Google Scholar] [CrossRef]
- Bourgot, I.; Primac, I.; Louis, T.; Noël, A.; Maquoi, E. Reciprocal Interplay Between Fibrillar Collagens and Collagen-Binding Integrins: Implications in Cancer Progression and Metastasis. Front. Oncol. 2020, 10, 1488. [Google Scholar] [CrossRef]
- Esposito, M.; Yerly, L.; Shukla, P.; Herme, V.; Sella, F.; Balazs, Z.; Evelyn Lattmann, E. COL10A1 expression distinguishes a subset of cancer-associated fibroblasts present in the stroma of high-risk basal cell carcinoma. Br. J. Dermatol. 2024, 191, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Zhu, G.; Zhu, W.; Wang, J.; Ouyang, X.; Yang, K.; Zhong, J. Oncogenic mechanisms of COL10A1 in cancer and clinical challenges (Review). Oncol. Rep. 2024, 52, 162. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Deshmukh, T. Do tensile and shear forces exerted on cells influence mechanotransduction through stored energy considerations? BIOCELL 2024, 48, 525–540. [Google Scholar] [CrossRef]
- Rømer, A.M.A.; Thorseth, M.L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef]
- Zhang, Q.; An, Z.Y.; Jiang, W.; Jin, W.L.; He, X.Y. Collagen code in tumor microenvironment: Functions, molecular mechanisms, and therapeutic implications. Biomed. Pharmacother. 2023, 166, 115390. [Google Scholar] [CrossRef]
- De Martino, D.; Bravo-Cordero, J.J. Collagens in Cancer: Structural Regulators and Guardians of Cancer Progression. Cancer Res. 2023, 83, 1386–1392. [Google Scholar] [CrossRef]
- Sahai, E.; Wyckoff, J.; Philippar, U.; Segall, J.E.; Gertler, F.; Condeelis, J. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 2005, 5, 14. [Google Scholar] [CrossRef]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 26, 821–828. [Google Scholar] [CrossRef]
- Li, X.; Dao, M.; Lykotrafitis, G.; Karniadakis, G.E. Biomechanics and biorheology of red blood cells in sickle cell anemia. J. Biomech. 2017, 50, 34–41. [Google Scholar] [CrossRef]
- Bruckner, B.R.; Janshoff, A. Importance of integrity of cell-cell junctions for the mechanics of confluent MDCK II cell. Sci. Rep. 2018, 8, 14117. [Google Scholar] [CrossRef]
- Van Damme, T.; Colige, A.; Syx, D.; Giunta, C.; Lindert, U.; Rohrbach, M.; Aryani, O.; Alanay, Y.; Simsek-Kiper, P.Ö.; Kroes, H.Y.; et al. Expanding the clinical and mutational spectrum of the Ehlers–Danlos syndrome, dermatosparaxis type. Genet. Med. 2016, 18, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Houdusse, A.; Sweeney, H.L. How Myosin Generates Force on Actin Filaments. Trends Biochem. Sci. 2016, 41, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, I.; Cantelli, G.; Bruce, F.; Sanz-Moreno, V. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Research 2016, 5, 783. [Google Scholar] [CrossRef]
- Karantza, V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 2011, 30, 127–138. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Patel, A.; Nadaminti, H. Use of Optical Coherence Tomography Images to Differentiate Between Normal Skin, Skin Lesions, and Melanoma: A Pilot Study. J. Cancer Sci. Therapy 2024, 4, 1751. [Google Scholar]
- Silver, F.H.; Deshmukh, T.; Ryan, N.; Romm, A.; Nadiminti, H. “Fingerprinting” Benign and Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography: Differentiation among Cancerous Lesion Types Based on the Presence of New Cells, Blood Vessels, and Fibrosis. Biomolecules 2022, 12, 1332. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, F.H.; Deshmukh, T.; Kollipara, G.; Patel, A. Noninvasive Screening of Basal Cell Carcinomas: A Comparison of the Structure and Physical Properties of Large and Small Nodular Lesions Using Vibrational OCT and Histopathology. Onco 2025, 5, 23. https://doi.org/10.3390/onco5020023
Silver FH, Deshmukh T, Kollipara G, Patel A. Noninvasive Screening of Basal Cell Carcinomas: A Comparison of the Structure and Physical Properties of Large and Small Nodular Lesions Using Vibrational OCT and Histopathology. Onco. 2025; 5(2):23. https://doi.org/10.3390/onco5020023
Chicago/Turabian StyleSilver, Frederick H., Tanmay Deshmukh, Gayathri Kollipara, and Aanal Patel. 2025. "Noninvasive Screening of Basal Cell Carcinomas: A Comparison of the Structure and Physical Properties of Large and Small Nodular Lesions Using Vibrational OCT and Histopathology" Onco 5, no. 2: 23. https://doi.org/10.3390/onco5020023
APA StyleSilver, F. H., Deshmukh, T., Kollipara, G., & Patel, A. (2025). Noninvasive Screening of Basal Cell Carcinomas: A Comparison of the Structure and Physical Properties of Large and Small Nodular Lesions Using Vibrational OCT and Histopathology. Onco, 5(2), 23. https://doi.org/10.3390/onco5020023








