AlphaGlue: A Novel Conceptual Delivery Method for α Therapy
Abstract
1. Introduction
1.1. AlphaGlue
1.2. DNA Damage
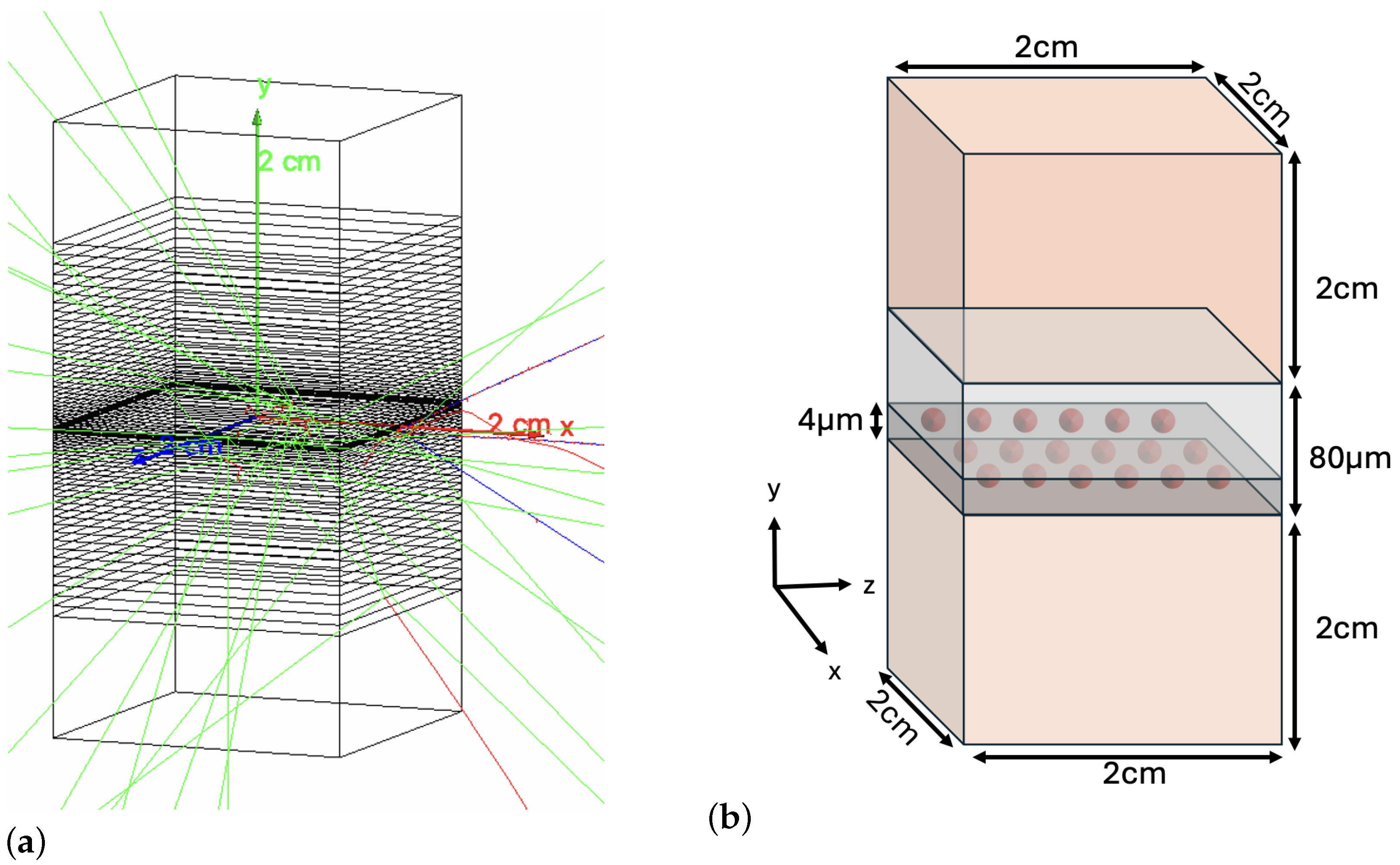
2. Materials and Methods
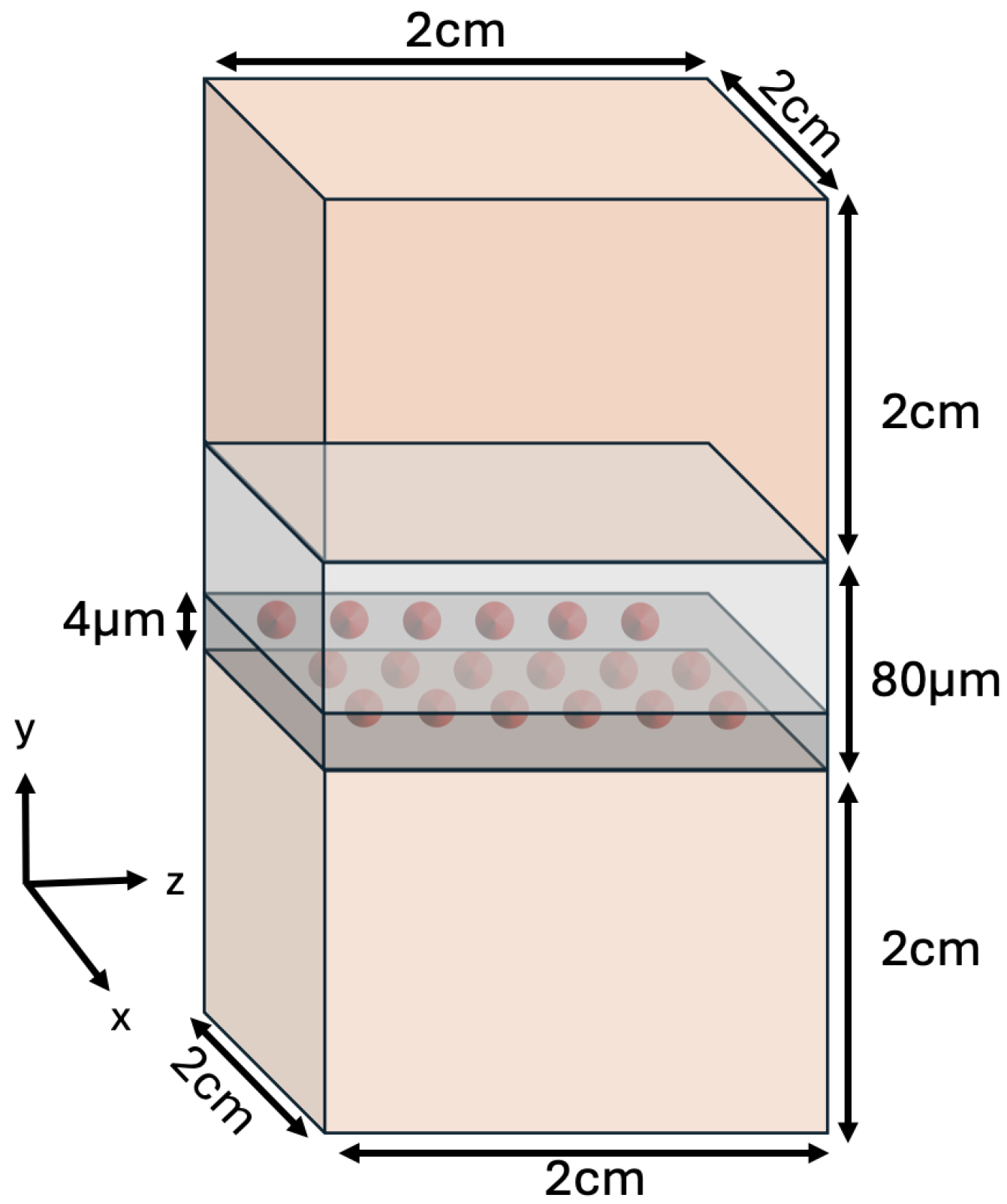
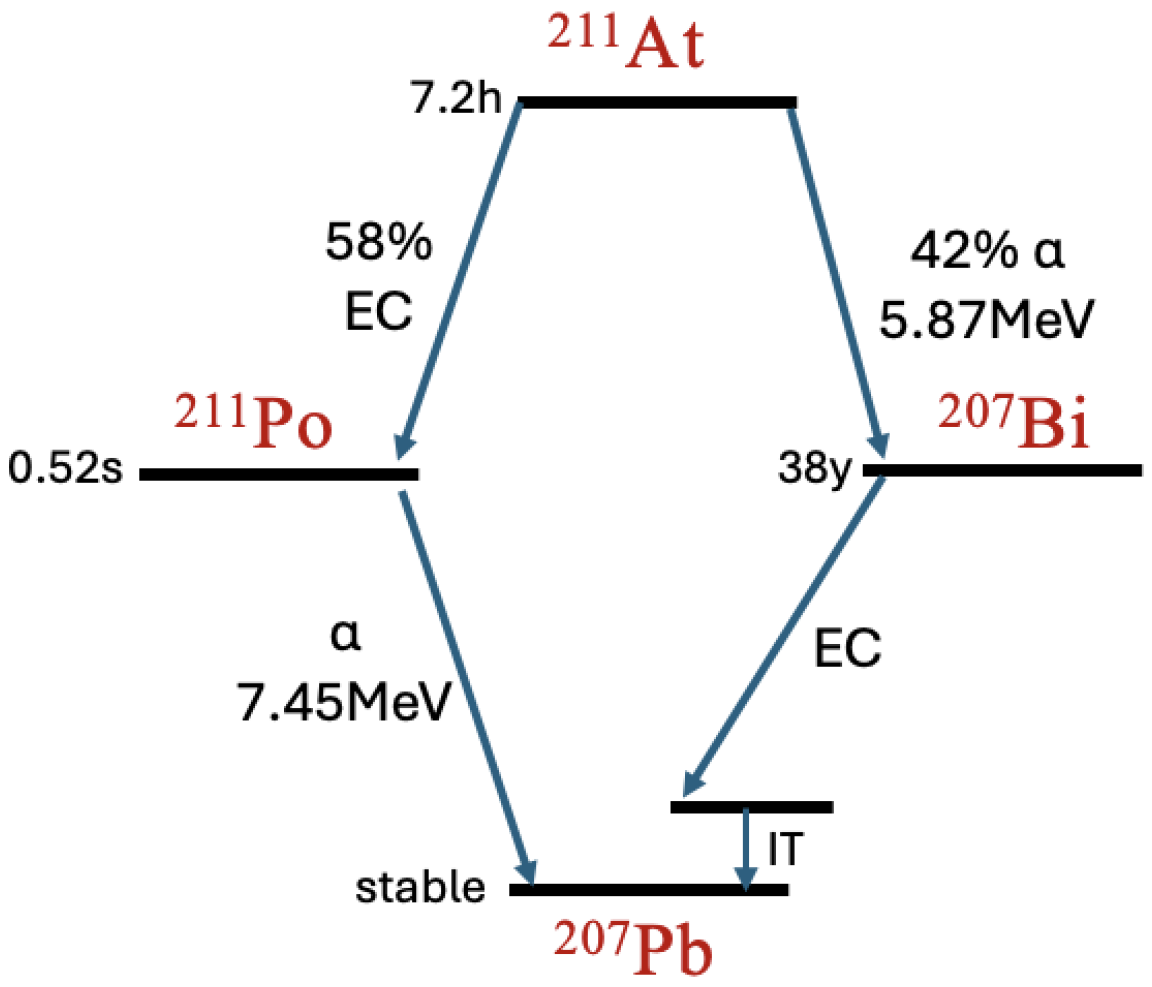
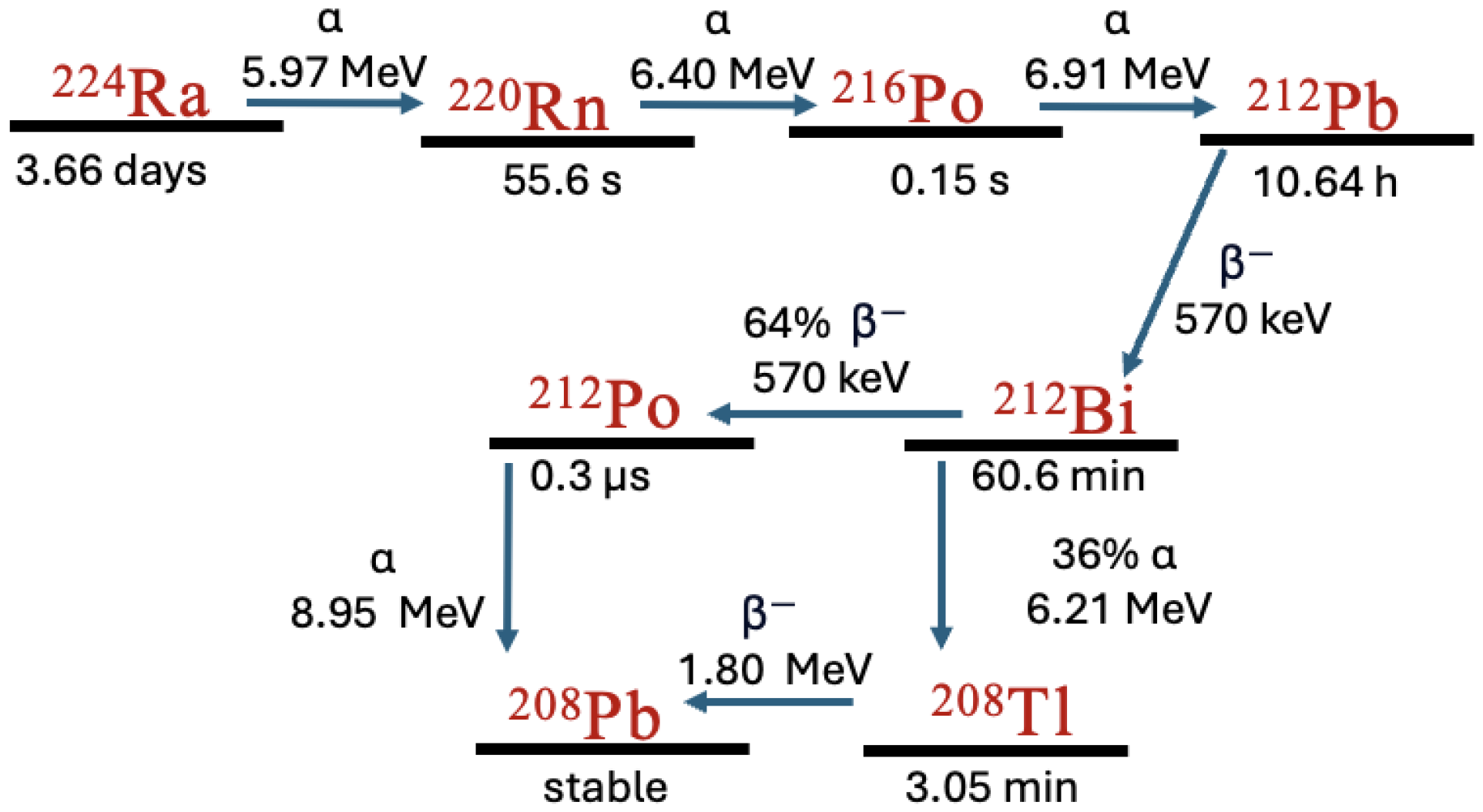
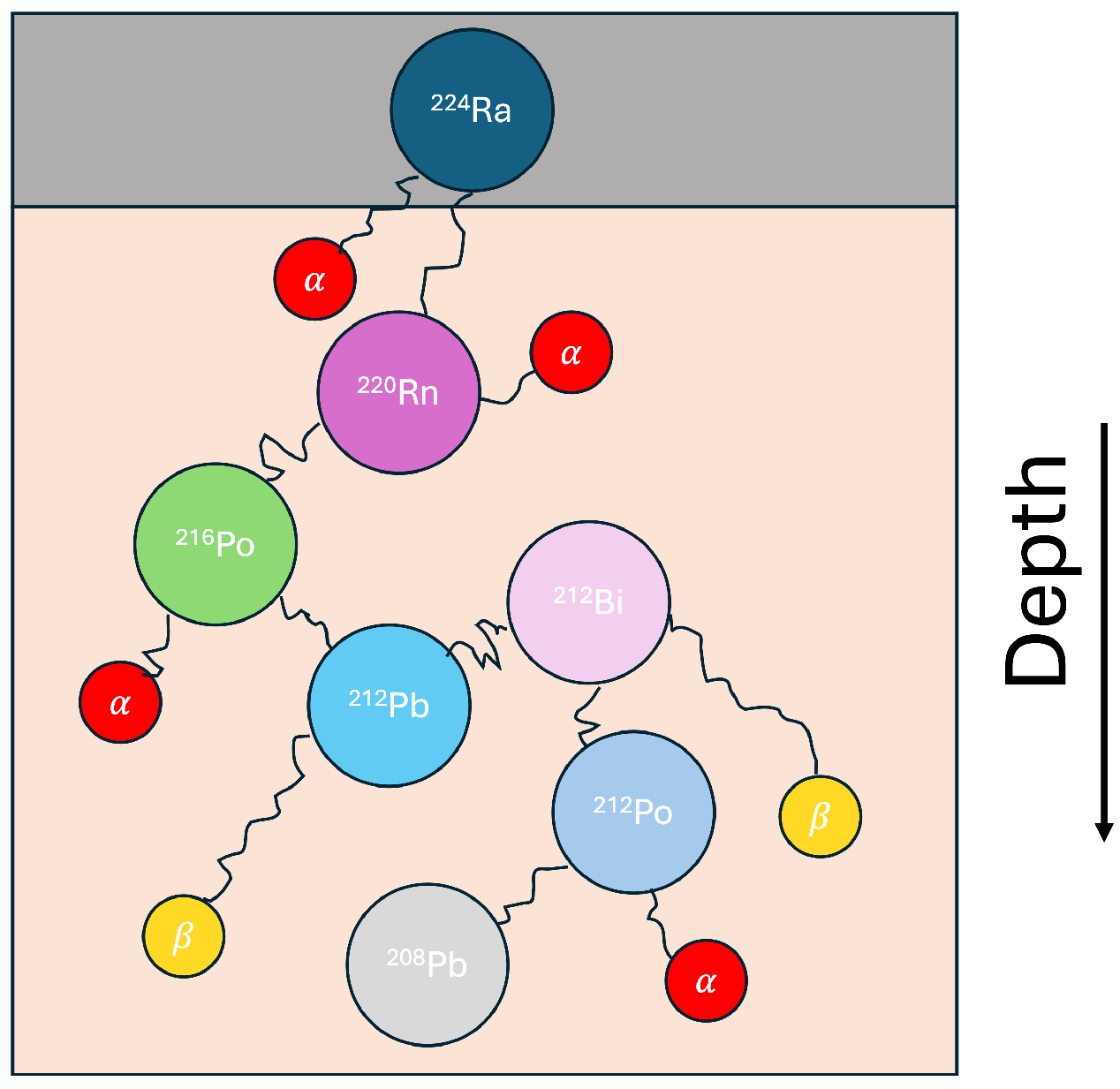
2.1. Decay Simulation
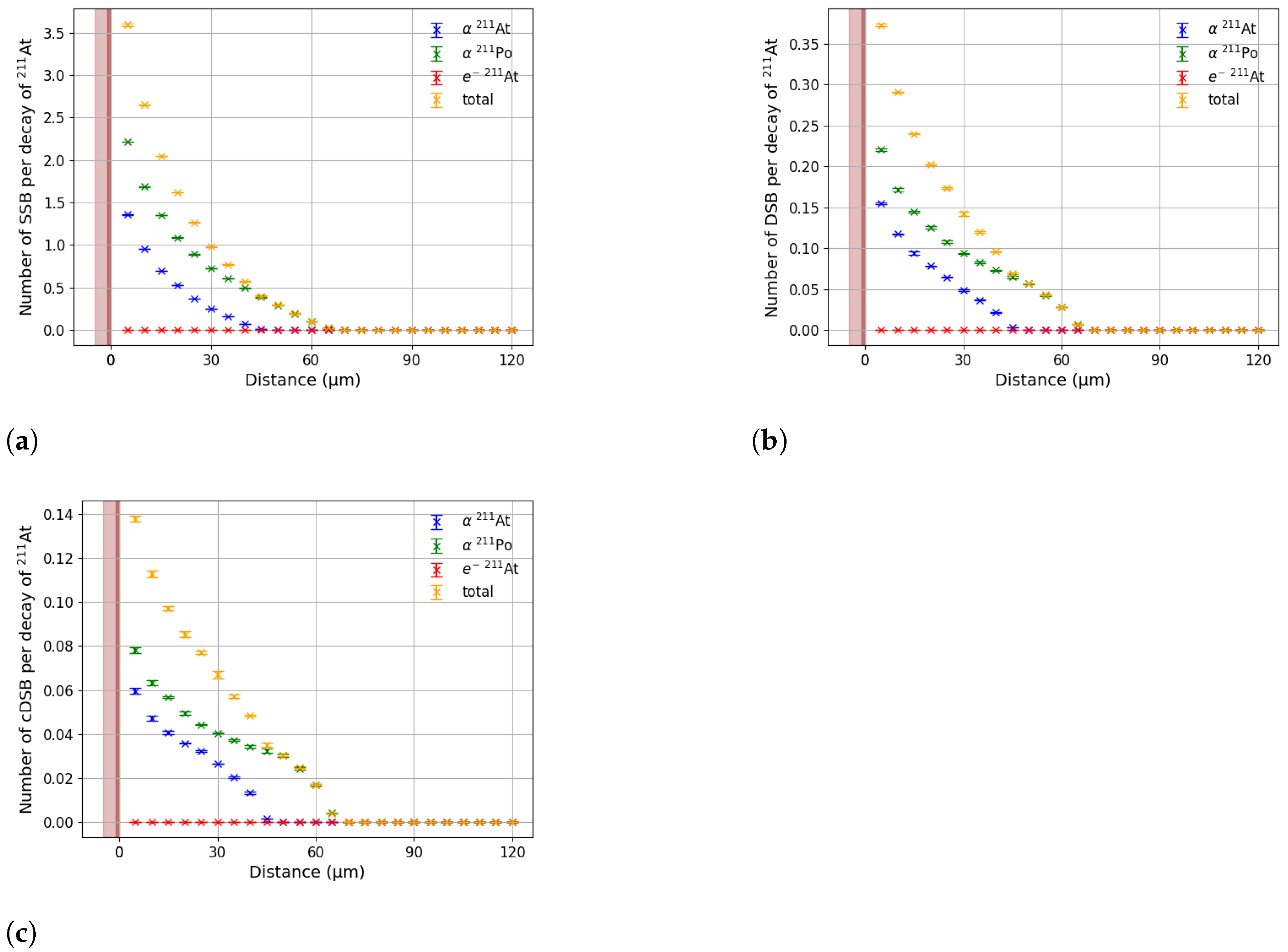
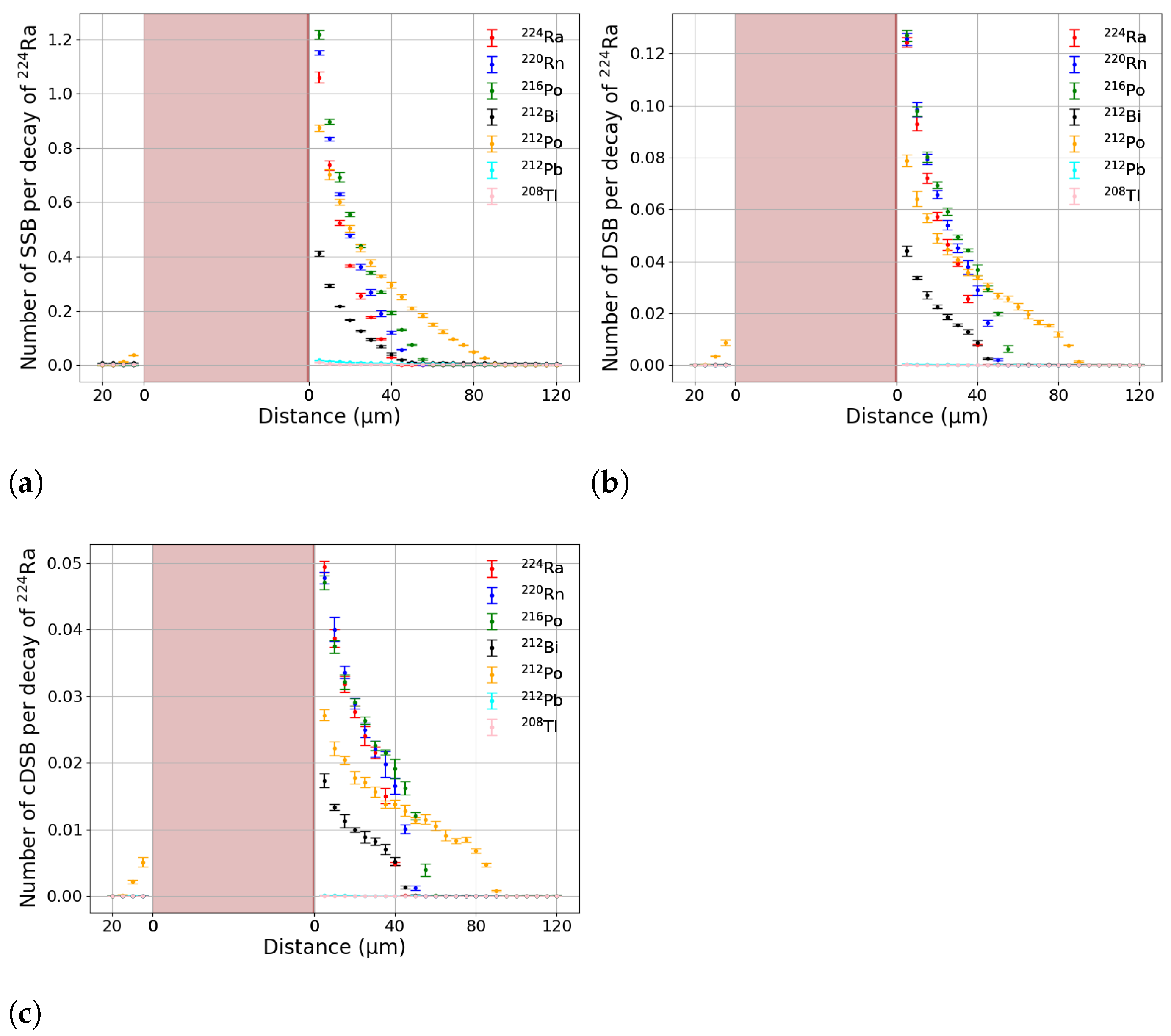
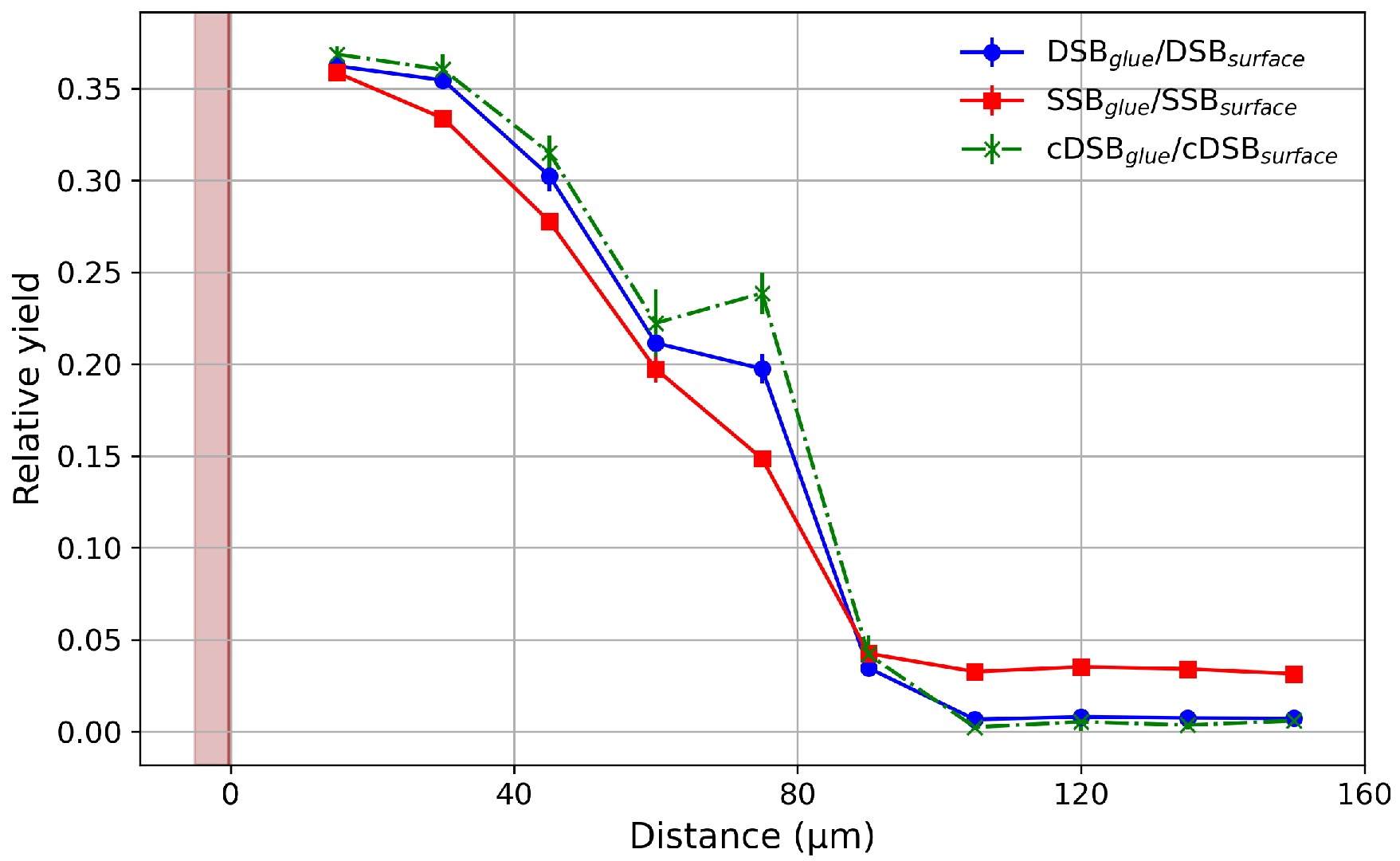
2.2. DNA Damage Simulation
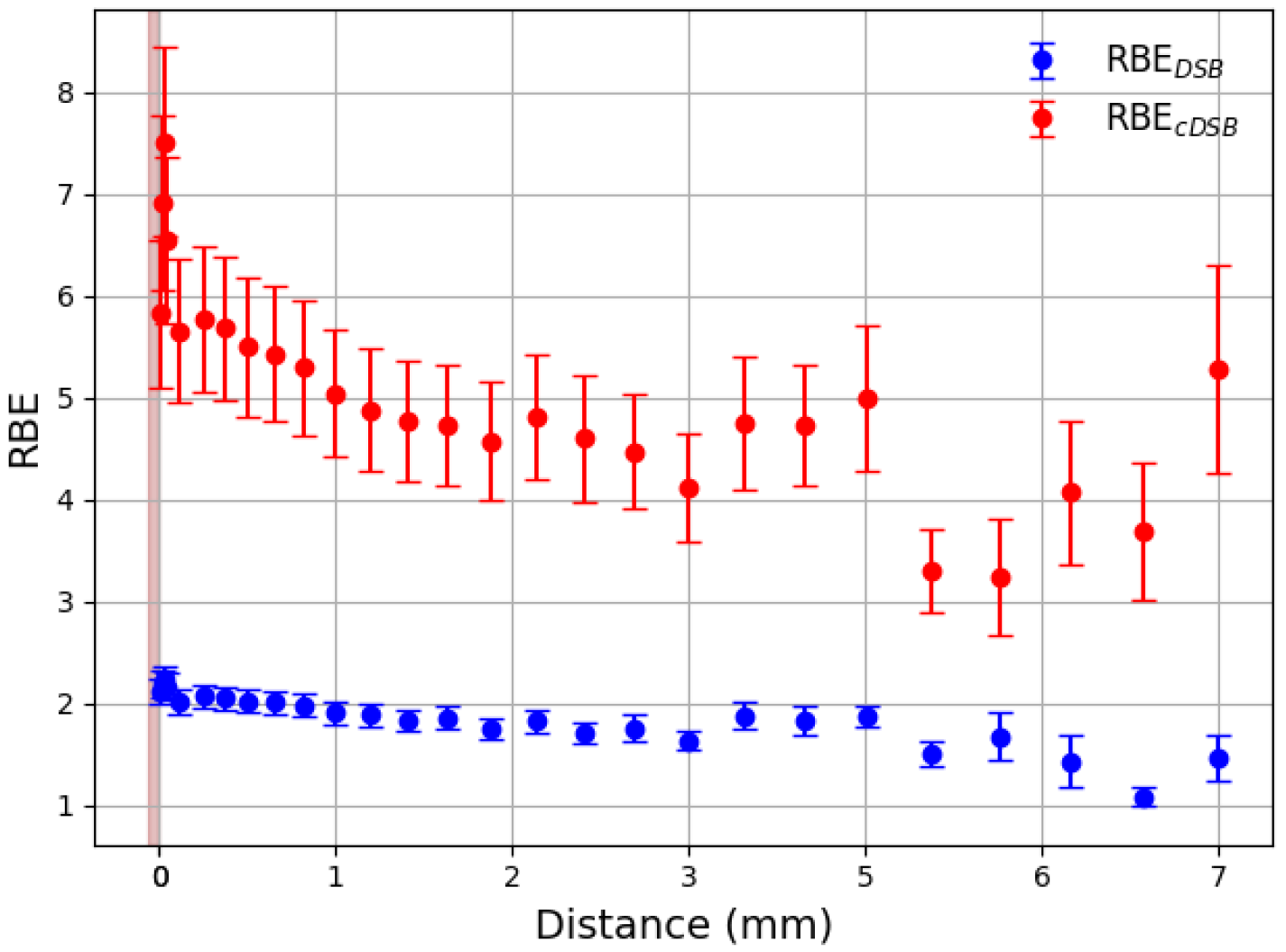
3. Results
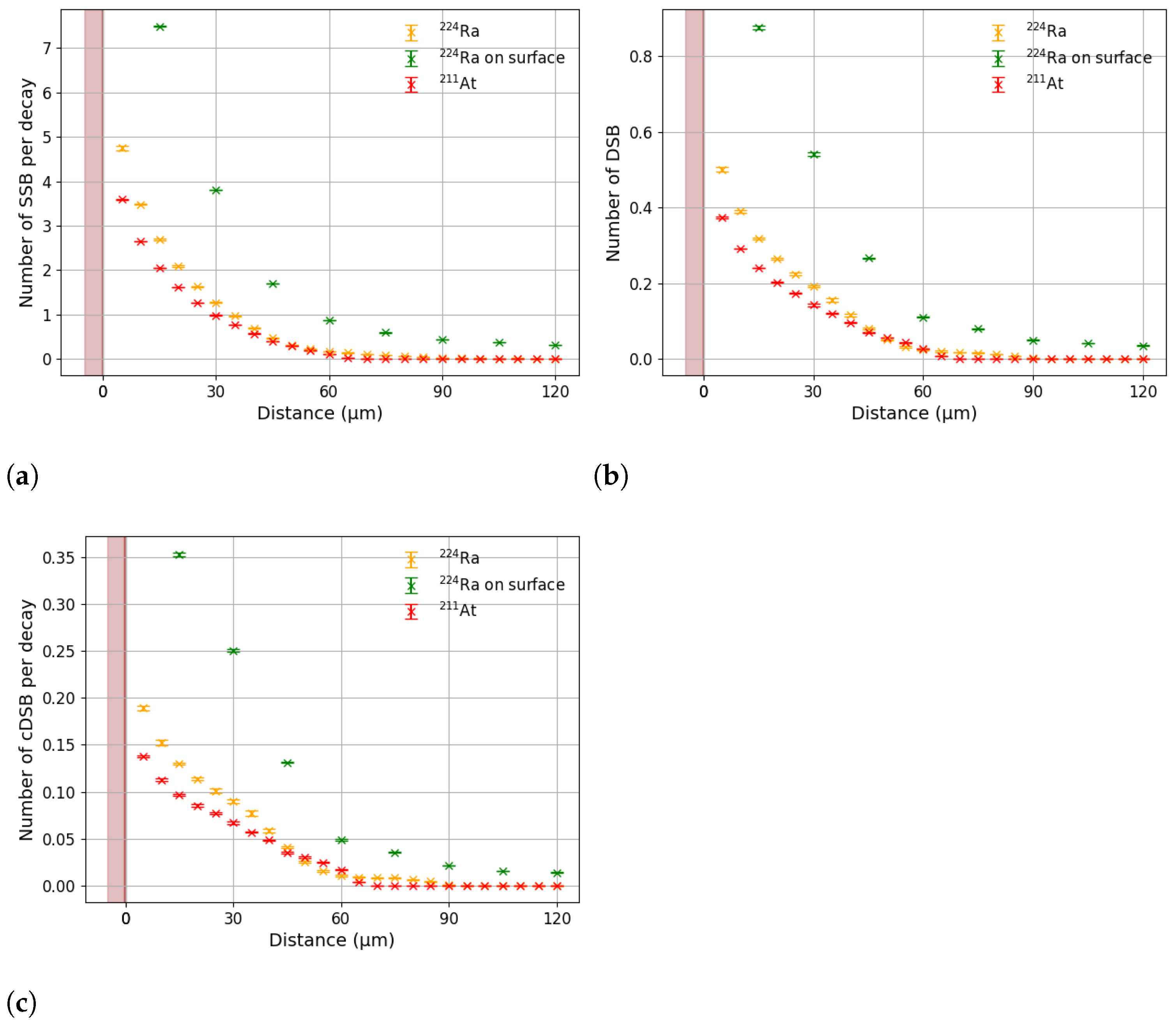
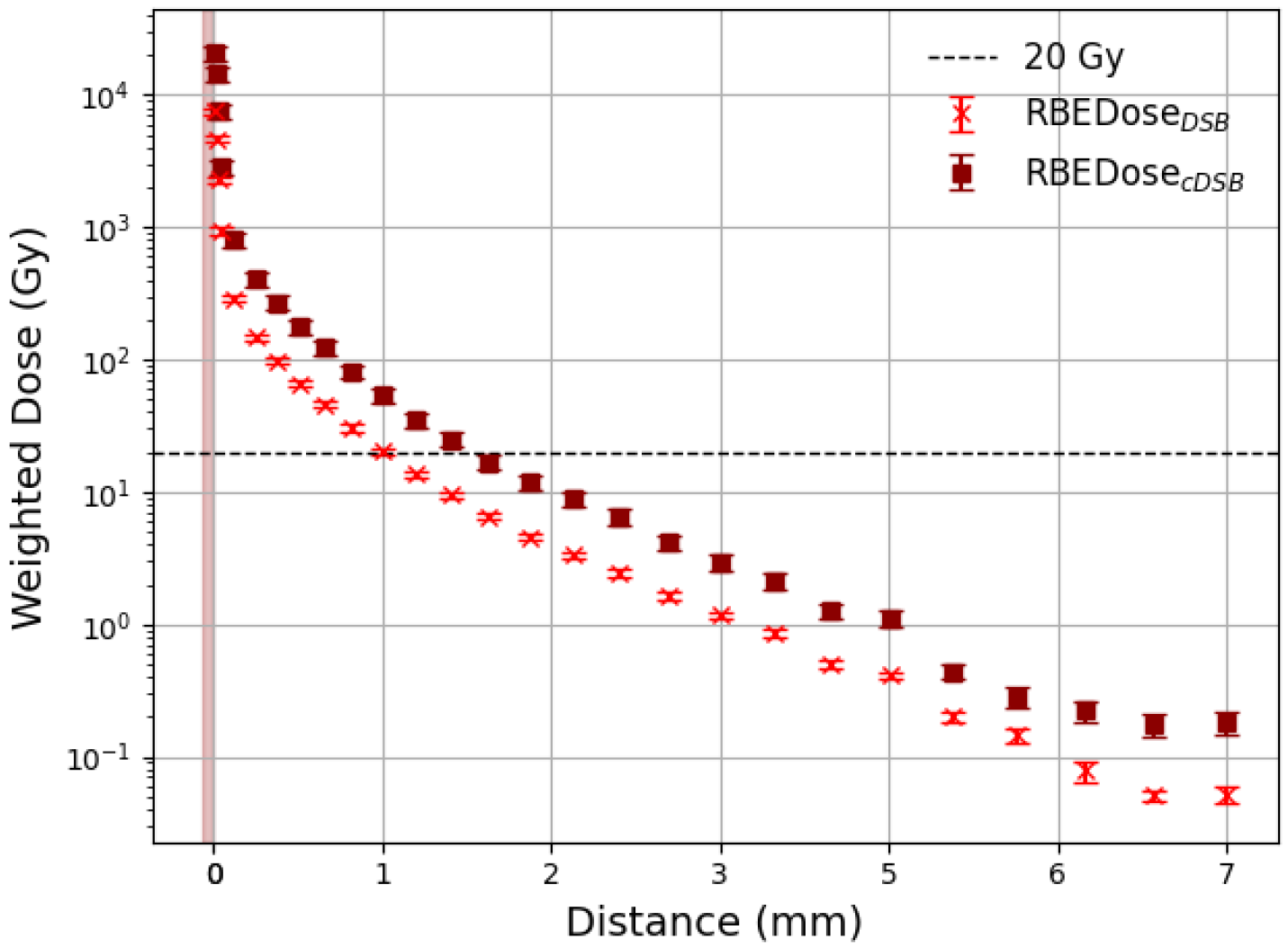
3.1. Effective Treatment Depth
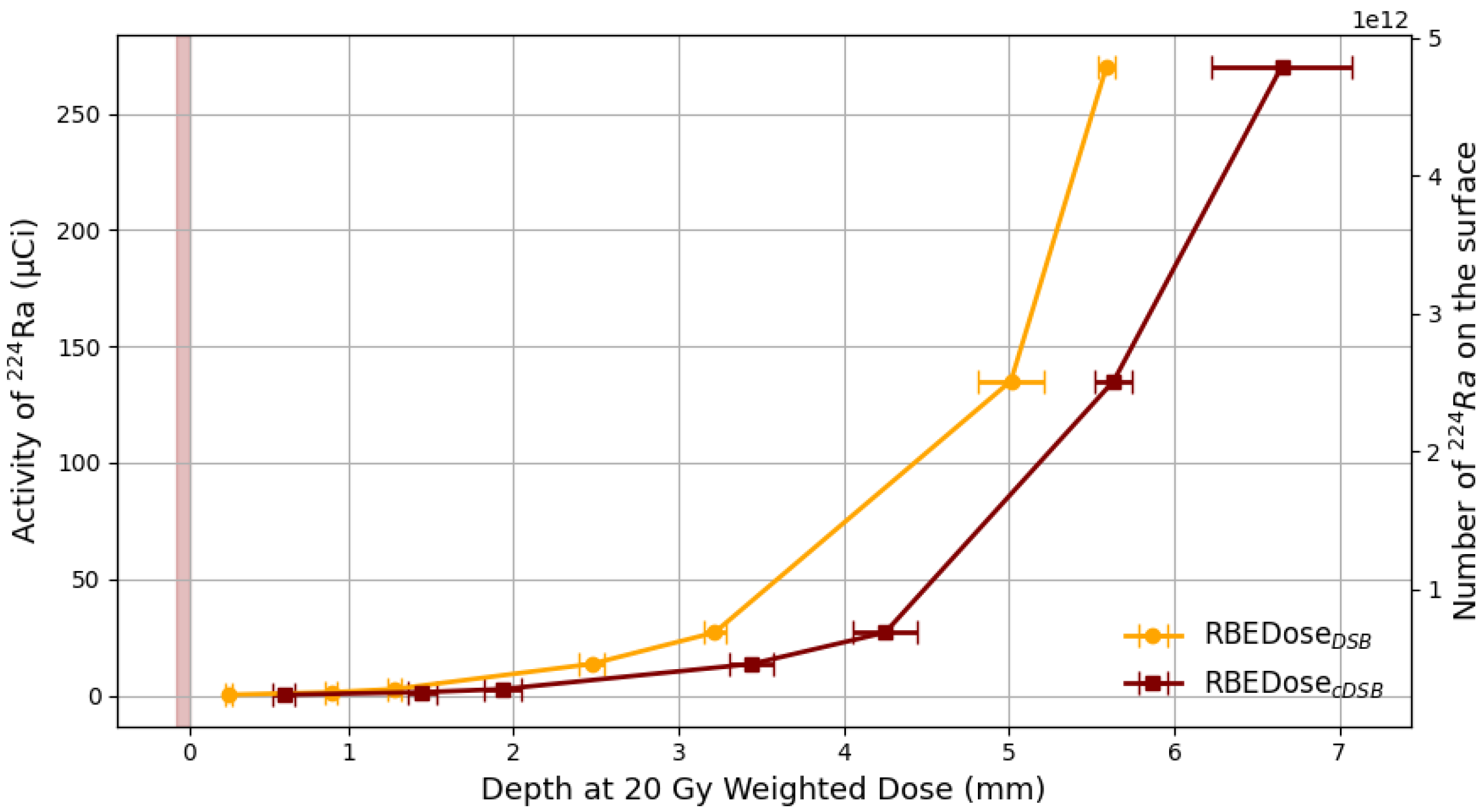
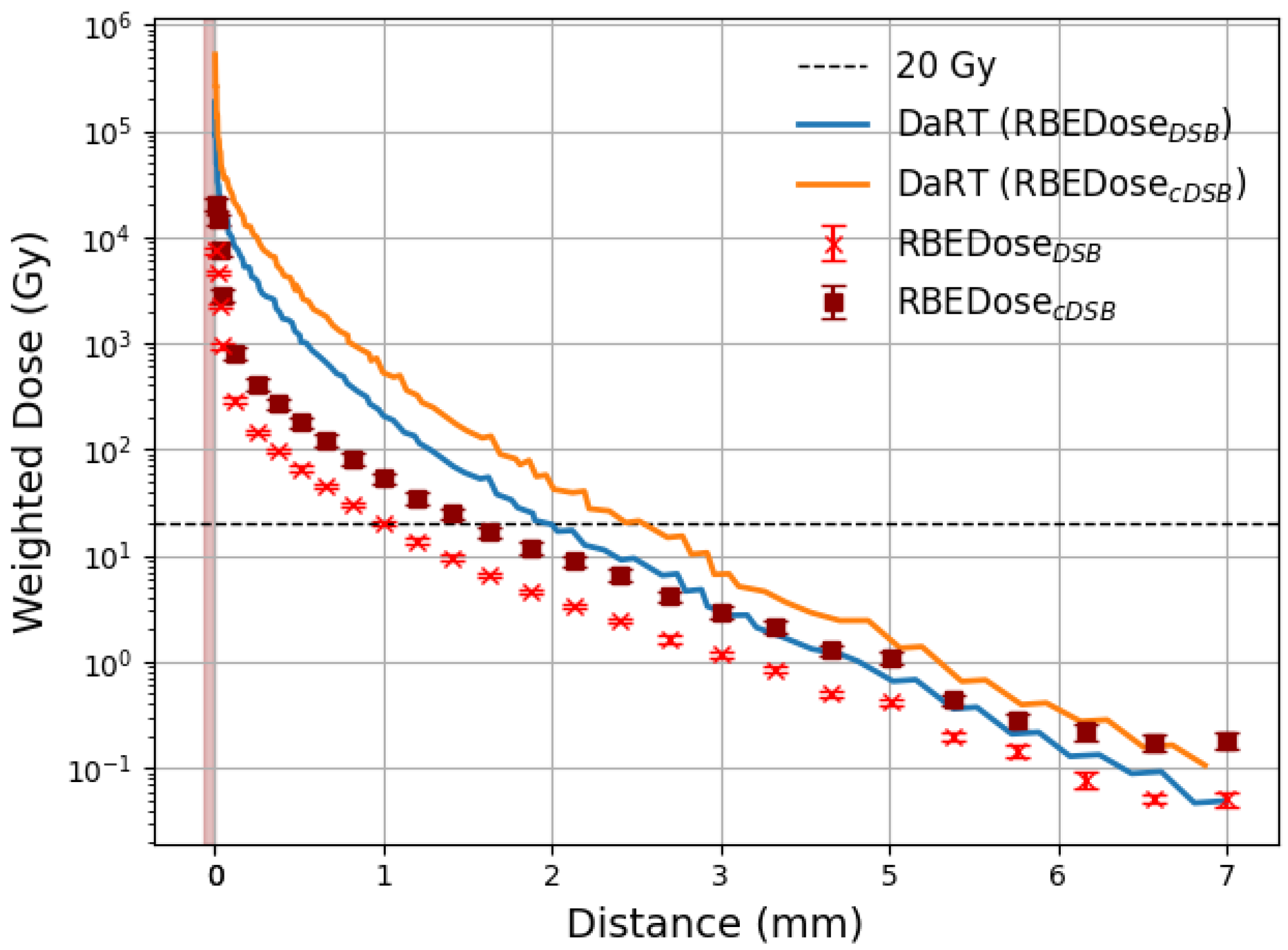
3.2. Comparison with DaRT
4. Discussion
Looking Towards the Future
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LET | linear energy transfer |
| TAT | Targeted Alpha Therapy |
| DaRT | Diffusing -emitters Radiation Therapy |
| SSB | Single Strand Break |
| DSB | Double Strand Break |
| cDSB | Complex Double Strand Break |
| RBE | relative biological effectiveness |
References
- Zhang, J.; Qin, S.; Yang, M.; Zhang, X.; Zhang, S.; Yu, F. Alpha-emitters and targeted alpha therapy in cancer treatment. iRADIOLOGY 2023, 1, 245–261. [Google Scholar] [CrossRef]
- Jang, A.; Kendi, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Sartor, O. Targeted alpha-particle therapy: A review of current trials. Int. J. Mol. Sci. 2023, 24, 11626. [Google Scholar] [CrossRef]
- Jalloul, W.; Ghizdovat, V.; Stolniceanu, C.; Ionescu, T.; Grierosu, I.; Pavaleanu, I.; Moscalu, M.; Stefanescu, C. Targeted Alpha Therapy: All We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide. Pharmaceuticals 2023, 16, 1679. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, L.; Liao, T.; Gong, W.; Zhang, C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 796657. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.H.; Bauer, D.; Michel, A.L.; Carter, L.M.; Lewis, J.S. Targeted Alpha Therapy with 225Ac-Macropa-Isatuximab for CD38-positive Hematological Malignancies. bioRxiv 2024. [Google Scholar]
- Schatz, C.; Zitzmann-Kolbe, S.; Moen, I.; Klotz, M.; Nair, S.; Stargard, S.; Bjerke, R.M.; Biseth, K.W.; Feng, Y.Z.; Indrevoll, B.; et al. Preclinical Efficacy of a PSMA-Targeted Actinium-225 Conjugate (225Ac-Macropa-Pelgifatamab): A Targeted Alpha Therapy for Prostate Cancer. Clin. Cancer Res. 2024, 30, 2531–2544. [Google Scholar] [CrossRef]
- Li, L.; Rousseau, J.; Jaraquemada-Peláez, M.d.G.; Wang, X.; Robertson, A.; Radchenko, V.; Schaffer, P.; Lin, K.S.; Bénard, F.; Orvig, C. 225Ac-H4py4pa for Targeted Alpha Therapy. Bioconjug. Chem. 2021, 32, 1348–1363. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; VanderWalde, N.A.; Ballo, M.T.; Patra, P.; Cohen, G.N.; Damato, A.L.; Barker, C.A. Feasibility and Safety of Diffusing Alpha-Emitter Radiation Therapy for Recurrent or Unresectable Skin Cancers. JAMA Netw. Open 2023, 6, e2312824. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Lecavalier-Barsoum, M.; Ma, K.; Santos Dutra, M.; Kaitoukov, Y.; Bahoric, B.; Tomic, N.; Dinelle, F.; Enger, S.; Batist, G.; et al. Feasibility and safety of endoscopic ultrasound-guided diffusing alpha emitter radiation therapy for advanced pancreatic cancer: Preliminary data. Endosc. Int. Open 2024, 12, E1085–E1091. [Google Scholar] [CrossRef]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front. Med. 2023, 9, 1076210. [Google Scholar] [CrossRef] [PubMed]
- De Sio, C.; Ballisat, L.; Beck, L.; Guatelli, S.; Sakata, D.; Shi, Y.; Duan, J.; Sabah, L.A.; Velthuis, J.; Rosenfeld, A. Targeted alpha therapies using 211At: A Geant4 simulation of dose and DNA damage. Phys. Medica 2025, 129, 104860. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, D.; Guleria, A.; Chakravarty, R. Production, purification and formulation of nanoradiopharmaceutical with 211At: An emerging candidate for targeted alpha therapy. Nucl. Med. Biol. 2024, 138–139, 108947. [Google Scholar] [CrossRef]
- Batra, V.; Samanta, M.; Makvandi, M.; Groff, D.; Martorano, P.; Elias, J.; Ranieri, P.; Tsang, M.; Hou, C.; Li, Y.; et al. Preclinical Development of 211At meta-astatobenzylguanidine (211At-MABG) as an Alpha Particle Radiopharmaceutical Therapy for Neuroblastoma. Clin. Cancer Res. 2022, 28, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Kashiyama, R.; Watanabe, H.; Akasaka, T.; Fujimoto, H.; Murakami, M.; Ooe, K.; Toyoshima, A.; Nakashima, K.; Ono, M. Feasibility of Targeted Alpha Therapy for Alzheimer’s Disease Using 211At-labeled Agent Targeting Amyloid-β Aggregates. ChemRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Bellia, S.; Feliciani, G.; Del Duca, M.; Monti, M.; Turri, V.; Sarnelli, A.; Romeo, A.; Kelson, I.; Keisari, Y.; Popovtzer, A.; et al. Clinical evidence of abscopal effect in cutaneous squamous cell carcinoma treated with diffusing alpha emitters radiation therapy: A case report. J. Contemp. Brachyther. 2019, 11, 449–457. [Google Scholar] [CrossRef]
- Popovtzer, A.; Rosenfeld, E.; Mizrachi, A.; Bellia, S.R.; Ben-Hur, R.; Feliciani, G.; Sarnelli, A.; Arazi, L.; Deutsch, L.; Kelson, I.; et al. Initial Safety and Tumor Control Results From a “First-in-Human” Multicenter Prospective Trial Evaluating a Novel Alpha-Emitting Radionuclide for the Treatment of Locally Advanced Recurrent Squamous Cell Carcinomas of the Skin and Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 571–578. [Google Scholar] [CrossRef]
- Yang, G.; Harrison, L. A Hard Target Needs a Sharper DaRT. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 152–153. [Google Scholar] [CrossRef]
- Popovtzer, A.; Mizrachi, A.; D’Andrea, M.; VanderWalde, N.A.; Kurman, N.; Rosenfeld, E.; Ben-Hur, R.; Bellia, S.R.; Feliciani, G.; Silvern, D.; et al. Extended Follow-Up Outcomes from Pooled Prospective Studies Evaluating Efficacy of Interstitial Alpha Radionuclide Treatment for Skin and Head and Neck Cancers. Cancers 2024, 16, 2312. [Google Scholar] [CrossRef]
- Yoshimura, R.; Toda, K.; Watanabe, H.; Miura, M.; Notake, R.; Murakami, N.; Igaki, H.; Nakamura, S.; Umezawa, R.; Kadoya, N.; et al. Efficacy and safety of diffusing alpha-emitter radiation therapy (DaRT) for head and neck cancer recurrence after radiotherapy. Int. J. Clin. Oncol. 2025, 30, 893–903. [Google Scholar] [CrossRef]
- Pashazadeh, A.; Landes, R.; Boese, A.; Kreissl, M.; Klopfleisch, M.; Friebe, M. Superficial skin cancer therapy with Y-90 microspheres: A feasibility study on patch preparation. Skin Res. Technol. 2020, 26, 25–29. [Google Scholar] [CrossRef]
- Finger, P. Yttrium-90 Episcleral Plaque Brachytherapy for Choroidal Melanoma. J. Vitreoretin. Dis. 2024, 8, 210–214. [Google Scholar] [CrossRef]
- Baxi, S.; Vohra, S.; Hong, A.; Mulholland, N.; Heuschkel, M.; Dahlhoff, G.; Cardaci, G.; Mirzaei, S.; Sathekge, M. Effectiveness and Patient Experiences of Rhenium Skin Cancer Therapy for Nonmelanoma Skin Cancer: Interim Results from the EPIC-Skin Study. J. Nucl. Med. 2024, 65, 1450–1455. [Google Scholar] [CrossRef]
- Cipriani, C.; Desantis, M.; Dahlhoff, G.; Brown, S.D., III; Wendler, T.; Olmeda, M.; Pietsch, G.; Eberlein, B. Personalized irradiation therapy for NMSC by rhenium-188 skin cancer therapy: A long-term retrospective study. J. Dermatol. Treat. 2022, 33, 969–975. [Google Scholar] [CrossRef]
- Cardaci, G.; Baxi, S.; Vohra, S.; Allison, C.; Hong, A.; Mulholland, N.; Sathekge, M.; Mokoala, K.; Heuschkel, M.; Tietze, J.; et al. Efficacy, Safety, and Patient Reported Outcomes of Rhenium-Skin Cancer Therapy for Non-Melanoma Skin Cancer: 1-Year Results from the EPIC-Skin Study. Adv. Radiat. Oncol. 2025, 10, 101802. [Google Scholar] [CrossRef] [PubMed]
- Krönert, M.; Schwarzenböck, S.; Kurth, J.; Heuschkel, M.; Krause, B.J.; Emmert, S.; Tietze, J.K. Patient-Orientated Evaluation of Treatment of Non-Melanoma Skin Cancer with Rhenium-188 Compared to Surgery. Healthcare 2024, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Hari Hara Sudhan, N.; Pandit, N.; Kumari, R.; Sunitha, V. Non-invasive treatment of recalcitrant keloids with contact brachytherapy using Phosphorus 32 skin patch and its effectiveness: A pilot study. J. Nucl. Med. 2023, 64 (Suppl. 1), 579. [Google Scholar]
- Jiang, P.; Purtskhvanidze, K.; Kandzia, G.; Neumann, D.; Luetzen, U.; Siebert, F.; Roider, J.; Dunst, J. 106Ruthenium eye plaque brachytherapy in the management of medium sized uveal melanoma. Radiat. Oncol. 2020, 15, 183. [Google Scholar] [CrossRef]
- Antohe, M.; Coman, A.; Turcu, G.; Nedelcu, R.I.; Brinzea, A.; Balaban, M.; Moroianu, A.; Manea, L.; Hulea, I.; Balasescu, E.; et al. The prognostic significance of the clinical and histological parameters in primary cutaneous melanoma patients. Med. Pharm. Rep. 2022, 95, 229–235. [Google Scholar] [CrossRef]
- Chen, M.L.; de Vere Hunt, I.J.; John, E.M.; Weinstock, M.A.; Swetter, S.M.; Linos, E. Differences in Thickness-Specific Incidence and Factors Associated With Cutaneous Melanoma in the US From 2010 to 2018. JAMA Oncol. 2022, 8, 755–759. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Phillips Helman, W.; Ross, A.B. Critical review of rate constants for reacitons of hydrated electrons. J. Phys. Chem. Ref. Data 1988, 17. [Google Scholar] [CrossRef]
- Ivanchenko, V.; Jones, F.; Jun, S.; Kaitaniemi, P.; Karakatsanis, N. Recent developments in Geant4. Nucl. Instruments Methods Phys. Res. A 2016, 835, 186–225. [Google Scholar]
- Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Dubois, P.A.; Asai, M.; Barrand, G.; Capra, R.; Chauvie, S.; Chytracek, R.; et al. Geant4 developments and applications. IEEE Trans. Nucl. Sci. 2006, 53, 270–278. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4–a simulation toolkit. Nucl. Instruments Methods Phys. Res. Sect. A 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Incerti, S.; Baldacchino, G.; Bernal, M.; Capra, R.; Champion, C.; Francis, Z.; Guèye, P.; Mantero, A.; Mascialino, B.; Moretto, P.; et al. The Geant4-DNA project. Int. J. Model. Simul. Sci. Comput. 2010, 1, 157–178. [Google Scholar] [CrossRef]
- Incerti, S.; Ivanchenko, A.; Karamitros, M.; Mantero, A.; Moretto, P.; Tran, H.; Mascialino, B.; Champion, C.; Ivanchenko, V.; Bernal, M.; et al. Comparison of Geant4 very low energy cross section models with experimental data in water. Med. Phys. 2010, 37, 4692–4708. [Google Scholar] [CrossRef]
- Bernal, M.A.; Bordage, M.C.; Brown, J.M.C.; Davídková, M.; Delage, E.; El Bitar, Z.; Enger, S.A.; Francis, Z.; Guatelli, S.; Ivanchenko, V.N.; et al. Track structure modeling in liquid water: A review of the Geant4-DNA very low energy extension of the Geant4 Monte Carlo simulation toolkit. Phys. Medica 2015, 31, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Incerti, S.; Kyriakou, I.; Bernal, M.; Bordage, M.; Francis, Z.; Guatelli, S.; Ivanchenko, V.; Karamitros, M.; Lampe, N.; Lee, S.B.; et al. Geant4-DNA example applications for track structure simulations in liquid water: A report from the Geant4-DNA Project. Med. Phys. 2018, 45, e722–e739. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Archer, J.; Baldacchino, G.; Brown, J.M.; Chappuis, F.; Cirrone, G.A.P.; Desorgher, L.; Dominguez, N.; Fattori, S.; Guatelli, S.; et al. Review of chemical models and applications in Geant4-DNA: Report from the ESA BioRad III Project. Med. Phys. 2024, 51, 5873–5889. [Google Scholar] [CrossRef]
- Kyriakou, I.; Sakata, D.; Tran, H.N.; Perrot, Y.; Shin, W.G.; Lampe, N.; Zein, S.; Bordage, M.C.; Guatelli, S.; Villagrasa, C.; et al. Review of the Geant4-DNA simulation toolkit for radiobiological applications at the cellular and DNA level. Cancers 2021, 14, 35. [Google Scholar] [CrossRef]
- Adjei, D.; Trinh, N.D.; Mostafavi, M. Application of Geant4-DNA for simulating water radiolysis induced by Auger electron-emitting radionuclides. J. Radiat. Res. 2023, 64, 369–378. [Google Scholar] [CrossRef]
- Thomson, W. Using VARSKIN+v1.2 to estimate dose from direct skin contamination with radionuclides 223Ra, 212 Pb and 225 Ac; considerations for Nuclear Medicine staff and associated Personal Protective Equipment (PPE). Nucl. Med. Commun. 2024, 45, 159–168. [Google Scholar]
- Hamby, D.; Mangini, C.; Luitjens, J.; Boozer, D.; Tucker, Z.; Rose, C.; Flora, R. VARSKIN+ 1.0 A Computer Code for Skin Contamination and Dosimetry Assessments. Available online: https://www.nrc.gov/docs/ML2120/ML21208A020.pdf (accessed on 6 September 2025).
- Yagi, M.; Wakisaka, Y.; Takeno, J.; Kanada, S.; Tsubouchi, T.; Hamatani, N.; Maruo, H.; Takashina, M.; Ishii, T.; Kanai, T.; et al. Dosimetric impact of stopping power for human bone porosity with dual-energy computed tomography in scanned carbon-ion therapy treatment planning. Sci. Rep. 2024, 14, 17440. [Google Scholar] [CrossRef]
- Arce, P.; Bolst, D.; Bordage, M.C.; Brown, J.; Cirrone, P.; Cortés-Giraldo, M.A.; Cutajar, D.; Cuttone, G.; Desorgher, L.; Dondero, P.; et al. Report on G4-Med, a Geant4 benchmarking system for medical physics applications developed by the Geant4 Medical Simulation Benchmarking Group. Med. Phys. 2021, 48, 19–56. [Google Scholar] [CrossRef]
- Ballisat, L.; De Sio, C.; Beck, L.; Guatelli, S.; Sakata, D.; Shi, Y.; Duan, J.; Velthuis, J.; Rosenfeld, A. Dose and DNA damage modelling of diffusing alpha-emitters radiation therapy using Geant4. Phys. Medica 2024, 121, 103367. [Google Scholar] [CrossRef]
- Arazi, L. Diffusing alpha-emitters radiation therapy: Approximate modeling of the macroscopic alpha particle dose of a point source. Phys. Med. Biol. 2020, 65, 015015. [Google Scholar] [CrossRef]
- Chatzipapas, K.; Dordevic, M.; Zivkovic, S.; Tran, N.H.; Lampe, N.; Sakata, D.; Petrovic, I.; Ristic-Fira, A.; Shin, W.G.; Zein, S.; et al. Geant4-DNA simulation of human cancer cells irradiation with helium ion beams. Phys. Medica 2023, 112, 102613. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Jost, D. How epigenome drives chromatin folding and dynamics, insights from efficient coarse-grained models of chromosomes. PLoS Comput. Biol. 2018, 14, e1006159. [Google Scholar] [CrossRef] [PubMed]
- Ballisat, L.; Beck, L.; De Sio, C.; Guatelli, S.; Sakata, D.; Incerti, S.; Tran, H.N.; Duan, J.; Maclean, K.; Shi, Y.; et al. In-silico calculations of DNA damage induced by α-particles in the 224Ra DaRT decay chain for a better understanding of the radiobiological effectiveness of this treatment. Phys. Medica 2023, 112, 102626. [Google Scholar] [CrossRef]
- Nikjoo, H.; O’neoll, P.; Goodhead, D.T.; Terrissol, M. Computational modelling of low-energy electron-induced DNA damage by early physical and chemical events. Int. J. Radiat. Biol. 1997, 71, 467–483. [Google Scholar] [CrossRef]
- Abbotts, R.; Wilson, D.M., III. Coordination of DNA single strand break repair. Free Radic. Biol. Med. 2017, 107, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Schipler, A.; Iliakis, G. DNA double-strand–break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic Acids Res. 2013, 41, 7589–7605. [Google Scholar] [CrossRef] [PubMed]
- Cannan, W.J.; Pederson, D.S. Mechanisms and consequences of double-strand DNA break formation in chromatin. J. Cell. Physiol. 2016, 231, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.G.; Sakata, D.; Lampe, N.; Belov, O.; Tran, N.H.; Petrovic, I.; Ristic-Fira, A.; Dordevic, M.; Bernal, M.A.; Bordage, M.C.; et al. A Geant4-DNA evaluation of radiation-induced DNA damage on a human fibroblast. Cancers 2021, 13, 4940. [Google Scholar] [CrossRef]
- Sakata, D.; Suzuki, M.; Hirayama, R.; Abe, Y.; Muramatsu, M.; Sato, S.; Belov, O.; Kyriakou, I.; Emfietzoglou, D.; Guatelli, S.; et al. Performance evaluation for repair of HSGc-C5 carcinoma cell using Geant4-DNA. Cancers 2021, 13, 6046. [Google Scholar] [CrossRef]
- Mady, M.M.; Fathy, M.M.; Youssef, T.; Khalil, W.M. Biophysical characterization of gold nanoparticles-loaded liposomes. Phys. Medica 2012, 28, 288–295. [Google Scholar] [CrossRef]
- Friedland, W.; Jacob, P.; Bernhardt, P.; Paretzke, H.G.; Dingfelder, M. Simulation of DNA damage after proton irradiation. Radiat. Res. 2003, 159, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sakata, D.; Hirayama, R.; Shin, W.G.; Belli, M.; Tabocchini, M.A.; Stewart, R.D.; Belov, O.; Bernal, M.A.; Brown, J.M.; et al. Prediction of DNA rejoining kinetics and cell survival after proton irradiation for V79cells using Geant4-DNA. Phys. Medica 2023, 105, 102508. [Google Scholar] [CrossRef]
- Fedorchenko, D.; Alani, S. Simulation of particle release for Diffusing Alpha-Emitters Radiation Therapy. Appl. Radiat. Isot. 2023, 197, 110825. [Google Scholar] [CrossRef]
- Jomah, J.; Elsafi, R.A.; Ali, K.S.A.E.; Abdullah, R.; Gelidan, A.G. Nasal skin thickness measurements using computed tomography in an adult Saudi population. Plast. Reconstr. Surg.–Glob. Open 2019, 7, e2450. [Google Scholar] [CrossRef]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025. [Google Scholar] [CrossRef]
- ICRU. ICRU Report 93: Prescribing, Recording, and Reporting Light Ion Beam Therapy. J. ICRU 2016, 16, 37–58. [Google Scholar]
- Mathuthu, M.; Mdziniso, N.W.; Asres, Y.H. Dosimetric evaluation of cobalt-60 teletherapy in advanced radiation oncology. J. Radiother. Pract. 2019, 18, 88–92. [Google Scholar] [CrossRef]
- European Medicines Agency. Xofigo, radium 223Ra dichloride. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xofigo (accessed on 4 September 2025).
- Deshayes, E.; Roumiguie, M.; Thibault, C.; Beuzeboc, P.; Cachin, F.; Hennequin, C.; Huglo, D.; Rozet, F.; Kassab-Chahmi, D.; Rebillard, X.; et al. Radium 223 dichloride for prostate cancer treatment. Drug Des. Dev. Ther. 2017, 11, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Xofigo: EPAR-Product Information. 2013. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xofigo (accessed on 4 September 2025).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Sabah, L.; Ballisat, L.; De Sio, C.; Dobrowolska, M.; Chambers, A.; Duan, J.; Guatelli, S.; Sakata, D.; Shi, Y.; Velthuis, J.; et al. AlphaGlue: A Novel Conceptual Delivery Method for α Therapy. BioMedInformatics 2025, 5, 58. https://doi.org/10.3390/biomedinformatics5040058
Abu Sabah L, Ballisat L, De Sio C, Dobrowolska M, Chambers A, Duan J, Guatelli S, Sakata D, Shi Y, Velthuis J, et al. AlphaGlue: A Novel Conceptual Delivery Method for α Therapy. BioMedInformatics. 2025; 5(4):58. https://doi.org/10.3390/biomedinformatics5040058
Chicago/Turabian StyleAbu Sabah, Lujin, Laura Ballisat, Chiara De Sio, Magdalena Dobrowolska, Adam Chambers, Jinyan Duan, Susanna Guatelli, Dousatsu Sakata, Yuyao Shi, Jaap Velthuis, and et al. 2025. "AlphaGlue: A Novel Conceptual Delivery Method for α Therapy" BioMedInformatics 5, no. 4: 58. https://doi.org/10.3390/biomedinformatics5040058
APA StyleAbu Sabah, L., Ballisat, L., De Sio, C., Dobrowolska, M., Chambers, A., Duan, J., Guatelli, S., Sakata, D., Shi, Y., Velthuis, J., & Rosenfeld, A. (2025). AlphaGlue: A Novel Conceptual Delivery Method for α Therapy. BioMedInformatics, 5(4), 58. https://doi.org/10.3390/biomedinformatics5040058





