Abstract
Background: Pleurotus salmoneostramineus is acknowledged as a reliable source of high-quality protein, with its protein concentrates, hydrolysates, and peptides potentially offering health benefits to humans. However, studies validating the medicinal effects of P. salmoneostramineus proteins, particularly the pink chromoprotein, are currently absent. Methods: This study explores anticancer peptides from the chromoprotein of P. salmoneostramineus, evaluating their ability to bind UCP2 via in silico analysis. Additionally, it assesses the protein hydrolysate from P. salmoneostramineus (PSPs) effect on HepG2 cell proliferation and mitochondrial metabolism, focusing on uncoupling protein activity. Results: Eight peptides were identified as potential UCP2 inhibitors. According to mACPpred2.0 and CSM-peptides servers, the peptides TSMQSSL, QEGQKL, SEDSGEA, and GRNSL exhibit promising anticancer properties. These anticancer peptides yielded the following docking scores (kcal/mol) when tested against UCP2: TSMQSSL (−166.75), QEGQKL (−126.06), SEDSGEA (−99.93), and GRNSL (−137.93). Molecular dynamics simulations have shown that the peptides establish stable interactions with UCP2 through salt bridges, hydrophobic interactions, and hydrogen bonds, implying that hydrogen bonding with RRR88 and FVW92 causes conformational changes in UCP2. Moreover, the outcomes of this study indicated that PSPs possess an antiproliferative effect on HepG2 cells and lower mitochondrial bioenergetics, especially UCP2 activity. Conclusions: These findings suggest that peptides from P.salmoneostramineus can inhibit UCP2, offering a promising approach for cancer prevention, playing therapeutic roles in treatment, and providing a basis for designing peptide-based cancer therapies.
1. Introduction
Hepatocellular carcinoma (HCC) ranks among the foremost causes of cancer-related mortality globally, being the fifth-leading cause of cancer death in males and the seventh in females. The primary contributors to HCC include obesity, excessive alcohol intake, tobacco use, and hepatitis virus infections. While most of these risk factors are preventable, the survival rate for HCC remains below 20% [1]. Diverse studies suggest that mitochondrial dysfunction, mitochondrial stress responses, and mitoribosomal defects induced by mitoribosomal protein aberrations in cancer cells are closely associated with tumor growth and metastasis via ROS production in the mitochondria of damaged hepatocytes, metabolic reprogramming, and mitohormetic responses. Therefore, mitochondria represent potential targets for the creation of innovative anticancer therapies [2,3,4,5]. In human hepatoma HepG2 cells, impaired mitochondrial function inhibits the expression of hypoxia-inducible factor-1α (HIF-1α), a crucial transcription factor that regulates cellular responses to low oxygen levels by activating AMPK signaling and inhibiting the mTOR pathway [6].
Recent studies suggest that uncoupling protein-2 (UCP2), an anion carrier located in the inner mitochondrial membrane, could play an important role as a potential target for the development of novel inhibition strategies in cancer treatments [7]. Further, some UCPs are also involved in proton transport by fatty acid cycling across the membrane [8,9]. Elevated expression levels of UCP2 have been reported in various types of leukemia, as well as in malignancies affecting the breast, ovaries, bladder, esophagus, pancreas, kidneys, testes, lungs, prostate, and skin [10]. Recently, UCP2 overexpression has been found to regulate the sensitivity of hepatocellular carcinoma (HCC) cells to antitumor drugs, such as gemcitabine (GEM), and to be negatively correlated with miR-214 expression [11]. Moreover, UCP2 levels were found to increase in correlation with the progression of neoplastic alterations throughout the colon adenoma–carcinoma sequence [12].
Targeting UCP2 in combination with conventional treatments (chemotherapy, radiotherapy, immunotherapy) could promote a beneficial response in patients. However, there are drugs such as genipin or rosiglitazone; their specific mechanism of action on UCP2 is unknown [13]. Furthermore, genipin can lead to cell death in different cancer cell lines, such as gastric, oral, and cervical, by initiating apoptosis and autophagy. It operates by influencing pathways such as mitochondrial dysfunction, JAK/STAT3, and autophagy, which ultimately results in reduced cell growth and heightened apoptotic cell death [14]. Therefore, the design and synthesis of new molecules targeting UCP2 should be a priority in the coming years to counteract tumor progression. Furthermore, the use of biologically active compounds has proven to be a widely used alternative to limit tumor cell growth.
The varieties of oyster mushrooms grown worldwide consist of Pleurotus ostreatus (Oyster mushroom), P. florida (Florida oysters), P. pulmonarius (Lung oysters), P. eryngii (King oysters), P. citrinopileatus (Yellow oysters), P. salmoneostramineus (Pink oysters), and P. cystidiosus (Abalone oysters). Studies indicate that these fungi are rich in different antioxidants, phenolic compounds, flavonoids, proteins, and polysaccharides, all of which enhance their antioxidant effects and medicinal properties [15]. Recent research indicates that protein concentrates derived from Pleurotus spp. mushrooms contain a high amount of hydrophobic amino acids, and the enzymatic hydrolysis of these proteins can enhance antioxidant activity by as much as 10 times. Furthermore, protein fractions under 3 kDa molecular weight demonstrate the greatest antioxidant activities [16]. Consequently, the proteins found in Pleurotus can be utilized to generate biopeptides (BPs).
BPs can inhibit cancer at all stages of the disease, and offer advantages such as their greater affinity, target-specific effects, reducing toxicity, and superior tissue penetration, in comparison to the side effects of chemotherapeutic molecules [17]. Typically, these molecules remain inactive within their parent proteins and are liberated through enzymatic reactions. Despite advances in proteomics, the identification and characterization of anticancer peptides remains a challenge due to the laborious and time-consuming nature of conventional methods; however, bioinformatics tools now enable the easy design of these peptides to target some proteins of interest, including the amino acid sequence, structure, and interaction partners of many oncogenic proteins.
The pink protein derived from the fungus Pleurotus salmoneostramineus (PsPCP) features unique primary sequences that bear minimal similarity to known proteins and displays a red hue in aqueous solutions [18,19]. It is also established that its expression varies throughout the fungus’s life cycle [20,21]. However, the potential biopeptides of this protein, which may possess significant therapeutic properties, remain unidentified. Consequently, this research seeks to characterize the peptides resulting from the in silico proteinase K-mediated hydrolysis of P. salmoneostramineus chromoprotein and evaluate their anticancer potential through molecular docking and dynamics simulations focused on UCP2. Furthermore, the bioinformatics findings were corroborated using cellular oxygen consumption (OCR) assays conducted with the Seahorse XFe96 Extracellular Flux Analyzer on the HepG2 cell line, both in the presence and absence of genipin or P. salmoneostramineus protein hydrolysate (PSPs).
2. Materials and Methods
In this study, in silico analysis enabled the prediction of a wide range of parameters relevant to the identification of bioactive peptides (BPs) and the assessment of their biological activity. For this reason, web-based tools were used to examine the fundamental physicochemical properties of the peptides. To predict the activity of target molecules, quantitative structure–activity relationship (QSAR) modeling, molecular docking, and molecular dynamics simulations were employed (Figure 1A). Furthermore, an in vitro approach using HepG2 cell culture was used to validate the in silico results by determining the effect of the obtained P. salmoneotramineus protein hydrolysate on mitochondrial parameters and cell viability (Figure 1B).
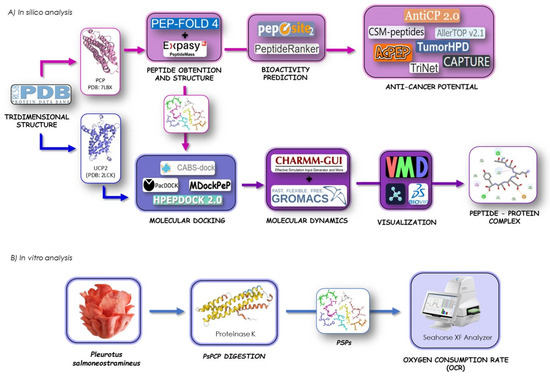
Figure 1.
(A) General workflow of in vitro and in silico analysis for protein digestion, peptide obtention, and interaction study of P. salmoneotramineus peptides and UCP-2 complex by molecular docking, molecular dynamics, and (B) effect of P. salmoneotramineus hydrolysate on oxygen consumption rate (OCR) in HepG2 cell line.
2.1. In Silico Cromoprotein Hydrolysis and Peptide Characterization
The Peptide Mass tool from Expasy (SIB Swiss Institute of Bioinformatics; https://web.expasy.org/peptide_mass/) (accessed on 23 November 2024) was used to determine the sequences, positions, and masses of 8 peptides derived from the proteolytic cleavage of the pink-colored protein (PCP) (PDB: 8II8) (PDB—Protein Data Bank; Research Collaboratory for Structural Bioinformatics; https://www.rcsb.org/structure/8II8) (accessed on 23 November 2024) by proteinase K [22]. Additionally, the 3D structures of the obtained peptides were predicted using the PEP-FOLD4 online server (https://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD4/) (accessed on 23 November 2024) [23].
2.2. Prediction of Anticancer Potential of Peptides
The overall bioactivity of the peptides was estimated using tools available in PeptideRanker (University College Dublin, Ireland; http://distilldeep.ucd.ie/PeptideRanker/, accessed on 23 November 2024) [24], a tool that can order a set of peptides and based on their function–structure models, assign the scores within the range of 0–1, with higher scores indicating higher activity. Peptides with PeptideRanker scores greater than 0.5 were analyzed using Pepsite2 (University of Heidelberg, Germany; http://pepsite2.russelllab.org) (accessed on 23 November 2024) [25], which allows computing the potential interaction between the peptide and target enzymes.
The CSM-peptides server (University of Queensland, Australia; https://biosig.lab.uq.edu.au/csm_peptides/) (accessed on 25 November 2024) was used to classify peptides by therapeutic activity including anti-angiogenic, anti-bacterial, anticancer, anti-inflammatory, anti-viral, cell penetrating, quorum sensing, and surface binding [26]. Subsequently, AllerTOP2 was employed for in silico allergy prediction based on the physicochemical features of proteins (Medical University of Sofia) https://www.ddg-pharmfac.net/allertop_test/, (accessed on 25 November 2024) [27] and ToxinPred was used to predict peptides’ toxicity using a data set of 1805 toxic ones (CSIR-Institute of Microbial Technology, Chandigarh, India; http://crdd.osdd.net/raghava/toxinpred/) (accessed on 28 November 2024) [28]. Additionally, TumorHPD, a web server for predicting and designing tumor-homing peptides (Institute of Microbial Technology, Chandigarh, India; http://osddlinux.osdd.net/raghava/tumorhpd/) (accessed on 28 November 2024) [29], was used to evaluate peptide interactions relevant to cancer targeting. AcPEP was employed to predict anticancer peptide activity across six different cancer types (breast, cervix, colon, lung, prostate, and skin) by sequence-based machine learning methods (University of Macau, Taipa, Macau, China; https://app.cbbio.online/acpep/home/, accessed on 23 November 2024) [30]. Additional computational tools, such as the Comprehensive Anti-Cancer Peptide Predictor (German Research Center for Artificial Intelligence; https://sds_genetic_analysis.opendfki.de/CAPTURE/) (accessed on 28 November 2024), PreTP-Stack (Liu Lab, Beijing Institute of Technology, China; http://bliulab.net/PreTP-Stack/, accessed on 23 November 2024) [31], Triad-Complex based Network (Trinet) (Shandong University, Weihai; http://liulab.top/TriNet/server, accessed on 23 November 2024), and AntiCP-2 (Raghava Lab at the International Institute of Information Technology (IIIT) Delhi; https://webs.iiitd.edu.in/raghava/anticp2/predict.php) (accessed on 30 November 2024) were also used. These tools are designed to predict peptides with potential anticancer properties, aiming to identify peptide sequences that can interact with cancer cells and exert therapeutic effects such as inducing apoptosis (programmed cell death), inhibiting tumor growth, or modulating immune responses.
2.3. Prediction of Peptide–Protein Interactions and Molecular Docking
To assess interactions between the peptides and their respective receptors, receptor grids were generated for the prepared protein structures, ensuring peptide binding within predicted active sites. These grids were generated with default values for van der Waals scaling factor (1.00) and charge cutoff of 0.25. A cubic search space, centered on the centroid of the active site residues predicted by PepSite 2, was generated for each receptor.
The UCP2 model was obtained from the deposited NMR structure (PDB ID: 2LCK). The peptide structures were derived from their amino acid sequences and converted to SMILES format using PepSMI (NovoPro Bioscience Inc.; https://www.novoprolabs.com/tools/convert-peptide-to-smiles-string) (accessed on 30 November 2024), followed by conformational optimization using cheminformatics tools and databases for pharmacology (Université Côte d’Azur; https://chemoinfo.ipmc.cnrs.fr/) (accessed on 30 November 2024) [32].
Docking simulations of peptides to UCP2 were performed using PacDock, HPEPDOCK 2.0, MDockPeP, and CABS-dock. PacDock is a novel web service for the fully automated detection and visualization of relevant non-covalent protein–ligand contacts in 3D structures (University of Bologna, Italy; https://pegasus.lbic.unibo.it/pacdock/PacVIEW_Receptor_Ligand_Interactions.html) (accessed on 5 December 2024) [33]. The PEPDOCK 2.0 (http://huanglab.phys.hust.edu.cn/hpepdock/) (accessed on 5 December 2024) server predicts the complex structure between a protein and a peptide through a hierarchical flexible peptide docking approach by fast conformational modeling and orientational sampling of peptides [34]. MDockPeP server (Zou Lab, University of Missouri; https://zougrouptoolkit.missouri.edu/mdockpep/index.html) (accessed on 5 December 2024) generates protein–peptide complex structures starting with the protein structure and the peptide sequence [35]. Each docking server generated at least 10 models, and the protein–peptide complexes with the highest binding scores were selected for further analysis. Moreover, CABS-dock (Laboratory of Computational Biology; https://biocomp.chem.uw.edu.pl/CABSdock/) (accessed on 5 December 2024) provides a comprehensive library of trajectories, enabling the assessment of ligand interaction dynamics and complex stability throughout the simulation [36]. Visualization of all peptide–protein complexes was performed in PyMOL 3.0.3 (Schrödinger; https://www.pymol.org/) (accessed on 5 December 2024), while ligand–receptor residual and atomic interactions were determined by BIOVIA Discovery Studio Visualizer (Dassault Systemes; https://www.3ds.com/) (accessed on 5 December 2024).
2.4. Molecular Dynamics of Peptide–UCP2 Complexes
To gain a deeper understanding of the interactions between peptide ligands and UCP2, membrane protein molecular dynamics (MD) simulations were performed using the best docking-derived models positioned within the protein channel. Three systems were generated: membrane–UCP2, membrane–UCP2 + PCP–peptide QEGQKL, and membrane–UCP2 + PCP–peptide PGSHPV. Firstly, the UCP2 (2LCK) structure was prepared in PyMOL 3.0.3, where all arginines and lysines were set in their protonated forms, histidines and cysteines in their neutral forms, glutamates and aspartates in their deprotonated forms. Then, the respective ligand was incorporated into a ligand–protein complex. Subsequently, a membrane–protein model was constructed using the Membrane Builder module of CHARMM-GUI (Lehigh University; http://www.charmm-gui.org/) (accessed on 20 December 2024) [37,38,39]. The system was parametrized with the CHARMM36m force field and TIP3P water model [40,41]. The membrane was composed of 230 DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) molecules (115 in the upper leaflet and 115 in the lower leaflet), 22,907 water molecules, and 0.15 M KCl to neutralize the system. All production and analysis of MD simulations were conducted in a total simulation of 50 nanoseconds in the GROMACS 2022.6 software package and visualized with the VMD (Visualize Molecular Dynamics) program (University of Illinois at Urbana-Champaign; https://www.ks.uiuc.edu/Research/vmd/; https://www.charmm-gui.org/) (accessed on 20 December 2024) [42,43]. Temperature coupling was performed using the velocity-rescaling (V-rescale) thermostat, with separate coupling groups for the solute, membrane, and solvent, each set to a reference temperature of 303.15 K. Pressure was controlled using a cell-rescaling (C-rescale) barostat with a reference pressure of 1.0 bar. Also, pressure coupling was set to semiisotropic, allowing the box to deform uniformly in the x-y plane and independently in z, showing natural fluctuations in membrane thickness and area. Periodic boundary conditions (PBCs) were applied throughout the entire simulation. After all, the system was recentered for visualization and trajectory analysis.
2.5. Preparation of the Protein Hydrolysate from P. salmoneostramineus (PSPs)
To obtain protein hydrolysate from P. salmoneostramineus (PSPs), we used dry mushrooms cultivated at Taretan, Michoacán, México (19°20′00″ North and 101°55′00″ West) [16,44]. The commercial protease used in this study was proteinase K produced by the fungus Tritirachium album Limber (Promega MC500B). P. salmoneostramineus was isolated by alkaline solubilization and acid deposition methods. Alkaline solubilization was carried out at 40 °C for 2 h at pH 9 and the solid–liquid ratio was 1:80. After centrifuging at 5000 rpm for 15 min, the supernatant was adjusted to pH 3.5. The precipitated protein was collected and dissolved in water to confirm that the mass fraction of a solution was 8%. Proteinase K was used for protein digestion at 45 °C for 2 h to obtain PSPs. The protease was inactivated by heating at 95 °C for 15 min. Ethanol was added while stirring until the final concentration was 60%. Ultimately, the degree of hydrolysis reached 60%. The supernatant was collected after centrifugation and filtered through a 0.45 μm microporous membrane. The PSPs were collected after freeze-drying.
2.6. Cell Culture
HepG2 cells were grown in 75 cm2 flasks in Dulbecco’s modified Eagle’s medium (DMEM) (5 mM glucose) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin and kept at 37 °C with 5% CO2. Media was changed every third day until confluence was reached. Then, cells were divided into three groups: control (C); 30 µM genipin (G); and 150 µg/mL P. salmoneostramineus protein hydrolysate (PSPs). The cells were treated with indicated concentrations of G or PSPs for 24 h, and cells without compound treatment served as a control for all analyses. Cells were detached with trypsin and resuspended in DMEM to inactivate trypsin, then centrifuged at 600× g for 10 min to recover the cellular pellet.
2.7. Oxygen Consumption Rate (OCR)
The XFe96 Extracellular Flux Analyzer (Seahorse XF, Seahorse Bioscience Inc., Santa Clara, CA, USA) is a 24-well device that monitors the oxygen consumption rate (OCR) in real time. Sensor cartridges, which include a fluorescence probe, were soaked in XF Calibrant (Agilent Technologies) the day prior to the assays, following the manufacturer’s guidelines, and were loaded with freshly prepared inhibitors rotenone and antimycin A, 30 min before conducting the assay. Before initiating the XF assay, the biosensors were calibrated separately using the standard automated procedure.
OCR measurements were conducted using the XF96 Extracellular Flux analyzer (Seahorse Bioscience). Briefly, cells were seeded at 3 × 105/well in XF96 cell culture plates and were incubated for 24 h in a humidified 37 °C incubator with 5% CO2. Before starting, growth medium was replaced with appropriate assay medium. All experiments were performed at 37 °C. Each measurement cycle consisted of a mixing time. OCR data points referred to the average rates during the measurement cycles. All compounds were prepared at appropriate concentrations in the desired assay medium and adjusted to pH 7.4. In a typical experiment, 3 baseline measurements were taken prior to the addition of any compound, and 3 response measurements were taken after the addition of each compound. The OCR was reported as absolute rates (pmoles/min/protein O.D).
Usually, at the conclusion of each assay, cells were separated by treatment with 0.25% trypsin (Invitrogen, Waltham, MA, USA), and the count and proportion of viable cells were assessed using a trypan blue exclusion assay with a ViCell (Beckman-Coulter, Fullerton, CA, USA). The cell viability of all treated samples was comparable to control cells in acute assays that lasted one hour or less.
2.8. Protein Quantification Assay
At the end of the Seahorse experiments, samples were lysed through Dounce–Potter homogenization into 100 µL of ice-cold RIPA buffer (Sigma-Aldrich, Merck, Toluca, Mexico) and kept at −20 °C until analysis. The protein quantification assay was performed with Pierce™BCA Protein Assay Kit (ThermoFisher Scientific, Mexico City, Mexico) following the manufacturer’s instructions.
2.9. Statistical Analysis
The results from three separate experiments were gathered and statistically evaluated using one-way analysis of variance (one-way ANOVA), followed by Tukey’s honestly significant difference (HSD). A probability of p < 0.05 suggested significant statistical differences.
3. Results
3.1. Pink Chromoprotein Hydrolysis and Peptide Physiochemical Parameters
The peptides selected for this study were obtained by in silico digestion of the Pleurotus chromoprotein with proteinase K with the PeptideMass server (Table 1). In addition, its reactivity was analyzed with the Peptide Ranker server. The PGSHPV peptide turned out to be the most reactive while the SQTTKESPSA peptide was more stable. The half-life of the peptides was assessed using the HLP web server. According to the said server, most of the peptides identified in this work have a high half-life. GRNSL shows a low half-life of less than 1.3 sec, while the peptides PGSHPV, QEGQKL, and SEDSGEA demonstrated a high half-life (Table 1). The AllerTop v2.1 web server predicts that peptides SQTTKESPSA, PQEDKSA, TSQESY, and PGSHPV are not potential allergens. However, peptides TSMQSSL, QEGQKL, and SEDSGEA are potentially allergenic. Finally, GRNSL was not analyzed since a minimum sequence of six residues is required to be reliably analyzed by AllerTop v2.1. Further, we employed the ToxinPred server to examine the toxicity of peptides. The results suggest that GRNSL was the only one with the potential to be toxic.

Table 1.
Physicochemical properties of P. salmoneostramineus chromoprotein biopeptides.
3.2. Peptide Therapeutic Bioactivity and Anticancer Potential
The CSM-peptides platform serves as an effective resource for identifying therapeutic peptides across eight distinct categories: anti-angiogenic (AAP), anti-bacterial (ABP), anticancer (ACP), cell penetrating (CPP), anti-inflammatory (AIP), anti-viral (AVP), quorum sensing (QSP), and surface binding (SBP). The likelihood scores (ps) indicate the classification of peptides into these categories. The possible therapeutic activities of eight peptides from P. salmoneostramineus are shown in Table 2. All peptides can have multiple activities where anti-angiogenic, anticancer, anti-inflammatory, and quorum sensing activities stand out. Only four peptides presented anticancer activity (TSMQSSL, QEGQKL, SEDSGEA, and GRNSL) and two of them anti-angiogenic activity (SQTTKESPSA and PQEDKSA). However, none of these have anti-bacterial and anti-viral activity. Finally, it is interesting to mention that TSMQSSL, PGSHPV, and GRNSL present very high quorum sensing activity. Therefore, these other activities could be analyzed in depth in subsequent studies.

Table 2.
Therapeutic activity prediction for PCP peptides by CSM-peptides.
Tumor-homing peptides (THPs) are short peptides (3 to 15 amino acids), which specifically recognize and bind to tumor cells or tumor vasculature. To confirm that anticancer activity is present in all peptides and which of them are THPs, we used other servers available online (SCMTHP, mACPred, TumorHPD, PreTP-Stack, TriNet, AntiCP, and CAPTURE). The CAPTURE server identified the potential target tissues for these eight BPs. Specifically, SQTTKESPSA and SEDSGEA were predicted to have an effect over prostate and breast cancer cells, respectively, while TSQESY and QEGQKL were associated with activity against skin ones. The rest of the peptides (PQEDKSA, TSMQSSL, PGSHPV, and GRNSL) were linked to colon-affected cells (Table 3). Although the PreTP-Stack and TriNet servers only recognized PGSHPV as an anticancer peptide, mACPred determined TSMQSSL, QEGQKL, PGSHPV, and GRNSL as having high anticancer potential (<0.5) (Table 3). Furthermore, only the last two of these were recognized as THPs by SCMTHP and TumorHPD. Conversely, AntiCP 2.0 indicated that TSMQSSL and SEDSGEA were categorized as non-anticancer peptides (Table 3).

Table 3.
Classification as THPs and anticancer potential activity for P. salmoneostramineus chromoprotein peptides.
3.3. Prediction of the GDP Binding Site in UCP2 Structure
UCP2 is an antioxidant that inhibits the production of ROS in mitochondria [45]. It has been shown that lipid and protein oxidation are increased in HepG2 cells exposed to ROS, while this increase significantly reduced the expression of UCP2 under the same conditions. UCP2 can limit the oxidative damage of HepG2 cells under oxidative stress, thereby improving cell function and anti-apoptotic ability [46].
UCP2 is inhibited by purine nucleotides [47,48]. There is one mechanism proposed for UCP1 inhibition by purine nucleotides in the late 1990s [49]. According to this mechanism, three arginines (R83, R182, and R276), which are localized in the central cavity of UCP1, bind the phosphate groups of di- and tri-phosphate purine nucleotides, leading to a conformational change in protein and inhibition of proton transport. This regulatory mechanism has generally been extended to other UCPs, because these arginines are conserved in all UCP homologs. In mouse UCP2, residues Arg88, Arg185, Arg279, and Lys141 are important for mediated inhibition [50,51].
In this study, GDP binds deeply in the central cavity of UCP2, with the diphosphate moieties positioned to interact with the positive electrostatic potential at the base, generated by R88, R185, and R279 (the arginine triplet) (Figure 2). GDP forms salt bridge and hydrogen bond interactions with the side chains of multiple residues, many of which are functionally important. The β-phosphate of GDP forms two salt bridges to R185. The α-phosphate of GDP forms a salt bridge to R279, a contact point of the substrate-binding site in other UCPs. The GDP ribose group forms a hydrogen bond to R279 and the guanine group forms two salt bridges to D28 and hydrogen bond to K141.
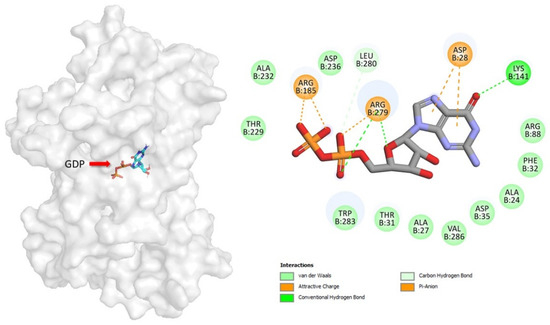
Figure 2.
Geometric positions of key GDP–residue interactions into UCP2 structure. The UCP2 residues are indicated by colored circles, while the GDP is indicated by element-colored sticks. Hydrogen bonds are represented as green dashed lines, and salt bridge interactions are indicated with dark yellow.
3.4. Peptide Affinity for UCP2 and Molecular Docking
After identifying promising anticancer peptides, we must investigate the mechanism by which they exhibit bioactivities. Molecular docking is an in silico method for explaining the interaction of ligands (BPs) and receptors (target proteins) at the atom level [52]. We used servers (PepSite2, MDockPeP, and HPEPDOCK 2.0) to perform a molecular docking analysis of peptides based on anticancer, toxicity, and allergic predictions. The PepSite2 server informed us that all peptides bind to the UCP2, although QEGQKL has the highest chance, but this was a preliminary conclusion (Table 4). MDockPeP and HPEPDOCK 2.0, on the other hand, calculate peptide–receptor binding energy based on bioactivity study. MDockPEP (ITScorePeP) reveals that the SQTTKESPSA peptide has the best binding energy, while HPEPDOCK 2.0 indicates that the TSMQSSL peptide has the highest affinity to the receptor (−166.75 kcal/mol), with PGSHPV coming in second (−164.64 kcal/mol) (Table 4).

Table 4.
Molecular docking between UCP2 and P. salmoneostramineus chromoprotein peptides.
Based on the above information, we decided to use the crystallographic structure of UCP2 to determine if the peptides with anticancer property could interact with this mitochondrial protein. To gain more insight into how UCP2 interferes with QEGQKL and PGSHPV, molecular docking was applied to elucidate interactions between the peptide and receptor. The UCP2 showed the greatest peptide binding affinity of −166.75 kcal/mol with TSMQSSL (Table 4). In Figure 3A, the molecular docking results showed that the QEGQKL peptide could bind UCP2 tightly by hydrogen bonding and hydrophobic interactions. The 5-oxo group of glutamine 1 of the peptide forms a hydrogen bond with Ala24 and the amino group at position 2 of glutamine forms a salt bridge with Phe92 of UCP2, while glutamine 4 of the peptide interacts with residue Asp28 of UCP2 by a hydrogen bond and the amino group at position 5 of glutamine 4 of the peptide forms three carbon hydrogen bonds with residues Asp28, Phe32, and Thr31 of UCP2. In addition, the carboxyl group of leucine 6 of the peptide formed a salt bridge or cation-Pi with Arg185 and Trp283 of UCP2 (Figure 3A), while the PGSHPV peptide shows hydrogen bonds and alkyl interaction with the UCP2. The pyrolidine group of proline 1 of the peptide forms a salt bridge with residue D138 of UCP2. In addition, D138 forms three hydrogen bonds with the amide bonds of the Gly2, Ser3, and Hys 4 residues of the peptide and the 1H-imidazol-4-yl group of histidine 4 forms a hydrogen bond with D138 of UCP2, while the pyrolidine group of proline 5 of the peptide has alkyl and Vander walls interactions with Phe92 of UCP2. Finally, the valine residue 6 of the peptide forms three alkyl interactions through its branched chain with Leu99, Val95, and Phe92 (Figure 3B). It is important to mention that the QEGQKL peptide binds in the same cavity of the guanine binding site and residues Arg185 and Arg279 are critical for the binding of both the peptide and GDP (compare Figure 2 with 3A). Therefore, this QEGQKL peptide could have the same inhibitory action as GDP.
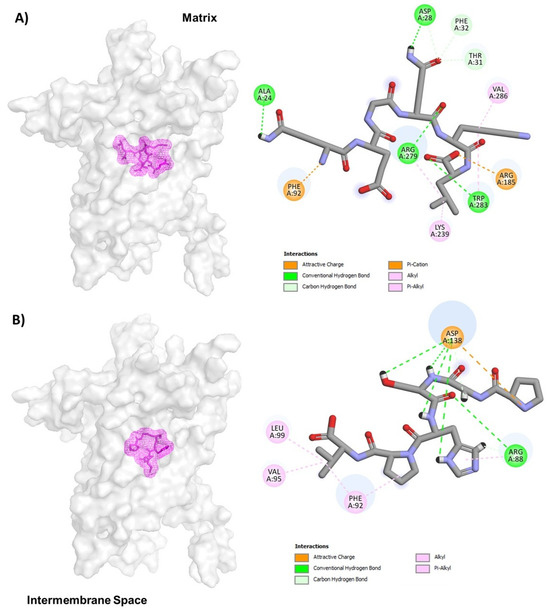
Figure 3.
Representation of UCP-2 molecular docking for QEGQKL (A) and PGSHPV (B) peptides. The conformations with the lowest docking energy were selected for demonstration. UCP2 is shown in surface representation (A,B), whereas peptides are shown as mesh representation in pink.
3.5. Peptide Translocation Mechanism Through UCP2
The blind molecular modeling analysis showed that the peptides have multiple binding sites inside the UCP2. This pattern indicates that the translocation of the peptides toward the nucleotide binding site occurs through several tunnels or channels. Considering the role of UCP2 as a transporter of C4 metabolites from the mitochondrial matrix to the intermembrane space, it is possible that the reported peptides use a mechanism like those metabolites. To demonstrate this hypothesis, molecular docking and molecular dynamics approaches were used to assess the conformational changes in UCP2. Figure 4A shows that the decapeptide SQTTKESPSA (yellow) has multiple docking poses that, when considered sequentially, suggest a potential path from the mitochondrial matrix to the nucleotide binding site. Surface interactions with Arg40, Arg60, and Arg71 appear to facilitate initial binding, while deeper interactions with Arg88, Arg 185, and Arg279 may stabilize the peptide within the UCP2 tunnel. In contrast, the heptapeptide PQEDKSA (pink) docking poses showed that it could go from the cytosolic side and get into the nucleotide binding site in the center of UCP2 (Figure 4B). In these poses, the hydrophobic residues (Ala24, Phe92, Val142, Gly178, and Val286) and hydrophilic residues (Asp28, Asp35, Lys141, Arg185, Lys239, and Arg279) may guide the peptide toward the central binding site. These docking results highlight key UCP2 residues that can allow both peptides to make different interactions around the protein channel from both sides of the mitochondrial membrane of cancer cells. It is important to note that molecular docking provides static snapshots of peptide–receptor interactions. Then, we decided to run molecular dynamic simulations to fully understand the binding process and conformational changes involved.
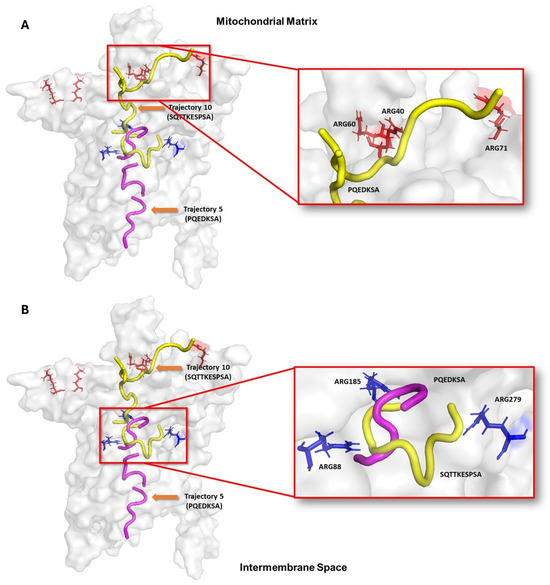
Figure 4.
Representation of peptide translocation through the UCP-2 channel. Ligands are shown in yellow and purple; and active site residues are shown in orange and blue bars. Trajectories were built from the results of molecular modeling with the HPEPDOCK 2.0, MDockPeP, and CabsDock servers. (A) shows how peptide SQTTKESPSA initially interacts with arginines 40, 60 and 71 on the mitochondrial matrix side and can subsequently position itself in the central part of UCP structure. (B) Peptide PQEDKSA initially binds to the cytosolic side and moves toward the same central region of the protein to interact with arginines 88 and 185.
3.6. Molecular Dynamics Simulations for Peptide–UCP2 Complexes
Several studies have suggested that the conformational changes between the open and closed forms may regulate the catalytic activity of UCP2. The NMR structure of UCP2 is known to be fully open and water-permeable, which is expected to be incompatible with controlled proton transport [53]. Our results suggest that both the core and gate elements undergo substantial conformational changes during the binding of the peptide to the c state (Figure 5). The control (blue) exhibits the c state, with distances of 9.2 Å between lysine 141 and glutamate 35 and 8.4 Å between glutamate 138 and lysine 239 (see Figure 5A). The PGSHPV peptide interacts with the UCP2 tunnel, causing a conformational shift to the m state. The distances between lysine 141 and aspartate 35 were 7.6 Å and between aspartate 138 and lysine 239 were 5.5 Å, indicating a closed conformation (pink) (see Figure 5B).
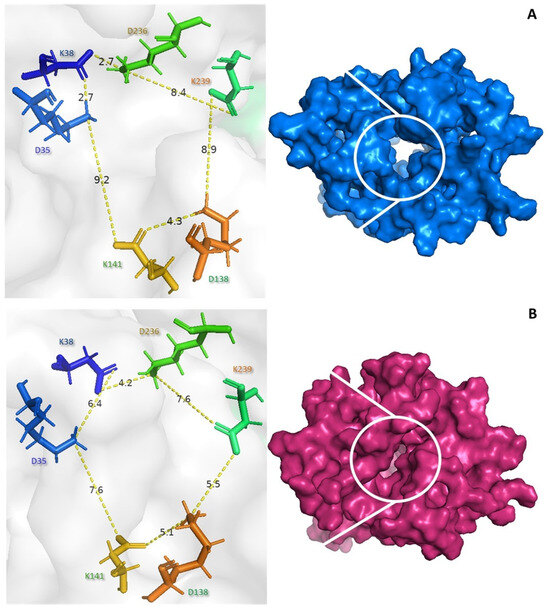
Figure 5.
Conformational changes in UCP2 by the PGSHPV peptide binding. Surface view of the free UCP2 (A) and peptide–UCP2 complex (B) viewed from the mitochondrial matrix.
In the MD simulations, it is possible to estimate how distinct parts of a molecule fluctuate at equilibrium and experience structural dynamic differences throughout a period of time [54]. Therefore, MD simulations were carried out to further characterize the dynamic behavior of the QEGQKL peptide in complex with UCP2. Data showed that according to the Root Mean Square Deviation (RMSD) plots, the complex was stable during the simulation time after a period of stabilization during the first 20 ns (Figure 6A). On the other hand, the Root Mean Square Fluctuations (RMSF) plot demonstrated important fluctuations in topological domains in the mitochondrial intermembrane and matrix (Figure 6B). The topological domains contain important residues for the opening and closing of the tunnel; it is of note that Arg20 and Arg21 are both in this region. Arg20 participates in the regulation site, and the high mobility of this region is consistent with that reported in other UCPs. Radius of gyration (RG) and Solvent-Accessible Surface Area (SASA) plots indicated that the complex protein remained in a compact state, which further confirms the stability of the binding (Figure 6C,D).
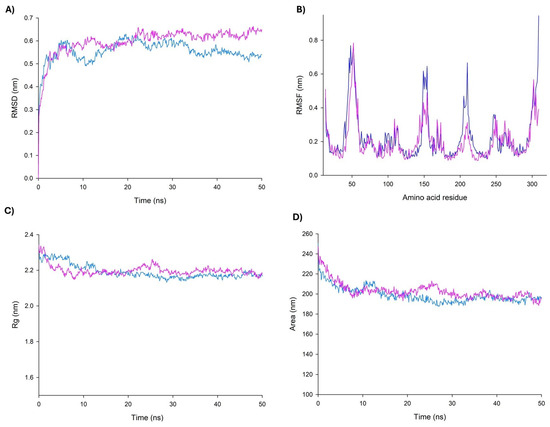
Figure 6.
Graphical parameters of molecular dynamics simulation for UCP2–peptide complex. Comparison of changes in RMSD (A), RMSF (B), RG (C), and SASA (D) values of UCP2 (blue) and UCP2–peptide (pink) along the simulation time (50 ns).
3.7. Effect of PSPs on Uncoupling Activity in HepG2 Cells
Hydrolysates and peptides isolated from food proteins have shown antiproliferative effects on cancer cells. The bioactive peptides from cowhide collagen (BPCC) significantly inhibited the cell viability of HepG2 cells. In addition, BPCC induced a decrease in mitochondrial membrane potential (MMP) in HepG2 cells. Therefore, the present finding proved that BPCC encompasses significant antioxidant activity and anticancer property on HepG2 cells and can be used as alternative food antioxidants for cancer prevention benefits [55].
We employed the Seahorse XFe96 analyzer to evaluate the impact of PSPs on the oxygen consumption rate (OCR) in HepG2 cells. The three groups’ OCR values were all adjusted to the total protein concentration, and the parameters that were examined were substrate oxidation and proton leak, which regulate basal respiration (State II). Basal respiration shows the energetic demand of cells under basal conditions and the oxygen consumption of basal respiration used to meet ATP synthesis, which results in mitochondrial proton leak. ATP-linked respiration is reflected by the decrease in the OCR following the injection of the ATP synthase inhibitor oligomycin, which is the portion of basal respiration. The remaining basal respiration not coupled to ATP synthesis after oligomycin injection represents the proton leak, which can be a sign of UCP activity. Maximal respiration represents the maximum capacity that the electron respiratory chain can achieve. The maximal oxygen consumption rate is measured by the injection of the uncoupler FCCP. Spare respiration is the difference between the maximal and basal respiration, which reflects the capability of the cells to respond to changes in energetic demand and indicates the fitness of the cells. Non-mitochondrial respiration is oxygen consumption due to oxidation reactions other than mitochondria after the injection of rotenone and antimycin A. On the other hand, genipin has been used to study the biology of UCP2, and it has been used in a number of experiments to block UCP2 in cancer cells. As Kreiter et al. have shown, genipin interacts with arginine residues in the UCP channel, resulting in a decrease in proton transport function in the presence of long-chain fatty acids [56]. Then, we used genipin as a control to compare the effects of P. salmoneostramineus protein hydrolysate by proteinase K.
To elucidate the potential mechanisms that contribute to the anticancer effects of PSPs, we found that genipin significantly lowered the viability of HepG2 cells by 61.6 ± 4.3%. In a similar manner, PSPs resulted in a 35% ± 7.6% reduction in the viability of HepG2 cells. To investigate the effects of genipin and PSPs on cellular metabolism, we measured the oxygen consumption rate (OCR), which is an indicator of mitochondrial respiration in HepG2 cells (Figure 7). The results demonstrate that genipin had a statistically significant adverse impact on the OCR of HepG2 cells, negatively affecting all mitochondrial respiration processes. Additionally, PSPs were shown to decrease basal respiration, ATP-linked respiration, and spare respiration.
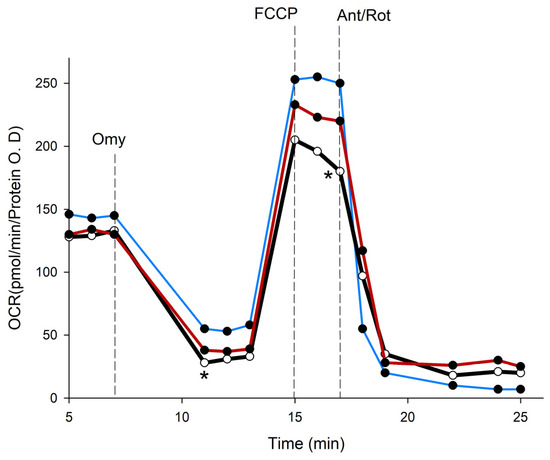
Figure 7.
Absolute change in mitochondrial function in intact HepG2 cells (blue stroke) and treated with genipin 30 µM (red stroke) and PSPs 150 µg/mL (black stroke). Arrow indicates the subsequent addition of oligomycin (Omy, 1 µM) at 8 min inhibits ATP production, resulting in a decrease in the oxygen consumption rate (OCR). The OCR increases in all treatments following the addition of FCCP (0.5 µM) at 15 min (uncoupled state). Electron transport chain inhibitors mix [Antimycin A (0.5 µM) and Rotenone, (0.5 µM)] decrease the oxygen consumption rates to very low levels, inhibiting total mitochondrial respiration at 25 min. * significant difference against control p ≤ 0.05 and p ≤ 0.001.
Basal respiration in cells treated with either genipin or PSPs was reduced compared to the control cells. In the case of ATP-linked respiration, it was not affected by the presence of genipin. In contrast, cells exposed to PSPs exhibited an 11% increase in the respiratory rate relative to the controls (Table 5). Notably, a proton leak, which is indicative of UCP activity, was significantly affected by both treatments, showing a 93% reduction with genipin and a 98% reduction with PSPs. Maximal respiration was similarly reduced in both treated groups compared to the control cells. Furthermore, PSPs completely abolished the spare respiratory capacity (SRC; Table 5). Finally, non-mitochondrial respiration was elevated in cells treated with genipin and PSPs relative to the control (Table 5). These findings suggest that the peptides present in PSPs may resemble the bioenergetic effects of genipin.

Table 5.
Impact of genipin and PSPs on OCR in HepG2 cells.
4. Discussion
Metabolic changes accompany liver carcinogenesis. During the progression of liver cancer, gluconeogenesis, detoxication, bile acid metabolism, and other typical hepatocyte metabolic functions are decreased, and these metabolic changes are accompanied by an increase in tumor progression [57]. In addition, liver cancer cells require much energy for growth. Mitochondrial metabolism in cancer cells is often altered to balance energy production with the need for biosynthetic precursors required for rapid cell proliferation. These alterations can include changes in the tricarboxylic acid (TCA) cycle and mitochondrial oxidative phosphorylation (OXPHOS), tailored to support the anabolic and catabolic demands of the tumor [58].
In malignant cells, the TCA cycle is substantially altered to fulfill the elevated demands for energy and biosynthetic precursors necessary for rapid growth and proliferation [59]. Reconfiguring the TCA cycle aids cancer cells in sustaining redox stability. The intermediates derived from the TCA cycle are important for the production of nicotinamide adenine dinucleotide phosphate (NADPH), a key reducing equivalent that is vital for addressing oxidative stress and enhancing reductive biosynthesis [60].
Mitochondrial carriers are a family of proteins that transport a diverse range of nucleotides, amino acids, inorganic ions, fatty acids, keto acids, and cofactors across the inner mitochondrial membrane [61]. Mitochondrial uncoupling proteins (UCPs) are a part of the large family of mitochondrial solute carriers (SLC25s), concentrated in the inner mitochondrial membrane that carries protons from the intermembrane space to the matrix. Over the last decade, a better understanding of UCP2’s biochemical and physiological functions has revealed that it plays a role in protecting cells from oxidative stress, regulating tumor progression through changes in glycolytic, oxidative, and calcium metabolism, and increasing antitumor immunity in the tumor microenvironment to limit cancer growth. With these pleiotropic roles, UCP2 can be thought of as a potential tumor biomarker that could be targeted positively or negatively, depending on the type, metabolic status, and stage of the tumor, in combination with conventional chemotherapy or immunotherapy to control tumor development and increase response to treatment [13].
Genipin has been demonstrated to have hepatoprotective function, acting as an efficient antioxidant and inhibitor of mitochondrial UCP2, and has also been linked to strong anticancer effects [62]. Then, high UCP2 expression may restrict elesclomol’s antitumor efficacy by inhibiting ROS responses, which can be countered by co-treatment with genipin; combining elesclomol and genipin may be a viable method for treating malignancies with high UCP2 [63]. Three arginines are key in the inhibition of UCPs by genipin and demonstrate that the molecular mechanisms of UCP inhibition by genipin and purine nucleotides might be similar [56]. However, its specificity for UCP2 is uncertain, and the underlying mechanism of action remains unknown. Our results (Table 5) confirm that genipin is able to inhibit the growth of HepG2 cells by altering their mitochondrial metabolism and these findings are in agreement with previous reports [62]. Similarly, PSPs inhibit UCP activity and affect other parameters of mitochondrial bioenergetics in HepG2 cells (Figure 7). These results agree with the observed reduction in cell proliferation in UCP2-silenced cells may account for the diminished demand for cellular ATP. The findings suggest that UCP2 does not operate as an uncoupling protein; rather, they support its role as a C4-metabolite carrier that is involved in the metabolic adaptations of proliferating cells [64]. Finally, UCP2 loss in PDAC cells reduced glutamine catabolism, increased ROS, and induced a shortage of aspartate, but only in KRAS mutant lines, supporting the fact that UCP2 is vital for glutamine-dependent tumors such as PDAC [65].
Although PSPs are a complex mixture of peptides, we consider that chromoprotein represents more than 60% of the total protein of the fungus and is therefore the main source of biopeptides in PSPs. Therefore, the bioinformatic data confirm that anticancer peptides obtained from the in silico hydrolysis of P. salmoneostramineus chromoprotein are potentially useful for the treatment of colon, skin, prostate, and breast cancer. Eight novel bioactive peptides, namely, SQTTKESPSA, PQEDKSA, TSMQSSL, TSQESY, QEGQKL, SEDSGEA, PGSHPV, and GRNSL, were identified within the P. salmoneostramineus chromoprotein through enzymatic digestion using proteinase K. Peptides TSQESY, PQEDKSA, and SEDSGEA exhibit a net negative charge at physiological pH. In contrast, GRNSL exhibits a positive charge, and the other four peptides are uncharged. Furthermore, the peptides have a molecular weight between 545.6 and 1035.08 g/mol and none of them can penetrate the cell according to the MLCCP 2.0 and CellPPD servers. However, TSMQSSL, PGSHPV, and GRNSL exhibited the characteristic of lodging in tumors according to the SCMTHP and TUMORHPD servers. It has been reported that THPs contain several typical motifs like arginine–glycine–aspartic acid (RGD) and asparagine–glycine–arginine (NGR) peptides, which specifically bind to the molecules on the surface of cancer cells or tumor blood vessels [66].
It is important to note that the peptides used in this study can present multiple activities that must be studied. For example, TSMQSSL, PGSHPV, and GRNSL peptides present very high anti-quorum sensing activity (Table 2). The quorum-sensing peptides are mainly secreted by Gram-positive bacteria and can “communicate” with human cells. Some sensing peptides have been shown to promote angiogenesis, tumor cell invasion, and metastasis of colon cancer as well as of breast cancer cells. On the other hand, quorum-sensing cyclodipeptides produced by bacteria and fungi have shown significant antitumor activities [67]. Therefore, it is an area of opportunity that should be explored in depth.
On the other hand, five peptides also possess anti-inflammatory activity. Inflammation is often associated with the development and progression of cancer (Table 2). The cells responsible for cancer-associated inflammation are genetically stable and thus are not subjected to the rapid emergence of drug resistance; therefore, the targeting of inflammation represents an attractive strategy both for cancer prevention and for cancer therapy. Tumor-extrinsic inflammation is caused by many factors, including bacterial and viral infections, autoimmune diseases, obesity, tobacco smoking, asbestos exposure, and excessive alcohol consumption, all of which increase cancer risk and stimulate malignant progression. In contrast, cancer-intrinsic or cancer-elicited inflammation can be triggered by cancer-initiating mutations and can contribute to malignant progression through the recruitment and activation of inflammatory cells. Both extrinsic and intrinsic inflammations can result in immunosuppression, thereby providing a preferred background for tumor development [68]. In addition, our results agree with various studies that have shown that extracts derived from oyster mushrooms are rich in polysaccharides like β-glucan and other macro molecules that have an antiproliferative effect against cancer cell lines, without harming the normal cells [69].
The results in Table 3 suggest that the peptides could be more effective for skin or colon cancer. These results correlate with the findings reported by Jedinak in 2008 who reported that P. ostreatus (oyster mushroom) suppressed the proliferation of breast cancer (MCF-7, MDA-MB-231) and colon cancer (HT-29, HCT-116) cells, without affecting the proliferation of epithelial mammary MCF-10A and normal colon FHC cells [70]. Moreover, MCF-7 cells were attenuated in a concentration-dependent manner by the administration of anthraquinone purified from P. ostreatus compared with the standard anticancer drug paclitaxel [71].
Finally, these peptides showed significant anticancer activity and uncoupling protein 2 (UCP2) inhibitory activity. Molecular docking analyses elucidated various interaction mechanisms between these peptides and the UCP2 receptor protein, including hydrogen bonding and hydrophobic interactions. This suggested that the peptides effectively suppress UCP2 by forming hydrogen bonds with the active pockets of mouse UCP2 with a high affinity. These results were correlated in part with the inhibitory effect of proton leak by PSPs (Table 5). Nonetheless, we did not conduct peptide separation and identification to verify whether the PSPs exclusively contain the eight identified peptides or additional ones. Consequently, it is essential to purify and characterize the chromoprotein peptides to ascertain if they interact solely with the UCP or if they also engage with other mitochondrial targets that may enhance anticancer effects.
5. Conclusions
In the present study, the effect of the peptides derived from the P. salmoneostramineus chromoprotein against UCP2 related to cancer was demonstrated by in silico testing. PeptideRanker, ToxinPred, AllerTOP v2.1, Pepsite2, and HPEPDOCK2.0 were used to predict the bioactive potential, toxicity, allergenicity characteristic, binding interactions, and probable binding conformations, respectively. It can be concluded that QEGQKL, PGSHPV, and GRNSL are biopeptides with UCP2 inhibitory activity in silico. Moreover, molecular dynamics simulations were used to study the stability and intermolecular interactions of peptides and UCP2 complexes to reveal the mechanism of peptides in this study. Peptides blocked UCP2 mainly through salt bridge, hydrophobic, and hydrogen bonding interactions. The conformational flexibility of the complex system during the simulation time indicated that peptides affected the conformational changes in UCP2 through strong interactions of hydrogen bonds with active domain residues Arg185 and Lys239. The mechanism of peptide inhibition and conformational behavior of UCP2 were investigated using MD simulations. The initial channel of the UCP2 structure undergoes conformational changes as the peptides reach the nucleotide binding site to form a funnel by closing the cytosolic side. The results also showed that PSPs reduced HepG2 cell viability in a time- and dose-dependent manner. To our understanding, this is the first report of the antiproliferative activity of these biomolecules on liver cancer cells. This study opened a new avenue for PsPCP and derived peptides as components in the development of nutraceutical formulations for treatment against cancer.
Author Contributions
Conceptualization, E.S.-C.; methodology, E.K.V.-G.; software, A.T.-V. and C.A.-D.; writing—original draft preparation, E.S.-C.; writing—review and editing, M.A.V.-S., G.G.-A. and E.K.V.-G., supervision, N.B., E.S.-C. and A.T.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Humanidades y Tecnologias (CONAHCyT), grants number 322074 to A.T.V. and CF-2023-I-2312 to C.A.D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the paper. Further inquiries can be directed to the corresponding author.
Acknowledgments
We express our sincere thanks to Francisco Javier Juárez Castañeda for technical assistance with molecular dynamic analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANT2 | Adenine nucleotide translocator 2 |
| BPs | Bioactive peptides |
| HCC | Hepatocellular carcinoma |
| MD | Molecular dynamics |
| MTPs | Mitochondria-targeted peptides |
| PCP | Pink chromoprotein |
| RG | Radius of gyration |
| RMSD | Root Mean Square Deviation |
| RMSF | Root Mean Square Fluctuations |
| SASA | Solvent-Accessible Surface Area |
| SLC25s | Mitochondrial solute carriers |
| THPs | Tumor-homing peptides |
| UCP2 | Uncoupling protein 2 |
| PSPs | Protein hydrolysate from P. salmoneostramineus |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Lee, Y.-K.; Min, S.; Woo, H.G.; Wang, H.J.; Yoon, G. Mitoribosome Defect in Hepatocellular Carcinoma Promotes an Aggressive Phenotype with Suppressed Immune Reaction. iScience 2020, 23, 101247. [Google Scholar] [CrossRef] [PubMed]
- Elhinnawi, M.A.; Boushra, M.I.; Hussien, D.M.; Hussein, F.H.; Abdelmawgood, I.A. Mitochondria’s Role in the Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma. Stem Cell Rev. Rep. 2024, 21, 198–210. [Google Scholar] [CrossRef]
- Komza, M.; Chipuk, J.E. Mitochondrial Metabolism: A Moving Target in Hepatocellular Carcinoma Therapy. J. Cell Physiol. 2025, 240, e31441. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wang, C.-H.; Wu, L.-C.; Hsia, C.-Y.; Chi, C.-W.; Yin, P.-H.; Chang, C.-J.; Sung, M.-T.; Wei, Y.-H.; Lu, S.-H. Mitochondrial Dysfunction Represses HIF-1α Protein Synthesis through AMPK Activation in Human Hepatoma HepG2 Cells. Biochim. Biophys. Acta General. Subj. 2013, 1830, 4743–4751. [Google Scholar] [CrossRef]
- Baffy, G. Mitochondrial Uncoupling in Cancer Cells: Liabilities and Opportunities. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 655–664. [Google Scholar] [CrossRef]
- Ježek, P.; Engstová, H.; Žáčková, M.; Vercesi, A.E.; Costa, A.D.T.; Arruda, P.; Garlid, K.D. Fatty Acid Cycling Mechanism and Mitochondrial Uncoupling Proteins. Biochim. Biophys. Acta Bioenerg. 1998, 1365, 319–327. [Google Scholar] [CrossRef]
- Liang, L.-Y.; Patel, O.; Janes, P.W.; Murphy, J.M.; Lucet, I.S. Eph Receptor Signalling: From Catalytic to Non-Catalytic Functions. Oncogene 2019, 38, 6567–6584. [Google Scholar] [CrossRef]
- Ayyasamy, V.; Owens, K.M.; Desouki, M.M.; Liang, P.; Bakin, A.; Thangaraj, K.; Buchsbaum, D.J.; LoBuglio, A.F.; Singh, K.K. Cellular Model of Warburg Effect Identifies Tumor Promoting Function of UCP2 in Breast Cancer and Its Suppression by Genipin. PLoS ONE 2011, 6, e24792. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Xu, K.; Dong, J. Dynamic Regulation of Uncoupling Protein 2 Expression by MicroRNA-214 in Hepatocellular Carcinoma. Biosci. Rep. 2016, 36, e00335. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, M.; Resnick, M.B.; Konkin, T.A.; Routhier, J.; Wands, J.R.; Baffy, G. Expression of Uncoupling Protein-2 in Human Colon Cancer. Clin. Cancer Res. 2004, 10, 6203–6207. [Google Scholar] [CrossRef] [PubMed]
- Luby, A.; Alves-Guerra, M.-C. UCP2 as a Cancer Target through Energy Metabolism and Oxidative Stress Control. Int. J. Mol. Sci. 2022, 23, 15077. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.J.; Jeong, S.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Na, Y.J.; Park, S.H.; Jeong, Y.A.; Kim, B.G. Genipin Induces Mitochondrial Dysfunction and Apoptosis via Downregulation of Stat3/Mcl-1 Pathway in Gastric Cancer. BMC Cancer 2019, 19, 739. [Google Scholar] [CrossRef]
- Thillaimaharani, K.A.; Sharmila, K.; Thangaraju, P.; Karthick, M.; Kalaiselvam, M. Studies on Antimicrobial and Antioxidant Properties of Oyster Mushroom Pleurotus Florida. Int. J. Pharm. Sci. Res. 2013, 4, 1540. [Google Scholar]
- de Carvalho Cavenaghi, D.F.L.; de Barros, W.M.; de Castro, R.J.S. Protein Hydrolysates from Pleurotus Spp. Mushrooms as a Source of Antioxidant Peptides and Their Stability after Gastrointestinal Digestion. Food Humanit. 2025, 4, 100519. [Google Scholar] [CrossRef]
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive Peptides in the Management of Lifestyle-Related Diseases: Current Trends and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 4593–4606. [Google Scholar] [CrossRef]
- Takekuma, S.; Takekuma, H.; Matsubara, Y.; Inaba, K.; Yoshida, Z. A Novel Mushroom Pigment: Isolation and Characterization. J. Am. Chem. Soc. 1994, 116, 8849–8850. [Google Scholar] [CrossRef]
- Shirasaka, N.; Yamaguchi, Y.; Yoshioka, S.; Fukuta, Y.; Terashita, T. Characterization of the Pigments of Pleurotus salmoneostramineus L. Vass. Mushroom Sci. Biotechnol. 2012, 20, 147–153. [Google Scholar]
- Fukuta, Y.; Kamei, K.; Matsui, A.; Fuji, Y.; Onuma, H.; Shirasaka, N. Gene Cloning of the Pink-Colored Protein from Pleurotus salmoneostramineus (PsPCP) and Its Species-Specific Chromoprotein Are Effective for Colorization of the Fruit Body. Biosci. Biotechnol. Biochem. 2019, 83, 1354–1361. [Google Scholar] [CrossRef]
- Ihara, M.; Tsuchida, N.; Sumida, M.; Himiyama, T.; Kitayama, T.; Shirasaka, N.; Fukuta, Y. Crystal Structure of the Native Chromoprotein from Pleurotus salmoneostramineus Provides Insights into the Pigmentation Mechanism. J. Agric. Food Chem. 2024, 72, 17626–17632. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Lindskog, I.; Gasteiger, E.; Bairoch, A.; Sanchez, J.; Hochstrasser, D.F.; Appel, R.D. Detailed Peptide Characterization Using PEPTIDEMASS–a World-Wide-Web-accessible Tool. Electrophoresis 1997, 18, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Murail, S.; de Vries, S.; Derreumaux, P.; Tuffery, P. PEP-FOLD4: A PH-Dependent Force Field for Peptide Structure Prediction in Aqueous Solution. Nucleic Acids Res. 2023, 51, W432–W437. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [PubMed]
- Trabuco, L.G.; Lise, S.; Petsalaki, E.; Russell, R.B. PepSite: Prediction of Peptide-Binding Sites from Protein Surfaces. Nucleic Acids Res. 2012, 40, W423–W427. [Google Scholar] [CrossRef]
- Rodrigues, C.H.M.; Garg, A.; Keizer, D.; Pires, D.E.V.; Ascher, D.B. CSM-peptides: A Computational Approach to Rapid Identification of Therapeutic Peptides. Protein Sci. 2022, 31, e4442. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v. 2—A Server for In Silico Prediction of Allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Consortium, O.S.D.D.; Raghava, G.P.S. In Silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, P.; Gautam, A.; Chaudhary, K.; Kumar, R.; Chauhan, J.S.; Tyagi, A.; Raghava, G.P.S. Computational Approach for Designing Tumor Homing Peptides. Sci. Rep. 2013, 3, 1607. [Google Scholar] [CrossRef]
- Chen, J.; Cheong, H.H.; Siu, S.W.I. XDeep-AcPEP: Deep Learning Method for Anticancer Peptide Activity Prediction Based on Convolutional Neural Network and Multitask Learning. J. Chem. Inf. Model. 2021, 61, 3789–3803. [Google Scholar] [CrossRef]
- Yan, K.; Lv, H.; Wen, J.; Guo, Y.; Xu, Y.; Liu, B. PreTP-Stack: Prediction of Therapeutic Peptide Based on the Stacked Ensemble Learning. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 20, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Riniker, S.; Landrum, G.A. Better Informed Distance Geometry: Using What We Know to Improve Conformation Generation. J. Chem. Inf. Model. 2015, 55, 2562–2574. [Google Scholar] [CrossRef] [PubMed]
- Carbone, J.; Ghidini, A.; Romano, A.; Gentilucci, L.; Musiani, F. PacDOCK: A Web Server for Positional Distance-Based and Interaction-Based Analysis of Docking Results. Molecules 2022, 27, 6884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. HPEPDOCK: A Web Server for Blind Peptide–Protein Docking Based on a Hierarchical Algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Xu, X.; Yan, C.; Zou, X. MDockPeP: An Ab-initio Protein–Peptide Docking Server. J. Comput. Chem. 2018, 39, 2409–2413. [Google Scholar] [CrossRef]
- Kurcinski, M.; Badaczewska-Dawid, A.; Kolinski, M.; Kolinski, A.; Kmiecik, S. Flexible Docking of Peptides to Proteins Using CABS-dock. Protein Sci. 2020, 29, 211–222. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Im, W. Automated Builder and Database of Protein/Membrane Complexes for Molecular Dynamics Simulations. PLoS ONE 2007, 2, e880. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M. CHARMM-GUI Membrane Builder toward Realistic Biological Membrane Simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Jo, S.; MacKerell, A.D.; Klauda, J.B.; Im, W. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. Biophys. J. 2016, 110, 641a. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; De Groot, B.L.; Grubmüller, H.; MacKerell Jr, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Nicoleta, S.; Alina, H.; Silvius, S.; Gabriela, R. Thermal Treatment Can Modify the Susceptibility of Whey Protein Concentrate to Enzymatic Hydrolysis. Innov. Rom. Food Biotechnol. 2010, 7, 30–36. [Google Scholar]
- Deng, S.; Yang, Y.; Han, Y.; Li, X.; Wang, X.; Li, X.; Zhang, Z.; Wang, Y. UCP2 Inhibits ROS-Mediated Apoptosis in A549 under Hypoxic Conditions. PLoS ONE 2012, 7, e30714. [Google Scholar] [CrossRef]
- Collins, P.; Jones, C.; Choudhury, S.; Damelin, L.; Hodgson, H. Increased Expression of Uncoupling Protein 2 in HepG2 Cells Attenuates Oxidative Damage and Apoptosis. Liver Int. 2005, 25, 880–887. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S. Superoxide Activates Mitochondrial Uncoupling Proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Azzu, V.; Brand, M.D. The On-off Switches of the Mitochondrial Uncoupling Proteins. Trends Biochem. Sci. 2010, 35, 298–307. [Google Scholar] [CrossRef]
- Modrianský, M.; Murdza-Inglis, D.L.; Patel, H.V.; Freeman, K.B.; Garlid, K.D. Identification by Site-Directed Mutagenesis of Three Arginines in Uncoupling Protein That Are Essential for Nucleotide Binding and Inhibition. J. Biol. Chem. 1997, 272, 24759–24762. [Google Scholar] [CrossRef]
- Berardi, M.J.; Shih, W.M.; Harrison, S.C.; Chou, J.J. Mitochondrial Uncoupling Protein 2 Structure Determined by NMR Molecular Fragment Searching. Nature 2011, 476, 109–113. [Google Scholar] [CrossRef]
- Zhu, R.; Rupprecht, A.; Ebner, A.; Haselgrübler, T.; Gruber, H.J.; Hinterdorfer, P.; Pohl, E.E. Mapping the Nucleotide Binding Site of Uncoupling Protein 1 Using Atomic Force Microscopy. J. Am. Chem. Soc. 2013, 135, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.; Santhanam, R.K.; Wang, C.; Gao, X.; Chen, Y.; Wang, C.; Xu, L.; Chen, H. Bioactive Peptide with Antioxidant and Anticancer Activities from Black Soybean [Glycine max (L.) Merr.] Byproduct: Isolation, Identification and Molecular Docking Study. Eur. Food Res. Technol. 2019, 245, 677–689. [Google Scholar] [CrossRef]
- Zoonens, M.; Comer, J.; Masscheleyn, S.; Pebay-Peyroula, E.; Chipot, C.; Miroux, B.; Dehez, F. Dangerous Liaisons between Detergents and Membrane Proteins. The Case of Mitochondrial Uncoupling Protein 2. J. Am. Chem. Soc. 2013, 135, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Purohit, R. Multi-target Approach against SARS-CoV-2 by Stone Apple Molecules: A Master Key to Drug Design. Phytother. Res. 2024, 38, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhai, Y.; Zhang, Y.; He, M.; Wang, X.; Yu, S.; Xiao, H.; Song, Y. Antioxidant and Anti-HepG2 Cell Activities of a Novel Bioactive Peptide from Cowhide Collagen in Vitro. J. Future Foods 2024, 4, 248–257. [Google Scholar] [CrossRef]
- Kreiter, J.; Rupprecht, A.; Zimmermann, L.; Moschinger, M.; Rokitskaya, T.I.; Antonenko, Y.N.; Gille, L.; Fedorova, M.; Pohl, E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019, 117, 1845–1857. [Google Scholar] [CrossRef]
- Lin, J.; Rao, D.; Zhang, M.; Gao, Q. Metabolic Reprogramming in the Tumor Microenvironment of Liver Cancer. J. Hematol. Oncol. 2024, 17, 6. [Google Scholar] [CrossRef]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting Mitochondrial Metabolism for Precision Medicine in Cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and Function of the Mammalian Tricarboxylic Acid Cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Palmieri, F. The Mitochondrial Transporter Family SLC25: Identification, Properties and Physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S. Genipin, an Inhibitor of UCP2 as a Promising New Anticancer Agent: A Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5637. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, Y.S.; Jung, K.-H.; Park, J.W.; Lee, K.-H. Genipin Enhances the Antitumor Effect of Elesclomol in A549 Lung Cancer Cells by Blocking Uncoupling Protein-2 and Stimulating Reactive Oxygen Species Production. Oncol. Lett. 2020, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Sánchez-Martín, C.; Pujol-Morcillo, A.; Martín-Ruiz, M.; de Los Santos, P.; Lobato-Alonso, D.; Oliver, E.; Rial, E. Role of UCP2 in the Energy Metabolism of the Cancer Cell Line A549. Int. J. Mol. Sci. 2023, 24, 8123. [Google Scholar] [CrossRef]
- Raho, S.; Capobianco, L.; Malivindi, R.; Vozza, A.; Piazzolla, C.; De Leonardis, F.; Gorgoglione, R.; Scarcia, P.; Pezzuto, F.; Agrimi, G. KRAS-Regulated Glutamine Metabolism Requires UCP2-Mediated Aspartate Transport to Support Pancreatic Cancer Growth. Nat. Metab. 2020, 2, 1373–1381. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Membrane-Active Peptides and Their Potential Biomedical Application. Pharmaceutics 2023, 15, 2091. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. The Role of Sensing Peptides in the Cross-Talk between Microbiota and Human Cancer Cells. Mini Rev. Med. Chem. 2018, 18, 1567–1571. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising Anticancer Activity of Polysaccharides and Other Macromolecules Derived from Oyster Mushroom (Pleurotus sp.): An Updated Review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef]
- Jedinak, A.; Sliva, D. Pleurotus ostreatus Inhibits Proliferation of Human Breast and Colon Cancer Cells through P53-Dependent as Well as P53-Independent Pathway. Int. J. Oncol. 2008, 33, 1307–1313. [Google Scholar]
- Jayaprakash, B.; Suresh, A.R.; Thiruvengadam, R.; Alharbi, N.S.; Kadaikunnan, S.; Sankaran, S.; Thiruvengadam, M.; Senthil, R.; Venkidasamy, B. Evaluation of Oyster Mushroom (Pleurotus ostreatus)-Derived Anthraquinone on the Induction of Apoptosis and Suppression of MMP-2 and MMP-9 Expression in Breast Cancer Cells. Int. J. Med. Sci. 2024, 21, 1016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).