Anticancer Effects of Pleurotus salmoneostramineus Protein Hydrolysate on HepG2 Cells and In Silico Characterization of Structural Effects of Chromoprotein-Derived Peptides on the Mitochondrial Uncoupling Protein 2 (UCP2)
Abstract
1. Introduction
2. Materials and Methods
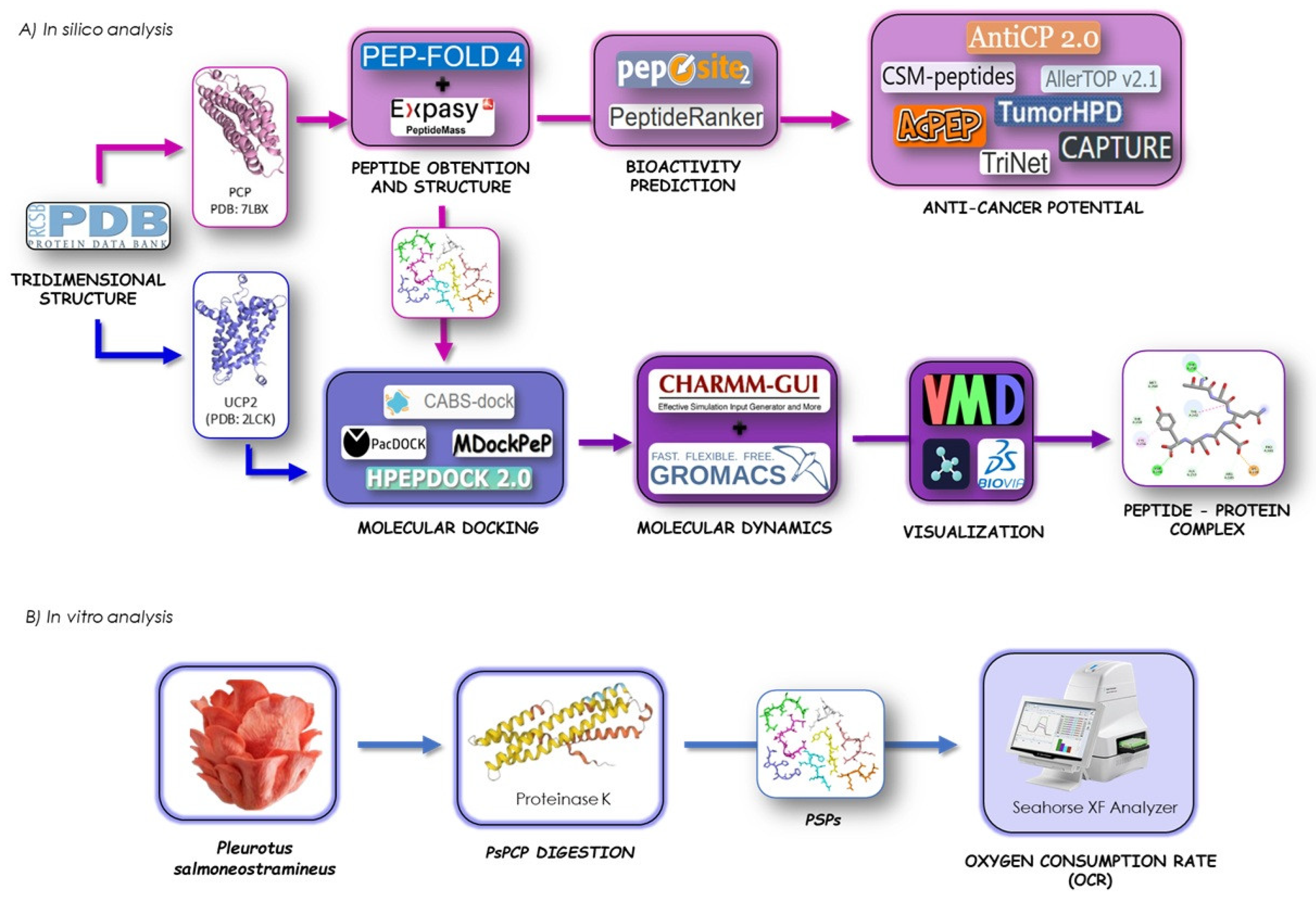
2.1. In Silico Cromoprotein Hydrolysis and Peptide Characterization
2.2. Prediction of Anticancer Potential of Peptides
2.3. Prediction of Peptide–Protein Interactions and Molecular Docking
2.4. Molecular Dynamics of Peptide–UCP2 Complexes
2.5. Preparation of the Protein Hydrolysate from P. salmoneostramineus (PSPs)
2.6. Cell Culture
2.7. Oxygen Consumption Rate (OCR)
2.8. Protein Quantification Assay
2.9. Statistical Analysis
3. Results
3.1. Pink Chromoprotein Hydrolysis and Peptide Physiochemical Parameters
3.2. Peptide Therapeutic Bioactivity and Anticancer Potential
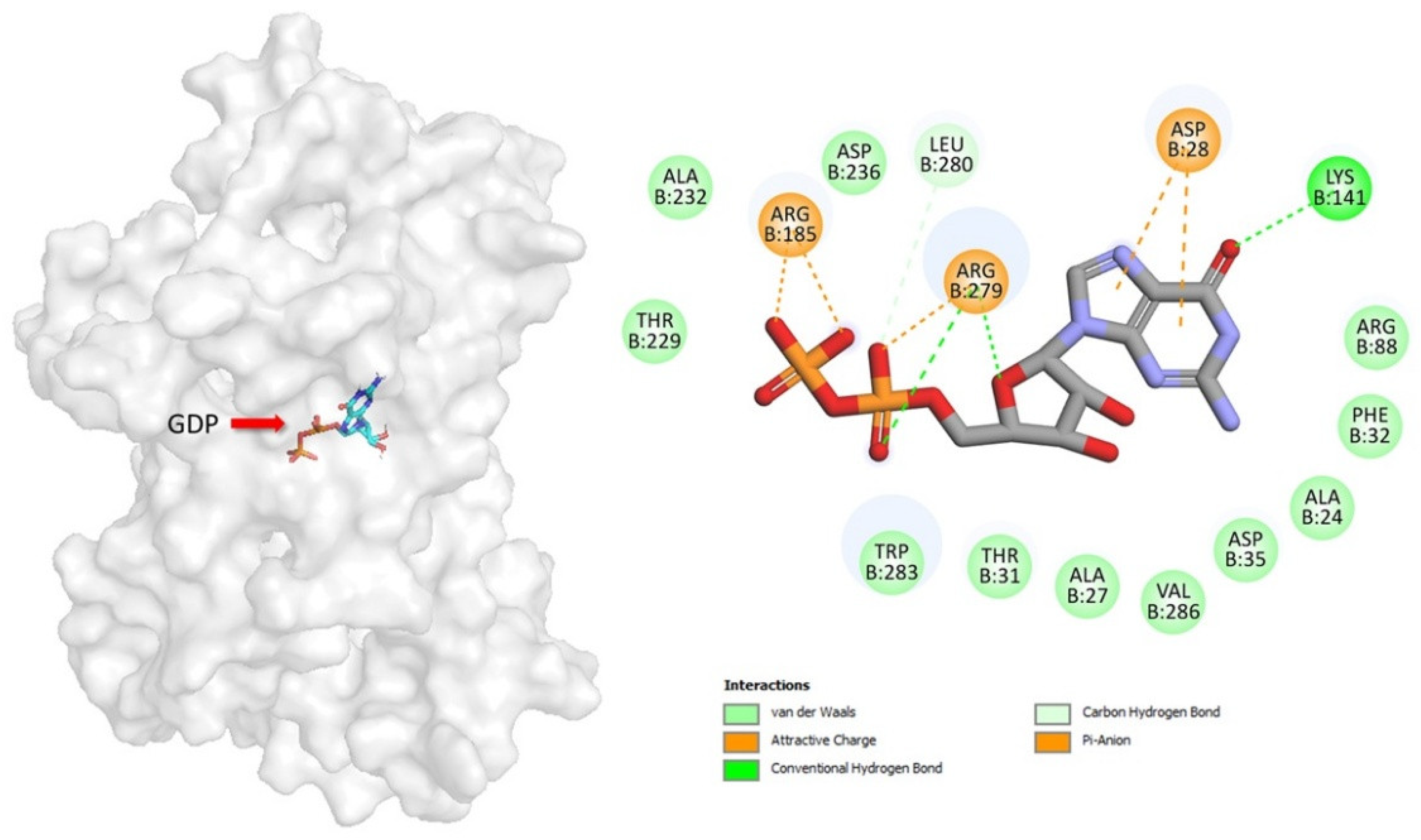
3.3. Prediction of the GDP Binding Site in UCP2 Structure
3.4. Peptide Affinity for UCP2 and Molecular Docking
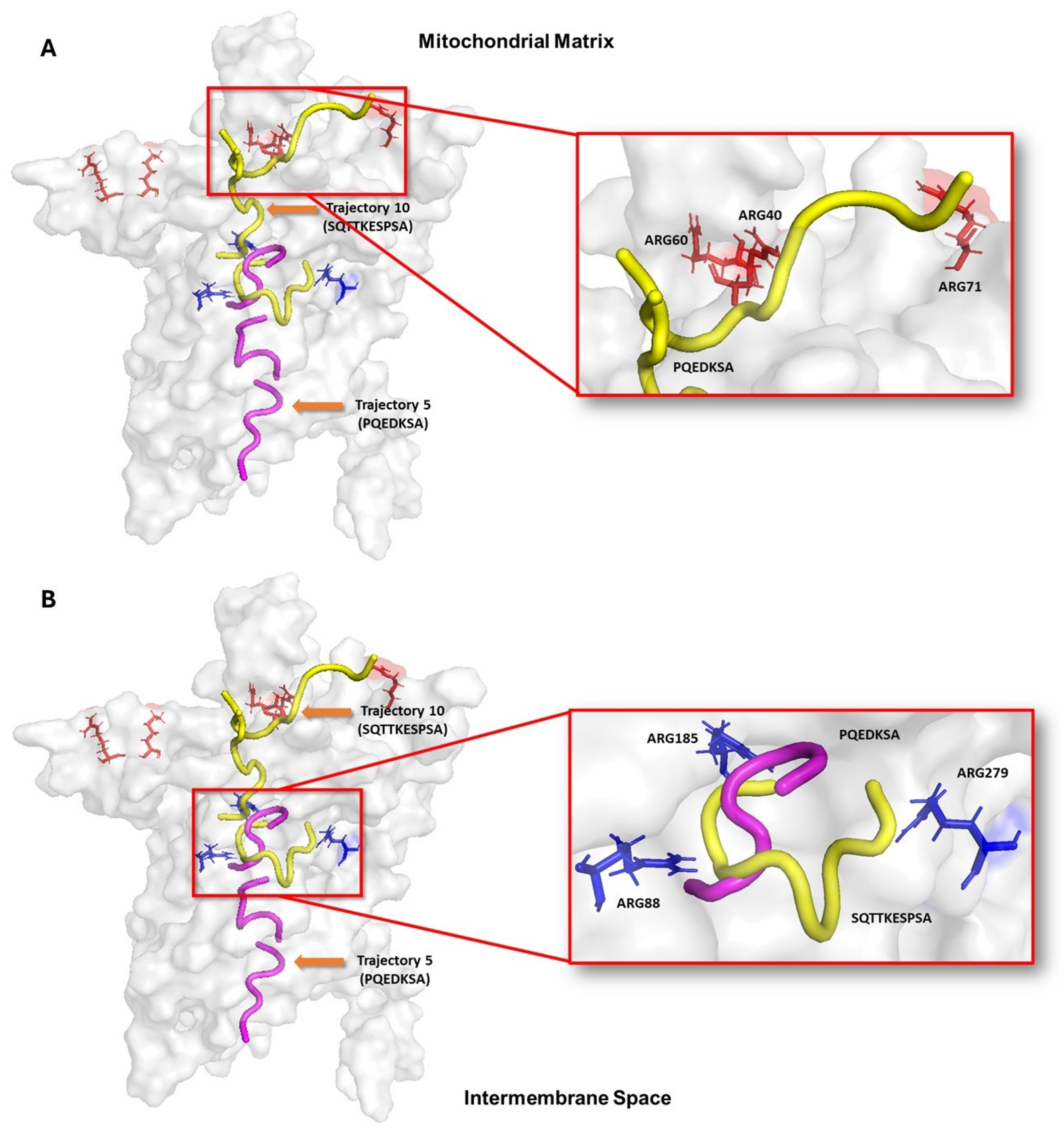
3.5. Peptide Translocation Mechanism Through UCP2
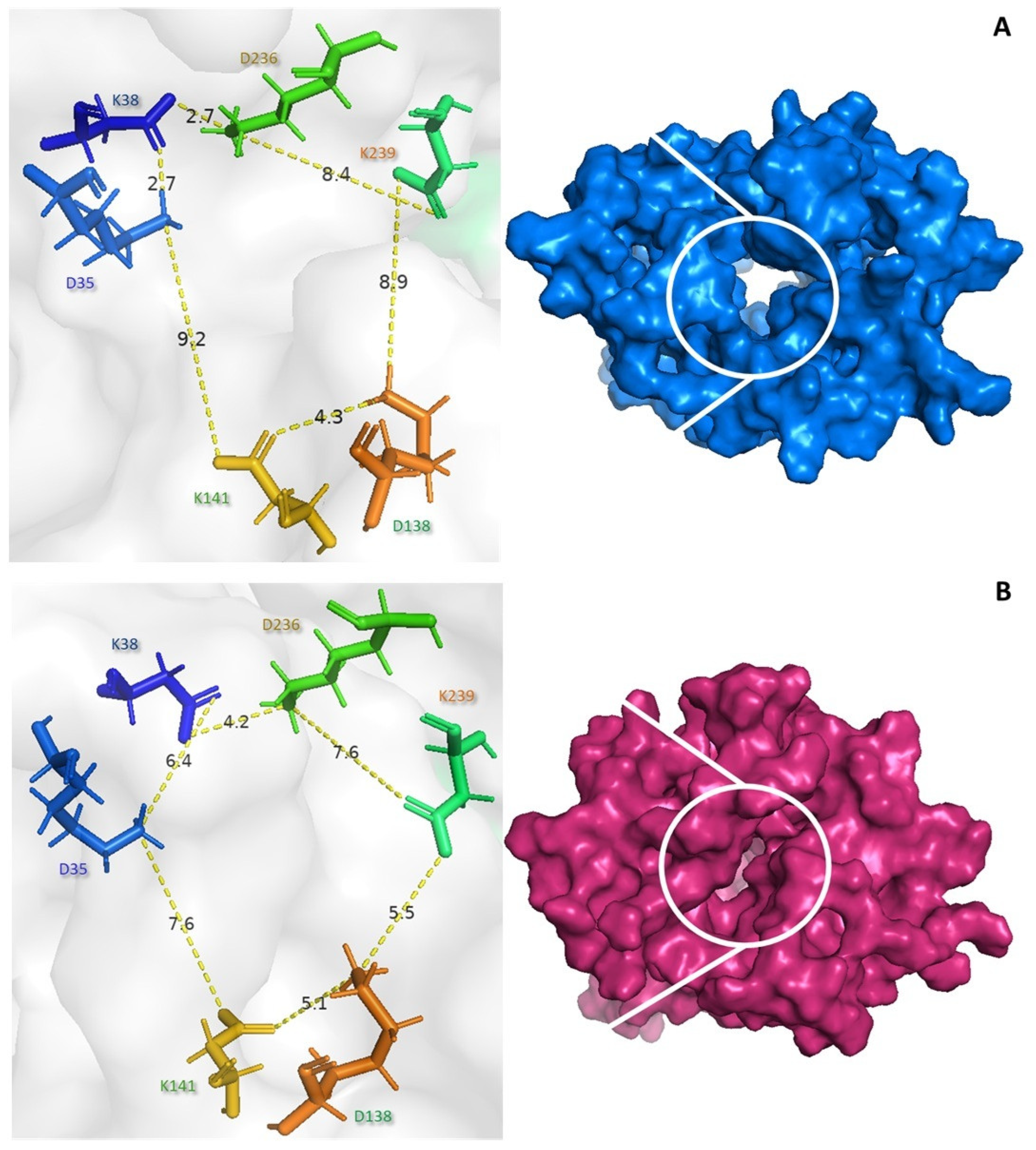
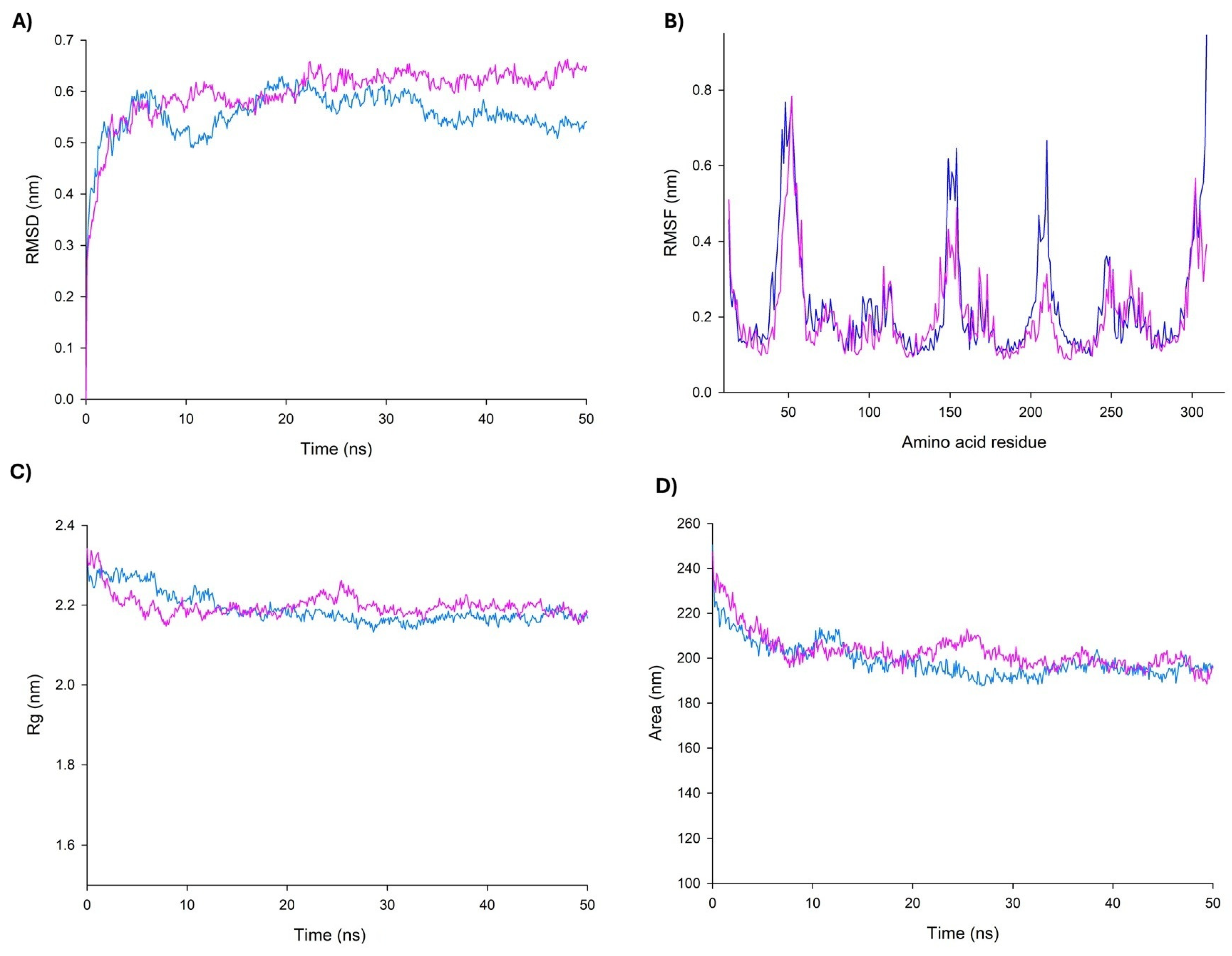
3.6. Molecular Dynamics Simulations for Peptide–UCP2 Complexes
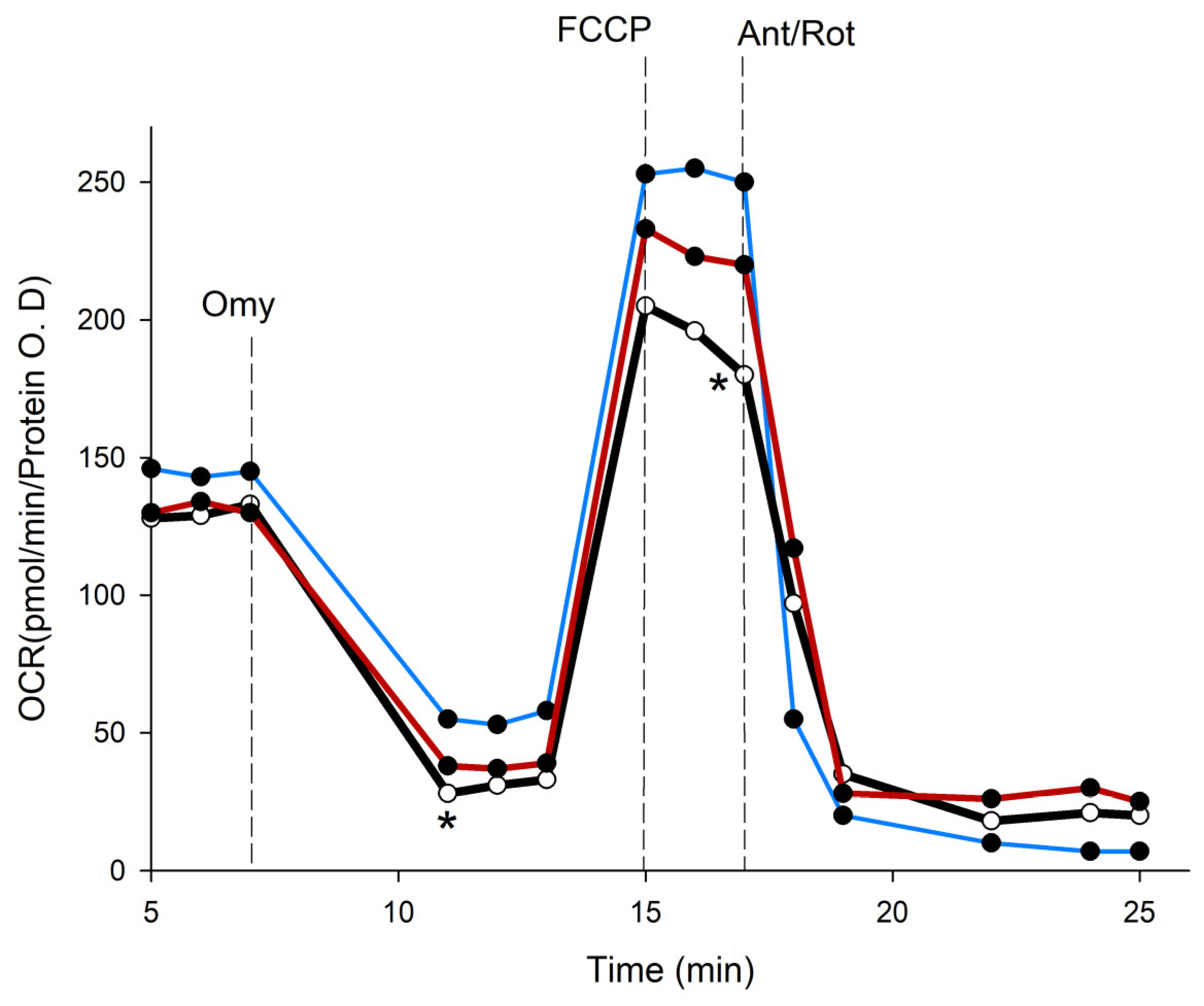
3.7. Effect of PSPs on Uncoupling Activity in HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANT2 | Adenine nucleotide translocator 2 |
| BPs | Bioactive peptides |
| HCC | Hepatocellular carcinoma |
| MD | Molecular dynamics |
| MTPs | Mitochondria-targeted peptides |
| PCP | Pink chromoprotein |
| RG | Radius of gyration |
| RMSD | Root Mean Square Deviation |
| RMSF | Root Mean Square Fluctuations |
| SASA | Solvent-Accessible Surface Area |
| SLC25s | Mitochondrial solute carriers |
| THPs | Tumor-homing peptides |
| UCP2 | Uncoupling protein 2 |
| PSPs | Protein hydrolysate from P. salmoneostramineus |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Lee, Y.-K.; Min, S.; Woo, H.G.; Wang, H.J.; Yoon, G. Mitoribosome Defect in Hepatocellular Carcinoma Promotes an Aggressive Phenotype with Suppressed Immune Reaction. iScience 2020, 23, 101247. [Google Scholar] [CrossRef] [PubMed]
- Elhinnawi, M.A.; Boushra, M.I.; Hussien, D.M.; Hussein, F.H.; Abdelmawgood, I.A. Mitochondria’s Role in the Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma. Stem Cell Rev. Rep. 2024, 21, 198–210. [Google Scholar] [CrossRef]
- Komza, M.; Chipuk, J.E. Mitochondrial Metabolism: A Moving Target in Hepatocellular Carcinoma Therapy. J. Cell Physiol. 2025, 240, e31441. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wang, C.-H.; Wu, L.-C.; Hsia, C.-Y.; Chi, C.-W.; Yin, P.-H.; Chang, C.-J.; Sung, M.-T.; Wei, Y.-H.; Lu, S.-H. Mitochondrial Dysfunction Represses HIF-1α Protein Synthesis through AMPK Activation in Human Hepatoma HepG2 Cells. Biochim. Biophys. Acta General. Subj. 2013, 1830, 4743–4751. [Google Scholar] [CrossRef]
- Baffy, G. Mitochondrial Uncoupling in Cancer Cells: Liabilities and Opportunities. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 655–664. [Google Scholar] [CrossRef]
- Ježek, P.; Engstová, H.; Žáčková, M.; Vercesi, A.E.; Costa, A.D.T.; Arruda, P.; Garlid, K.D. Fatty Acid Cycling Mechanism and Mitochondrial Uncoupling Proteins. Biochim. Biophys. Acta Bioenerg. 1998, 1365, 319–327. [Google Scholar] [CrossRef]
- Liang, L.-Y.; Patel, O.; Janes, P.W.; Murphy, J.M.; Lucet, I.S. Eph Receptor Signalling: From Catalytic to Non-Catalytic Functions. Oncogene 2019, 38, 6567–6584. [Google Scholar] [CrossRef]
- Ayyasamy, V.; Owens, K.M.; Desouki, M.M.; Liang, P.; Bakin, A.; Thangaraj, K.; Buchsbaum, D.J.; LoBuglio, A.F.; Singh, K.K. Cellular Model of Warburg Effect Identifies Tumor Promoting Function of UCP2 in Breast Cancer and Its Suppression by Genipin. PLoS ONE 2011, 6, e24792. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Xu, K.; Dong, J. Dynamic Regulation of Uncoupling Protein 2 Expression by MicroRNA-214 in Hepatocellular Carcinoma. Biosci. Rep. 2016, 36, e00335. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, M.; Resnick, M.B.; Konkin, T.A.; Routhier, J.; Wands, J.R.; Baffy, G. Expression of Uncoupling Protein-2 in Human Colon Cancer. Clin. Cancer Res. 2004, 10, 6203–6207. [Google Scholar] [CrossRef] [PubMed]
- Luby, A.; Alves-Guerra, M.-C. UCP2 as a Cancer Target through Energy Metabolism and Oxidative Stress Control. Int. J. Mol. Sci. 2022, 23, 15077. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.J.; Jeong, S.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Na, Y.J.; Park, S.H.; Jeong, Y.A.; Kim, B.G. Genipin Induces Mitochondrial Dysfunction and Apoptosis via Downregulation of Stat3/Mcl-1 Pathway in Gastric Cancer. BMC Cancer 2019, 19, 739. [Google Scholar] [CrossRef]
- Thillaimaharani, K.A.; Sharmila, K.; Thangaraju, P.; Karthick, M.; Kalaiselvam, M. Studies on Antimicrobial and Antioxidant Properties of Oyster Mushroom Pleurotus Florida. Int. J. Pharm. Sci. Res. 2013, 4, 1540. [Google Scholar]
- de Carvalho Cavenaghi, D.F.L.; de Barros, W.M.; de Castro, R.J.S. Protein Hydrolysates from Pleurotus Spp. Mushrooms as a Source of Antioxidant Peptides and Their Stability after Gastrointestinal Digestion. Food Humanit. 2025, 4, 100519. [Google Scholar] [CrossRef]
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive Peptides in the Management of Lifestyle-Related Diseases: Current Trends and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 4593–4606. [Google Scholar] [CrossRef]
- Takekuma, S.; Takekuma, H.; Matsubara, Y.; Inaba, K.; Yoshida, Z. A Novel Mushroom Pigment: Isolation and Characterization. J. Am. Chem. Soc. 1994, 116, 8849–8850. [Google Scholar] [CrossRef]
- Shirasaka, N.; Yamaguchi, Y.; Yoshioka, S.; Fukuta, Y.; Terashita, T. Characterization of the Pigments of Pleurotus salmoneostramineus L. Vass. Mushroom Sci. Biotechnol. 2012, 20, 147–153. [Google Scholar]
- Fukuta, Y.; Kamei, K.; Matsui, A.; Fuji, Y.; Onuma, H.; Shirasaka, N. Gene Cloning of the Pink-Colored Protein from Pleurotus salmoneostramineus (PsPCP) and Its Species-Specific Chromoprotein Are Effective for Colorization of the Fruit Body. Biosci. Biotechnol. Biochem. 2019, 83, 1354–1361. [Google Scholar] [CrossRef]
- Ihara, M.; Tsuchida, N.; Sumida, M.; Himiyama, T.; Kitayama, T.; Shirasaka, N.; Fukuta, Y. Crystal Structure of the Native Chromoprotein from Pleurotus salmoneostramineus Provides Insights into the Pigmentation Mechanism. J. Agric. Food Chem. 2024, 72, 17626–17632. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Lindskog, I.; Gasteiger, E.; Bairoch, A.; Sanchez, J.; Hochstrasser, D.F.; Appel, R.D. Detailed Peptide Characterization Using PEPTIDEMASS–a World-Wide-Web-accessible Tool. Electrophoresis 1997, 18, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Murail, S.; de Vries, S.; Derreumaux, P.; Tuffery, P. PEP-FOLD4: A PH-Dependent Force Field for Peptide Structure Prediction in Aqueous Solution. Nucleic Acids Res. 2023, 51, W432–W437. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [PubMed]
- Trabuco, L.G.; Lise, S.; Petsalaki, E.; Russell, R.B. PepSite: Prediction of Peptide-Binding Sites from Protein Surfaces. Nucleic Acids Res. 2012, 40, W423–W427. [Google Scholar] [CrossRef]
- Rodrigues, C.H.M.; Garg, A.; Keizer, D.; Pires, D.E.V.; Ascher, D.B. CSM-peptides: A Computational Approach to Rapid Identification of Therapeutic Peptides. Protein Sci. 2022, 31, e4442. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v. 2—A Server for In Silico Prediction of Allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Consortium, O.S.D.D.; Raghava, G.P.S. In Silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, P.; Gautam, A.; Chaudhary, K.; Kumar, R.; Chauhan, J.S.; Tyagi, A.; Raghava, G.P.S. Computational Approach for Designing Tumor Homing Peptides. Sci. Rep. 2013, 3, 1607. [Google Scholar] [CrossRef]
- Chen, J.; Cheong, H.H.; Siu, S.W.I. XDeep-AcPEP: Deep Learning Method for Anticancer Peptide Activity Prediction Based on Convolutional Neural Network and Multitask Learning. J. Chem. Inf. Model. 2021, 61, 3789–3803. [Google Scholar] [CrossRef]
- Yan, K.; Lv, H.; Wen, J.; Guo, Y.; Xu, Y.; Liu, B. PreTP-Stack: Prediction of Therapeutic Peptide Based on the Stacked Ensemble Learning. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 20, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Riniker, S.; Landrum, G.A. Better Informed Distance Geometry: Using What We Know to Improve Conformation Generation. J. Chem. Inf. Model. 2015, 55, 2562–2574. [Google Scholar] [CrossRef] [PubMed]
- Carbone, J.; Ghidini, A.; Romano, A.; Gentilucci, L.; Musiani, F. PacDOCK: A Web Server for Positional Distance-Based and Interaction-Based Analysis of Docking Results. Molecules 2022, 27, 6884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. HPEPDOCK: A Web Server for Blind Peptide–Protein Docking Based on a Hierarchical Algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Xu, X.; Yan, C.; Zou, X. MDockPeP: An Ab-initio Protein–Peptide Docking Server. J. Comput. Chem. 2018, 39, 2409–2413. [Google Scholar] [CrossRef]
- Kurcinski, M.; Badaczewska-Dawid, A.; Kolinski, M.; Kolinski, A.; Kmiecik, S. Flexible Docking of Peptides to Proteins Using CABS-dock. Protein Sci. 2020, 29, 211–222. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Im, W. Automated Builder and Database of Protein/Membrane Complexes for Molecular Dynamics Simulations. PLoS ONE 2007, 2, e880. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M. CHARMM-GUI Membrane Builder toward Realistic Biological Membrane Simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Jo, S.; MacKerell, A.D.; Klauda, J.B.; Im, W. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. Biophys. J. 2016, 110, 641a. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; De Groot, B.L.; Grubmüller, H.; MacKerell Jr, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Nicoleta, S.; Alina, H.; Silvius, S.; Gabriela, R. Thermal Treatment Can Modify the Susceptibility of Whey Protein Concentrate to Enzymatic Hydrolysis. Innov. Rom. Food Biotechnol. 2010, 7, 30–36. [Google Scholar]
- Deng, S.; Yang, Y.; Han, Y.; Li, X.; Wang, X.; Li, X.; Zhang, Z.; Wang, Y. UCP2 Inhibits ROS-Mediated Apoptosis in A549 under Hypoxic Conditions. PLoS ONE 2012, 7, e30714. [Google Scholar] [CrossRef]
- Collins, P.; Jones, C.; Choudhury, S.; Damelin, L.; Hodgson, H. Increased Expression of Uncoupling Protein 2 in HepG2 Cells Attenuates Oxidative Damage and Apoptosis. Liver Int. 2005, 25, 880–887. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S. Superoxide Activates Mitochondrial Uncoupling Proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Azzu, V.; Brand, M.D. The On-off Switches of the Mitochondrial Uncoupling Proteins. Trends Biochem. Sci. 2010, 35, 298–307. [Google Scholar] [CrossRef]
- Modrianský, M.; Murdza-Inglis, D.L.; Patel, H.V.; Freeman, K.B.; Garlid, K.D. Identification by Site-Directed Mutagenesis of Three Arginines in Uncoupling Protein That Are Essential for Nucleotide Binding and Inhibition. J. Biol. Chem. 1997, 272, 24759–24762. [Google Scholar] [CrossRef]
- Berardi, M.J.; Shih, W.M.; Harrison, S.C.; Chou, J.J. Mitochondrial Uncoupling Protein 2 Structure Determined by NMR Molecular Fragment Searching. Nature 2011, 476, 109–113. [Google Scholar] [CrossRef]
- Zhu, R.; Rupprecht, A.; Ebner, A.; Haselgrübler, T.; Gruber, H.J.; Hinterdorfer, P.; Pohl, E.E. Mapping the Nucleotide Binding Site of Uncoupling Protein 1 Using Atomic Force Microscopy. J. Am. Chem. Soc. 2013, 135, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.; Santhanam, R.K.; Wang, C.; Gao, X.; Chen, Y.; Wang, C.; Xu, L.; Chen, H. Bioactive Peptide with Antioxidant and Anticancer Activities from Black Soybean [Glycine max (L.) Merr.] Byproduct: Isolation, Identification and Molecular Docking Study. Eur. Food Res. Technol. 2019, 245, 677–689. [Google Scholar] [CrossRef]
- Zoonens, M.; Comer, J.; Masscheleyn, S.; Pebay-Peyroula, E.; Chipot, C.; Miroux, B.; Dehez, F. Dangerous Liaisons between Detergents and Membrane Proteins. The Case of Mitochondrial Uncoupling Protein 2. J. Am. Chem. Soc. 2013, 135, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Purohit, R. Multi-target Approach against SARS-CoV-2 by Stone Apple Molecules: A Master Key to Drug Design. Phytother. Res. 2024, 38, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhai, Y.; Zhang, Y.; He, M.; Wang, X.; Yu, S.; Xiao, H.; Song, Y. Antioxidant and Anti-HepG2 Cell Activities of a Novel Bioactive Peptide from Cowhide Collagen in Vitro. J. Future Foods 2024, 4, 248–257. [Google Scholar] [CrossRef]
- Kreiter, J.; Rupprecht, A.; Zimmermann, L.; Moschinger, M.; Rokitskaya, T.I.; Antonenko, Y.N.; Gille, L.; Fedorova, M.; Pohl, E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019, 117, 1845–1857. [Google Scholar] [CrossRef]
- Lin, J.; Rao, D.; Zhang, M.; Gao, Q. Metabolic Reprogramming in the Tumor Microenvironment of Liver Cancer. J. Hematol. Oncol. 2024, 17, 6. [Google Scholar] [CrossRef]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting Mitochondrial Metabolism for Precision Medicine in Cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and Function of the Mammalian Tricarboxylic Acid Cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Palmieri, F. The Mitochondrial Transporter Family SLC25: Identification, Properties and Physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S. Genipin, an Inhibitor of UCP2 as a Promising New Anticancer Agent: A Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5637. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, Y.S.; Jung, K.-H.; Park, J.W.; Lee, K.-H. Genipin Enhances the Antitumor Effect of Elesclomol in A549 Lung Cancer Cells by Blocking Uncoupling Protein-2 and Stimulating Reactive Oxygen Species Production. Oncol. Lett. 2020, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Sánchez-Martín, C.; Pujol-Morcillo, A.; Martín-Ruiz, M.; de Los Santos, P.; Lobato-Alonso, D.; Oliver, E.; Rial, E. Role of UCP2 in the Energy Metabolism of the Cancer Cell Line A549. Int. J. Mol. Sci. 2023, 24, 8123. [Google Scholar] [CrossRef]
- Raho, S.; Capobianco, L.; Malivindi, R.; Vozza, A.; Piazzolla, C.; De Leonardis, F.; Gorgoglione, R.; Scarcia, P.; Pezzuto, F.; Agrimi, G. KRAS-Regulated Glutamine Metabolism Requires UCP2-Mediated Aspartate Transport to Support Pancreatic Cancer Growth. Nat. Metab. 2020, 2, 1373–1381. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Membrane-Active Peptides and Their Potential Biomedical Application. Pharmaceutics 2023, 15, 2091. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. The Role of Sensing Peptides in the Cross-Talk between Microbiota and Human Cancer Cells. Mini Rev. Med. Chem. 2018, 18, 1567–1571. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising Anticancer Activity of Polysaccharides and Other Macromolecules Derived from Oyster Mushroom (Pleurotus sp.): An Updated Review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef]
- Jedinak, A.; Sliva, D. Pleurotus ostreatus Inhibits Proliferation of Human Breast and Colon Cancer Cells through P53-Dependent as Well as P53-Independent Pathway. Int. J. Oncol. 2008, 33, 1307–1313. [Google Scholar]
- Jayaprakash, B.; Suresh, A.R.; Thiruvengadam, R.; Alharbi, N.S.; Kadaikunnan, S.; Sankaran, S.; Thiruvengadam, M.; Senthil, R.; Venkidasamy, B. Evaluation of Oyster Mushroom (Pleurotus ostreatus)-Derived Anthraquinone on the Induction of Apoptosis and Suppression of MMP-2 and MMP-9 Expression in Breast Cancer Cells. Int. J. Med. Sci. 2024, 21, 1016. [Google Scholar] [CrossRef]

| Peptide Sequence | Mass (g/mol) | Position (Res. Number) | Hydrophilicity | Peptide Ranker | Half-Life (sec) |
|---|---|---|---|---|---|
| SQTTKESPSA | 1035.49 | 59–68 | 0.5 | 0.055 | 1.634 |
| PQEDKSA | 774.36 | 37–43 | 1.29 | 0.083 | 1.672 |
| TSMQSSL | 753.34 | 164–170 | −0.34 | 0.149 | 1.378 |
| TSQESY | 714.29 | 49–54 | 0.18 | 0.061 | 2.148 |
| QEGQKL | 702.37 | 129–134 | 0.77 | 0.184 | 2.616 |
| SEDSGEA | 694.25 | 194–200 | 1.3 | 0.092 | 2.810 |
| PGSHPV | 593.30 | 140–145 | −0.28 | 0.382 | 2.581 |
| GRNSL | 546.29 | 19–23 | 0.34 | 0.304 | 1.284 |
| Peptide Sequence | Activity Prediction | |||||
|---|---|---|---|---|---|---|
| AAP | ABP | ACP | AIP | AVP | QSP | |
| SQTTKESPSA | 0.52 | --------- | ------- | ------- | --------- | -------- |
| PQEDKSA | 0.56 | --------- | ------- | -------- | --------- | -------- |
| TSMQSSL | ---------- | --------- | 0.65 | 0.55 | --------- | 0.93 |
| TSQESY | ---------- | --------- | ------- | 0.99 | --------- | 0.55 |
| QEGQKL | ---------- | --------- | 0.79 | 0.64 | --------- | 0.51 |
| SEDSGEA | 0.50 | --------- | 0.53 | ------- | --------- | ------- |
| PGSHPV | 0.54 | --------- | ------- | ------- | --------- | 0.96 |
| GRNSL | 0.56 | --------- | 0.56 | ------- | --------- | 1.00 |
| Peptide Sequence | Multiple Peptide Servers | ||||||
|---|---|---|---|---|---|---|---|
| SCMTHP | mACPpred | TumorHPD | PreTP-Stack | TriNet | AntiCP 2.0 | CAPTURE | |
| SQTTKESPSA | NON-THP (257.8) | ACP (0.529) | NON-THP (−0.33) | ACP (0.5) | 0.0 | AntiCP (0.49) | prostate |
| PQEDKSA | NON-THP (223.8) | ACP (0.551) | NON-THP (−1.94) | ACP (0.5) | 0.0 | AntiCP (0.45) | colon |
| TSMQSSL | THP (315.5) | ACP (0.655) | THP (0.81) | ACP (0.5) | 0.0 | Non-AntiCP (0.38) | colon |
| TSQESY | NON-THP (250.3) | ACP (0.587) | NON-THP (−0.38) | ACP (0.5) | 0.0 | AntiCP (0.53) | skin |
| QEGQKL | NON-THP (173.1) | ACP (0.722) | NON-THP (−0.99) | ACP (0.5) | 0.0 | AntiCP (0.7) | skin |
| SEDSGEA | NON-THP (195.4) | ACP (0.575) | NON-THP (−1.35) | ACP (0.5) | 0.0 | Non-AntiCP (0.21) | breast |
| PGSHPV | THP (361.3) | ACP (0.817) | THP (0.86) | ACP (0.7) | 1.0 | AntiCP (0.69) | colon |
| GRNSL | THP (346.2) | ACP (0.789) | THP (0.94) | ACP (0.5) | 0.0 | AntiCP (0.5) | colon |
| Peptide Sequence | PepSite2 p-Value | ITScorePeP | HPEPDOCK 2.0 |
|---|---|---|---|
| SQTTKESPSA | 0.02 | −116.4 | −156.04 |
| PQEDKSA | 0.09 | −102.0 | −137.38 |
| TSMQSSL | 0.05 | −102.9 | −166.75 |
| TSQESY | 0.03 | −108.4 | −148.36 |
| QEGQKL | 0.02 | −101.8 | −126.06 |
| SEDSGEA | 0.16 | −83.0 | −99.93 |
| PGSHPV | 0.02 | −89.0 | −164.64 |
| GRNSL | 0.04 | −82.8 | −137.93 |
| Parameters | Control (pmol/min/Protein O.D) | Genipin (30 µM) | PSPs (150 µg/mL) |
|---|---|---|---|
| Basal | 133.66 ± 3.57 | 115.6 ± 2.1 * | 111.3 ± 1.52 * |
| ATP-linked Resp | 95.7 ± 4.8 | 96.2 ± 1.5 | 106.8 ± 3.2 * |
| Proton Leak (UCP) | 33.7 ± 4.9 | 2.9 ± 3.73 ** | 2.56 ± 0.4 ** |
| Max Resp | 243.25 ± 3.6 | 196.25 ± 6.75 ** | 180.8 ± 4.1 ** |
| SRC | 102.5 ± 3.8 | 81.25 ± 6.9 ** | 56.17 ± 3.6 ** |
| Non-Mito Resp | 8.65 ± 0.4 | 21.6 ± 3.5 ** | 18.52 ± 0.8 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura-García, E.K.; Valdez-Solana, M.A.; Avitia-Domínguez, C.; García-Arenas, G.; Téllez-Valencia, A.; Balagurusamy, N.; Sierra-Campos, E. Anticancer Effects of Pleurotus salmoneostramineus Protein Hydrolysate on HepG2 Cells and In Silico Characterization of Structural Effects of Chromoprotein-Derived Peptides on the Mitochondrial Uncoupling Protein 2 (UCP2). BioMedInformatics 2025, 5, 29. https://doi.org/10.3390/biomedinformatics5020029
Ventura-García EK, Valdez-Solana MA, Avitia-Domínguez C, García-Arenas G, Téllez-Valencia A, Balagurusamy N, Sierra-Campos E. Anticancer Effects of Pleurotus salmoneostramineus Protein Hydrolysate on HepG2 Cells and In Silico Characterization of Structural Effects of Chromoprotein-Derived Peptides on the Mitochondrial Uncoupling Protein 2 (UCP2). BioMedInformatics. 2025; 5(2):29. https://doi.org/10.3390/biomedinformatics5020029
Chicago/Turabian StyleVentura-García, Erica K., Mónica A. Valdez-Solana, Claudia Avitia-Domínguez, Guadalupe García-Arenas, Alfredo Téllez-Valencia, Nagamani Balagurusamy, and Erick Sierra-Campos. 2025. "Anticancer Effects of Pleurotus salmoneostramineus Protein Hydrolysate on HepG2 Cells and In Silico Characterization of Structural Effects of Chromoprotein-Derived Peptides on the Mitochondrial Uncoupling Protein 2 (UCP2)" BioMedInformatics 5, no. 2: 29. https://doi.org/10.3390/biomedinformatics5020029
APA StyleVentura-García, E. K., Valdez-Solana, M. A., Avitia-Domínguez, C., García-Arenas, G., Téllez-Valencia, A., Balagurusamy, N., & Sierra-Campos, E. (2025). Anticancer Effects of Pleurotus salmoneostramineus Protein Hydrolysate on HepG2 Cells and In Silico Characterization of Structural Effects of Chromoprotein-Derived Peptides on the Mitochondrial Uncoupling Protein 2 (UCP2). BioMedInformatics, 5(2), 29. https://doi.org/10.3390/biomedinformatics5020029






