Abstract
Background: Exosomes are a group of extracellular vesicles that are released by almost all mammalian cell types and engage in intracellular communication. Studies conducted in recent years have shown that exosomes are involved in a variety of diseases, where they may act as “vehicles” for the transmission of biomolecules and biomolecular information. Amyloidoses constitute a critical subgroup of these diseases, caused by extracellular deposition or intracellular inclusions of insoluble protein fibrils in cells and tissues. However, how exosomes are involved in these diseases remains largely unexplored. Methods: To detect possible links between amyloid proteins and exosomes, protein data from amyloidosis-isolated exosomes were collected and visualized using biological networks. Results: This biomedical informatics approach for the analysis of interaction networks, in combination with the existing literature, highlighted the involvement of exosomes in amyloidosis while strengthening existing hypotheses regarding their mechanism of action. Conclusion: This work is focused on exosomes from patients with Alzheimer’s disease and identifies important amyloidogenic proteins found in exosomes. These proteins can be used for future research in the field of exosome-based biomarkers of amyloidosis and potential prognostic or preventive approaches.
1. Introduction
Exosomes are small extracellular vesicles (EVs) of 50–130 nm in diameter, secreted from intracellular endosomal departments called multivesicular bodies (MVBs), which contain intraluminal vesicles. Instead of fusing with lysosomes and other acidic departments, multivesicular bodies may fuse with the plasma membrane, resulting in the secretion of intraluminal vesicles referred to as exosomes [1,2,3]. Exosomes typically include cytosolic cargo, such as proteins, peptides, lipid derivatives, and RNA. They are surrounded by a lipid bilayer of endosomal origin that serves for the safe transport of enclosed and surface materials in short and long distances within tissues or between them by flowing in biological fluids. Their function depends on both the cell type of origin and the target (the recipient cell). Some of the most well-characterized functions include involvement in antigen presentation [4], blood coagulation [5], sperm maturation [6], RNA and protein transport [7], and cell communication and signaling [8]. However, exosomes also seem to play a significant role in pathological conditions such as cancer [9], autoimmune diseases [10], inflammation [11], infections [12,13], and metabolic [14] and cardiovascular diseases [15]. More specifically, there are specific proteins that implicate exosomes in a variety of diseases and pathological conditions, such as Alzheimer’s disease (AD) [16,17], Prion disease [18], Parkinson’s disease [19], tau protein-induced diseases [20], and Huntington’s disease [21]. These diseases are distinct by a specific feature, which is the deposition of amyloid fibrils [22].
In recent years, the ever-increasing number of studies on extracellular vesicles, specifically on exosomes, resulted in large amounts of data. Specialized databases have been created from those data for the storage and categorization of proteins, nucleic acids, lipids, and other components of extracellular vesicles. Three databases with EVs and/or exosomal data are available as follows: the ExoCarta (Version 6) [23], the EVpedia (2018 Version) [24], and the Vesiclepedia (Version 4.1) [25]. ExoCarta holds entirely exosomal data, while EVpedia and Vesiclepedia contain data on the more general group of EVs.
The ExoCarta database is a manually curated collection of exosomal proteins, RNAs, and lipids. It is freely available to the public, and its latest version [26] (7 October 2015) includes 41,860 protein entries (5402 unique proteins in Homo sapiens), more than 7540 RNAs, and 1116 lipid molecules. The data were isolated from approximately 286 “extracellular” studies and annotated according to the least experimental requirements set up by ISEV for extracellular vesicles. Each entry is represented by a specific ExoCarta number and includes information on the gene of origin, the type of tissue/cell from which the exosomes of each study were derived, the associated Gene Ontology annotation, information on the respective experiment, pathways in which the respective protein participates, and a list of protein–protein interactions.
EVpedia is a manually curated and integrated collection of data derived from high-throughput analyses of extracellular vesicles. Compared to ExoCarta and Vesiclepedia, EVpedia also includes data from prokaryotic organisms, along with a variety of tools, such as Gene Ontology enrichment analysis, network analysis of proteins and mRNAs, and comparison of vesicle data with rational identification. The latest version of EVpedia (30 April 2018) includes 14,192 research studies from 7373 principal investigators and 1114 high-throughput datasets from 722,551 isolated vesicular components.
Vesiclepedia is a manually curated collection of molecular data, found on all classes of EVs, including apoptotic bodies, exosomes, shedding microvesicles, and other subgroups. The main criterion for manual curation was the presence of these vesicles in the extracellular environment. It contains RNA, proteins, lipids, and metabolites that have been identified in EVs from both published and unpublished studies. The latest version of Vesiclepedia (15 August 2018) includes data from 1.254 studies on extracellular vesicles of 41 organisms, 349,988 protein entries (12,799 proteins in Homo sapiens), 38,146 RNA entries, and 639 lipid/metabolite entries. Vesiclepedia is freely accessible to the public and allows the user to search and isolate the cargo of extracellular vesicles based on various search criteria (e.g., organism, vesicle type, content type, source material). Additionally, the database offers information related to the isolation protocol/strategy and characterization of each extracellular vesicle, as well as to the biophysical and molecular properties of each isolated material.
Several neurodegenerative diseases are related to the activities of amyloid-like proteins, which create conformations that extend to healthy proteins. Some of them include AD [27] (amyloid-beta and tau), Parkinson’s disease [28] (alpha-synuclein), Huntington’s disease [29] (Huntingtin), and amyotrophic lateral sclerosis [30] (superoxide dismutase 1). Although these are not the only proteins found in pathological aggregates, they appear to be associated with the aggregation process and subsequent neurotoxicity. AmyCo [31] is a collection of protein data related to amyloidosis, including the precursor proteins (causative proteins) and co-deposited proteins of amyloid deposits and affected tissues or organs.
This study aimed to use bioinformatics tools and methods, such as protein–protein interaction networks and network analyses, to correlate the subgroup of exosomes with neurodegenerative diseases and specifically Alzheimer’s disease. Notably, a bibliometric analysis covering studies from 2002 to 2021 [13] has highlighted the significant progress and research interest in exosomes within the field of autoimmune diseases.
2. Materials and Methods
2.1. Exosomal and Amyloid Protein Database Collection
All exosomal data and information were collected from databases related to exosomes (ExoCarta) and EVs (Vesiclepedia, EVpedia), as well as from the literature (PubMed, 2025). For each database, a different pipeline was used to filter the proteins.
Although ExoCarta and Vesiclepedia hold useful information for individual exosomal proteins, EVpedia allows the user to extract entire protein datasets using specific keywords. For this reason, we used EVpedia to find protein datasets derived from studies related to neurodegenerative diseases and, more specifically, Alzheimer’s disease (AD). EVpedia was used for the in-depth search of datasets resulting from research studies of exosomes in AD. Initially, a list of 75 amyloidosis keywords (Table S1) was generated using the names of the diseases as described in the AmyCo database, along with the names of the related proteins. The keywords were inserted in the field/category Experiment [Protein]. In this category, it is possible to find specific proteomic datasets using keywords in a variety of fields (e.g., article title, PubMed ID, journal, a technique for isolating ΕVs) and characteristic criteria filters (e.g., super kingdom, species, type, tissue type, and in vitro and in vivo experiments). For the performed searches, we used the keywords mentioned above (Table S1), while the filters used were the same for all searches: (a) super kingdom → eukaryotes; (b) organism → Homo sapiens; (c) vesicle type → exosomes; (d) sample type → any. From a total of 1114 high-performance proteomic datasets in EVpedia, 2 datasets were isolated, which related to exosomal data from control proteome (631 proteins) and 4R0N tau-overexpressed M1C neuroblastoma cells (660 proteins), as described in the work of Saman et al. [32]. There was an important correlation between genes that were significantly downregulated in multiple forms of AD and proteins that had been recruited on exosomes from tau, which indicates the central involvement of tau secretion in AD cytopathogenesis.
Vesiclepedia in combination with the ExoCarta database was used to characterize AmyCo proteins as exosomal. The choice of AmyCo over the other databases [33,34,35,36,37,38] was based on the fact that AmyCo includes a comprehensive collection of amyloidogenic proteins, even for rare diseases, and it is frequently updated. Specifically, the 85 AmyCo proteins (precursor and co-deposited proteins) were searched in the two databases, and 72 out of the 85 were found in exosomes (Table S2). This list of proteins was used at a later stage for the visualization of the exosomal data. In this way, we managed to integrate more information into the final visualization and extract more accurate results.
2.2. Network Analysis
The collected proteins found in exosomes from patients were used to query the STRING database (12.0) [39,40], and their protein–protein interactions were obtained. The STRING database collects both functional associations and physical interactions, which include false positives. Therefore, a confidence score value of 0.9 was used to limit the dataset to interactions of the highest confidence, while no additional interactors were included. A less strict cutoff of 0.7 corresponding to high confidence was also used, and the network is provided in the Supplementary Materials (Table S7, Figure S1). Visualization of the interaction network from STRING was performed using Cytoscape (3.10.2) [41] and the stringApp tool (v2.1.0) [42]. Additionally, the Network Analyzer (4.5.0) [43] and Network Randomizer (v1.1.3) [44] were used to statistically analyze the network and to produce a random network of the same size. Lastly, a functional annotation of the nodes was performed using the stringApp tool, which relies on sources such as KEGG pathways, Gene Ontology, and more.
2.3. Bibliographic Search
Along with the database searches, an additional literature search using PubMed (until December 2022) was performed to further identify exosomal proteomic datasets related to neurodegenerative diseases, if any. Specific keywords (Table S3) were used to perform queries, and through manual curation of the results, proteomic data were isolated. At this stage, it should be noted that a considerable proportion of the results were derived from studies performed in the field of cancer, while several of the studies targeting neurodegenerative disorders did not provide the user with the proteomic data for further processing. Thus, after an extensive search, we did not find any additional datasets related to AD. Additionally, PubMed was used to detect critical proteins in the network (assigned with a green color) that were of literature interest regarding their importance in exosomes and AD.
3. Results
3.1. Data Analysis
To identify important proteins found in exosomes, a protein comparison between healthy and AD exosome datasets from EVpedia was made. The goal of the comparison was to find common and unique proteins between each dataset. Data comparison and extraction of the results were performed using a Python [45] script (version 3.7.13), which was constructed specifically for this process, and the results were recorded. Thus, according to the EVpedia datasets, the subsets “Proteins in Healthy” (631 proteins), “Proteins in Disease” (660 proteins), “Common Proteins” (35 proteins), “Proteins only in Healthy” (596 proteins), and “Proteins only in Disease” (625 proteins) were formed with their respective UniProt [46] accession numbers (ACs).
Afterwards, comparing the EVpedia resultant subsets with the Saman et al. 2014 sets [32], some differences were detected. In Saman et al.’s work, the “Proteins in Healthy” were 634, and the “Proteins in Disease” were 662, while the 35 common proteins were the same in both datasets.
3.2. Visualization of Interaction Network
3.2.1. Disease Protein Network
The protein–protein interactions of the category “Proteins in Disease state” (660 proteins) were obtained using the STRING database (Table S4). Less than half of these proteins (301) had at least one interaction in the network with the total number of edges being 471. As shown in Figure 1, while there is a large connected component, the majority of proteins are not part of it, indicating that these proteins have disparate functional roles or lack high confidence interactions. Proteins from the AmyCo database were found in the network, and six were connected (Q5XWF0, P02671, P68104, Q9UC33, P37840, and Q6NUR9), and one was isolated (Q8N177).
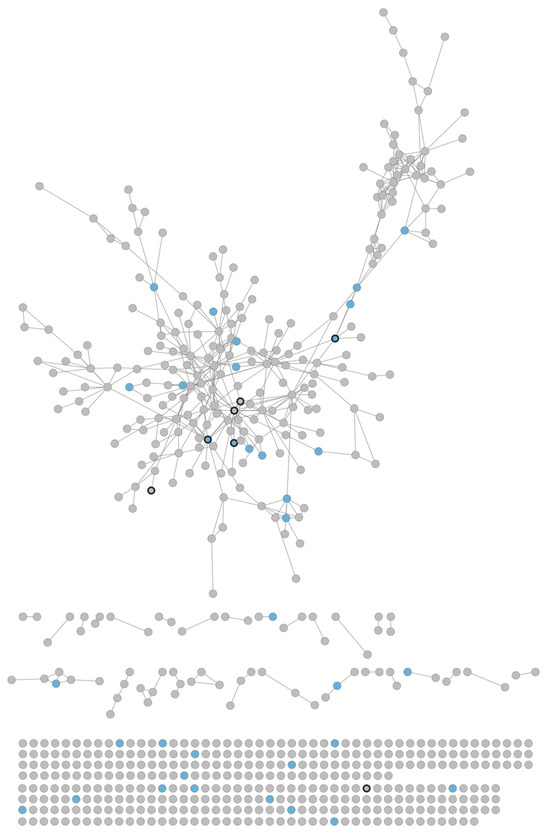
Figure 1.
Exosomal disease network. A total of 301 proteins/nodes participate in at least 1 of 471 interactions/edges. Most proteins are found only in the disease state (grey), while only a few are common in disease and healthy (blue). A large connected component exists, which contains six AmyCo proteins (dark border).
3.2.2. Statistical Analysis
The Cytoscape Network Analyzer (4.5.0) [43] (Table 1) plugin was used for the calculation of topological parameters. In addition, combining it with the Network Randomizer, an evaluation of the network as biological or random [47,48,49] was made. Most notable is the difference between the clustering coefficients, a measure of the networks’ tendency to form interconnected groups/clusters of nodes. The disease network has a much higher value than the random one. This is in accordance with what one would expect from a biological network [50]. Similarly, while the two networks have a similar average number of neighbors, the node degree distribution of the disease network resembles a scale-free network [51], with a small number of nodes having a large degree. This is a characteristic of biological networks. Lastly, the parameters related to the distance between nodes indicate that the disease network nodes are more distant than the random network nodes. This further indicates that the disease proteins are only distantly connected.

Table 1.
Topological parameters of the disease set network calculated with the Network Analyzer plugin.
Additionally, crucial elements in the disease protein–protein interaction (PPI) network, the most connected nodes, were identified.
Table 2 shows the top central nodes of the disease protein network with a degree greater than 11. These proteins, as central nodes, play a significant role in the network, as they are connected to many neighbors. Additionally, according to Zotenko et al. [52], the removal of these central nodes may cause the collapse of the network. Most of those central nodes also appear in the final Alzheimer’s disease network, described next (Figure 2).

Table 2.
The most central nodes of the disease set network (277 proteins | confidence score: 0.9 and experimental evidence) and the number of their interactions.
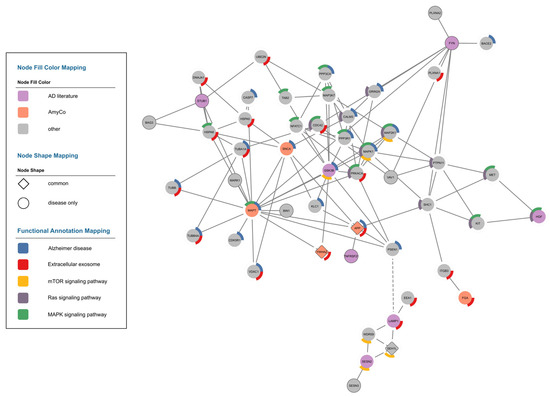
Figure 2.
Alzheimer’s disease network. The proteins of interest from the literature are depicted in purple, and the proteins from AmyCo are shown in orange. Only three proteins are also found in healthy samples, APP, YWHAZ, and SEH1L, and are shown with a diamond shape. A custom edge (dashed line) was added between LAMP1 and PSEN1 to represent an interaction discussed below. Finally, a mapping of some key functional annotations is shown around the nodes. Specifically, the annotations used are KEGG Pathways Alzheimer’s disease (hsa05010), mTOR signaling pathway (hsa04150), Ras signaling pathway (hsa04014), MAPK signaling pathway (hsa04010), and the GO Cellular Component Extracellular Exosome (GO:0070062).
3.2.3. Alzheimer’s Disease Network
An Alzheimer’s disease network was constructed using proteins of interest in the literature regarding their association with Alzheimer’s disease (P49841, O75509, P14210, P11279, P58004, Q9UNE7, and P06241). Additionally, the amyloidogenic proteins from AmyCo were included (P37840, P10636, and P02671). These proteins and their first neighbors were isolated from the network. In this way, the information was condensed without overloading the visualization of the network. While the STRING interactions shown here are of very high confidence, they might still represent false positives, and their evidence can be investigated individually for validation.
3.3. Functional Analysis
Firstly, the stringApp (v2.1.0) was used to find biological terms enriched in the disease network (Table S5). Most notably, a number of diseases were found among the KEGG Pathways terms, including Alzheimer’s disease (hsa05010) and the related conditions of Prion disease (hsa05020), Parkinson’s disease (hsa05012), Huntington’s disease (hsa05016), and amyotrophic lateral sclerosis (hsa05014). Furthermore, several GO Biological Processes relate to brain function, such as nervous system development (GO:0007399) and synaptic signaling (GO:0099536). Overall, these results are expected from a dataset of Alzheimer’s disease-related proteins. Similarly, the Alzheimer’s disease network was analyzed, and terms related to that subset were found (Table S6). Some key terms and their related nodes are visualized in Figure 2, specifically those for the GO Cellular Component Extracellular Exosome (GO:0070062) and for KEGG Pathways Alzheimer’s disease (hsa05010), mTOR signaling pathway (hsa04150), Ras signaling pathway (hsa04014), and MAPK signaling pathway (hsa04010). The first two terms are related to the core characteristics of the protein dataset, while the latter three terms are related to both the disease and/or exosomes. More specifically, evidence supports the possible involvement of the mTOR signaling pathway (has04150) in disease progression [53,54,55,56,57]. Another important and related pathway is the MAPK signaling pathway (hsa04010) [58,59,60] that, along with the Ras signaling pathway (hsa04014), has been implicated in the mechanistic role of exosomes [61].
4. Discussion
In this study, we investigated the contribution of exosomes to Alzheimer’s disease propagation from a biomedical informatics perspective. The collection of available data combined with their processing and visualization in the protein interaction network confirms to a satisfactory degree the contribution of exosomes in the pathogenesis of the disease.
Starting with the presence of amyloidogenic proteins in the network, we observed the amyloid-beta precursor protein (APP), alpha-synuclein (SNCA), microtubule-associated protein tau (MAPT), and fibrinogen alpha chain (FGA). The first three proteins are directly associated with AD [62,63,64], while the FGA is the main protein responsible for hereditary amyloidosis of the α-chain of fibrinogen [65].
It is argued that APP processing occurs mainly in the endosomes, in which exosomes are formed as intraluminal vesicles [66,67,68,69]. Analogously, APP, C-terminal fragments (CTF-α, CTF-β), and amyloid-beta have been found in varying amounts within exosomes from a variety of cell lines overexpressing APP, as well as in EVs of unknown origin isolated from mouse brains [70]. However, there is no clear consensus among the scientific community members on the different APP fragments associated with exosomes secreted by neurons. For the first time, Laulagnier et al. [71] showed that a subset of CD63-negative exosomes that are derived from inducible Tet-On mouse neuroblastoma cells (N2a—hAPPSWE) transport APP and CTFs in the dendrites of neurons via endocytosis.
Several studies have identified interactions between alpha-synuclein and tau, which may have implications for various neurodegenerative diseases [72,73,74]. For example, the carboxyl terminus of alpha-synuclein may directly interact with the microtubule-binding site of tau [75], while the presence of alpha-synuclein can induce the intracellular aggregation of tau [76]. The interactions between these two proteins could also be catalyzed by the serine/threonine kinase glycogen synthase kinase-3 beta (GSK3β) [3]. GSK3β participates in a variety of cellular signaling pathways that are related to growth, proliferation, immunity, and apoptosis. It is also capable of inducing the abnormal phosphorylation of tau in a variety of disease-associated sites [77,78,79,80,81,82]. Kozikowski et al. [83] used neuroblastoma SH-Y5Y cells stably expressing alpha-synuclein to identify novel inhibitors of GSK3 and found that selective inhibition of GSK3β not only prevented the phosphorylation of tau but also reduced alpha-synuclein levels. In the network, we identified the direct link between MAPT and GSK3β; however, alpha-synuclein appears to be distant from MAPT by two additional nodes. The presence of amyloid-beta may increase the activity of GSK3β, which, in turn, induces the phosphorylation of tau and the production of alpha-synuclein [70]. Subsequently, the overall aggregation of the three proteins leads to cellular dysfunction and, consequently, apoptosis. According to Danzer et al. [84], alpha-synuclein oligomers that are localized in exosomes appear to cause greater toxicity compared to free oligomers.
According to specific studies [85,86,87,88], TNFRSF21, or Death Receptor (DR6), plays a critical role in neuronal cell death, while, in some cell lines, it can lead to apoptosis. In conjunction with the above, Xu et al. [89] reported that amyloid-beta may affect the DR6/N-APP pathway by promoting DR6 expression, resulting in Ab-induced neuronal damage. Autophagy dysfunction may further exacerbate neuronal apoptosis by influencing this pathway. Exosomes may carry components like N-APP, which interact with DR6, thereby promoting neurodegeneration, as suggested by recent findings [90]. The appearance of DR6 exclusively in patients, where it is found directly connected to the APP node and indirectly with BACE1, supports the hypothesis of Xu et al. [89].
The presence of E3 ubiquitin-protein ligase CHIP (STUB1) in the network could attribute a protective role to exosomes against pathological elements of disease and beta-amyloid. Specifically, in AD, BACE1 activity is induced by oxidative stress and dysfunction of the ubiquitin-proteasome system (UPS), which is associated with the inactivity of p53 [91]. BACE1 seemed to be a novel substrate of STUB1 in HEK-APP cells [92], as it inhibits BACE1 levels via ubiquitination and proteasomal degradation and also stabilizes p53, thereby reducing the processing of APP.
The custom edge between Presenilin-1 (PSEN1) and Lysosomal-Associated Membrane Protein 1 (LAMP1) was derived from the experimentally proven interactions of the disease set network created to highlight the potential involvement of the lysosomal protein LAMP1 in disease pathogenesis. In the brains of mouse models of AD, expressing human APP and Presenilin-1, immunofluorescence staining identified amyloid-beta deposits that were circularly surrounded by a layer of LAMP1 [93]. The concomitant presence of elevated BACE1 levels already from the preliminary stages of the disease in dystrophic axons with APP deposits is probably due to the accumulation of lysosomes, “poor” in proteases. The function of LAMP1 is mainly related to the structural stability of the lysosomal compartment, while its presence is already noted in mature, late endosomes. However, given the origin of exosomes from late endosomes and the frequent localization of LAMP1 [4,94,95] in them (a potential biomarker), this finding implies the presence and/or involvement of exosomes in amyloid plaques [70]. Furthermore, the presence of Sestrin-2 (SESN2) that indirectly binds to LAMP1 probably confers and/or enhances a (the) protective role to exosomes, as both in physiological and pathological situations it regulates oxidative stress (ROS), autophagy, metabolism [96,97,98], and, probably, amyloid-beta toxicity [99].
5. Conclusions
This study aims to highlight the possible involvement of exosomes in a group of diseases called amyloidosis and, more specifically, in Alzheimer’s disease. The collection of available data, combined with their processing and visualization in protein interaction networks, confirms to a satisfactory degree the contribution of exosomes in the pathogenesis of AD. In particular, the analysis of the network and the subsequent justification of the results attribute a dual character to exosomes, which move between a protective role against disease toxicity and a pathogenic role, where they take part in the dissemination and exacerbation of diseases. Moreover, the potential protective role of proteins in exosome-mediated pathways needs more empirical evidence to be claimed. Furthermore, amyloidogenic proteins (alpha-synuclein (P37840), microtubule-associated protein tau (P10636), and fibrinogen alpha chain (P02671)) occupy a dynamic position in the network, as they connect to central nodes (glycogen synthase kinase-3 beta (P49841), amyloid-beta precursor protein (P05067), mitogen-activated protein kinase 1 (P28482)) that enable their involvement in network connectivity and communication with other important proteins. It should be noted that the lack of consensus regarding which amyloid precursor protein (APP) fragments are associated with exosomes across different cell types could lead to inconsistencies in interpretation and requires further experimental clarification. Additionally, while the interactions between amyloid-beta, tau, and alpha-synuclein are well-established, the role of exosomes in mediating these interactions should be examined through direct experimental approaches to assess the functional relevance of these protein aggregations in exosome trafficking.
This study aimed to integrate different sources of information to shed light on the potential role of exosomes in Alzheimer’s disease. It aims to provide a starting point for the application of network analyses to exosome-mediated communication in Alzheimer’s disease. It is important to consider the heterogeneity of exosome populations and the potential for contamination from other sources to minimize errors in identifying specific biomarkers with high confidence. It provides a comprehensive analysis of the available data on exosomes and Alzheimer’s disease and may serve as a scaffold for future research in this field. Thus, further investigation and experimental confirmation of the results are considered necessary and may lead to the identification of exosomal biomarkers for AD and preventive strategies for its elimination. It is necessary to highlight the dynamic nature of exosome composition and concomitant diseases to simplify data interpretation. Also, this work can be expanded to other neurodegenerative diseases or other diseases in the group of amyloidosis, as this type of biomedical approach can lead to biomarker detection.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedinformatics5020019/s1, Table S1: Amyloidosis keywords; Table S2: AmyCo proteins in exosomes; Table S3: Literature search keywords; Table S4: Protein–protein interactions; Table S5: Functional analysis of the whole network; Table S6: Functional analysis of the Alzheimer’s disease network; Table S7: Protein–protein interactions using 0.7 score; Figure S1: Exosomal disease network with 0.7 score.
Author Contributions
Conceptualization, V.A.I. and Z.I.L.; methodology, A.S. and A.E.A.; software, A.S.; validation, A.E.A., Z.I.L., M.H.A. and V.A.I.; formal analysis, A.S. and A.E.A.; investigation, A.S. and Z.I.L.; data curation, A.S. and Z.I.L.; writing—original draft preparation, A.S. and Z.I.L.; writing—review and editing, A.S., A.E.A., Z.I.L., M.H.A. and V.A.I.; visualization, A.S. and A.E.A.; supervision, V.A.I.; project administration, V.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Kawakami, F.; Suzuki, M.; Shimada, N.; Kagiya, G.; Ohta, E.; Tamura, K.; Maruyama, H.; Ichikawa, T. Stimulatory effect of alpha-synuclein on the tau-phosphorylation by GSK-3beta. FEBS J. 2011, 278, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Schoppet, M.; Schurgers, L.J.; Reynolds, J.L.; McNair, R.; Heiss, A.; Jahnen-Dechent, W.; Hackeng, T.M.; Schlieper, G.; Harrison, P.; et al. Prothrombin Loading of Vascular Smooth Muscle Cell-Derived Exosomes Regulates Coagulation and Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e22–e32. [Google Scholar] [CrossRef]
- Sullivan, R.; Saez, F.; Girouard, J.; Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 2005, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Batagov, A.O.; Kurochkin, I.V. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3’-untranslated regions. Biol. Direct 2013, 8, 12. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef]
- Withrow, J.; Murphy, C.; Liu, Y.; Hunter, M.; Fulzele, S.; Hamrick, M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2016, 18, 286. [Google Scholar] [CrossRef]
- Console, L.; Scalise, M.; Indiveri, C. Exosomes in inflammation and role as biomarkers. Clin. Chim. Acta 2019, 488, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Abrami, L.; Brandi, L.; Moayeri, M.; Brown, M.J.; Krantz, B.A.; Leppla, S.H.; van der Goot, F.G. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013, 5, 986–996. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. Knowledge Mapping of Exosomes in Autoimmune Diseases: A Bibliometric Analysis (2002–2021). Front. Immunol. 2022, 13, 939433. [Google Scholar] [CrossRef] [PubMed]
- Georgatzakou, H.T.; Pavlou, E.G.; Papageorgiou, E.G.; Papassideri, I.S.; Kriebardis, A.G.; Antonelou, M.H. The Multi-Faced Extracellular Vesicles in the Plasma of Chronic Kidney Disease Patients. Front. Cell Dev. Biol. 2020, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Belting, M.; Christianson, H.C. Role of exosomes and microvesicles in hypoxia-associated tumour development and cardiovascular disease. J. Intern. Med. 2015, 278, 251–263. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, S.; Lv, J.; Wang, X.; Afewerky, H.K.; Li, H.; Lu, Y. The emerging role of exosomes in Alzheimer’s disease. Ageing Res. Rev. 2021, 68, 101321. [Google Scholar] [CrossRef]
- Lakshmi, S.; Essa, M.M.; Hartman, R.E.; Guillemin, G.J.; Sivan, S.; Elumalai, P. Exosomes in Alzheimer’s Disease: Potential Role as Pathological Mediators, Biomarkers and Therapeutic Targets. Neurochem. Res. 2020, 45, 2553–2559. [Google Scholar] [CrossRef]
- Jackson, N.A.; Guerrero-Munoz, M.J.; Castillo-Carranza, D.L. The prion-like transmission of tau oligomers via exosomes. Front. Aging Neurosci. 2022, 14, 974414. [Google Scholar] [CrossRef]
- Li, K.L.; Huang, H.Y.; Ren, H.; Yang, X.L. Role of exosomes in the pathogenesis of inflammation in Parkinson’s disease. Neural Regen. Res. 2022, 17, 1898–1906. [Google Scholar] [CrossRef]
- Polanco, J.C.; Hand, G.R.; Briner, A.; Li, C.; Gotz, J. Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol. 2021, 141, 235–256. [Google Scholar] [CrossRef]
- Ananbeh, H.; Vodicka, P.; Kupcova Skalnikova, H. Emerging Roles of Exosomes in Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 4085. [Google Scholar] [CrossRef]
- Stroo, E.; Koopman, M.; Nollen, E.A.A.; Mata-Cabana, A. Cellular Regulation of Amyloid Formation in Aging and Disease. Front. Neurosci. 2017, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kang, B.; Kim, O.Y.; Choi, D.S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Hillen, H. The Beta Amyloid Dysfunction (BAD) Hypothesis for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1154. [Google Scholar] [CrossRef]
- Araki, K.; Yagi, N.; Aoyama, K.; Choong, C.J.; Hayakawa, H.; Fujimura, H.; Nagai, Y.; Goto, Y.; Mochizuki, H. Parkinson’s disease is a type of amyloidosis featuring accumulation of amyloid fibrils of alpha-synuclein. Proc. Natl. Acad. Sci. USA 2019, 116, 17963–17969. [Google Scholar] [CrossRef]
- McGowan, D.P.; van Roon-Mom, W.; Holloway, H.; Bates, G.P.; Mangiarini, L.; Cooper, G.J.; Faull, R.L.; Snell, R.G. Amyloid-like inclusions in Huntington’s disease. Neuroscience 2000, 100, 677–680. [Google Scholar] [CrossRef]
- Sasaki, S.; Iwata, M. Immunoreactivity of beta-amyloid precursor protein in amyotrophic lateral sclerosis. Acta Neuropathol. 1999, 97, 463–468. [Google Scholar] [CrossRef]
- Nastou, K.C.; Nasi, G.I.; Tsiolaki, P.L.; Litou, Z.I.; Iconomidou, V.A. AmyCo: The amyloidoses collection. Amyloid 2019, 26, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Saman, S.; Lee, N.C.; Inoyo, I.; Jin, J.; Li, Z.; Doyle, T.; McKee, A.C.; Hall, G.F. Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J. Alzheimers Dis. 2014, 40 (Suppl. S1), S47–S70. [Google Scholar] [CrossRef] [PubMed]
- Cruts, M.; Theuns, J.; Van Broeckhoven, C. Locus-specific mutation databases for neurodegenerative brain diseases. Hum. Mutat. 2012, 33, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.O.; Kim, W.Y.; Jeong, S.Y.; Oh, J.H.; Jho, S.; Bhak, J.; Kim, N.S. PDbase: A database of Parkinson’s disease-related genes and genetic variation using substantia nigra ESTs. BMC Genom. 2009, 10 (Suppl. S3), S32. [Google Scholar] [CrossRef]
- Lill, C.M.; Roehr, J.T.; McQueen, M.B.; Kavvoura, F.K.; Bagade, S.; Schjeide, B.M.; Schjeide, L.M.; Meissner, E.; Zauft, U.; Allen, N.C.; et al. Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: The PDGene database. PLoS Genet. 2012, 8, e1002548. [Google Scholar] [CrossRef]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef]
- Bodi, K.; Prokaeva, T.; Spencer, B.; Eberhard, M.; Connors, L.H.; Seldin, D.C. AL-Base: A visual platform analysis tool for the study of amyloidogenic immunoglobulin light chain sequences. Amyloid 2009, 16, 1–8. [Google Scholar] [CrossRef]
- Rowczenio, D.M.; Noor, I.; Gillmore, J.D.; Lachmann, H.J.; Whelan, C.; Hawkins, P.N.; Obici, L.; Westermark, P.; Grateau, G.; Wechalekar, A.D. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum. Mutat. 2014, 35, E2403–E2412. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- von Mering, C.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Assenov, Y.; Ramirez, F.; Schelhorn, S.E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Tosadori, G.; Bestvina, I.; Spoto, F.; Laudanna, C.; Scardoni, G. Creating, generating and comparing random network models with NetworkRandomizer. F1000Res 2016, 5, 2524. [Google Scholar] [CrossRef]
- Rossum, G.V.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- UniProt, C. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Dong, J.; Horvath, S. Understanding network concepts in modules. BMC Syst. Biol. 2007, 1, 24. [Google Scholar] [CrossRef]
- Fronczak, A.; Fronczak, P.; Holyst, J.A. Average path length in random networks. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2004, 70, 056110. [Google Scholar] [CrossRef]
- Pavlopoulos, G.A.; Secrier, M.; Moschopoulos, C.N.; Soldatos, T.G.; Kossida, S.; Aerts, J.; Schneider, R.; Bagos, P.G. Using graph theory to analyze biological networks. BioData Min. 2011, 4, 10. [Google Scholar] [CrossRef]
- Barabasi, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Albert, R. Scale-free networks in cell biology. J. Cell Sci. 2005, 118, 4947–4957. [Google Scholar] [CrossRef]
- Zotenko, E.; Mestre, J.; O’Leary, D.P.; Przytycka, T.M. Why do hubs in the yeast protein interaction network tend to be essential: Reexamining the connection between the network topology and essentiality. PLoS Comput. Biol. 2008, 4, e1000140. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Tanzi, R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 2005, 115, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Mueed, Z.; Tandon, P.; Maurya, S.K.; Deval, R.; Kamal, M.A.; Poddar, N.K. Tau and mTOR: The Hotspots for Multifarious Diseases in Alzheimer’s Development. Front. Neurosci. 2018, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging 2012, 4, 187–201. [Google Scholar] [CrossRef]
- Walpert, M.J.; Normando, E.M.; Annus, T.; Jennings, S.R.; Wilson, L.R.; Watson, P.; Zaman, S.H.; Cordeiro, M.F.; Holland, A.J. Age-related retinal thickness in Down’s syndrome: A high-risk population for dementia. Alzheimers Dement. 2019, 11, 744–751. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Cramer, P.E.; Varvel, N.H.; Reed-Geaghan, E.; Jiang, Q.; Szabo, A.; Herrup, K.; Lamb, B.T.; Landreth, G.E. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer’s disease. Neurobiol. Aging 2012, 33, 197.e21–197.e32. [Google Scholar] [CrossRef]
- Franco, R.; Martinez-Pinilla, E.; Navarro, G.; Zamarbide, M. Potential of GPCRs to modulate MAPK and mTOR pathways in Alzheimer’s disease. Prog. Neurobiol. 2017, 149–150, 21–38. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, H.G.; Raina, A.K.; Perry, G.; Smith, M.A. The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals 2002, 11, 270–281. [Google Scholar] [CrossRef]
- Agarwal, K.; Saji, M.; Lazaroff, S.M.; Palmer, A.F.; Ringel, M.D.; Paulaitis, M.E. Analysis of exosome release as a cellular response to MAPK pathway inhibition. Langmuir 2015, 31, 5440–5448. [Google Scholar] [CrossRef]
- Azoulay-Alfaguter, I.; Mor, A. Proteomic analysis of human T cell-derived exosomes reveals differential RAS/MAPK signaling. Eur. J. Immunol. 2018, 48, 1915–1917. [Google Scholar] [CrossRef]
- Horiguchi, T.; Uryu, K.; Giasson, B.I.; Ischiropoulos, H.; LightFoot, R.; Bellmann, C.; Richter-Landsberg, C.; Lee, V.M.; Trojanowski, J.Q. Nitration of tau protein is linked to neurodegeneration in tauopathies. Am. J. Pathol. 2003, 163, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Meyer, H.E.; Egensperger, R.; Marcus, K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer’s disease. Prog. Neurobiol. 2008, 85, 393–406. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Benson, M.D.; Liepnieks, J.; Uemichi, T.; Wheeler, G.; Correa, R. Hereditary renal amyloidosis associated with a mutant fibrinogen alpha-chain. Nat. Genet. 1993, 3, 252–255. [Google Scholar] [CrossRef]
- Das, U.; Scott, D.A.; Ganguly, A.; Koo, E.H.; Tang, Y.; Roy, S. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron 2013, 79, 447–460. [Google Scholar] [CrossRef]
- Sannerud, R.; Esselens, C.; Ejsmont, P.; Mattera, R.; Rochin, L.; Tharkeshwar, A.K.; De Baets, G.; De Wever, V.; Habets, R.; Baert, V.; et al. Restricted Location of PSEN2/gamma-Secretase Determines Substrate Specificity and Generates an Intracellular Abeta Pool. Cell 2016, 166, 193–208. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Milner, T.A.; Li, F.; Nam, E.E.; Edgar, M.A.; Yamaguchi, H.; Beal, M.F.; Xu, H.; Greengard, P.; Gouras, G.K. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 2002, 161, 1869–1879. [Google Scholar] [CrossRef]
- White, A.R.; Du, T.; Laughton, K.M.; Volitakis, I.; Sharples, R.A.; Xilinas, M.E.; Hoke, D.E.; Holsinger, R.M.; Evin, G.; Cherny, R.A.; et al. Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. J. Biol. Chem. 2006, 281, 17670–17680. [Google Scholar] [CrossRef]
- Daksh, R.; Mathew, M.S.; Bosco, A.M.; Sojan, C.; Tom, A.A.; Bojja, S.L.; Nampoothiri, M. The role of exosomes in diagnosis, pathophysiology, and management of Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2025, 754, 151526. [Google Scholar] [CrossRef]
- Laulagnier, K.; Javalet, C.; Hemming, F.J.; Chivet, M.; Lachenal, G.; Blot, B.; Chatellard, C.; Sadoul, R. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol. Life Sci. 2018, 75, 757–773. [Google Scholar] [CrossRef]
- Moussaud, S.; Jones, D.R.; Moussaud-Lamodiere, E.L.; Delenclos, M.; Ross, O.A.; McLean, P.J. Alpha-synuclein and tau: Teammates in neurodegeneration? Mol. Neurodegener. 2014, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Atya, H.B.; Sharaf, N.M.; Abdelghany, R.M.; El-Helaly, S.N.; Taha, H. Autophagy and exosomes; inter-connected maestros in Alzheimer’s disease. Inflammopharmacology 2024, 32, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chen, Z. Unveiling the Complex Role of Exosomes in Alzheimer’s Disease. J. Inflamm. Res. 2024, 17, 3921–3948. [Google Scholar] [CrossRef]
- Oikawa, T.; Nonaka, T.; Terada, M.; Tamaoka, A.; Hisanaga, S.; Hasegawa, M. α-Synuclein Fibrils Exhibit Gain of Toxic Function, Promoting Tau Aggregation and Inhibiting Microtubule Assembly. J. Biol. Chem. 2016, 291, 15046–15056. [Google Scholar] [CrossRef]
- Waxman, E.A.; Giasson, B.I. Induction of intracellular tau aggregation is promoted by alpha-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J. Neurosci. 2011, 31, 7604–7618. [Google Scholar] [CrossRef]
- Latimer, D.A.; Gallo, J.M.; Lovestone, S.; Miller, C.C.; Reynolds, C.H.; Marquardt, B.; Stabel, S.; Woodgett, J.R.; Anderton, B.H. Stimulation of MAP kinase by v-raf transformation of fibroblasts fails to induce hyperphosphorylation of transfected tau. FEBS Lett. 1995, 365, 42–46. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef]
- Rankin, C.A.; Sun, Q.; Gamblin, T.C. Tau phosphorylation by GSK-3beta promotes tangle-like filament morphology. Mol. Neurodegener. 2007, 2, 12. [Google Scholar] [CrossRef]
- Reynolds, C.H.; Gibb, G.M.; Lovestone, S. Tau phosphorylation both in vitro and in cells. Methods Mol. Med. 2000, 32, 375–393. [Google Scholar] [CrossRef]
- Takashima, A.; Noguchi, K.; Michel, G.; Mercken, M.; Hoshi, M.; Ishiguro, K.; Imahori, K. Exposure of rat hippocampal neurons to amyloid beta peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3 beta. Neurosci. Lett. 1996, 203, 33–36. [Google Scholar] [CrossRef]
- Woodgett, J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990, 9, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Gaisina, I.N.; Petukhov, P.A.; Sridhar, J.; King, L.T.; Blond, S.Y.; Duka, T.; Rusnak, M.; Sidhu, A. Highly potent and specific GSK-3beta inhibitors that block tau phosphorylation and decrease alpha-synuclein protein expression in a cellular model of Parkinson’s disease. ChemMedChem 2006, 1, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Danzer, K.M.; Kranich, L.R.; Ruf, W.P.; Cagsal-Getkin, O.; Winslow, A.R.; Zhu, L.; Vanderburg, C.R.; McLean, P.J. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 2012, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, J.T.; Bobe, J.; Goetz, F.W.; Johnson, A.L. Conservation of death receptor-6 in avian and piscine vertebrates. Biochem. Biophys. Res. Commun. 2001, 284, 1109–1115. [Google Scholar] [CrossRef]
- Eimon, P.M.; Kratz, E.; Varfolomeev, E.; Hymowitz, S.G.; Stern, H.; Zha, J.; Ashkenazi, A. Delineation of the cell-extrinsic apoptosis pathway in the zebrafish. Cell Death Differ. 2006, 13, 1619–1630. [Google Scholar] [CrossRef]
- Kasof, G.M.; Gomes, B.C. Livin, a novel inhibitor of apoptosis protein family member. J. Biol. Chem. 2001, 276, 3238–3246. [Google Scholar] [CrossRef]
- Pan, G.; Bauer, J.H.; Haridas, V.; Wang, S.; Liu, D.; Yu, G.; Vincenz, C.; Aggarwal, B.B.; Ni, J.; Dixit, V.M. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 1998, 431, 351–356. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Luo, Y.; Li, W.; Shan, Y.; Tan, X.; Zhu, C. Beta amyloid-induced upregulation of death receptor 6 accelerates the toxic effect of N-terminal fragment of amyloid precursor protein. Neurobiol. Aging 2015, 36, 157–168. [Google Scholar] [CrossRef]
- Soliman, H.M.; Ghonaim, G.A.; Gharib, S.M.; Chopra, H.; Farag, A.K.; Hassanin, M.H.; Nagah, A.; Emad-Eldin, M.; Hashem, N.E.; Yahya, G.; et al. Exosomes in Alzheimer’s Disease: From Being Pathological Players to Potential Diagnostics and Therapeutics. Int. J. Mol. Sci. 2021, 22, 10794. [Google Scholar] [CrossRef]
- Tamagno, E.; Bardini, P.; Obbili, A.; Vitali, A.; Borghi, R.; Zaccheo, D.; Pronzato, M.A.; Danni, O.; Smith, M.A.; Perry, G.; et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 2002, 10, 279–288. [Google Scholar] [CrossRef]
- Singh, A.K.; Pati, U. CHIP stabilizes amyloid precursor protein via proteasomal degradation and p53-mediated trans-repression of beta-secretase. Aging Cell 2015, 14, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Yuan, P.; Wu, Y.; Schrag, M.; Paradise, S.; Grutzendler, J.; De Camilli, P.; Ferguson, S.M. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc. Natl. Acad. Sci. USA 2015, 112, E3699–E3708. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.K.; Andrews, N.W.; Simon, S.M. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 2002, 159, 625–635. [Google Scholar] [CrossRef]
- Budanov, A.V.; Lee, J.H.; Karin, M. Stressin’ Sestrins take an aging fight. EMBO Mol. Med. 2010, 2, 388–400. [Google Scholar] [CrossRef]
- Ding, B.; Parmigiani, A.; Divakaruni, A.S.; Archer, K.; Murphy, A.N.; Budanov, A.V. Sestrin2 is induced by glucose starvation via the unfolded protein response and protects cells from non-canonical necroptotic cell death. Sci. Rep. 2016, 6, 22538. [Google Scholar] [CrossRef]
- Wang, J.M.; Liu, B.Q.; Li, C.; Du, Z.X.; Sun, J.; Yan, J.; Jiang, J.Y.; Wang, H.Q. Sestrin 2 protects against metabolic stress in a p53-independent manner. Biochem. Biophys. Res. Commun. 2019, 513, 852–856. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, S.D.; Wu, C.L.; Huang, S.S.; Yang, D.I. Induction of sestrin2 as an endogenous protective mechanism against amyloid beta-peptide neurotoxicity in primary cortical culture. Exp. Neurol. 2014, 253, 63–71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).