Experimental Study of Aqueous Foam Use for Heat Transfer Enhancement in Liquid Piston Gas Compression at Various Initial Pressure Levels
Abstract
1. Introduction
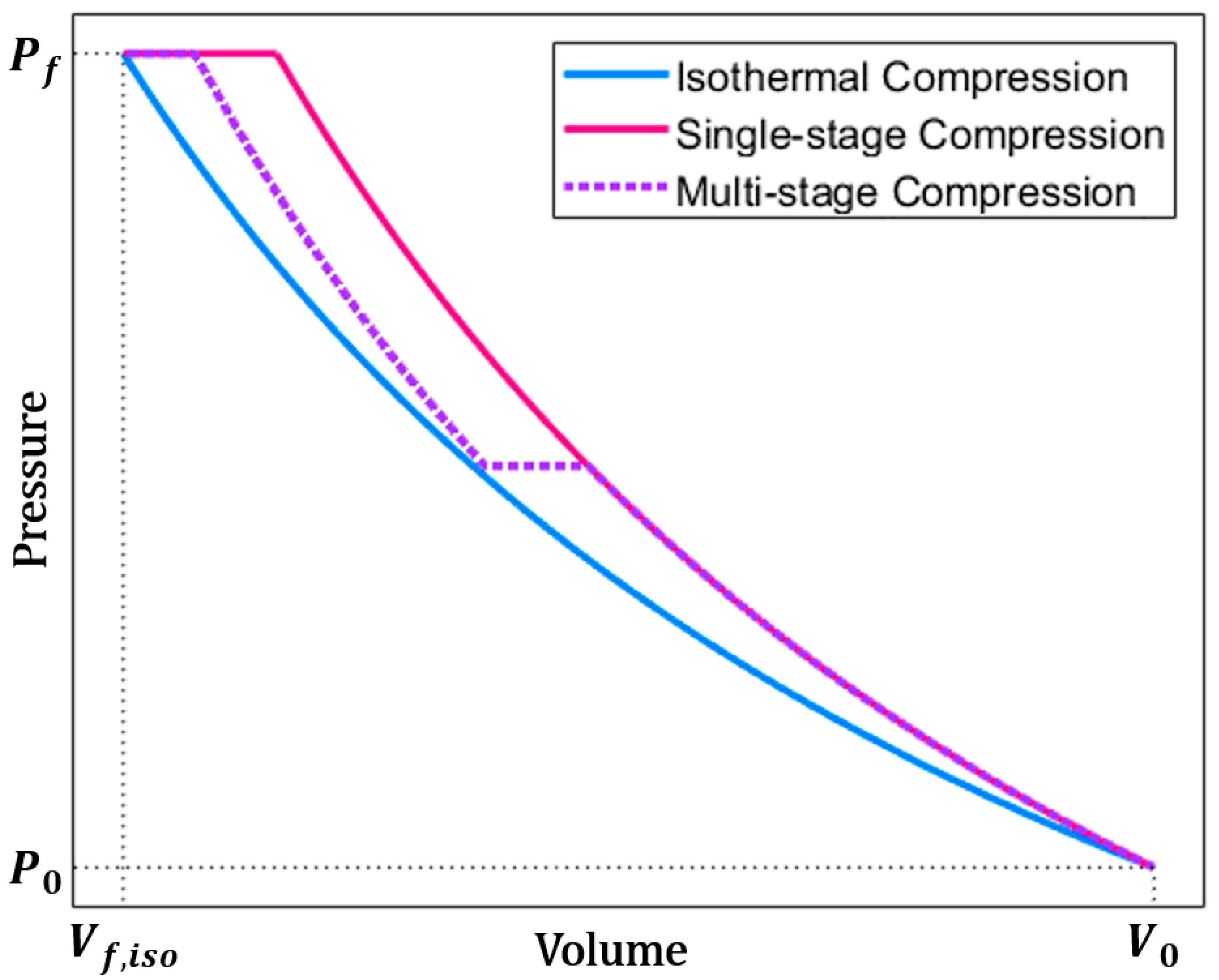
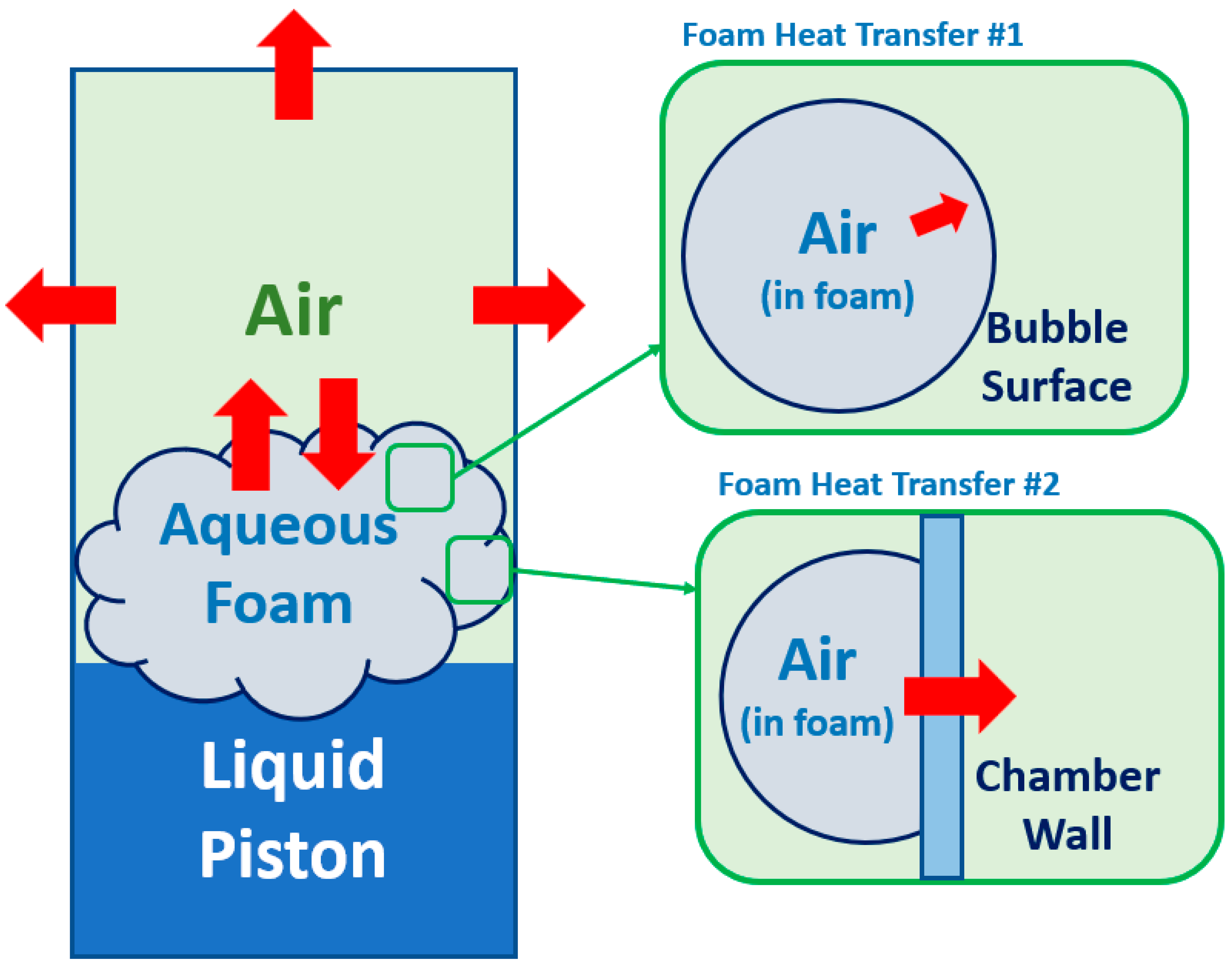
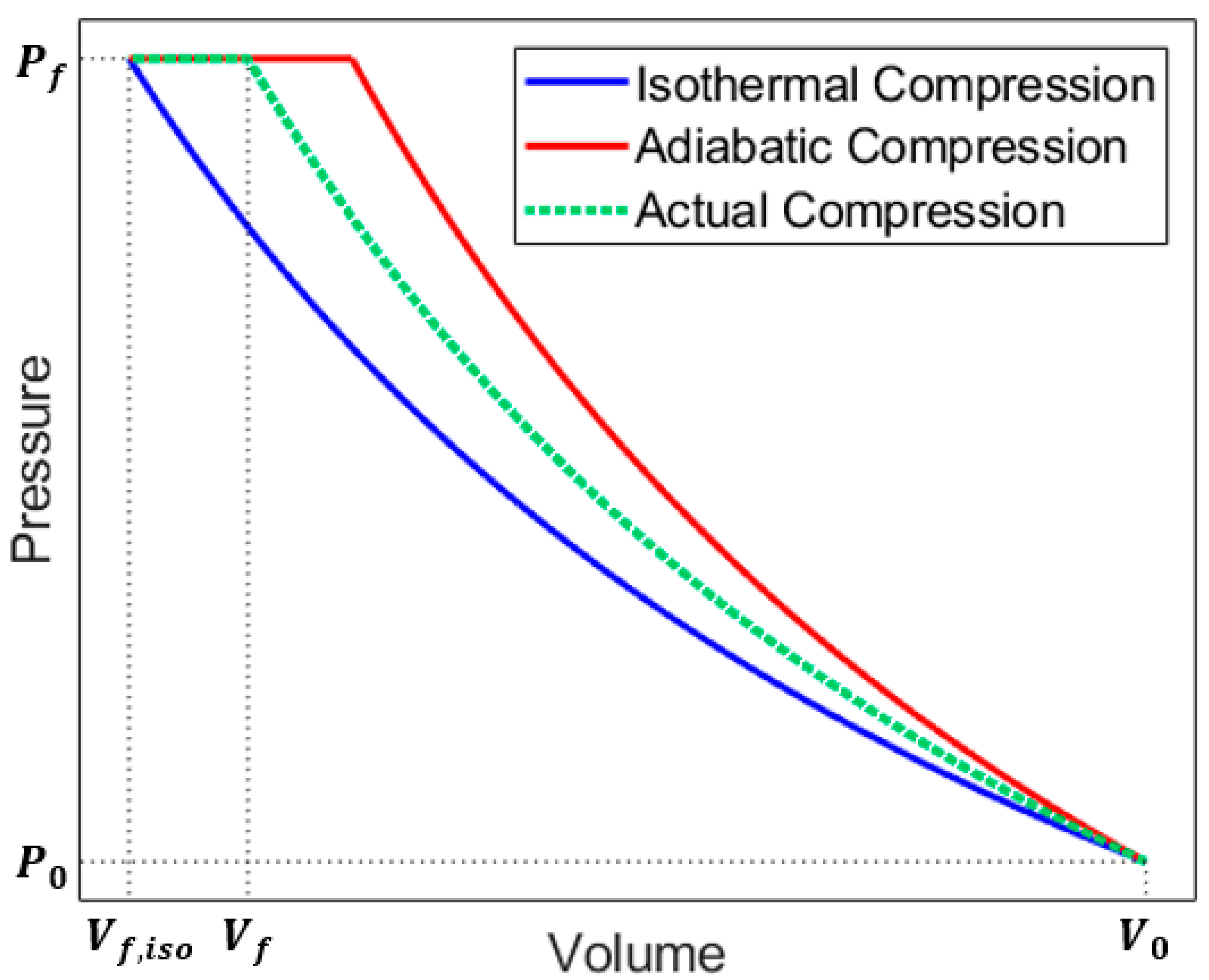
2. Analytical Modeling
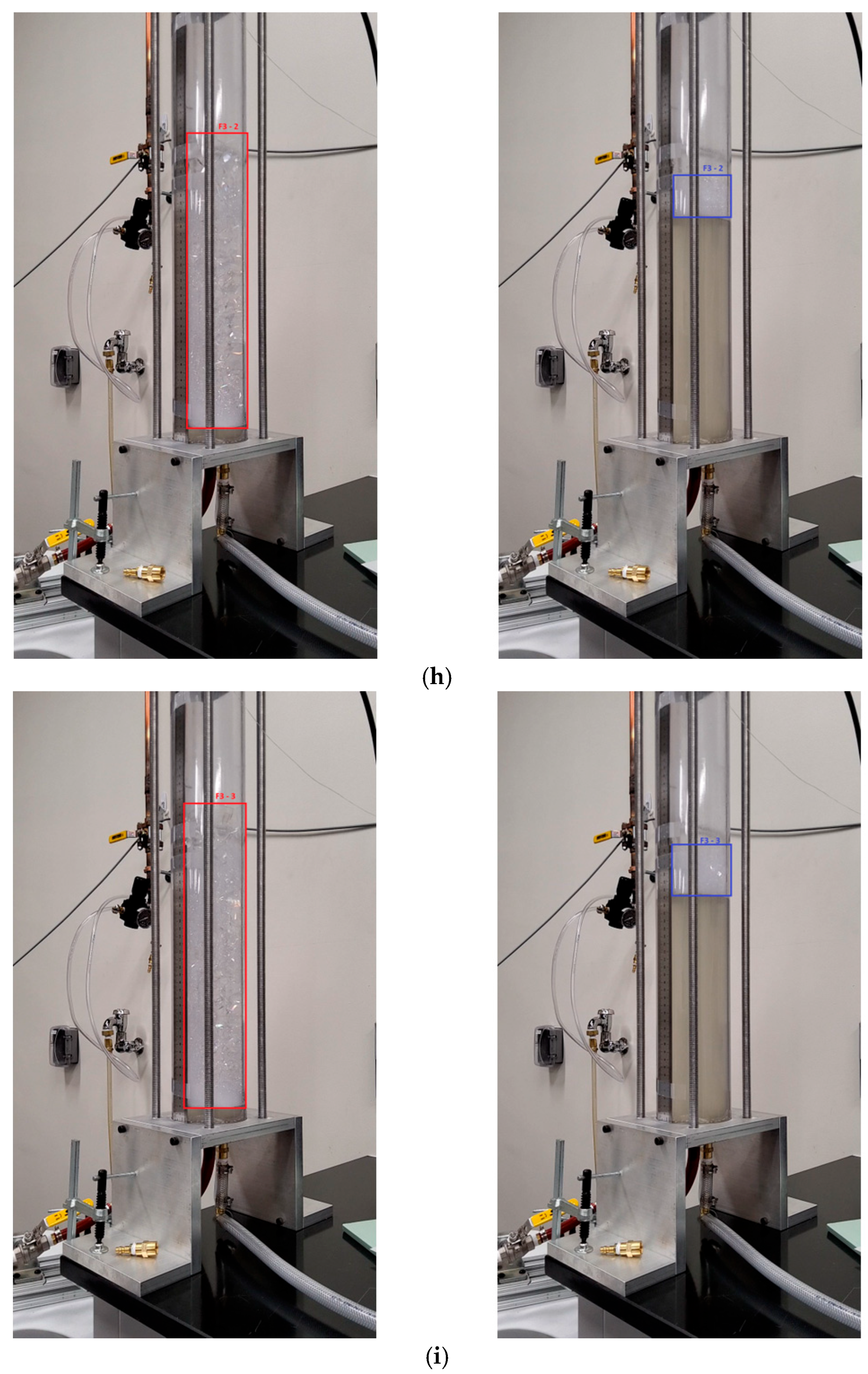
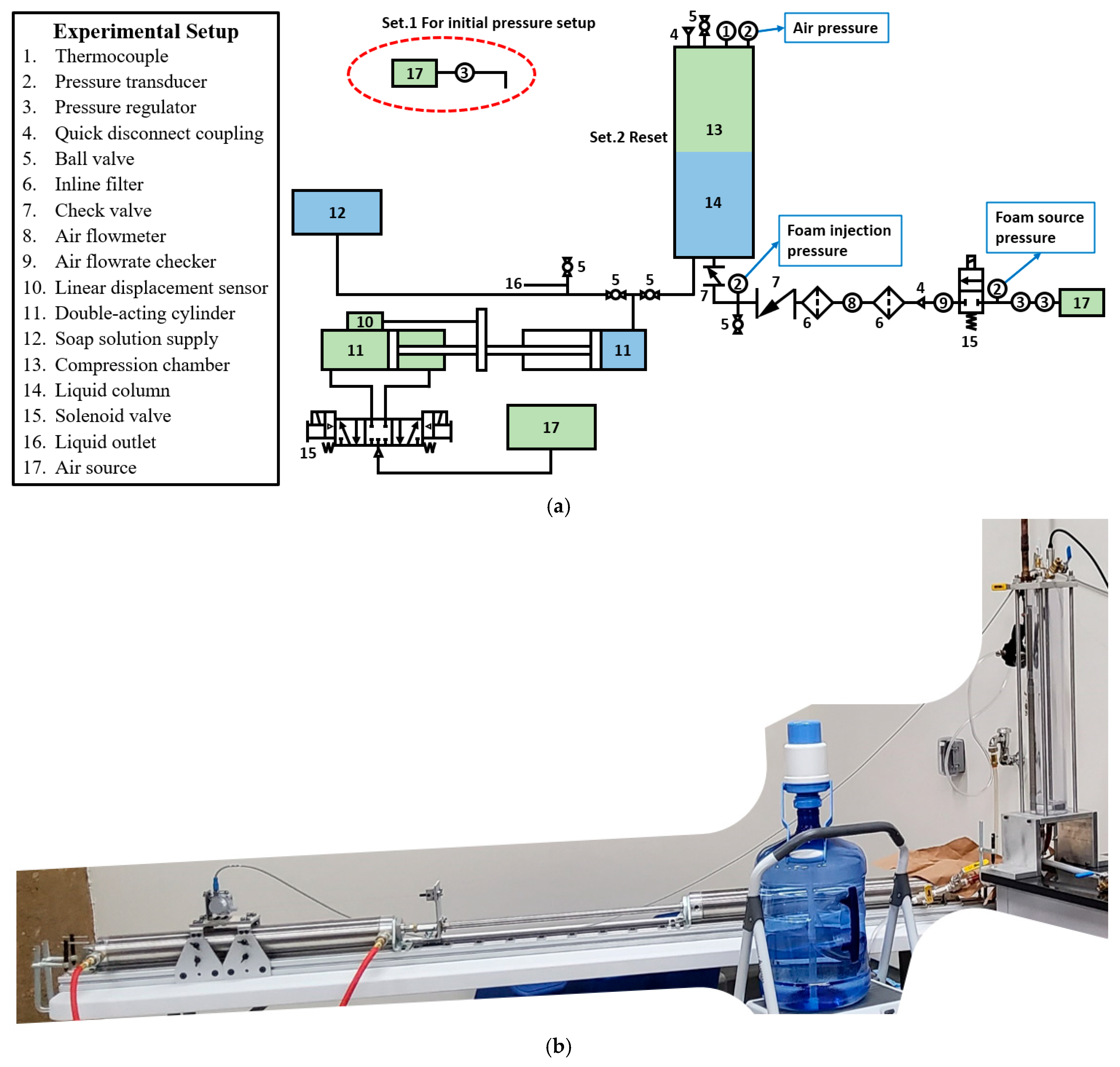
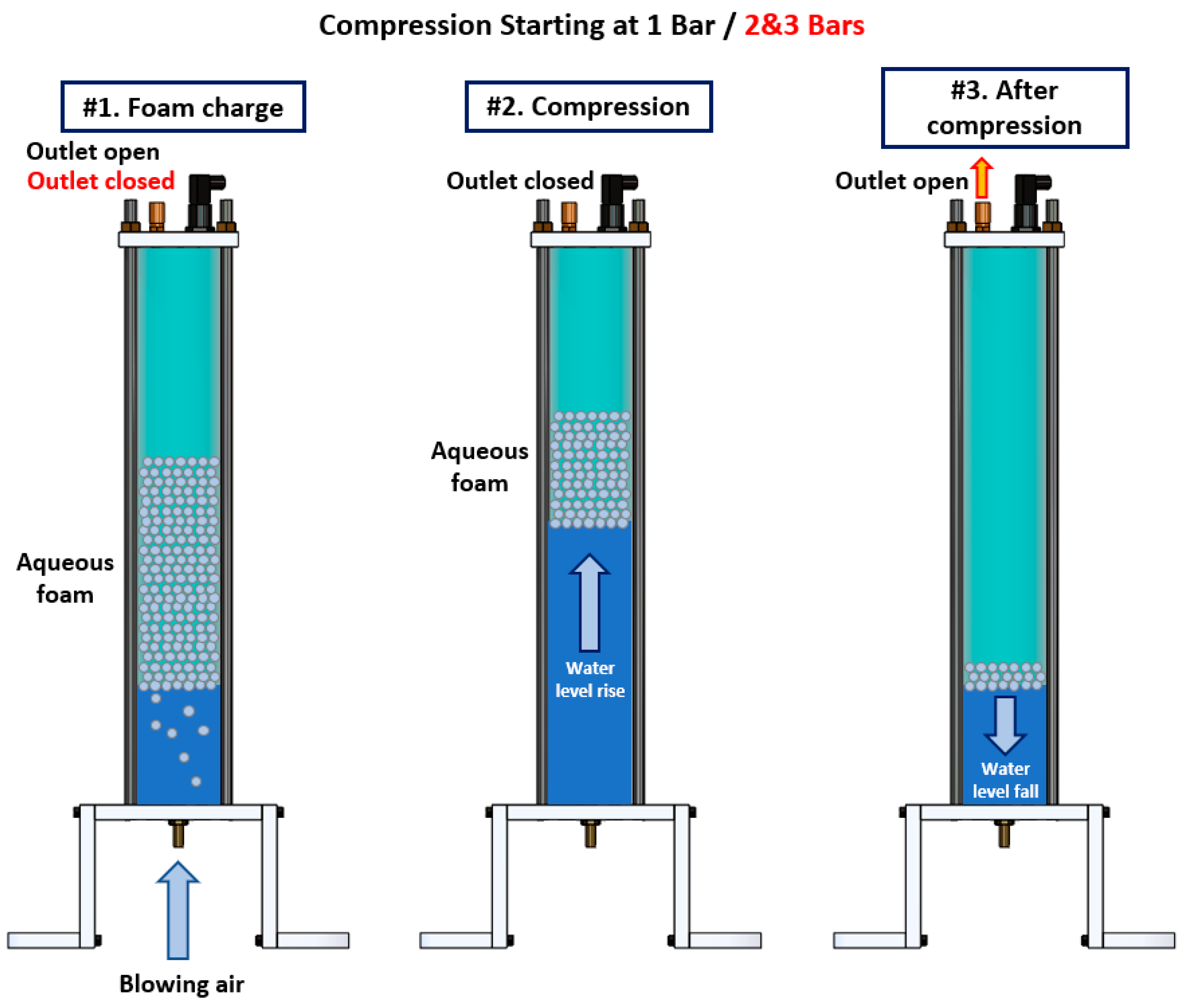
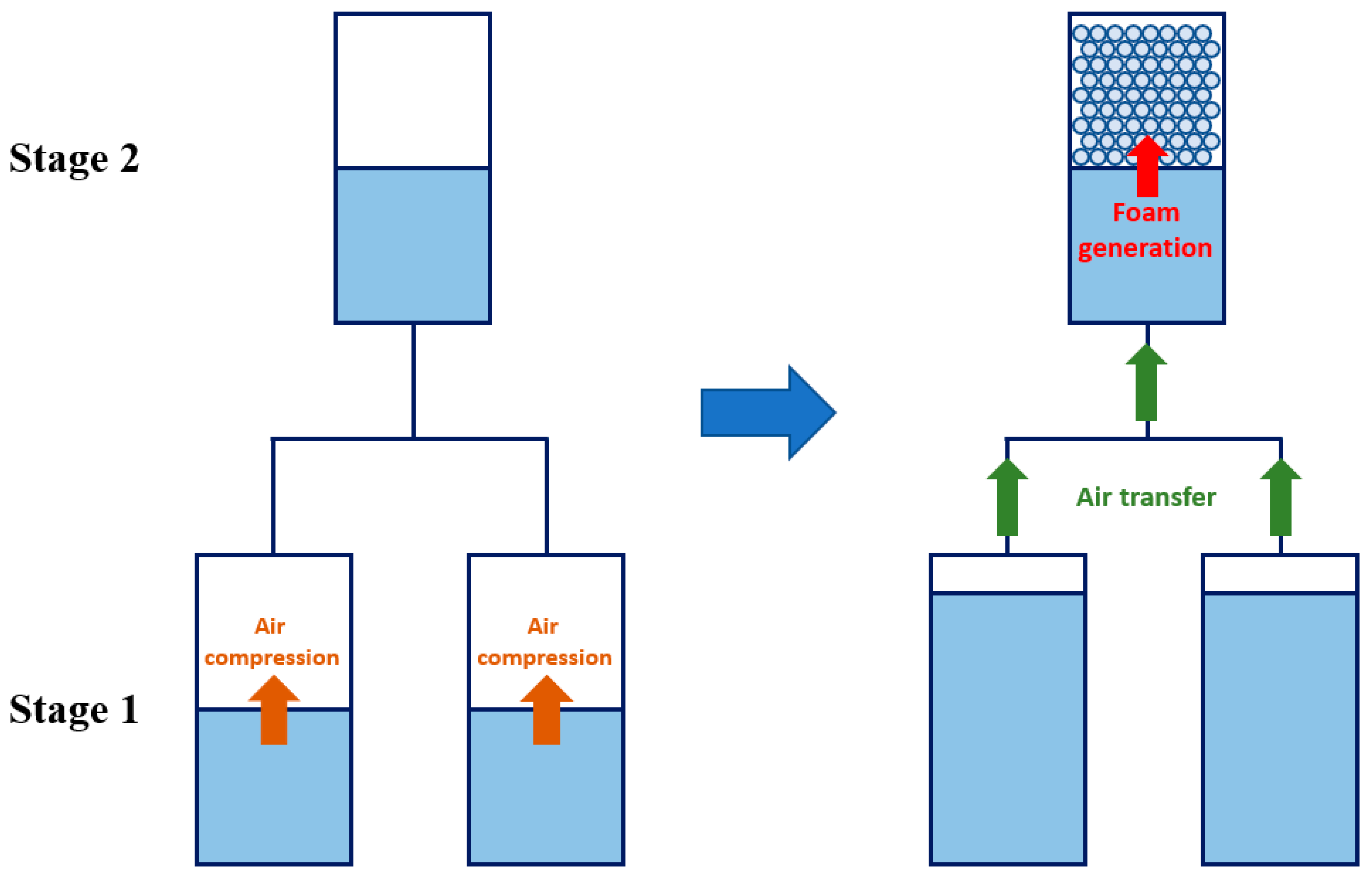
3. Methodology
4. Results and Analysis
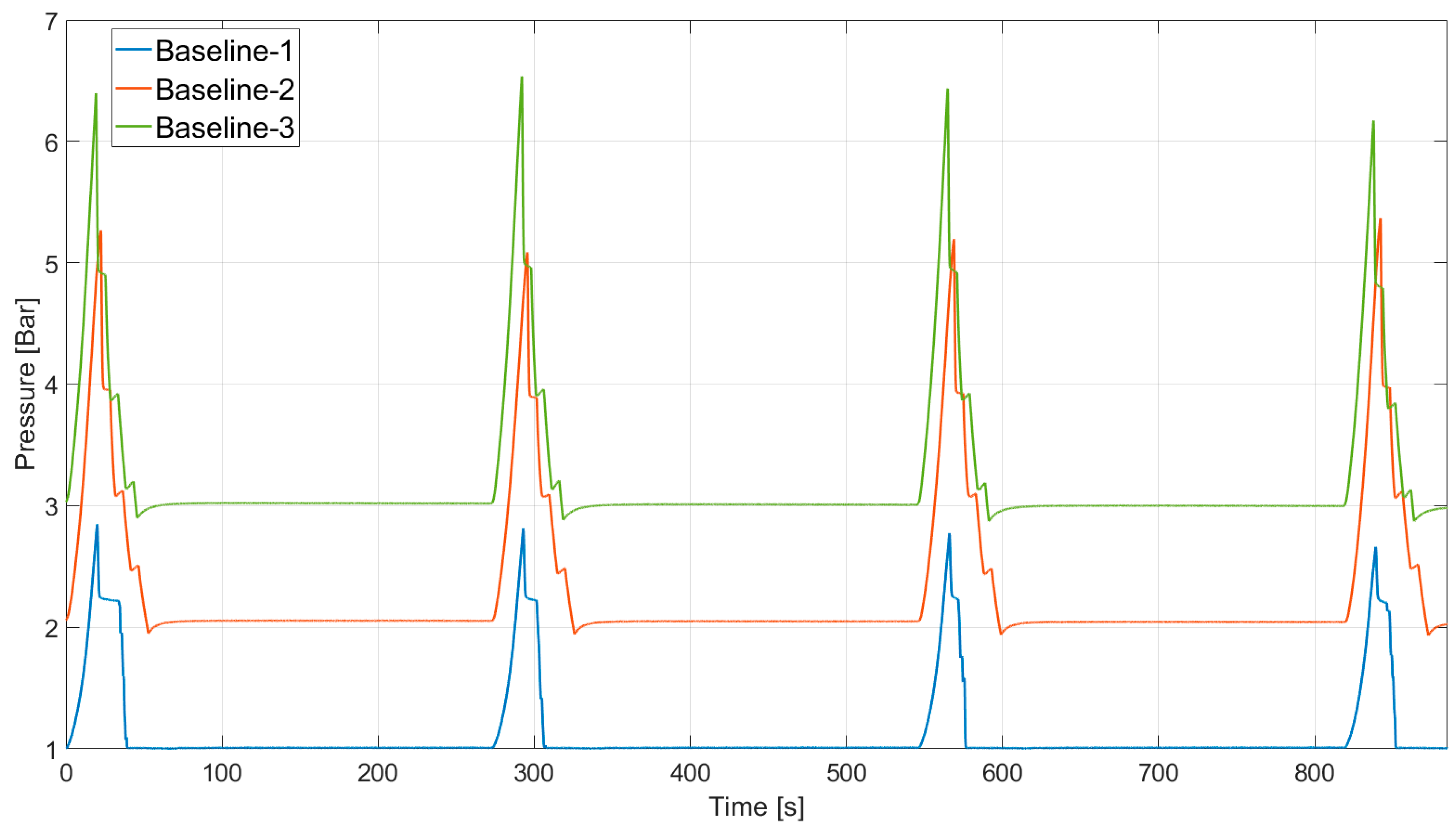
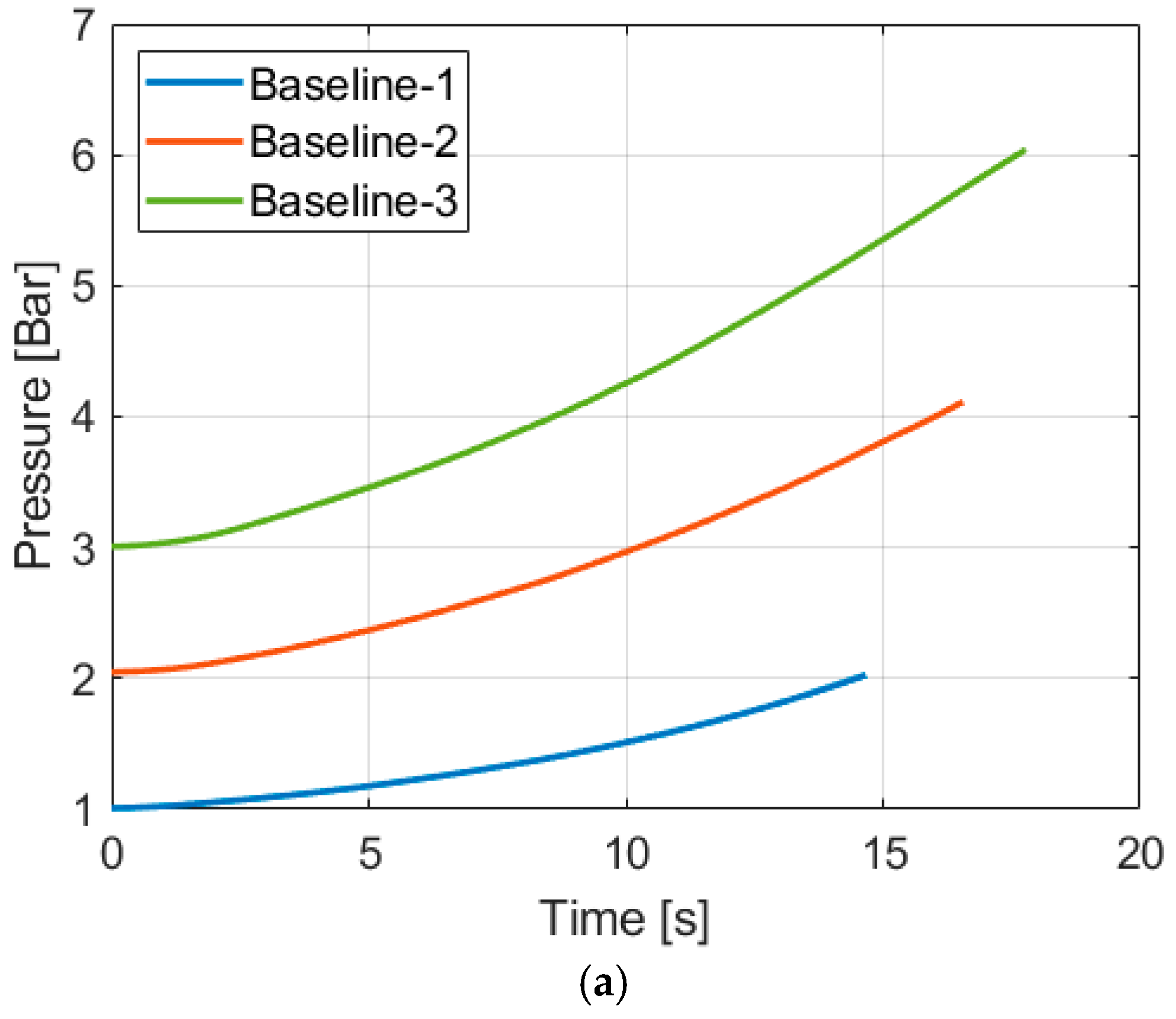
4.1. Baseline Compression
4.2. Compression with Aqueous Foam
4.3. Isothermal Efficiency of Compressions with and Without Aqueous Foam
4.4. Aqueous Foam Results Analysis
4.5. Suggestion for Applications in Real System and for Future Work
5. Conclusions
- For all the three pressure levels, the use of aqueous foam aided in achieving a better isothermal efficiency.
- In both cases, a higher initial pressure led to a decreased efficiency.
- Efficiency improvement from the foam decreased as the initial pressure increased, even though the differences were marginal.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Nomenclature | |
| Surface area of foam | |
| Surface area | |
| Specific heat | |
| Hydraulic diameter | |
| Final foam height | |
| Initial foam height | |
| Change in foam height | |
| Thermal conductivity of foam | |
| Thickness of single bubble | |
| Mass of air | |
| Nusselt number at entry | |
| Pressure | |
| Initial pressure | |
| Pressure of air | |
| Final pressure | |
| Pressure profile of isothermal process | |
| Prandtl number | |
| Rate of heat transfer | |
| Rate of aqueous foam heat transfer | |
| Rate of heat transfer of single bubble | |
| Reynold number | |
| Temperature of air | |
| Temperature of foam | |
| Temperature of surroundings | |
| Time | |
| Overall heat transfer coefficient | |
| Rate of internal energy | |
| Volume | |
| Initial volume | |
| Volume of air | |
| Final volume | |
| Final volume of isothermal process | |
| Volume profile of isothermal process | |
| Compression work | |
| Cooling work | |
| Isothermal compression work | |
| Rate of compression work | |
| Greek letters | |
| Isothermal compression efficiency | |
| Change in isothermal compression efficiency | |
| Abbreviations | |
| CAES | Compressed air energy storage |
| A-CAES | Adiabatic compressed air energy storage |
| D-CAES | Diabatic compressed air energy storage |
| I-CAES | Isothermal compressed air energy storage |
| Baseline-1 | Baseline compression starting at 1 bar |
| Baseline-2 | Baseline compression starting at 2 bars |
| Baseline-3 | Baseline compression starting at 3 bars |
| Foam-1 | Compression with foam starting at 1 bar |
| Foam-2 | Compression with foam starting at 2 bars |
| Foam-3 | Compression with foam starting at 3 bars |
| PHS | Pumped hydroelectric storage |
Appendix A
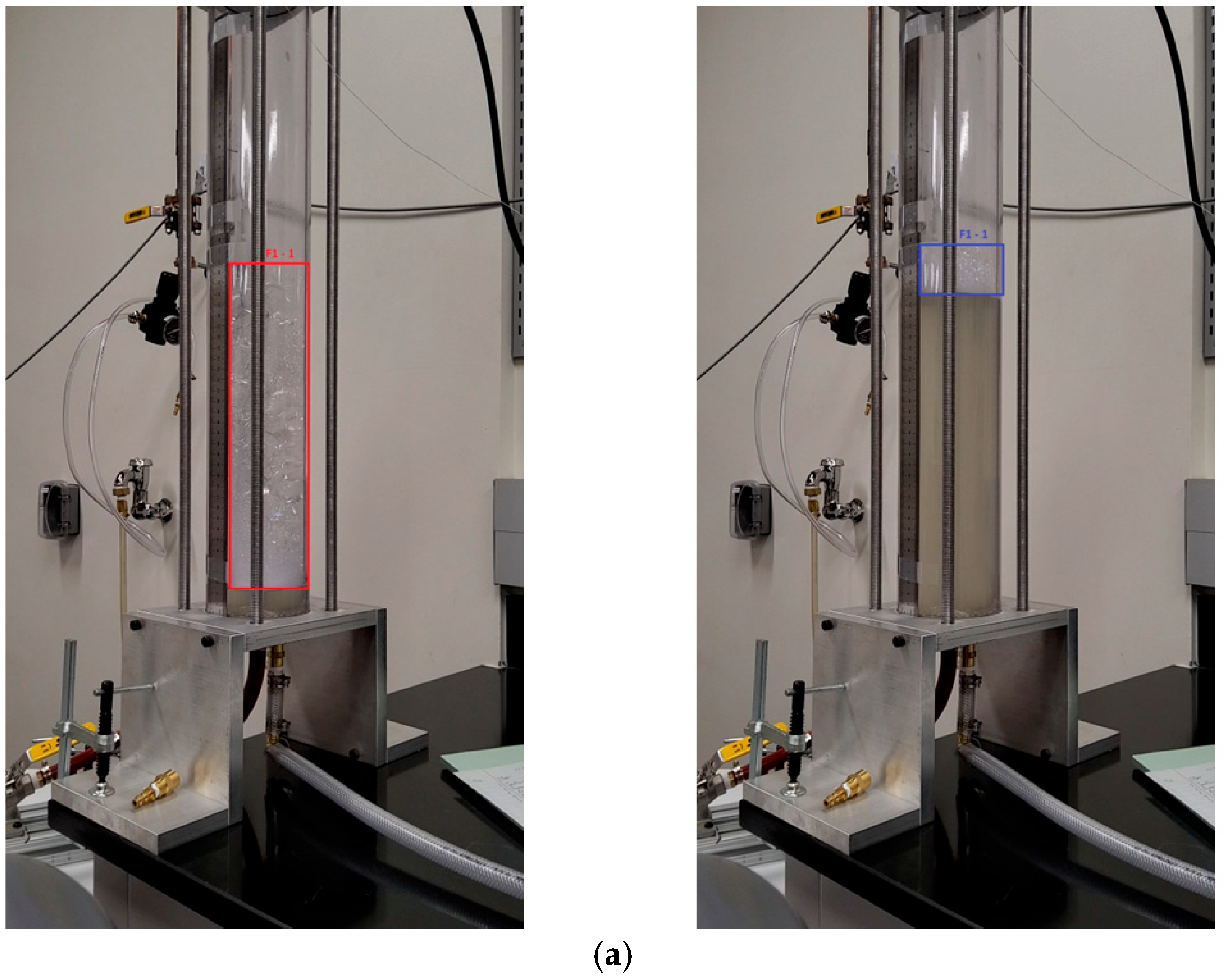
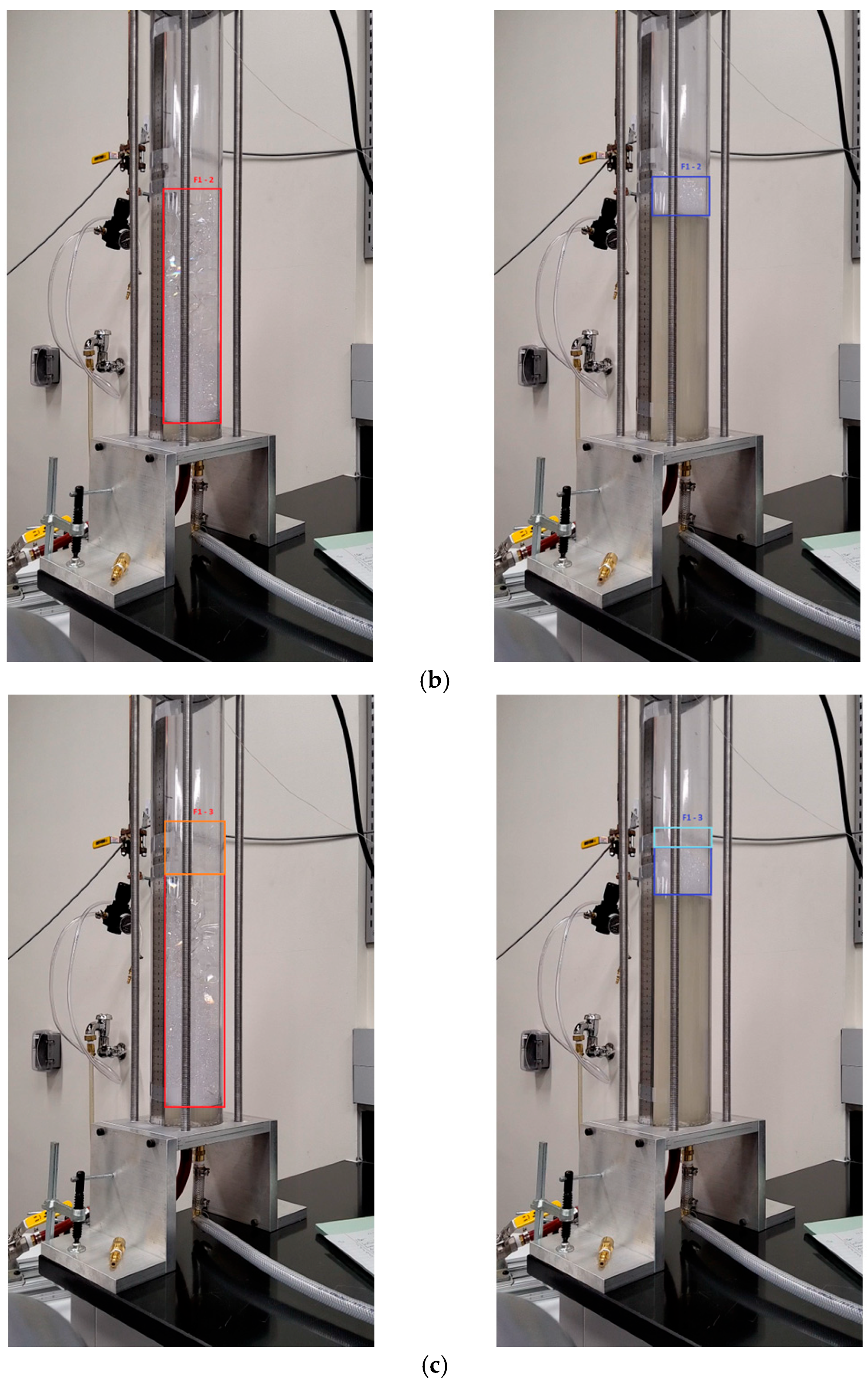
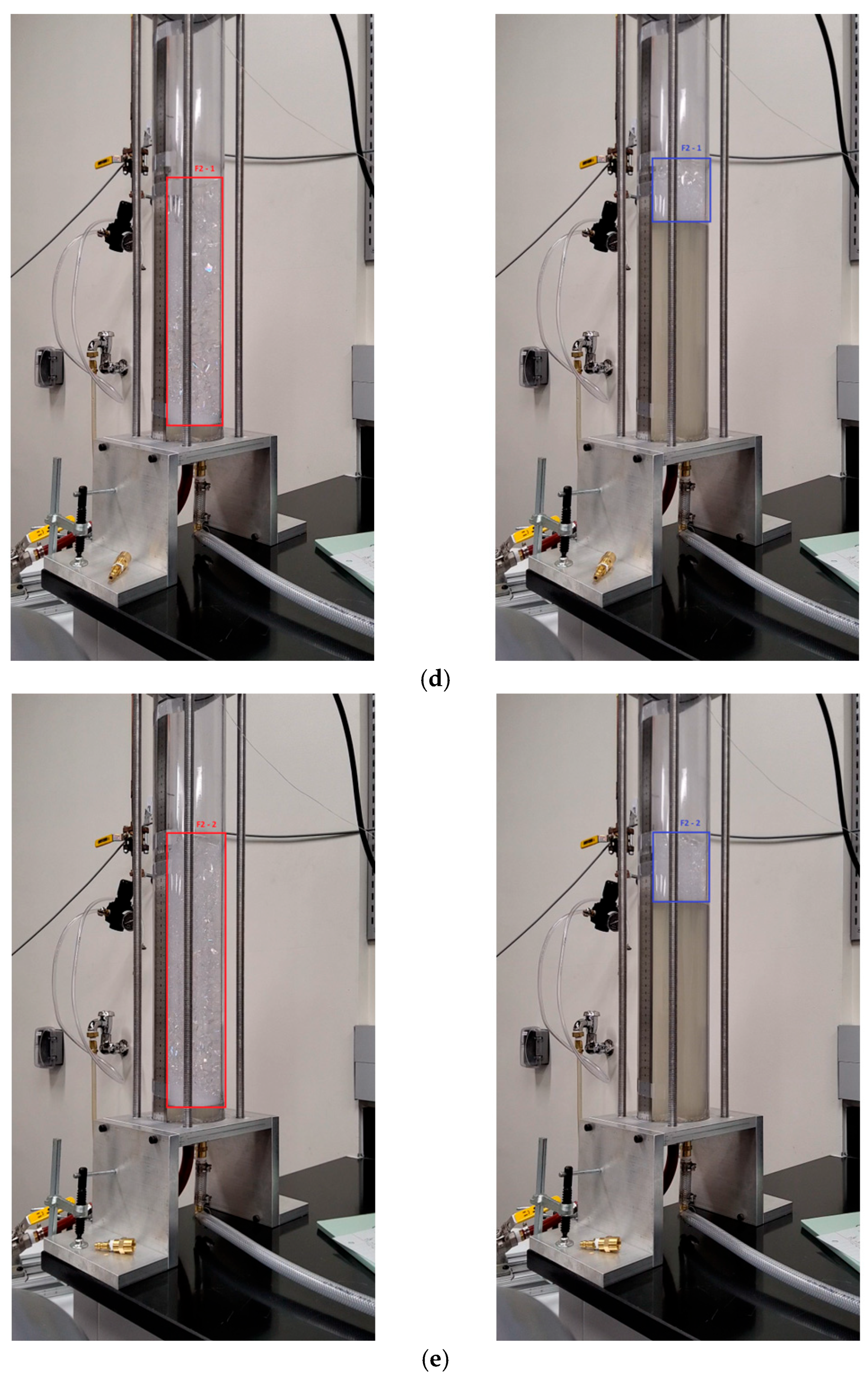
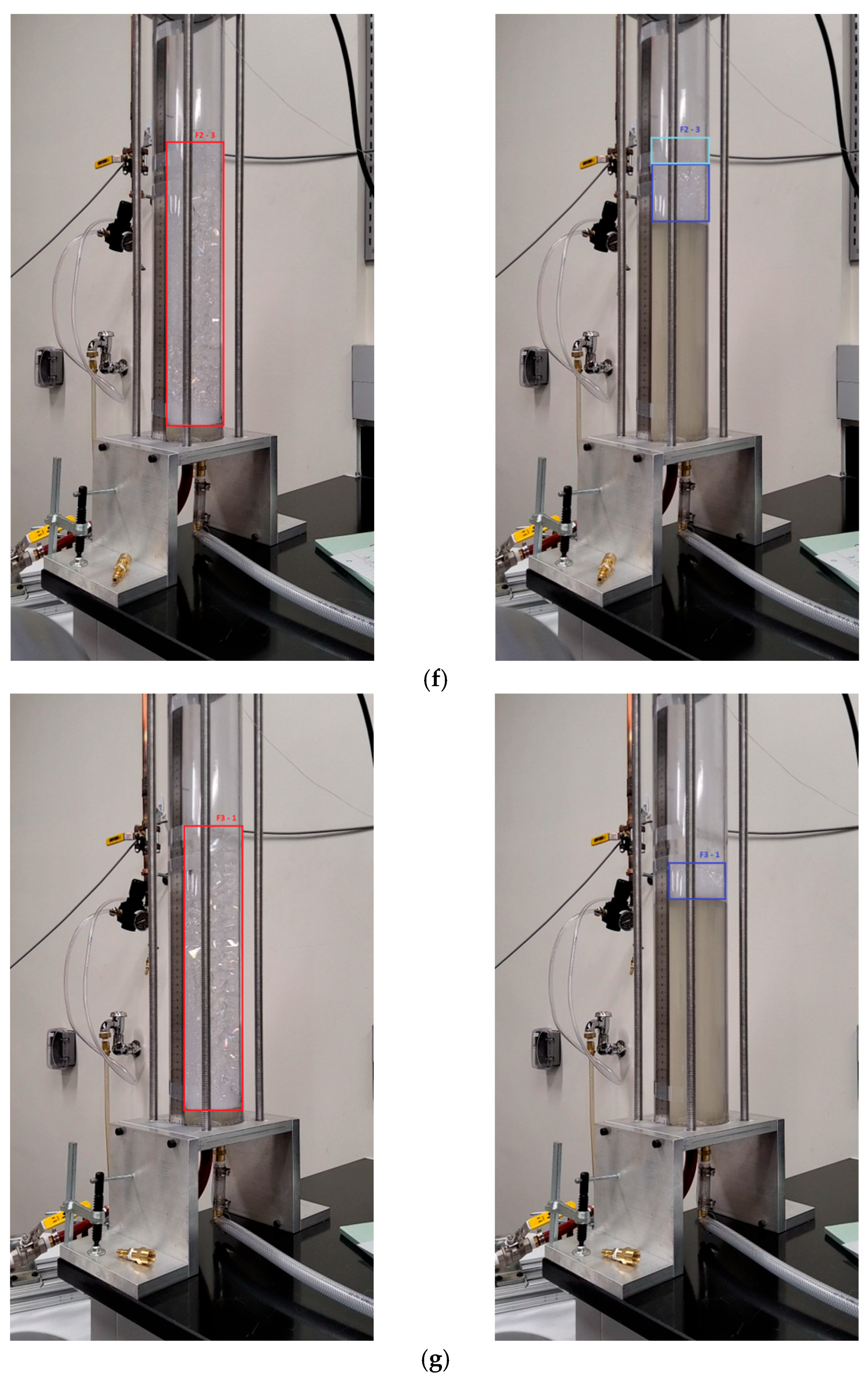
References
- Fossil Fuels, EESI Environmental and Energy Study Institute. Available online: https://www.eesi.org/topics/fossil-fuels/description (accessed on 21 June 2023).
- Frequently Asked Questions (FAQs)—What Is U.S. Electricity Generation by Energy Source? U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/tools/faqs/faq.php?id=427&t=3 (accessed on 21 June 2023).
- Molina, F.; López, I.; Dapelo, D. A proposal to Make Frankfurt am Main Carbon Free by 2050. In Technical Report for the Energy Transition in Frankfurt; Provadis School of International Management and Technology: Frankfurt, Germany, 2013. [Google Scholar] [CrossRef]
- YekiniSuberu, M.; Wazir Mustafa, M.; Bashir, N. Energy storage systems for renewable energy power sector integration and mitigation of intermittency. Renew. Sustain. Energy Rev. 2014, 35, 499–514. [Google Scholar] [CrossRef]
- Qin, C.; Loth, E.; Li, P.; Simon, T.; Van de Ven, J. Spray-cooling concept for wind-based compressed air energy storage. J. Renew. Sustain. Energy 2014, 6, 043125. [Google Scholar] [CrossRef]
- Wang, J.; Lu, K.; Ma, L.; Wang, J.; Dooner, M.; Miao, S.; Li, J.; Wang, D. Overview of compressed air energy storage and technology development. Energies 2017, 10, 991. [Google Scholar] [CrossRef]
- Lim, S.D.; Mazzoleni, A.P.; Park, J.; Ro, P.I.; Quinlan, B. Conceptual Design of Ocean Compressed Air Energy Storage System. Mar. Technol. Soc. J. 2013, 47, 70–81. [Google Scholar] [CrossRef]
- Evans, A.; Strezov, V.; Evans, T.J. Assessment of utility energy storage options for increased renewable energy penetration. Renew. Sustain. Energy Rev. 2012, 16, 4141–4147. [Google Scholar] [CrossRef]
- Pemberton, D.; Jewitt, J.; Pletka, R.; Fischbach, M.; Meyer, T.; Ward, M.; Bjorge, B.; Hargreaves, D.; Jordan, G. Mini-Compressed Air Energy Storage for Transmission Congestion Relief and Wind Shaping Applications; New York State Energy Research and Development Authority: Albany, NY, USA, 2008.
- Pimm, A.J.; Garvey, S.D.; de Jong, M. Design and testing of Energy Bags for underwater compressed air energy storage. Energy 2014, 66, 496–508. [Google Scholar] [CrossRef]
- Bennett, J.A.; Simpson, J.G.; Qin, C.; Fittro, R.; Koenig, G.M.; Clarens, A.F.; Loth, E. Techno-economic analysis of offshore isothermal compressed air energy storage in saline aquifers co-located with wind power. Appl. Energy 2021, 303, 117587. [Google Scholar] [CrossRef]
- Cavallo, A.J. Energy Storage Technologies for Utility Scale Intermittent Renewable Energy Systems. J. Sol. Energy Eng. 2001, 123, 387–389. [Google Scholar] [CrossRef]
- Cavallo, A. Controllable and affordable utility-scale electricity from intermittent wind resources and compressed air energy storage (CAES). Energy 2007, 32, 120–127. [Google Scholar] [CrossRef]
- Alirahmi, S.M.; Mousavi, S.B.; Razmi, A.R.; Ahmadi, P. A comprehensive techno-economic analysis and multi-criteria optimization of a compressed air energy storage (CAES) hybridized with solar and desalination units. Energy Convers. Manag. 2021, 236, 114053. [Google Scholar] [CrossRef]
- Wolf, D. Methods for Design and Application of Adiabatic Compressed Air Energy Storage Based on Dynamic Modeling; Laufen: Oberhausen, Germany, 2011. [Google Scholar]
- Tec-Science. Why Do Pressure and Temperature Increase During the Compression of a Gas? 2021. Available online: https://www.tec-science.com/thermodynamics/thermodynamic-processes-in-closed-systems/why-does-pressure-and-temperature-increase-during-the-compression-of-a-gas/ (accessed on 22 June 2023).
- Kim, Y.-M.; Lee, J.-H.; Kim, S.-J.; Favrat, D. Potential and Evolution of Compressed Air Energy Storage: Energy and Exergy Analyses. Entropy 2012, 14, 1501–1521. [Google Scholar] [CrossRef]
- Patil, V.C.; Ro, P.I. Energy and Exergy Analysis of Ocean Compressed Air Energy Storage Concepts. J. Eng. 2018, 2018, e5254102. [Google Scholar] [CrossRef]
- Van de Ven, J.D.; Li, P.Y. Liquid piston gas compression. Appl. Energy 2009, 86, 2183–2191. [Google Scholar] [CrossRef]
- Qin, C.; Loth, E. Liquid piston compression efficiency with droplet heat transfer. Appl. Energy 2014, 114, 539–550. [Google Scholar] [CrossRef]
- Qin, C.C.; Loth, E. Isothermal compressed wind energy storage using abandoned oil/gas wells or coal mines. Appl. Energy 2021, 292, 116867. [Google Scholar] [CrossRef]
- Patil, V.C.; Ro, P.I. Modeling of liquid-piston based design for isothermal ocean compressed air energy storage system. J. Energy Storage 2020, 31, 101449. [Google Scholar] [CrossRef]
- Zhang, C.; Saadat, M.; Li, P.Y.; Simon, T.W. Heat Transfer in a Long, Thin Tube Section of an Air Compressor: An Empirical Correlation from CFD and a Thermodynamic Modeling. In American Society of Mechanical Engineers Digital Collection; American Society of Mechanical Engineers: New York, NY, USA, 2013; pp. 1601–1607. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.Y.; Van de Ven, J.D.; Simon, T.W. Design analysis of a liquid-piston compression chamber with application to compressed air energy storage. Appl. Therm. Eng. 2016, 101, 704–709. [Google Scholar] [CrossRef]
- Yan, B.; Wieberdink, J.; Shirazi, F.; Li, P.Y.; Simon, T.W.; Van de Ven, J.D. Experimental study of heat transfer enhancement in a liquid piston compressor/expander using porous media inserts. Appl. Energy 2015, 154, 40–50. [Google Scholar] [CrossRef]
- Wieberdink, J.; Li, P.Y.; Simon, T.W.; Van de Ven, J.D. Effects of porous media insert on the efficiency and power density of a high pressure (210 bar) liquid piston air compressor/expander—An experimental study. Appl. Energy 2018, 212, 1025–1037. [Google Scholar] [CrossRef]
- Ren, T.; Xu, W.; Cai, M.; Wang, X.; Li, M. Experiments on Air Compression with an Isothermal Piston for Energy Storage. Energies 2019, 12, 3730. [Google Scholar] [CrossRef]
- Ren, T.; Xu, W.; Jia, G.-W.; Cai, M. A Novel Isothermal Compression Method for Energy Conservation in Fluid Power Systems. Entropy 2020, 22, 1015. [Google Scholar] [CrossRef] [PubMed]
- Weiqing, X.; Ziyue, D.; Xiaoshuang, W.; Maolin, C.; Guanwei, J.; Yan, S. Isothermal piston gas compression for compressed air energy storage. Int. J. Heat Mass Transf. 2020, 155, 119779. [Google Scholar] [CrossRef]
- Khaljani, M.; Vennard, A.; Harrison, J.; Surplus, D.; Murphy, A.; Mahmoudi, Y. Experimental and modelling analysis of efficiency enhancement in a liquid piston gas compressor using metal plate inserts for compressed air energy storage application. J. Energy Storage 2021, 43, 103240. [Google Scholar] [CrossRef]
- Patil, V.C.; Liu, J.; Ro, P.I. Efficiency improvement of liquid piston compressor using metal wire mesh for near-isothermal compressed air energy storage application. J. Energy Storage 2020, 28, 101226. [Google Scholar] [CrossRef]
- Patil, V.C.; Acharya, P.; Ro, P.I. Experimental investigation of water spray injection in liquid piston for near-isothermal compression. Appl. Energy 2020, 259, 114182. [Google Scholar] [CrossRef]
- Ahn, B.; Patil, V.C.; Ro, P.I. Effect of Integrating Metal Wire Mesh with Spray Injection for Liquid Piston Gas Compression. Energies 2021, 14, 3723. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; He, X.; Zhang, Y. Experimental and thermodynamic investigation on isothermal performance of large-scaled liquid piston. Energy 2022, 249, 123731. [Google Scholar] [CrossRef]
- Ahn, B.; Ro, P.I. Spray cooling technique in liquid piston gas compression and impact of air dissolution on efficiency evaluation at different pressure levels. J. Energy Storage 2024, 81, 110460. [Google Scholar] [CrossRef]
- Patil, V.C.; Ro, P.I. Experimental study of heat transfer enhancement in liquid piston compressor using aqueous foam. Appl. Therm. Eng. 2020, 164, 114441. [Google Scholar] [CrossRef]
- Patil, V.C. Efficiency Improvement Techniques in Liquid Piston Compressor for Ocean Compressed Air Energy Storage Application; North Carolina State University: Raleigh, NC, USA, 2019. [Google Scholar]
- Bollinger, B. Demonstration of Isothermal Compressed Air Energy Storage to Support Renewable Energy Production; SustainX, Inc.: Seabrook, NH, USA, 2015. [Google Scholar]
- Wieberdink, J. Increasing Efficiency and Power Density Of a Liquid Piston Air Compressor/Expander With Porous Media Heat Transfer Elements; University of Minnesota: Minneapolis, MN, USA, 2014. [Google Scholar]
- Marketwired, SustainX Begins Operating World’s First Grid-Scale Isothermal Compressed Air Energy Storage System, Yahoo Finance. 2013. Available online: http://finance.yahoo.com/news/sustainx-begins-operating-worlds-first-130000557.html (accessed on 27 June 2023).
- SustainX Delivers Compressed Air Energy Storage System, Nanalyze. 2014. Available online: https://www.nanalyze.com/2014/07/sustainx-delivers-compressed-air-energy-storage-system/ (accessed on 27 June 2023).
- POWER. First Megawatt-Scale Isothermal CAES Completion, POWER Magazine. 2013. Available online: https://www.powermag.com/first-megawatt-scale-isothermal-caes-completion/ (accessed on 27 June 2023).
- NRStor Advances CAES in Ontario, General Compression and SustainX Merges, Energy Storage World Forum. Available online: https://www.power-eng.com/energy-storage/long-duration-energy-storage/general-compression-sustainx-to-merge-as-gcx-energy-storage/ (accessed on 15 August 2025).
- Attia, J.A.; McKinley, I.M.; Moreno-Magana, D.; Pilon, L. Convective heat transfer in foams under laminar flow in pipes and tube bundles. Int. J. Heat Mass Transf. 2012, 55, 7823–7831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gylys, J.; Zdankus, T.; Gylys, M. Experimental investigation of heat transfer from inclined flat surface to aqueous foam. Int. J. Heat Mass Transf. 2014, 69, 230–236. [Google Scholar] [CrossRef]
- Herzhaft, B. Rheology of Aqueous Foams: A Literature Review of Some Experimental Works. Oil Gas Sci. Technol.—Rev. d’IFP Energies Nouv. 1999, 54, 587–596. [Google Scholar] [CrossRef]
- Larmignat, S.; Vanderpool, D.; Lai, H.K.; Pilon, L. Rheology of colloidal gas aphrons (microfoams). Colloids Surfaces A: Physicochem. Eng. Asp. 2008, 322, 199–210. [Google Scholar] [CrossRef]
- Ahn, B.; Ro, P.I. Experimental Investigation of Impacts of Initial Pressure Levels on Compression Efficiency and Dissolution in Liquid Piston Gas Compression. Energies 2023, 16, 1921. [Google Scholar] [CrossRef]
- Patil, V.C.; Acharya, P.; Ro, P.I. Experimental investigation of heat transfer in liquid piston compressor. Appl. Therm. Eng. 2019, 146, 169–179. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, B.; Wieberdink, J.; Li, P.; Van de Ven, J.; Loth, E.; Simon, T. Thermal analysis of a compressor for application to Compressed Air Energy Storage. Appl. Therm. Eng. 2014, 73, 1402–1411. [Google Scholar] [CrossRef]
- Ahn, B. Comprehensive Experimental Investigation of Liquid Piston Gas Compression for Energy Storage Applications. Ph.D. Thesis, Baylor University, Waco, TX, USA, 2023. [Google Scholar]
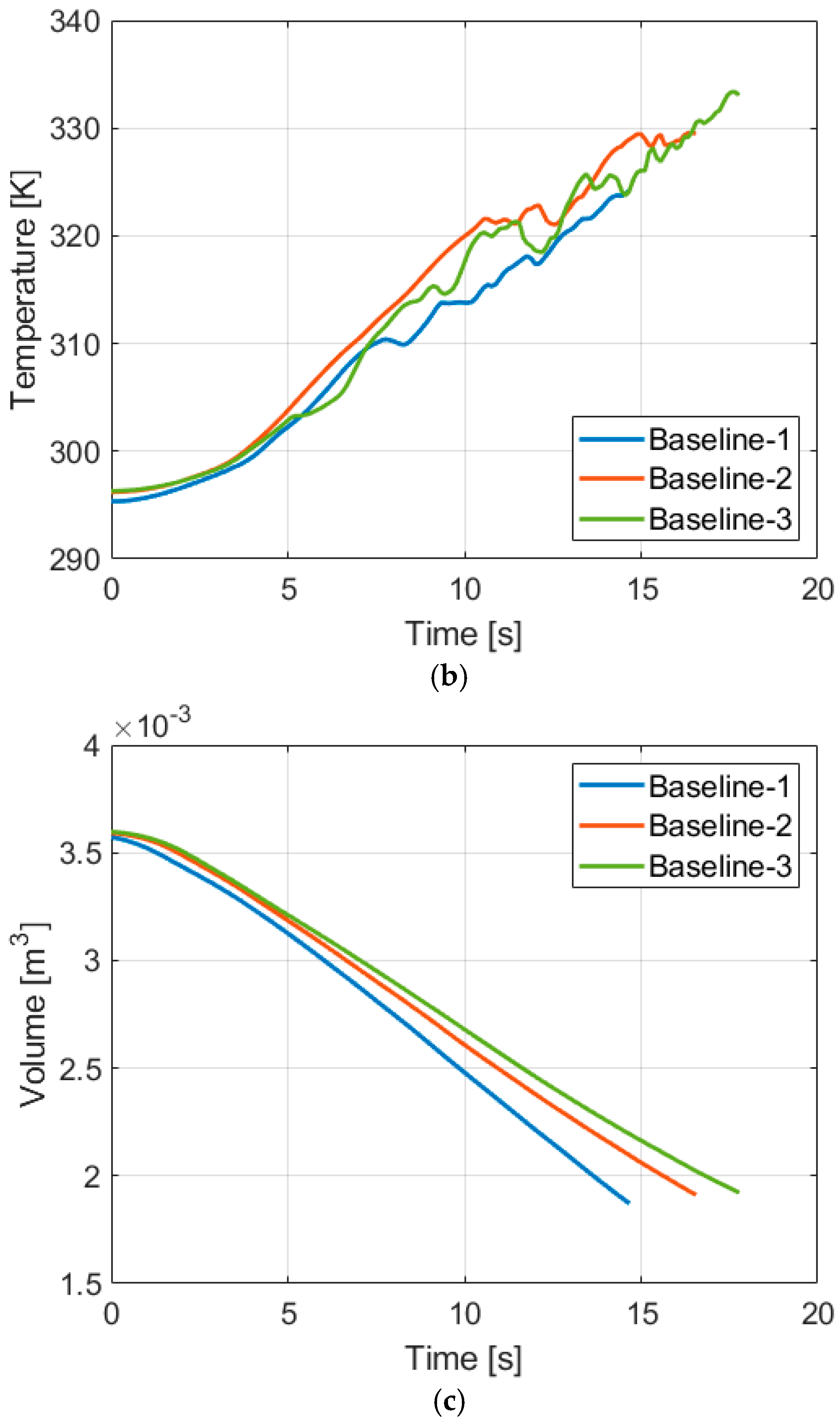
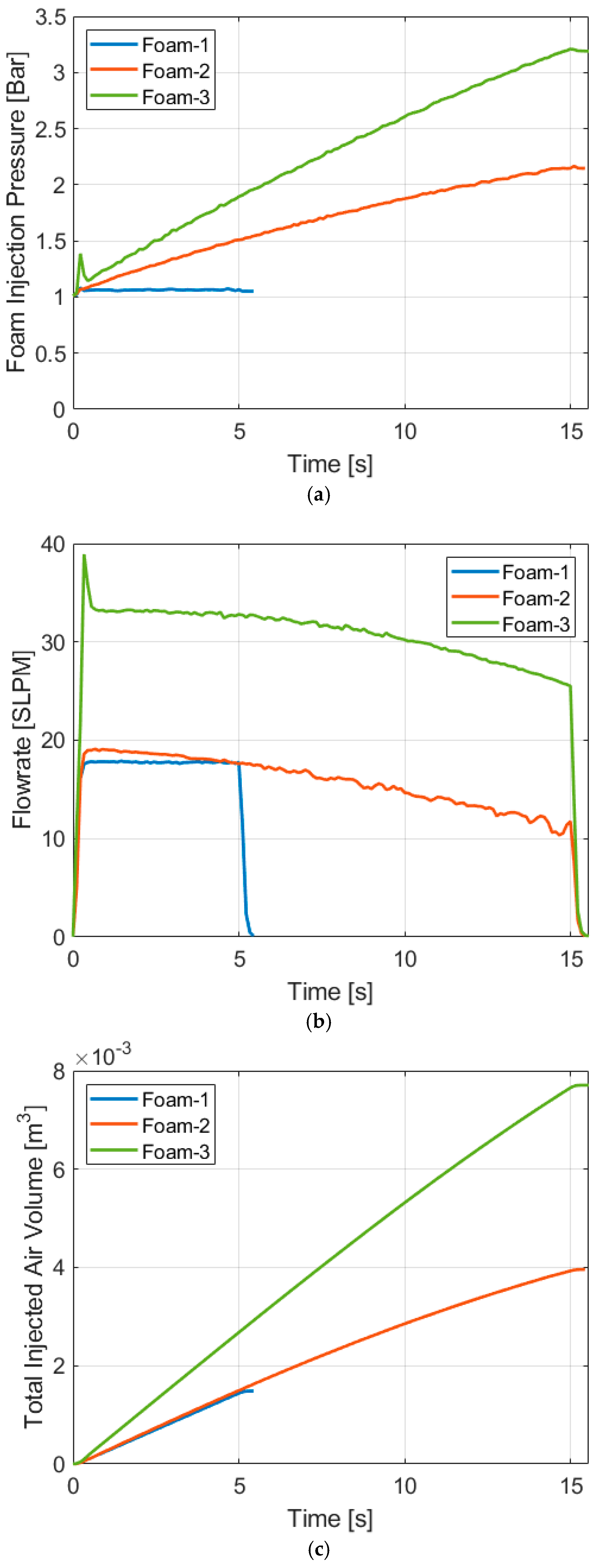
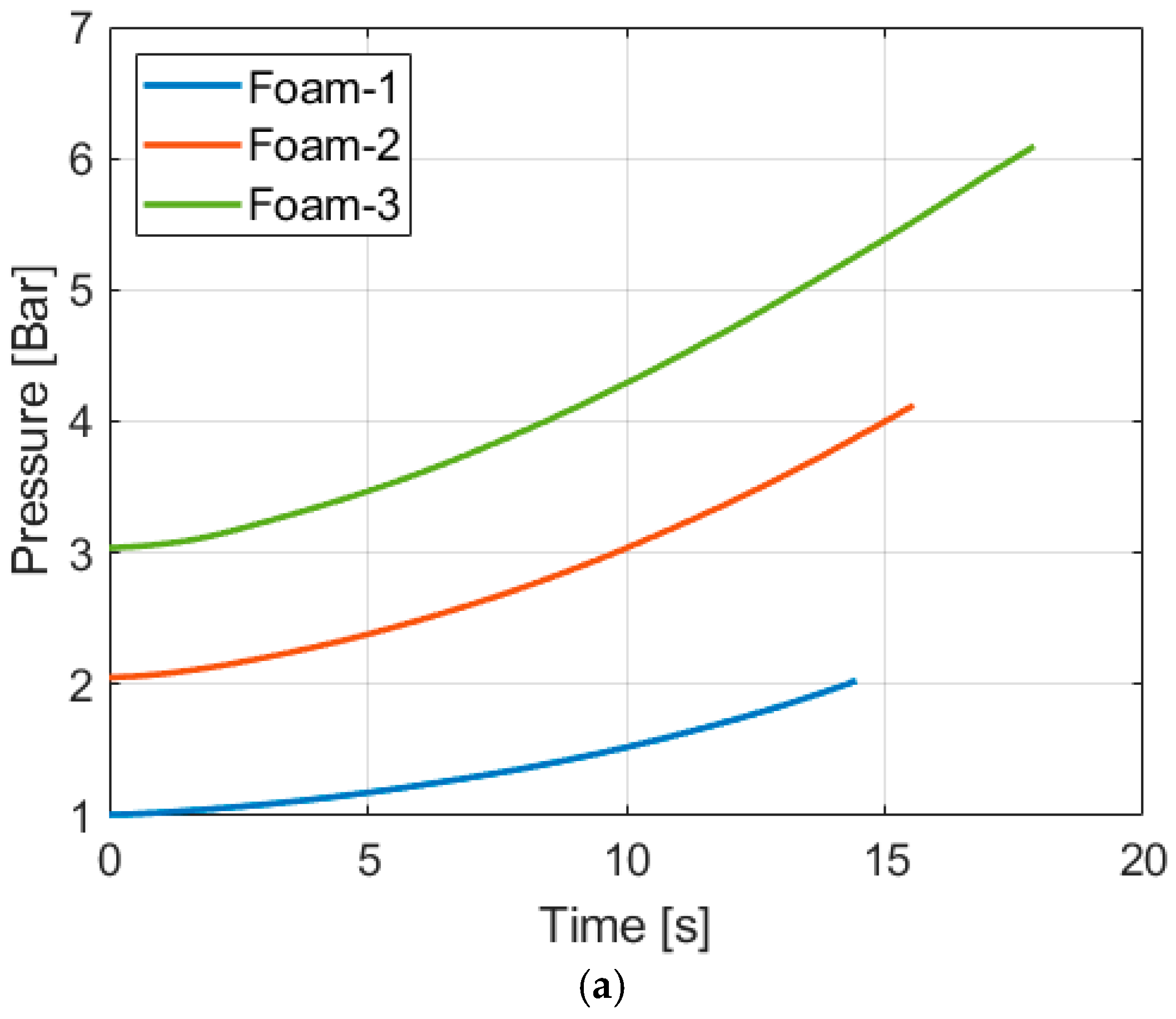

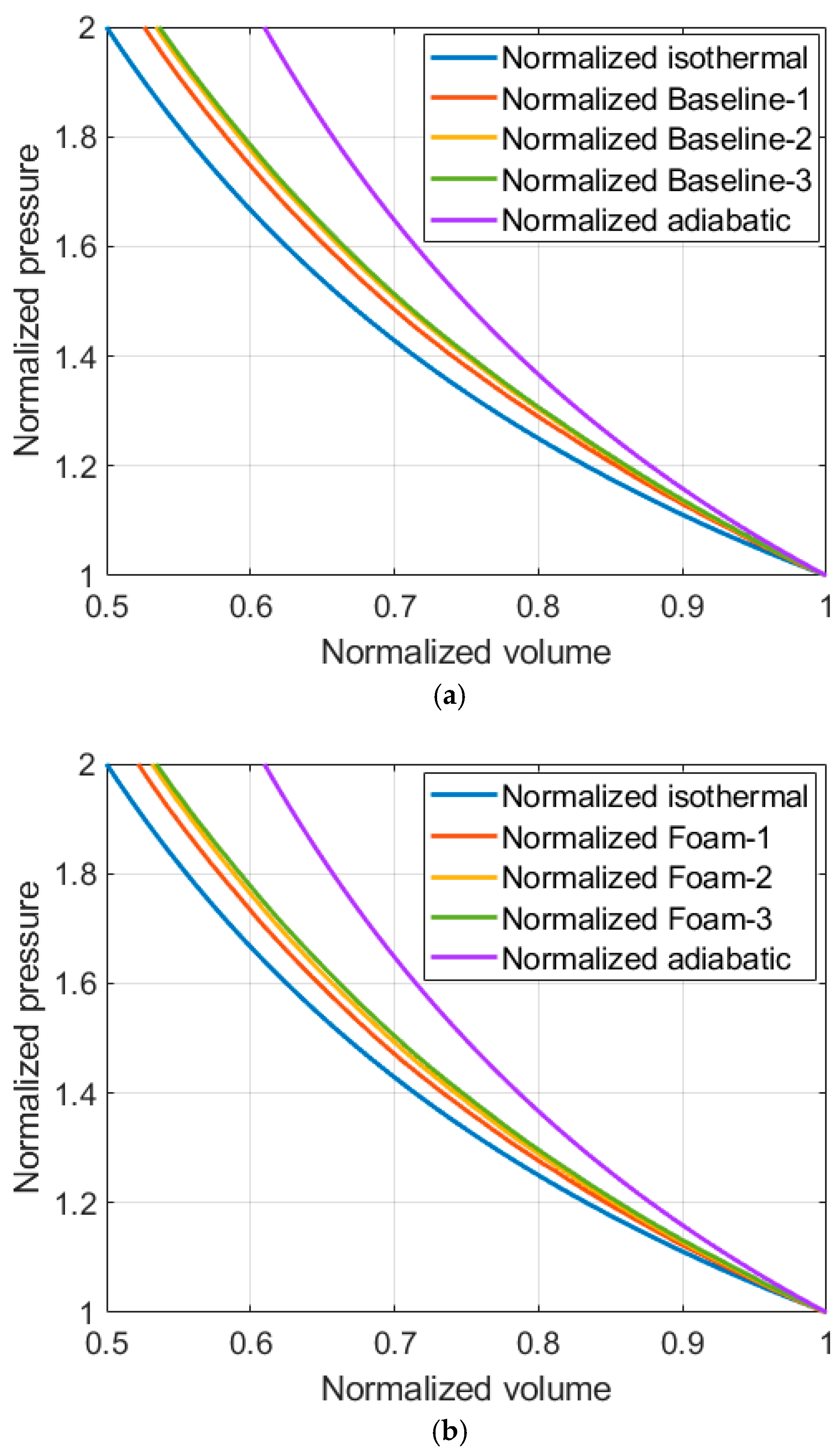
| Temperature Increase [K] | ||
|---|---|---|
| [Bar] | Baseline | Foam |
| 1 | 28.90 | 27.47 |
| 2 | 33.32 | 32.95 |
| 3 | 36.75 | 34.08 |
| Isothermal Efficiency [%] | |||
|---|---|---|---|
| Baseline | Foam | ||
| 1 Bar | 89.2 | 91.4 | 2.2 |
| 2 Bar | 86.1 | 88.2 | 2.1 |
| 3 Bar | 85.3 | 86.6 | 1.3 |
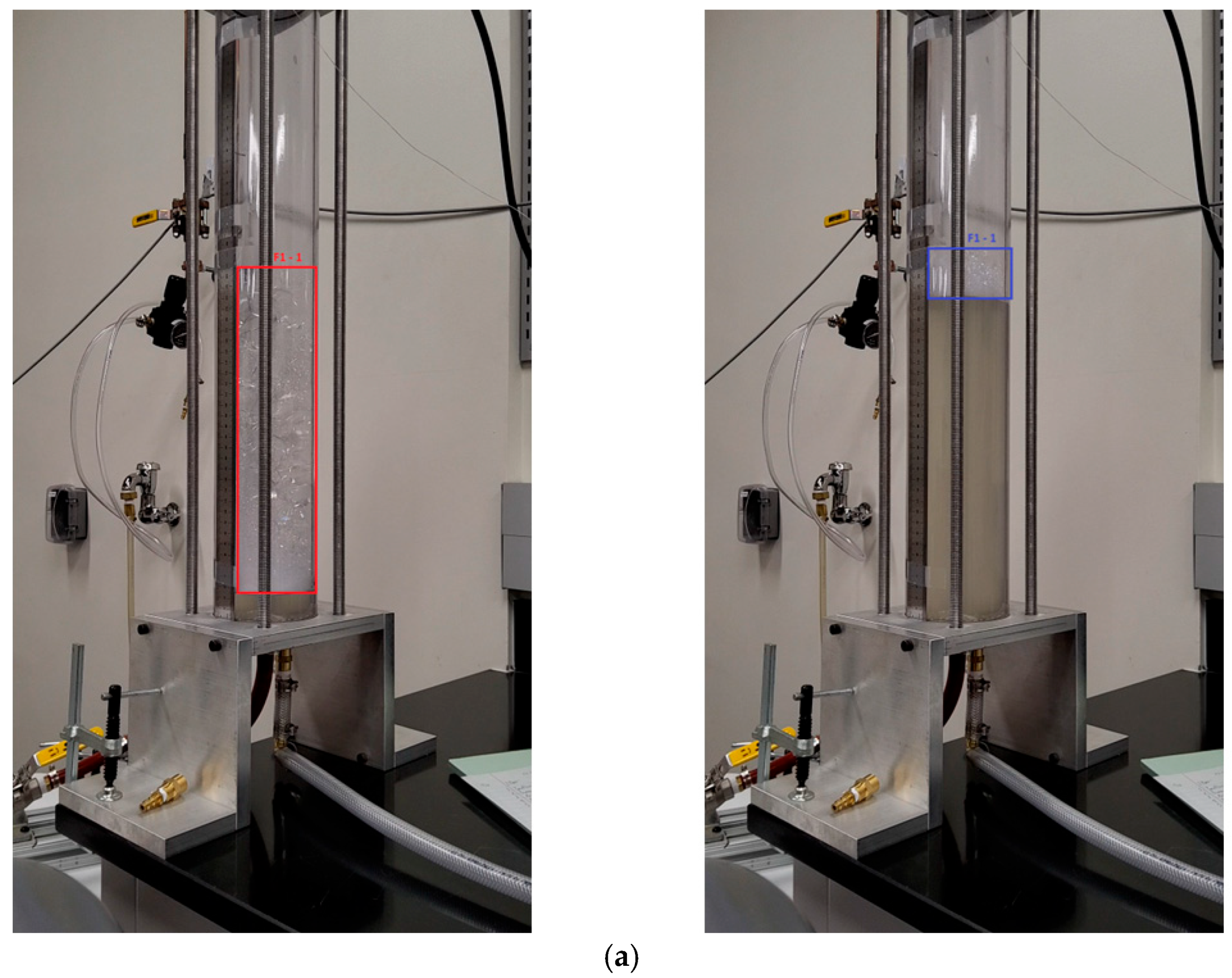
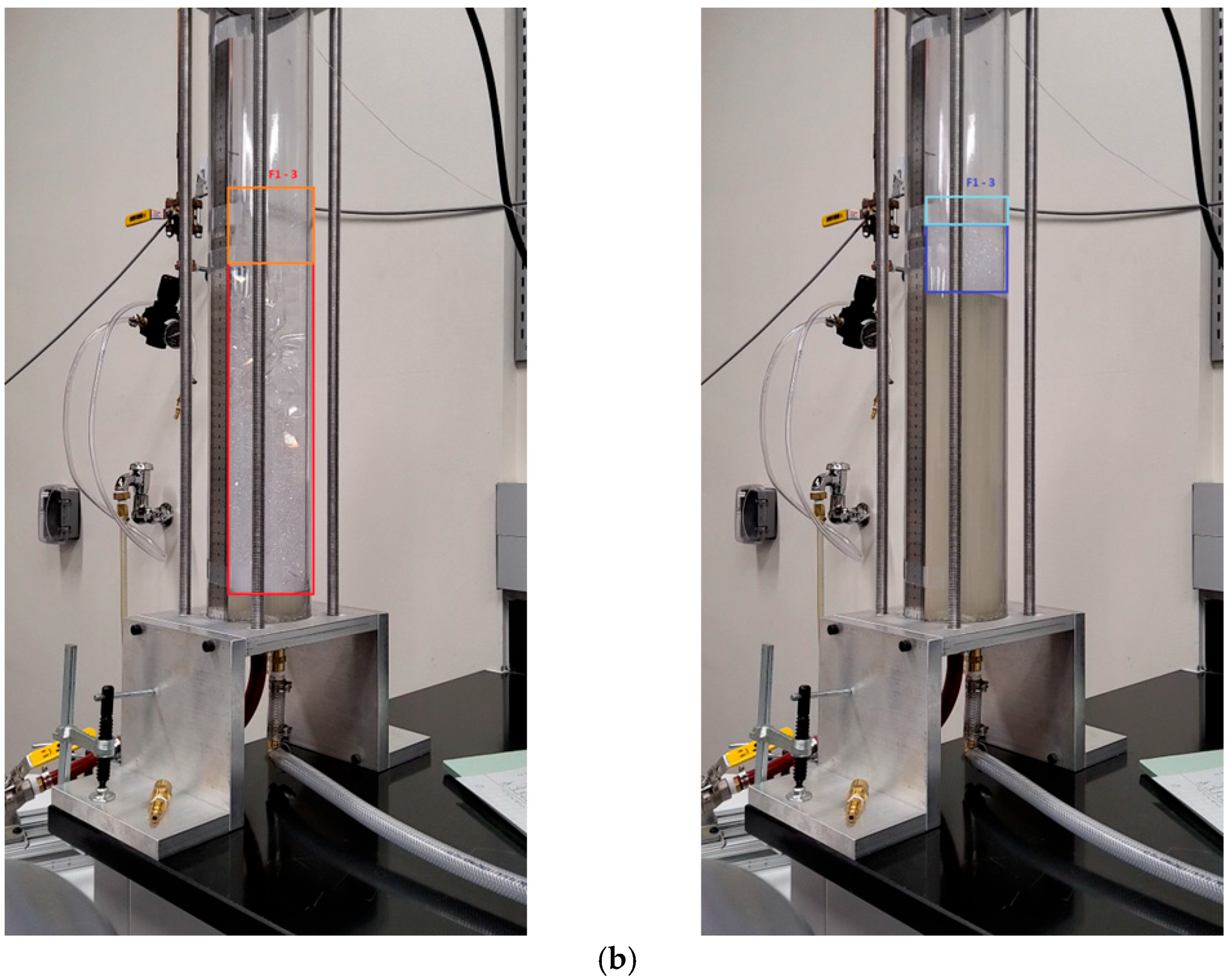
| Process | Stroke # | [mm] | [mm] | [mm] | [%] |
|---|---|---|---|---|---|
| Foam-1 | 1 | 300 | 45 | 255 | 91.23 |
| 2 | 305 | 50 | 255 | 91.23 | |
| 3 | 375 * | 85 * | 290 | 91.87 | |
| Foam-2 | 1 | 330 | 85 | 245 | 87.87 |
| 2 | 360 | 95 | 265 | 88.22 | |
| 3 | 375 | 110 * | 265 | 88.48 | |
| Foam-3 | 1 | 365 | 45 | 320 | 86.50 |
| 2 | 375 | 55 | 320 | 86.62 | |
| 3 | 385 | 65 | 320 | 86.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, B.; Schmetzer, M.; Ro, P.I. Experimental Study of Aqueous Foam Use for Heat Transfer Enhancement in Liquid Piston Gas Compression at Various Initial Pressure Levels. Thermo 2025, 5, 39. https://doi.org/10.3390/thermo5040039
Ahn B, Schmetzer M, Ro PI. Experimental Study of Aqueous Foam Use for Heat Transfer Enhancement in Liquid Piston Gas Compression at Various Initial Pressure Levels. Thermo. 2025; 5(4):39. https://doi.org/10.3390/thermo5040039
Chicago/Turabian StyleAhn, Barah, Macey Schmetzer, and Paul I. Ro. 2025. "Experimental Study of Aqueous Foam Use for Heat Transfer Enhancement in Liquid Piston Gas Compression at Various Initial Pressure Levels" Thermo 5, no. 4: 39. https://doi.org/10.3390/thermo5040039
APA StyleAhn, B., Schmetzer, M., & Ro, P. I. (2025). Experimental Study of Aqueous Foam Use for Heat Transfer Enhancement in Liquid Piston Gas Compression at Various Initial Pressure Levels. Thermo, 5(4), 39. https://doi.org/10.3390/thermo5040039






