Modelling the Impact of Temperature under Climate Change Scenarios on Native and Invasive Vascular Vegetation on the Antarctic Peninsula and Surrounding Islands
Abstract
1. Introduction
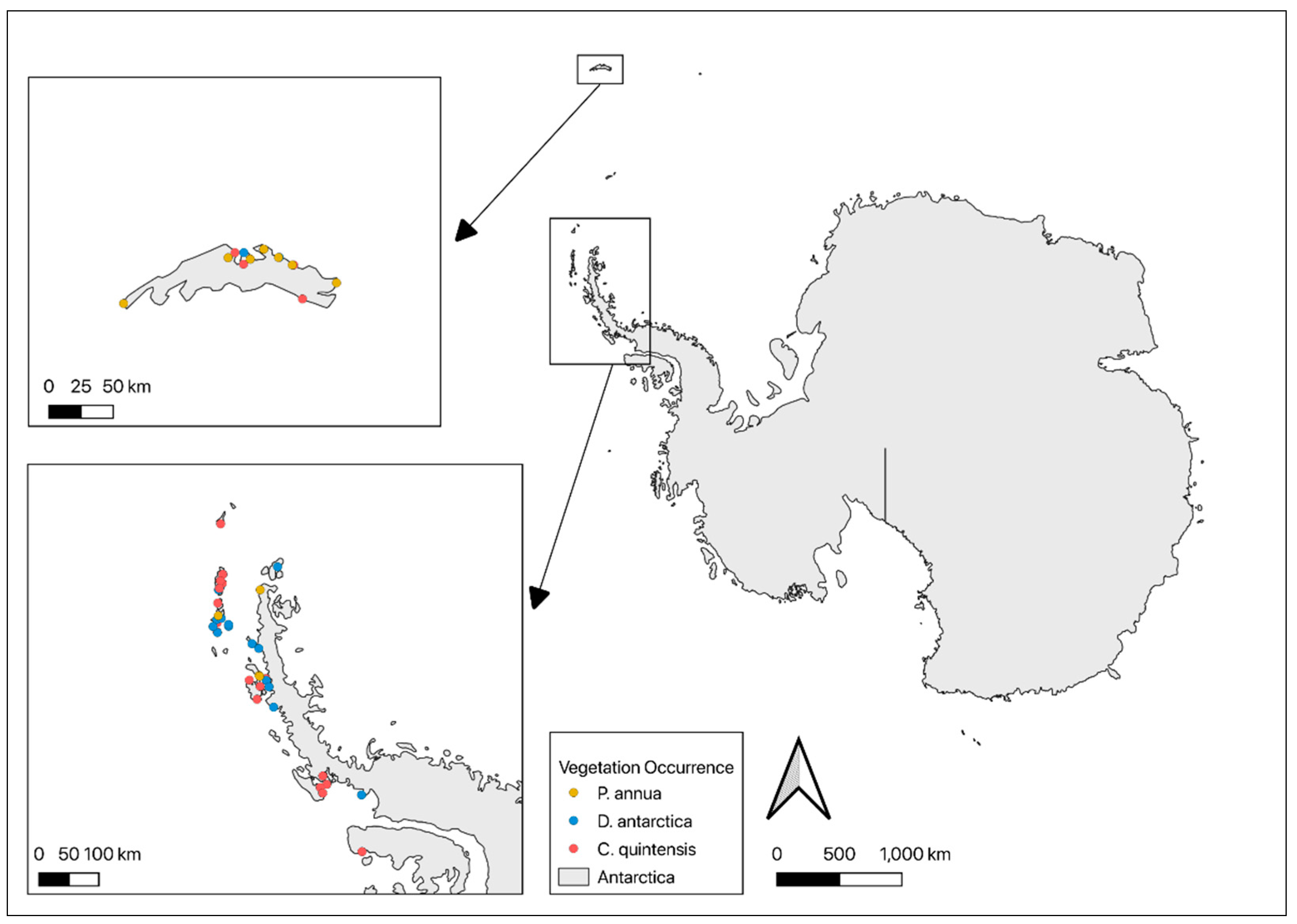
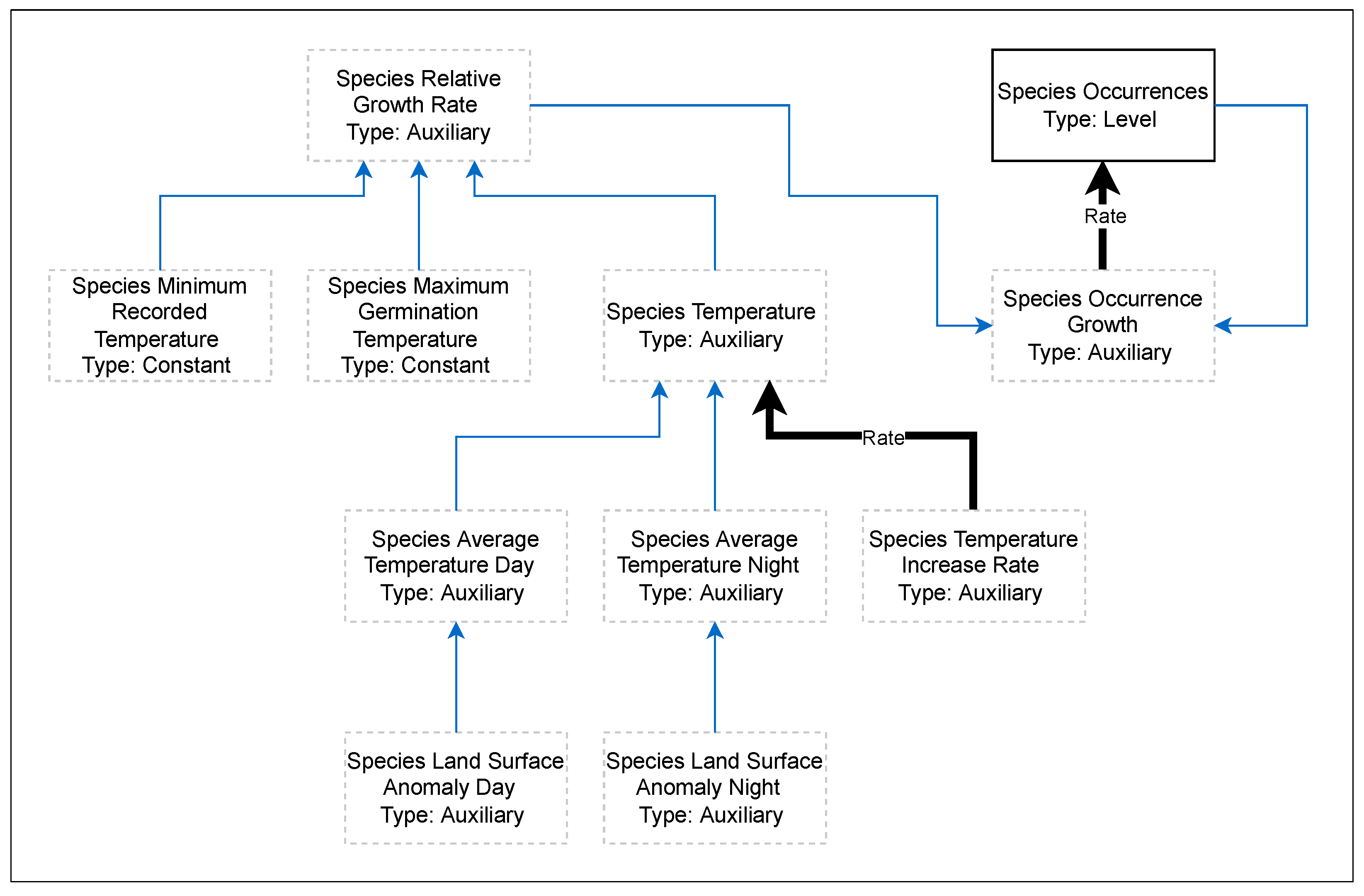
2. Materials and Methods
| Data Type | Date | Resolution | Source | Additional Information |
|---|---|---|---|---|
| Article | 2007–2008 2008–2009 | Chwedorzewska et al., 2014 [31] | The vegetation was recorded with spatial coordinates and included in the article. The coordinates were used to convert the vegetation record into a point shapefile. | |
| Article | 2007–2008 2009–2010 | Molina-Montenegro et al., 2012 [11] | The vegetation was recorded with spatial coordinates and included in the article. The coordinates were used to convert the vegetation record into a point shapefile. | |
| Human observation records | 2000–2022 | Global Biodiversity Information Facility [30] | Human Observation vegetation records were compiled by the Global Biodiversity Information Facility from various datasets. The coordinates included in the dataset were used to convert the vegetation record into a point shapefile. | |
| GeoTIFF | 2000–2022 | 1 km × 1 km | NASA Earth Observations [32] | The GeoTIFF shows the daytime temperature of the land. |
| GeoTIFF | 2000–2022 | 1 km × 1 km | NASA Earth Observations [32] | The GeoTIFF shows the nighttime temperature of the land. |
| GeoTIFF | 2000–2022 | 1 km × 1 km | NASA Earth Observations [32] | The GeoTIFF shows if the daytime surface temperature on the top 1 mm of land is warmer or colder than the average land surface temperature between 2000 and 2010. |
| GeoTIFF | 2000–2022 | 1 km × 1 km | NASA Earth Observations [32] | The GeoTIFF shows if the nighttime surface temperature on the top 1 mm of land is warmer or colder than the average land surface temperature between 2000 and 2010. |
3. Results
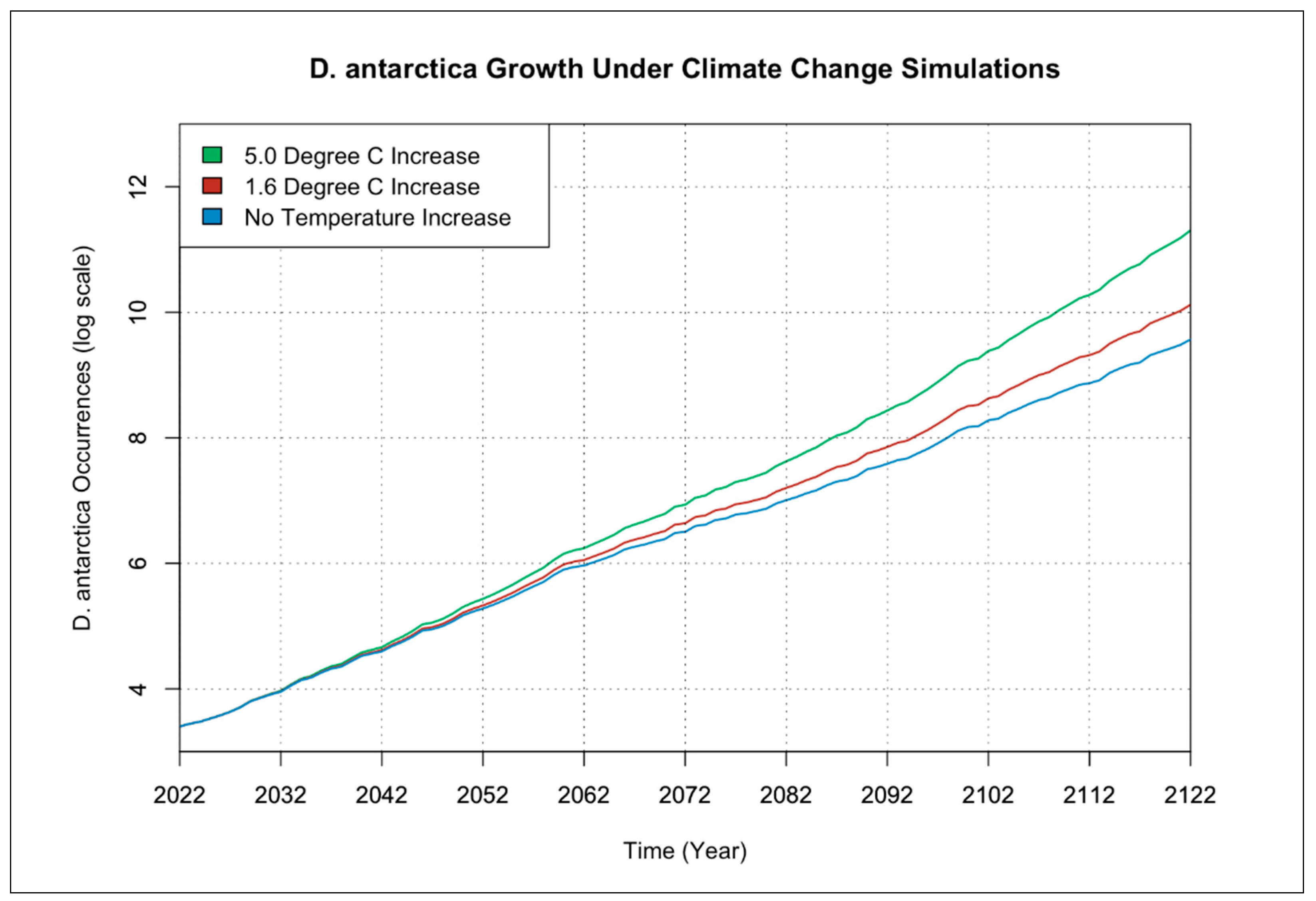
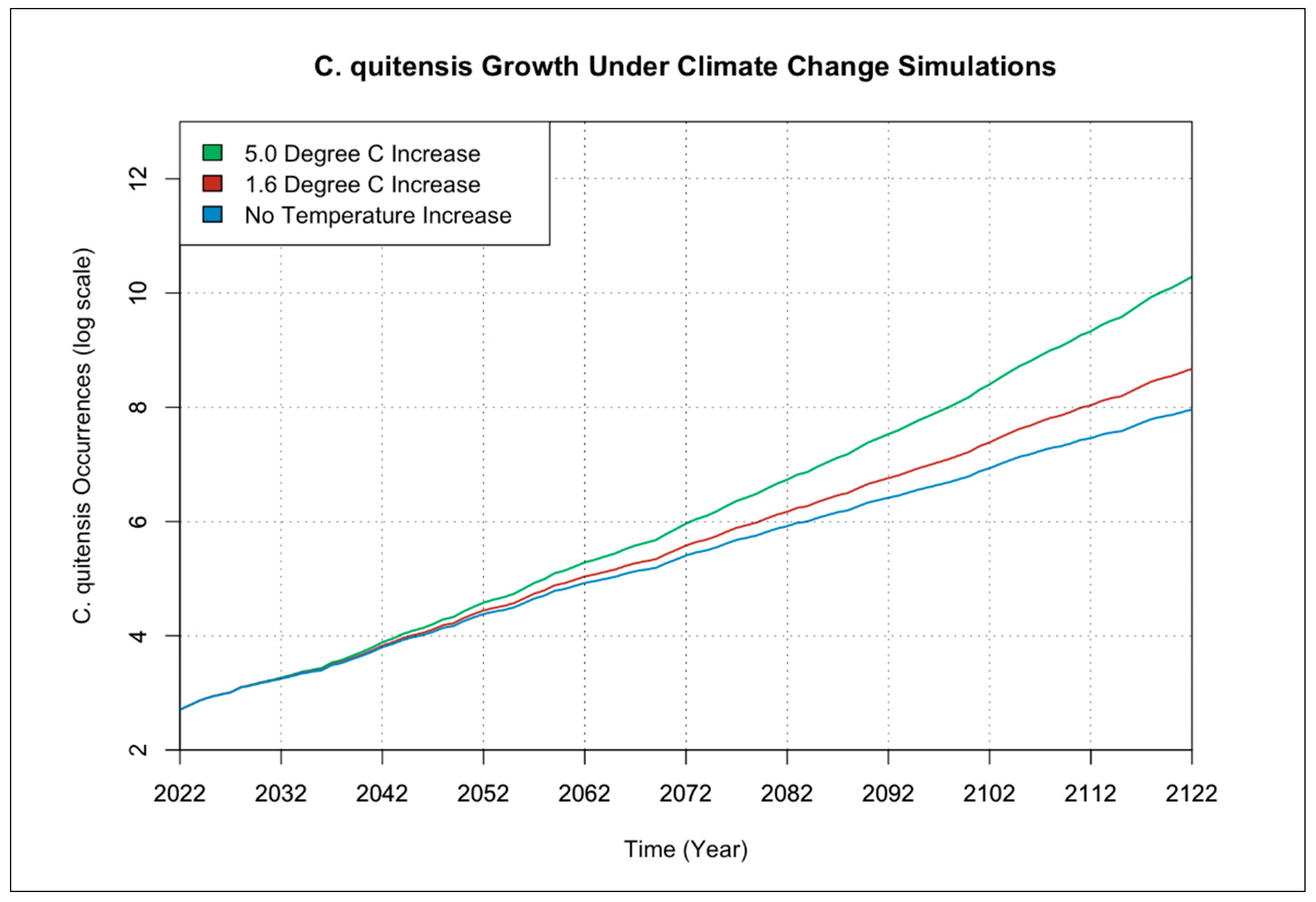
3.1. Individual Species Occurrence Increase under Climate Change Scenarios
3.2. Interactions of Species Occurrence Increase under Climate Change Scenarios
3.3. Relative Growth Rate per Temperature Curves
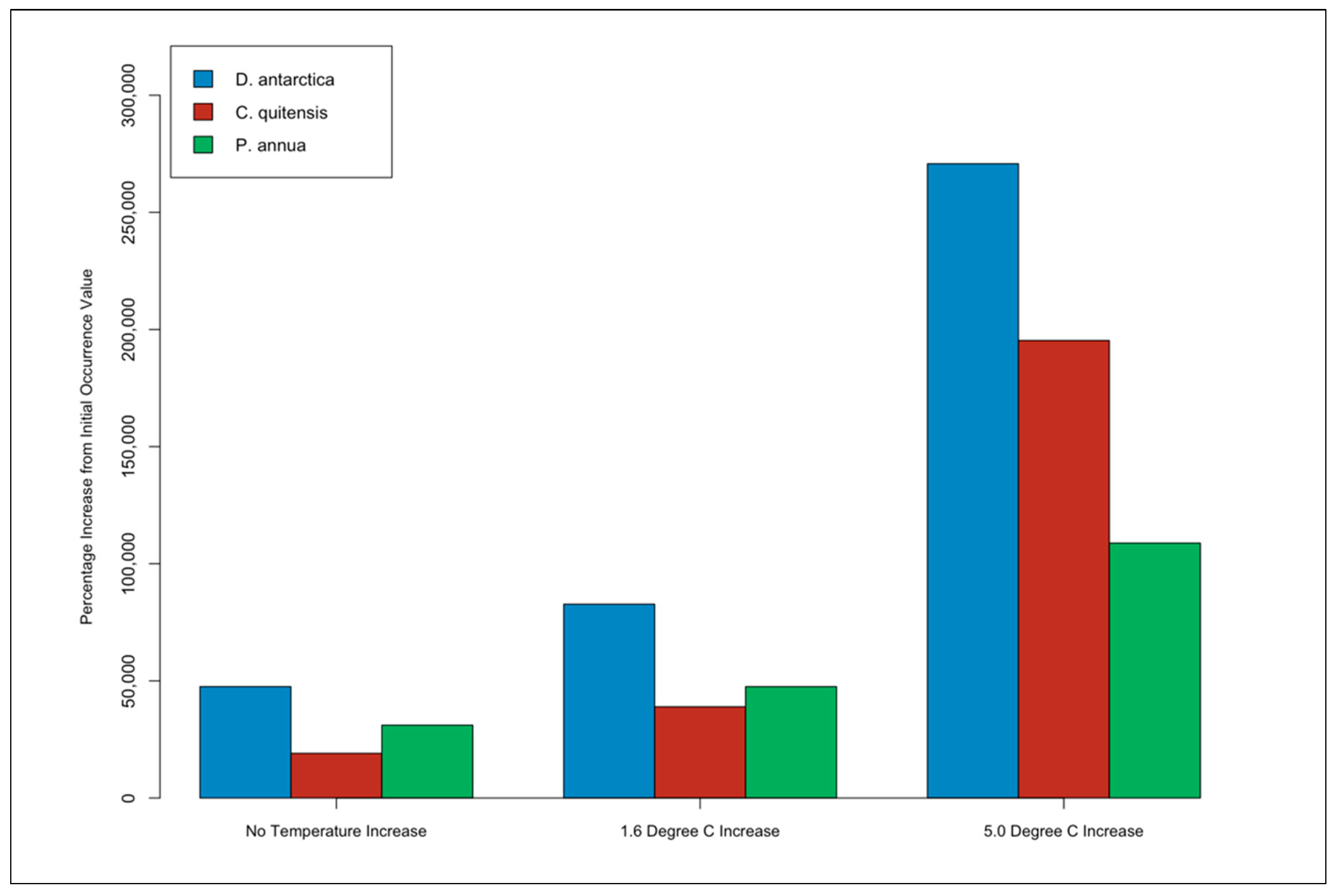
3.4. Species Occurrence Percentage Increase
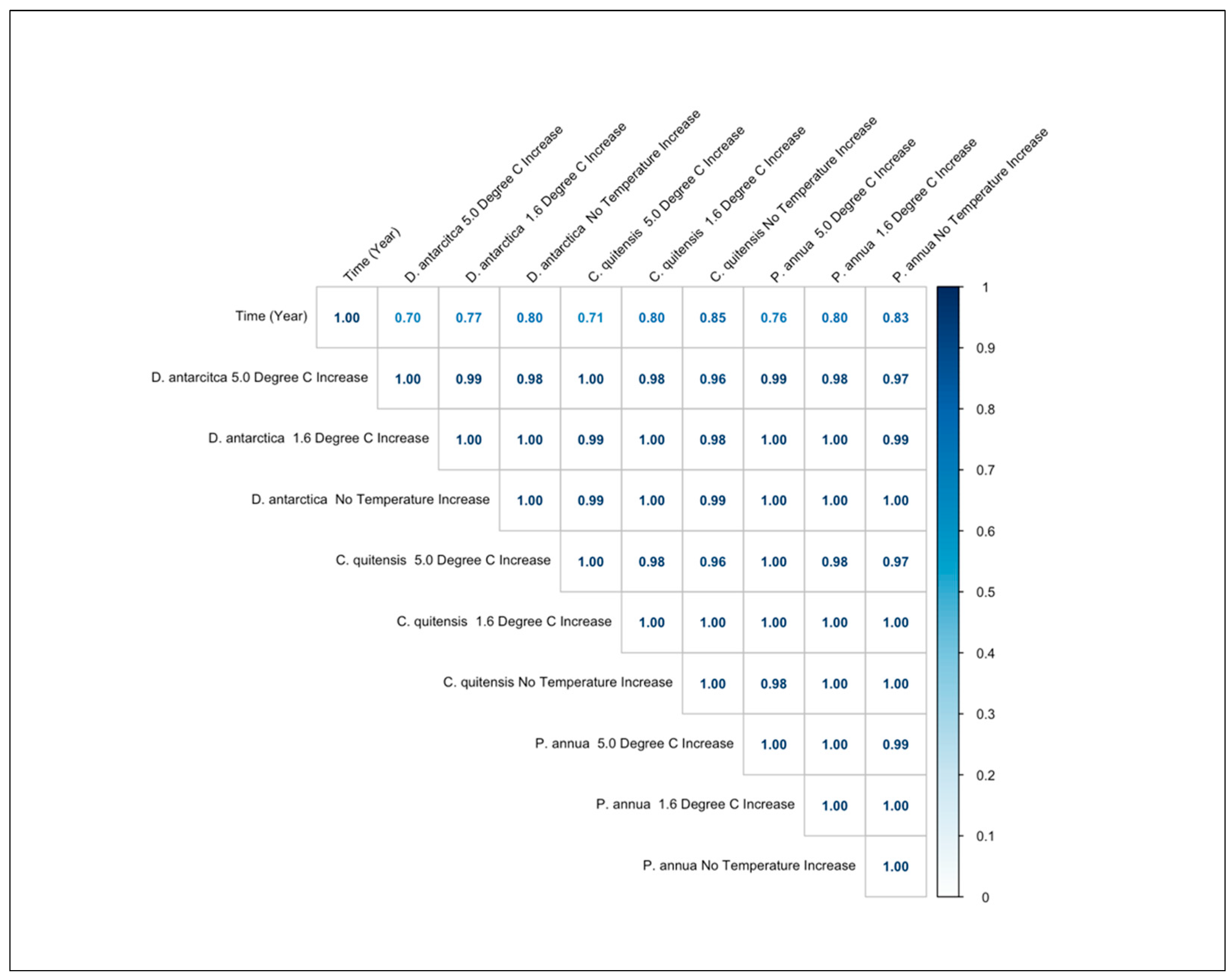
3.5. Correlation Coefficients of the Species Occurrence Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Descriptive Values of Model Parameters
| Description | Symbol | Model Value | Original Temperature Values | Units |
|---|---|---|---|---|
| D. antarcticaAverage Temperature Day | DAvgTempD | 15.944 | −9.006 | Degree Celsius |
| D. antarcticaAverage Temperature Night | DAvgTempN | 12.972 | −12.028 | Degree Celsius |
| C. quitensisAverage Temperature Day | CAvgTempD | 19.211 | −5.789 | Degree Celsius |
| C. quitensisAverage Temperature Night | CAvgTempN | 5.336 | −19.664 | Degree Celsius |
| P. annuaAverage Temperature Day | PAvgTempD | 25.873 | 0.873 | Degree Celsius |
| P. annuaAverage Temperature Night | PAvgTempN | 17.224 | −7.776 | Degree Celsius |
| D. antarcticaMinimum Recorded Temperature | DTmin | 1.648 | −23.352 | Degree Celsius |
| D. antarcticaMaximum Germination Temperature | DTmax | 50.000 | 25.000 | Degree Celsius |
| C. quitensisMinimum Recorded Temperature | CTmin | 4.121 | −20.879 | Degree Celsius |
| C. quitensisMaximum Germination Temperature | CTmax | 65.000 | 37.000 | Degree Celsius |
| P. annuaMinimum Recorded Temperature | PTmin | 11.264 | −13.736 | Degree Celsius |
| P. annuaMaximum Germination Temperature | PTmax | 67.000 | 22.000 | Degree Celsius |
| D. antarcticaOccurrence Initial Value | 30 | Species Occurrences | ||
| C. quitensisOccurrence Initial Value | 15 | Species Occurrences | ||
| P. annuaOccurrence Initial Value | 13 | Species Occurrences | ||
| D. antarcticaLand Surface Temperature Anomaly Day Minimum | −11.388 | Degree Celsius | ||
| D. antarcticaLand Surface Temperature Anomaly Day Maximum | 6.400 | Degree Celsius | ||
| D. antarcticaLand Surface Temperature Anomaly Day Seed | 10 | |||
| D. antarcticaLand Surface Temperature Anomaly Night Minimum | −6.024 | Degree Celsius | ||
| D. antarcticaLand Surface Temperature Anomaly Night Maximum | 8.941 | Degree Celsius | ||
| D. antarcticaLand Surface Temperature Anomaly Night Seed | 10 | |||
| C. quitensisLand Surface Temperature Anomaly Day Minimum | −4.424 | Degree Celsius | ||
| C. quitensisLand Surface Temperature Anomaly Day Maximum | 6.588 | Degree Celsius | ||
| C. quitensisLand Surface Temperature Anomaly Day Seed | 10 | |||
| C. quitensisLand Surface Temperature Anomaly Night Minimum | −3.012 | Degree Celsius | ||
| C. quitensisLand Surface Temperature Anomaly Night Maximum | 3.388 | Degree Celsius | ||
| C. quitensisLand Surface Temperature Anomaly Night Seed | 10 | |||
| P. annuaLand Surface Temperature Anomaly Day Minimum | −2.447 | Degree Celsius | ||
| P. annuaLand Surface Temperature Anomaly Day Maximum | 6.871 | Degree Celsius | ||
| P. annuaLand Surface Temperature Anomaly Day Seed | 10 | |||
| P. annuaLand Surface Temperature Anomaly Night Minimum | −2.353 | Degree Celsius | ||
| P. annuaLand Surface Temperature Anomaly Night Maximum | 0.753 | Degree Celsius | ||
| P. annuaLand Surface Temperature Anomaly Night Seed | 10 | |||
| Empirical Scaling Constant | c | 1 × 10−5 | ||
| Temperature Increase Rate 1 | 0.016 | Temperature /Year | ||
| Temperature Increase Rate 2 | 0.05 | Temperature/Year | ||
| Time | t | Year |
References
- Lee, J.R.; Raymond, B.; Bracegirdle, T.J.; Chades, I.; Fuller, R.A.; Shaw, J.D.; Terauds, A. Climate changes drives expansion of Antarctic ice-free habitat. Nature 2017, 547, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wouters, B.; Martin-Español, A.; Helm, V.; Flament, T.; van Wessem, J.M.; Ligtenberg, S.R.M.; van der Broeke, M.R.; Bamber, J.L. Dynamic thinning of glaciers on the Southern Antarctic Peninsula. Science 2015, 348, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.; Ivins, E.; Rignot, E.; Smith, B.; Van Den Broeke, M.; Velicogna, I.; Whitehouse, P.; Briggs, K.; Joughin, I.; Krinner, G.; et al. Mass balance of the Antarctic Ice Sheet from 1992 to 2017. Nature. 2018, 558, 219–222. [Google Scholar] [CrossRef]
- Rignot, E.; Mouginot, J.; Scheuchl, B.; van den Broeke, M.; van Wessem, M.J.; Morlighem, M. Four decades of Antarctic Ice Sheet mass balance from 1979–2017. Proc. Natl. Acad. Sci. USA 2019, 116, 1095–1103. [Google Scholar] [CrossRef]
- The Intergovernmental Panel on Climate Change. Available online: http://www.ipcc.ch (accessed on 18 August 2022).
- National Oceanic and Atmospheric Administration. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature-projections (accessed on 14 September 2022).
- Siegert, M.; Atkinson, A.; Banwell, A.; Brandon, M.; Convey, P.; Davies, B.; Downie, R.; Edwards, T.; Hubbard, B.; Marshall, G.; et al. The Antarctic Peninsula under 1.5 °C global warming scenario. Front. Environ. Sci. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Torres-Diaz, C.; Gallardo-Cerda, J.; Lavin, P.; Oses, R.; Carrasco-Urra, F.; Atala, C.; Acuna-Rodriguez, I.S.; Convey, P.; Monina-Montenegro, M.A. Biological interactions and simulated climate change modulates the ecophysiological performance of Colobanthus quitensis in the Antarctic ecosystem. PLoS ONE 2016, 1, e0164844. [Google Scholar] [CrossRef]
- British Antarctic Survey Natural Environmental Research Council. Available online: https://www.bas.ac.uk/about/antarctica/wildlife/plants/ (accessed on 15 September 2022).
- The Center for Agriculture and Bioscience International Invasive Species Compendium. Available online: https://www.cabi.org/isc/datasheet/42485 (accessed on 15 September 2022).
- Molina-Montenegro, M.A.; Carrasco-Urra, F.; Rodrigo, C.; Convey, P.; Valladares, F.; Gianoli, E. Occurrence of the non-native annual bluegrass on the Antarctic mainland and its negative effects on native plants. Conserv. Biol. 2012, 26, 717–723. [Google Scholar] [CrossRef]
- Vera, M.L. Colonization and demographic structure of Deschampsia antarctica and Colobanthus quitensis along an altitudinal gradient on Living- ston Island, South Shetland Islands, Antarctica. Polar Res. 2011, 1, 1–10. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Sanhueza, A.K.; Torres-Mellado, G.; Casanova-Katny, A. Competition between native Antarctic vascular plants and invasive Poa annua changes with temperature and soil nitrogen availability. Biol. Invasions. 2018, 20, 1597–1610. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Bergstrom, D.M.; Chewedorzewska, D.M.; Katerzyna, J.; Convey, P.; Chown, S.L. Increasing impacts by Antarctica’s most widespread invasive plant species as result of direct competition with native vascular plants. NeoBiota. 2019, 51, 19–40. [Google Scholar] [CrossRef]
- Atala, C.; Pertierra, L.R.; Aragon, P.; Carrasco-Urra, F.; Lavin, P.; Gallardo-Cerda, J.; Ricote-Martinezm, N.; Torres-Diaz, C.; Molina-Montenegro, M.A. Positive interactions among native and invasive vascular plants in Antarctica: Assessing the “nurse effect” at different spatial scales. Biol. Invasions. 2019, 21, 2819–2836. [Google Scholar] [CrossRef]
- Scheiter, S.; Higgins, S.I. Impacts of climate change on the vegetation of Africa: An adaptive dynamic vegetation modelling approach. Glob. Change Biol. 2009, 15, 2224–2246. [Google Scholar] [CrossRef]
- Goufan, S. Potential impacts of climate change on mixed broadleaved-Korean pine forest stand: A gap model approach. Clim. Change. 1996, 34, 263–268. [Google Scholar] [CrossRef]
- Georgio, V.; Renzo, M. An improved species distribution model for Scots pine and downy oak under future climate change in the NW Italian Alps. Ann. For. Sci. 2014, 72, 321–334. [Google Scholar] [CrossRef]
- Kerns, B.; Peterson, D.W. An Overview of Vegetation Models for Climate Change Impacts. Available online: www.fs.usda.gov/ccrc/topics/overview-vegetation-models (accessed on 5 May 2022).
- Franklin, J. Species distribution models in conservation biogeography: Developments and challenges. Divers. Distrib. 2013, 19, 1217–1223. [Google Scholar] [CrossRef]
- De Souza Muñoz, M.E.; De Giovanni, R.; De Siqueira, M.F.; Sutton, T.; Brewer, P.; Pereira, R.S.; Canhos, D.A.L.; Canhos, V.P. openModeller: A generic approach to species’ potential distribution modelling. Geoinformatica 2011, 15, 111–135. [Google Scholar] [CrossRef]
- Vensim. Available online: https://vensim.com/ (accessed on 7 November 2019).
- Abadi, L.S.K.; Shamsai, A.; Goharnejad, H. An analysis of the sustainability of basin water resources using Vensim model. KSCE J. Civ. Eng. 2015, 19, 1941–1949. [Google Scholar] [CrossRef]
- Krupanidhi, S.; Sai, N.M.; Leung, H.; Kineman, J.J. The leaf as a sustainable and renewable system. Syst. Res. Behav. Sci. 2017, 34, 564–576. [Google Scholar] [CrossRef]
- Maani, K. Decision-making for climate change adaptation: A systems thinking approach. Natl. Clim. Change Adapt. Res. Facil. Gold Coast 2013, 66. [Google Scholar]
- Sardo, M.S.; Jalalkamaali, N. A system dynamic approach for reservoir impact assessment on groundwater aquifer considering climate change scenario. Groundw. Sustain. Dev. 2022, 17, 100754. [Google Scholar] [CrossRef]
- Carrera-Villacrés, D.V.; Quinteros-Carabelí, J.A.; Gómez, A.J.; Solano, E.M.; Llumiquinga, G.E.; Burgos, C.A. Dynamic model for the management of water resource and water aptitude for irrigation of the Togllahuayco gorge in the Guangopolo micro-basin. In Proceedings of the 5th International Conference of Water Resource and Environment (WRE 2019), Macao, China, 16–19 July 2019; IOP Publishing Ltd: Bristol, England, 2019. [Google Scholar] [CrossRef]
- Odadaa, E.O.; Ochola, W.O.; Olago, D.O. Understanding future ecosystem changes in Lake Victoria basin using participatory local scenarios. Afr. J. Ecol. 2009, 47, 147–153. [Google Scholar] [CrossRef]
- Roriz, P.A.C.; Yanai, A.M.; Fearnside, P.M. Deforestation and carbon loss in Southwest Amazonia: Impact of Brazil’s revised forest code. Environ. Manag. 2017, 60, 367–382. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility. Free and Open Access to Biodiversity Data. Available online: https://www.gbif.org/ (accessed on 25 May 2022). [CrossRef]
- Chwedorzewska, K.J.; Gielwaanowska, I.; Olech, M.; Molina-Montenegro, M.A.; Wódkiewicz, M.; Galera, H. Poa annua in the maritime Antarctic: An overview. Polar Rec. 2014, 51, 637–643. [Google Scholar] [CrossRef]
- NASA Earth Observations (NEO). Available online: https://neo.gsfc.nasa.gov/ (accessed on 25 May 2022).
- Quantum GIS. Available online: https://www.qgis.org (accessed on 19 June 2020).
- van der Heide, T.; Roijackers, R.M.M.; van Nes, E.H.; Peeters, T.H.M. A simple equation for describing the temperature dependent growth of free-floating macrophytes. Aquat. Bot. 2006, 84, 171–175. [Google Scholar] [CrossRef]
- Kellmann-sopya, W.; Giewanowska, I. Germination capacity of five polar Caryophyllaceae and Poaceae species under different temperature conditions. Polar Biol. 2015, 38, 1753–1765. [Google Scholar] [CrossRef]
- Carroll, D.E.; Brosnan, J.T.; Trigiano, R.T.; Horvath, B.J.; Shekoofa, A.; Mueller, T.C. Current understanding of the Poa annua life cycle. Crop Sci. 2021, 61, 1527–1537. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 23 June 2020).
- Molina-Montenegro, M.A.; Pertierra, L.R.; Razeto-Barry, P.; Diaz, J.; Finot, V.L.; Torres-Diaz, C. A recolonization record of the invasive Poa annua in Paradise Bay, Antarctic Peninsula: Modeling of the potential spreading risk. Polar Biol. 2015, 38, 1091–1096. [Google Scholar] [CrossRef]
- Hughes, K.A.; Pertierra, L.R.; Molina-Montenegro, M.A.; Convey, P. Biological invasions in terrestrial Antarctica: What is the current status and can we respond? Biodivers. Conserv. 2015, 24, 1031–1055. [Google Scholar] [CrossRef]
- Galera, H.; Wódkiewicz, M.; Czyż, E.; Łapiński, S.; Elzbieta, M.; Pasik, M.; Rajner, M.; Bylina, P.; Chwedorzewska, K.J. First step to eradication of Poa annua L. from Point Thomas Oiasis (King George Island, South Shetlands, Antarctica). Polar Biol. 2017, 40, 939–945. [Google Scholar] [CrossRef]
- Frenot, Y.; Chown, S.L.; Whinam, J.; Selkirk, P.M.; Convey, P.; Skotnicki, M.; Bergstrom, D.M. Biological invasions in the Antarctic: Extent, impacts and implications. Biol. Rev. 2005, 80, 45–72. [Google Scholar] [CrossRef]
- Cannone, N.; Malfasi, F.; Favero-longo, S.E.; Guglielmin, M. Acceleration of climate warming and plant dynamics in Antarctica. Curr. Biol. 2022, 32, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Fowbert, J.A.; Smith, R.I.L. Rapid population increase in native vascular plants in the Argentine Island, Antarctic Peninsula. Arct. Antarct. Alp. Res. 1994, 26, 290–296. [Google Scholar] [CrossRef]
- Smith, R.I.L. Signy Island as a paradigm of biological and environmental change in Antarctic terrestrial ecosystems. In Antarctic Ecosystems; Kerry, K.R., Hempel, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 32–50. [Google Scholar] [CrossRef]
- Smith, R.I.L. Vascular plants as bioindicators of regional warming in Antarctica. Oecologia 1994, 99, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, R.P.; Khare, R. Influence of climate change on Antarctic flora. Polar Sci. 2018, 18, 94–101. [Google Scholar] [CrossRef]
- Sanhueza, C.; Vallejos, V.; Cavieres, L.A.; Saez, P.; Bravo, L.A.; Corcuera, L.J. Growing temperature affects seed germination of the antarctic plant Colobanthus quitensis (Kunth) Bartl (Caryophyllaceae). Polar Biol. 2017, 40, 449–455. [Google Scholar] [CrossRef]
- Fuentes-Lilo, E.; Cuba-Diaz, M.; Rifo, S. Morfo-physiological response of Colobanthus quitensis and Juncus bufonius under different simulations of climate change. Polar Sci. 2017, 11, 11–18. [Google Scholar] [CrossRef]
- Convey, P.; Peck, L.S. Antarctic environmental change and biological responses. Sci. Adv. 2019, 5, eaaz0888. [Google Scholar] [CrossRef]
- Cannone, N.; Guglielmin, M.; Malfasi, F.; Hubberten, H.W.; Wagner, F. Rapid soil and vegetation changes at regional scale in continental Antarctica. Geoderma 2021, 394, 115017. [Google Scholar] [CrossRef]
- Wódkiewicz, M.; Chwedorzewska, K.J.; Bednarek, P.T.; Znór, A.; Androsiuk, P.; Galera, H. How much of the invader’s genetic variability can slip between our fingers? A case study of secondary dispersal of Poa annua on King George Island (Antarctica). Ecol. Evol. 2017, 8, 592–600. [Google Scholar] [CrossRef]
- Duffy, G.A.; Lee, J.R. Ice-free area expansion compounds the non-native species threat to Antarctic terrestrial biodiversity. Biol. Conserv. 2019, 232, 253–257. [Google Scholar] [CrossRef]
- Maalfasi, F.; Convey, P.; Zaccara, S.; Cannone, N. Establishment and eradication of an alien plant species in Antarctica: Poa annua at Signy Island. Biodivers. Conserv. 2019, 29, 173–186. [Google Scholar] [CrossRef]
- Sierra-Almeida, A.; Cavieres, L.A.; Bravo, L.A. Warmer temperatures affect the in situ freezing resistance of the Antarctic vascular plants. Front. Plant. Sci. 2018, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Gooseff, M.N.; Wlostowski, A.; McKnight, D.M.; Jaros, C. Hydrologic connectivity and implications for ecosystem processes—Lessons from naked watersheds. Geomorphology 2017, 277, 63–71. [Google Scholar] [CrossRef]
- Prietzel, J.; Prater, I.; Hurtarte, L.C.C.; Hrbáček, F.; Klysubun, W.; Mueller, C.W. Site conditions and vegetation determine phosphorus and sulfer speciation in soils of Antarctica. Geochim. Cosmoshim. Acta 2019, 246, 339–362. [Google Scholar] [CrossRef]

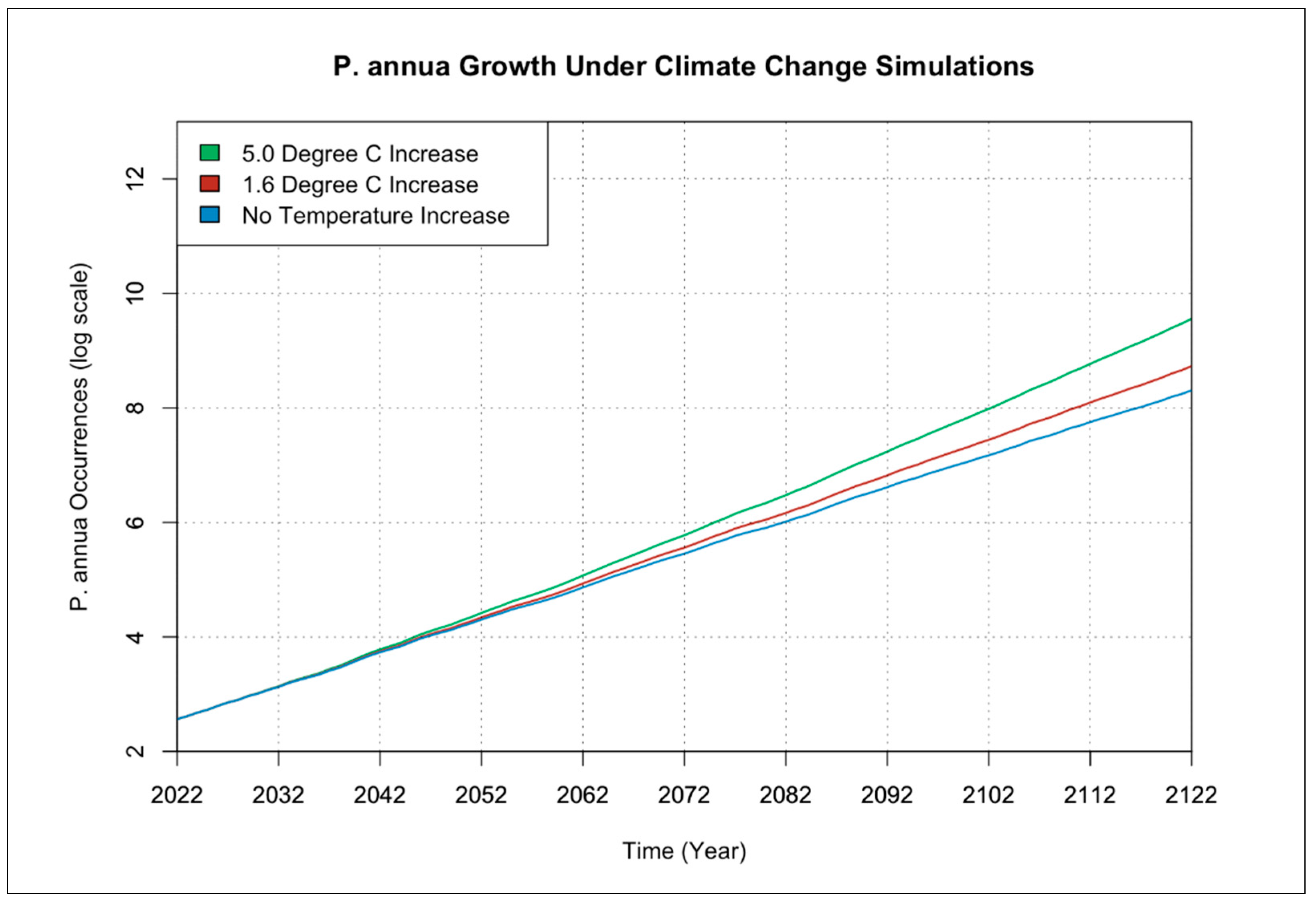



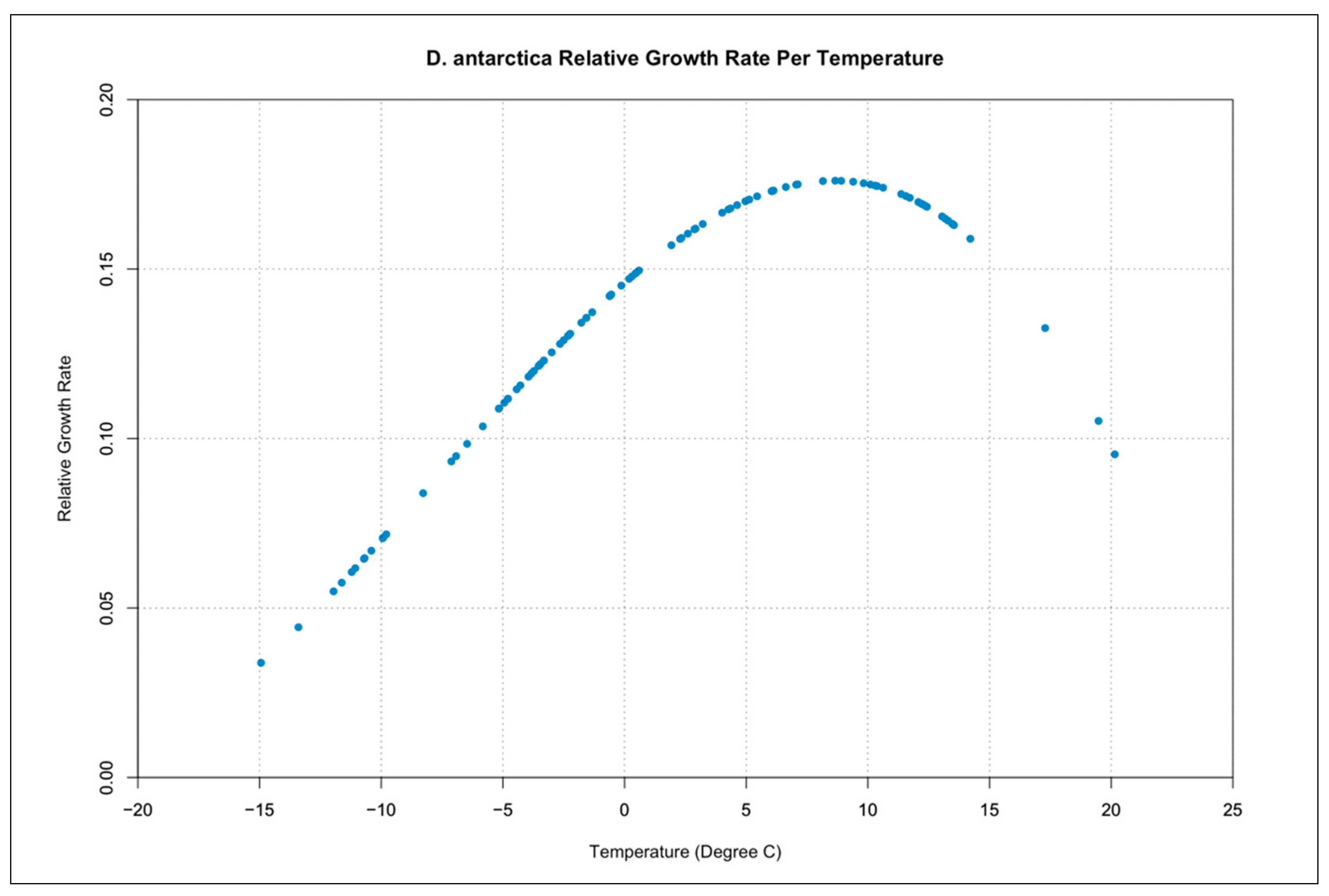
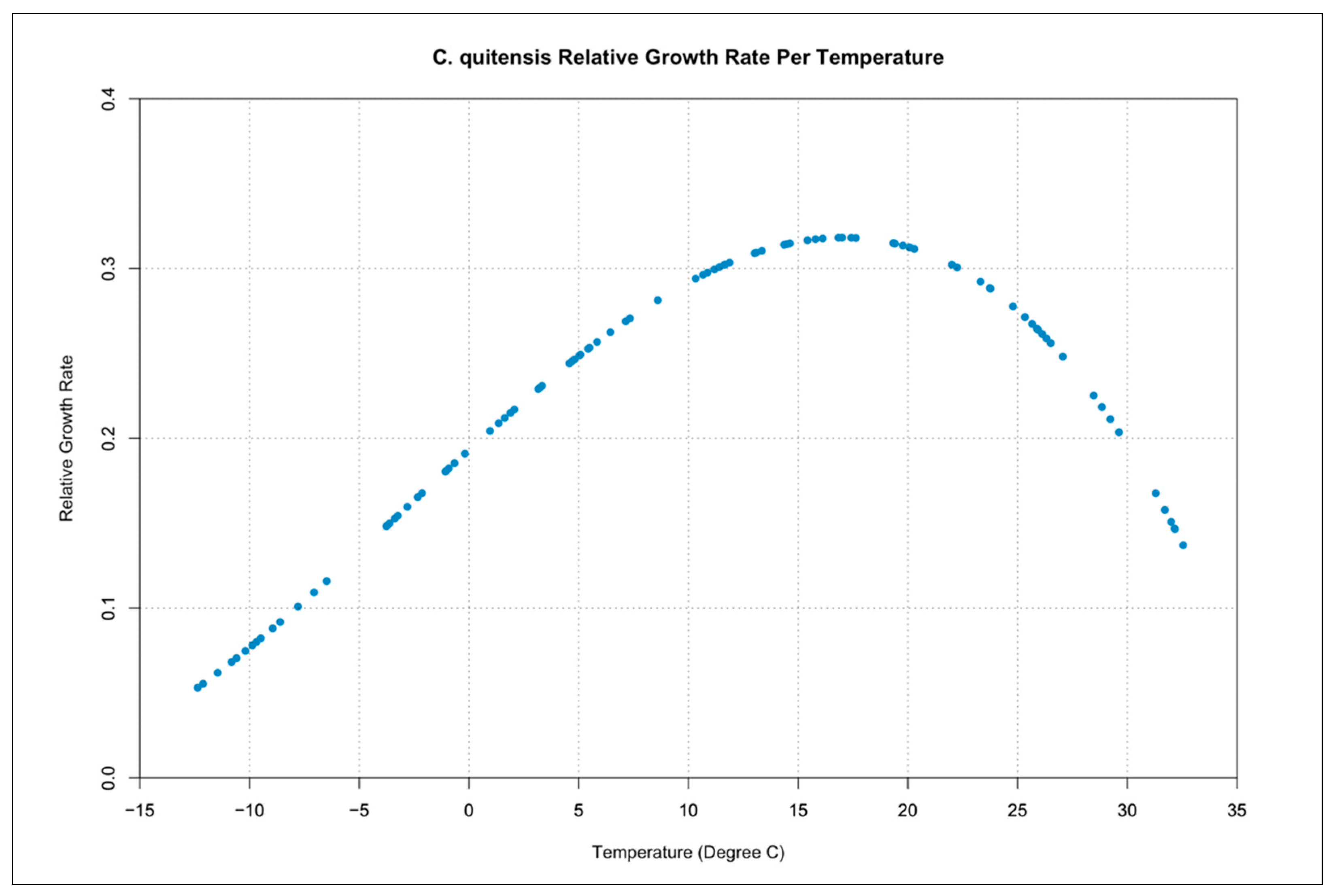
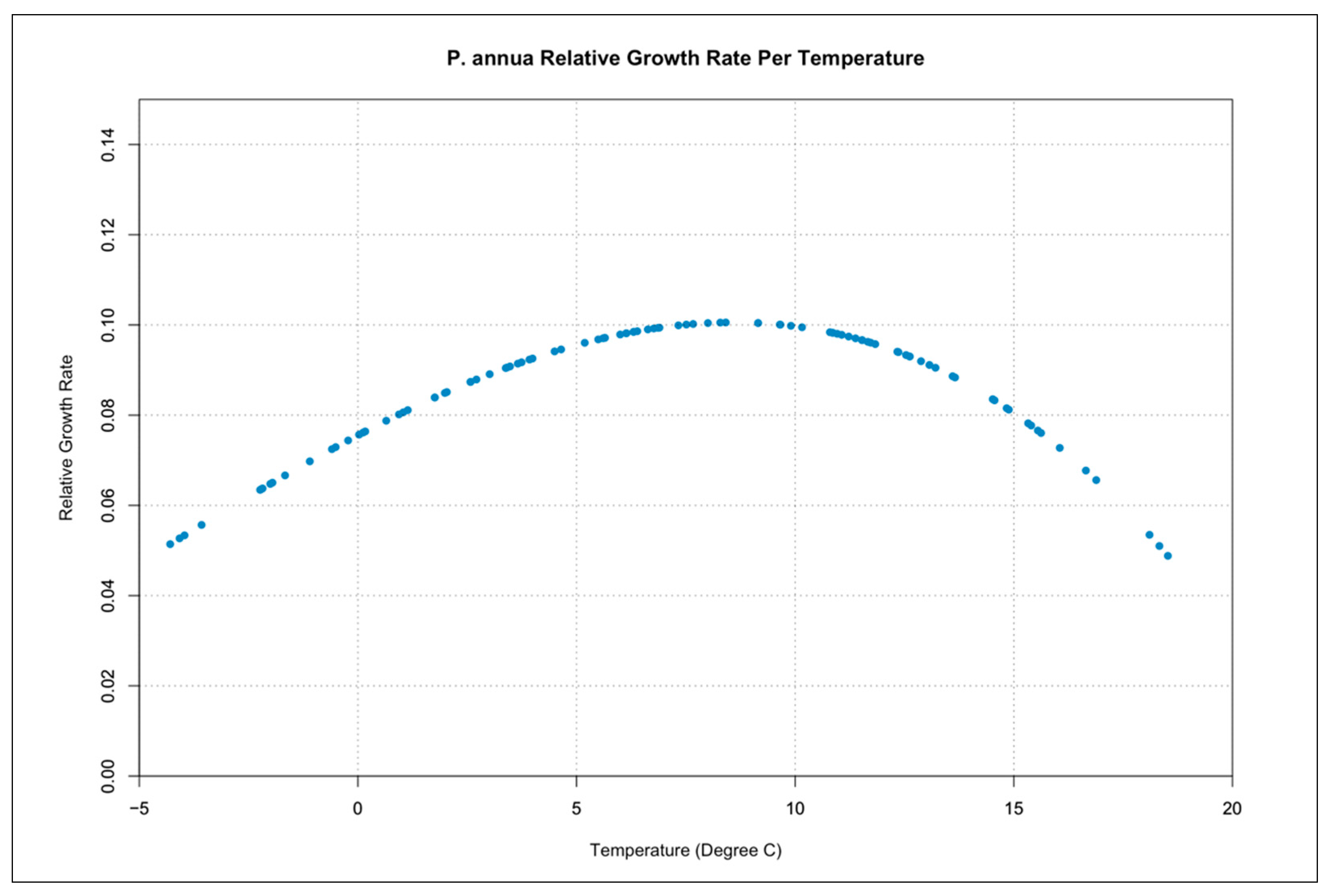
| Symbol | Description | Units |
|---|---|---|
| x | D. antarctica Relative Growth Rate | Growth/year |
| C. quitensis Relative Growth Rate | Growth/year | |
| P. annua Relative Growth Rate | Growth/year | |
| D. antarctica Occurrence Growth | Species Occurrences | |
| C. quitensis Occurrence Growth | Species Occurrences | |
| P. annua Occurrence Growth | Species Occurrences | |
| d | D. antarctica Occurrences | Species Occurrences |
| q | C. quitensis Occurrences | Species Occurrences |
| p | P. annua Occurrences | Species Occurrences |
| D. antarctica Land Surface Temperature Anomaly Day | Degree Celsius | |
| D. antarctica Land Surface Temperature Anomaly Night | Degree Celsius | |
| C. quitensis Land Surface Temperature Anomaly Day | Degree Celsius | |
| C. quitensis Land Surface Temperature Anomaly Night | Degree Celsius | |
| P. annua Land Surface Temperature Anomaly Day | Degree Celsius | |
| P. annua Land Surface Temperature Anomaly Night | Degree Celsius | |
| D. antarctica Temperature Increase Rate | Temperature/Year | |
| C. quitensis Temperature Increase Rate | Temperature/Year | |
| P. annua Temperature Increase Rate | Temperature/Year | |
| D. antarctica Ruling Temperature | Degree Celsius | |
| C. quitensis Ruling Temperature | Degree Celsius | |
| P. annua Ruling Temperature | Degree Celsius |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penfound, E.; Wellen, C.; Vaz, E. Modelling the Impact of Temperature under Climate Change Scenarios on Native and Invasive Vascular Vegetation on the Antarctic Peninsula and Surrounding Islands. Geomatics 2022, 2, 390-414. https://doi.org/10.3390/geomatics2040022
Penfound E, Wellen C, Vaz E. Modelling the Impact of Temperature under Climate Change Scenarios on Native and Invasive Vascular Vegetation on the Antarctic Peninsula and Surrounding Islands. Geomatics. 2022; 2(4):390-414. https://doi.org/10.3390/geomatics2040022
Chicago/Turabian StylePenfound, Elissa, Christopher Wellen, and Eric Vaz. 2022. "Modelling the Impact of Temperature under Climate Change Scenarios on Native and Invasive Vascular Vegetation on the Antarctic Peninsula and Surrounding Islands" Geomatics 2, no. 4: 390-414. https://doi.org/10.3390/geomatics2040022
APA StylePenfound, E., Wellen, C., & Vaz, E. (2022). Modelling the Impact of Temperature under Climate Change Scenarios on Native and Invasive Vascular Vegetation on the Antarctic Peninsula and Surrounding Islands. Geomatics, 2(4), 390-414. https://doi.org/10.3390/geomatics2040022







