From Thermal Conversion to Cathode Performance: Acid-Activated Walnut Shell Biochar in Li–S Batteries and Its Impact on Air Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Production
2.2. Biochar Characterization
2.3. Electrode Fabrication, Cell Assembly, and Electrochemical Characterization from Biochar
2.4. Gas Fraction Analysis
3. Results and Discussion
3.1. Biochar Characterization
3.2. Electrochemical Performance of Li–S Batteries
3.3. Gaseous Emissions
3.4. Air Pollution Implications
| Condition | VOC | τOH | τCl | τNO3 | POCP |
|---|---|---|---|---|---|
| Without acid activation | Hexanal | 9.92 h | 4.5 days | 2 days | 60.92 a |
| Benzene, methyl | 1.9 days | 23.1 days | 341 days | 63.7 b-44 c | |
| Octane | 1.4 days | 3 days | 126.5 days | 18.08 a-45.3 b-34 c-13 d | |
| With acid activation | Acetic acid | 17.3 days | 112.8 years | - | 9.7 b-9 c |
| Benzene, methyl | 1.9 days | 23.1 days | 341 days | 63.7 b-44 c | |
| Benzene, ethyl | 1.5 days | 10 days | 40.5 days | 73 b-46 c | |
| Benzene, 1,2-dimethyl | 6.21 h | 8.3 days | 61.4 days | 105.3 b-78 c-86 d | |
| Benzene | 9 days | 18,000.17 years | 2.1 years | 21.8 b-10 c |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bora, R.R.; Tao, Y.; Lehmann, J.; Tester, J.W.; Richardson, R.E.; You, F. Techno-Economic Feasibility and Spatial Analysis of Thermochemical Conversion Pathways for Regional Poultry Waste Valorization. ACS Sustain. Chem. Eng. 2020, 8, 5763–5775. [Google Scholar] [CrossRef]
- Ricciardi, P.; Cillari, G.; Miino, M.C.; Collivignarelli, M.C. Valorization of agro-industry residues in the building and environmental sector: A review. Waste Manag. Res. 2020, 38, 487–513. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, Q.; Li, W.; Ciais, P.; Wang, Y.-P.; Goll, D.S.; Zhu, L.; Zhao, Z.; Wang, J.; Wei, Y.; et al. Global soil organic carbon changes and economic revenues with biochar application. GCB Bioenergy 2022, 14, 364–377. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 12–22. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.A. Unravelling the Environmental Application of Biochar as Low-Cost Biosorbent: A Review. Appl. Sci. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, K.; Lv, J.; Zhang, J.; Peng, C.; Li, Y.; Gu, Z.; Song, X. Biochar amendments increase soil organic carbon storage and decrease global warming potentials of soil CH4 and N2O under N addition in a subtropical Moso bamboo plantation. For. Ecosyst. 2022, 9, 100054. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Li, J.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.; Wang, S.; Song, H.; et al. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon Res. 2022, 1, 4. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Theng, B.K.G.; Lee, X.; Zhang, X.; Chen, M.; Xu, P. Removal performance; mechanisms, and influencing factors of biochar for air pollutants: A critical review. Biochar 2022, 4, 30. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Activated carbon from biomass precursors using phosphoric acid: A review. Heliyon 2022, 8, e11940. [Google Scholar] [CrossRef]
- Jin, X.; Lee, J.H.; Choi, J.W. Catalytic co-pyrolysis of woody biomass with waste plastics: Effects of HZSM-5 and pyrolysis temperature on producing high-value pyrolytic products and reducing wax formation. Energy 2022, 239, 121739. [Google Scholar] [CrossRef]
- Meier, D.; Faix, O. State of the art of applied fast pyrolysis of lignocellulosic materials—A review. Bioresour. Technol. 1999, 68, 71–77. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Kravchenko, O.A.; Ananikov, V.P. Conversion of plant biomass to furan derivatives and sustainable access to the new generation of polymers. functional materials and fuels, Russ. Chem. Rev. 2017, 86, 357. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lee, G.B.; Kim, H.; Park, J.E. Influence of mixed methods on the surface area and gas products of activated carbon. Carbon Lett. 2020, 30, 603–611. [Google Scholar] [CrossRef]
- Gu, H.; Bergman, R.; Anderson, N.; Alanya-Rosenbaum, S. Life cycle assessment of activated carbon from woody biomass. Wood Fiber Sci. 2018, 50, 229–243. [Google Scholar] [CrossRef]
- Hadanu, R.; Apituley, D.A.N. Volatile Compounds Detected in Coconut Shell Liquid Smoke through Pyrolysis at a Fractioning Temperature of 350–420 °C. Makara J. Sci. 2016, 20, 95–100. [Google Scholar] [CrossRef]
- Aguirre, F.; Lobos, M.L.N.; Basto, M.A.L.; Teruel, M.A.; Moyano, E.L.; Blanco, M.B. Volatile Organic Compounds Released During the Fast Pyrolysis of Peanut Shells and Environmental Implications. Bull. Environ. Contam. Toxicol. 2022, 108, 1139–1146. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Liu, J.; Liu, B.; Wang, C.; Huang, Z.; Hu, L.; Ke, X.; Liu, L.; Shi, Z.; Guo, Z. Walnut shell—Derived activated carbon: Synthesis and its application in the sulfur cathode for lithium–sulfur batteries. J. Alloys Compd. 2017, 718, 373–378. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium–sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef]
- Lv, Z.-C.; Wang, P.-F.; Wang, J.-C.; Tian, S.-H.; Yi, T.-F. Key challenges, recent advances and future perspectives of rechargeable lithium-sulfur batteries. J. Ind. Eng. Chem. 2023, 124, 68–88. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Zhang, Q. A review of flexible lithium–sulfur and analogous alkali metal–chalcogen rechargeable batteries. Chem. Soc. Rev. 2017, 46, 5237–5288. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, B.; Zhai, X.; Li, H.; Jiang, Q. Review of Carbon Materials for Lithium-Sulfur Batteries. ChemistrySelect 2018, 3, 2245–2260. [Google Scholar] [CrossRef]
- Zaini, M.S.M.; Anuar, N.F.; Al-Junid, S.A.M.; Syed-Hassan, S.S.A. Agricultural biomass-based carbon cathode materials for lithium-sulfur batteries: A systematic review. Mater. Sci. Energy Technol. 2023, 6, 205–225. [Google Scholar] [CrossRef]
- Benítez, A.; González-Tejero, M.; Caballero, Á.; Morales, J. Almond Shell as a Microporous Carbon Source for Sustainable Cathodes in Lithium–Sulfur Batteries. Materials 2018, 11, 1428. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Li, H.; Pan, L.; Lu, T.; Zhan, Y.; Nie, C.; Sun, Z. A comparative study on electrosorptive behavior of carbon nanotubes and graphene for capacitive deionization. J. Electroanal. Chem. 2011, 653, 40–44. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; Poon, C.S. Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural. Bioresour. Technol. 2018, 267, 242–248. [Google Scholar] [CrossRef]
- Imwinkelried, G.; Fermanelli, C.S.; Teruel, M.A.; Saux, C.; Blanco, M.B. Comprehensive analysis of soybean residues pyrolysis products. J. Anal. Appl. Pyrolysis 2024, 177, 106367. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; El Hajjaji, S.; Szabó, T.; Trif, L.; Abbi, I.F.K.; Labjar, N.; Harmouche, L.; Shaban, A. Biomass valorization of walnut shell into biochar as a resource for electrochemical simultaneous detection of heavy metal ions in water and soil samples: Preparation, characterization, and applications. Arab. J. Chem. 2022, 15, 104252. [Google Scholar] [CrossRef]
- Raviolo, S.; Bracamonte, M.V.; Ramanzin, M.B.S.; Alburquenque, D.; Oliva, M.I.; Cometto, F.P.; Luque, G.L. Valorization of brewer’s spent grain via systematic study of KOH activation parameters and its potential application in energy storage. Biomass Bioenergy 2025, 192, 107494. [Google Scholar] [CrossRef]
- Sevilla, M.; Carro-Rodríguez, J.N.; Díez, N.; Fuertes, A.B. Straightforward synthesis of Sulfur/N, S-codoped carbon cathodes for Lithium-Sulfur batteries. Sci. Rep. 2020, 10, 4866. [Google Scholar] [CrossRef]
- Cherrington, R.; Liang, J. 2—Materials and Deposition Processes for Multifunctionality. In Design and Manufacture of Plastic Components for Multifunctionality; Goodship, V., Middleton, B., Cherrington, R., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 19–51. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Thermal behaviour of lignocellulosic material in the presence of phosphoric acid. Influence of the acid content in the initial solution. Carbon 2006, 44, 2347–2350. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710. [Google Scholar] [CrossRef]
- Benítez, A.; Morales, J.; Caballero, Á. Pistachio Shell-Derived Carbon Activated with Phosphoric Acid: A More Efficient Procedure to Improve the Performance of Li–S Batteries. Nanomaterials 2020, 10, 840. [Google Scholar] [CrossRef]
- Chen, H.; Xia, P.; Lei, W.; Pan, Y.; Zou, Y.; Ma, Z. Preparation of activated carbon derived from biomass and its application in lithium–sulfur batteries. J. Porous Mater. 2019, 26, 1325–1333. [Google Scholar] [CrossRef]
- Vélez, P.; Para, M.L.; Luque, G.L.; Barraco, D.; Leiva, E.P.M. Modeling of substitutionally modified graphene structures to prevent the shuttle mechanism in lithium-sulfur batteries. Electrochim. Acta 2019, 309, 402–414. [Google Scholar] [CrossRef]
- Vélez, P.; Rojas, M.D.C.; Velasco, J.; Para, M.L.; Barraco, D.; Leiva, E.P.M.; Luque, G.L. On the role of oxidized graphene interfaces in lithium sulfur batteries: Thermodynamic and kinetic aspects using density functional theory. Appl. Surf. Sci. 2021, 550, 149358. [Google Scholar] [CrossRef]
- Sahore, R.; Levin, B.D.A.; Pan, M.; Muller, D.A.; DiSalvo, F.J.; Giannelis, E.P. Design Principles for Optimum Performance of Porous Carbons in Lithium–Sulfur Batteries. Adv. Energy Mater. 2016, 6, 1600134. [Google Scholar] [CrossRef]
- Hu, X.; Guo, H.; Gholizadeh, M.; Sattari, B.; Liu, Q. Pyrolysis of different wood species: Impacts of C/H ratio in feedstock on distribution of pyrolysis products. Biomass Bioenergy 2019, 120, 28–39. [Google Scholar] [CrossRef]
- Park, Y.-K.; Yoo, M.L.; Heo, H.S.; Lee, H.W.; Park, S.H.; Jung, S.-C.; Park, S.-S.; Seo, S.-G. Wild reed of Suncheon Bay: Potential bio-energy source. Renew. Energy 2012, 42, 168–172. [Google Scholar] [CrossRef]
- Becidan, M.; Skreiberg, Ø.; Hustad, J.E. Products distribution and gas release in pyrolysis of thermally thick biomass residues samples. J. Anal. Appl. Pyrolysis 2007, 78, 207–213. [Google Scholar] [CrossRef]
- Krishna, B.B.; Singh, R.; Bhaskar, T. Effect of catalyst contact on the pyrolysis of wheat straw and wheat husk. Fuel 2015, 160, 64–70. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Tahir, M.H.; Zhao, Z.; Ren, J.; Rasool, T.; Naqvi, S.R. Thermo-kinetics and gaseous product analysis of banana peel pyrolysis for its bioenergy potential. Biomass Bioenergy 2019, 122, 193–201. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, G.; Xu, X.; Shen, D.; Jin, B. Study on carbonization of lignin by TG-FTIR and high-temperature carbonization reactor. Fuel Process. Technol. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Ojha, D.K.; Kumar, V.S.P.; Vinu, R. Analytical pyrolysis of bagasse and groundnut shell briquettes: Kinetics and pyrolysate composition studies. Bioresour. Technol. Rep. 2021, 15, 100784. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Hu, B.; Lu, G.; Wang, Y. Efficient catalytic conversion of lignocellulosic biomass into renewable liquid biofuels via furan derivatives. RSC Adv. 2014, 4, 31101–31107. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Mehrabian, R.; Scharler, R.; Obernberger, I. Kinetic scheme of biomass pyrolysis considering secondary charring reactions. Energy Convers. Manag. 2014, 87, 687–696. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.; Dong, C.; Zhang, Z.; Zhang, X.; Zhu, X. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Galloway, J.N.; Likens, G.E.; Keene, W.C.; Miller, J.M. The composition of precipitation in remote areas of the world. J. Geophys. Res. Ocean 1982, 87, 8771–8786. [Google Scholar] [CrossRef]
- Keene, W.C.; Mosher, B.W.; Jacob, D.J.; Munger, J.W.; Talbot, R.W.; Artz, R.S.; Maben, J.R.; Daube, B.C.; Galloway, J.N. Carboxylic acids in clouds at a high-elevation forested site in central Virginia. J. Geophys. Res. Atmos. 1995, 100, 9345–9357. [Google Scholar] [CrossRef]
- Khare, P.; Kumar, N.; Kumari, K.M.; Srivastava, S.S. Atmospheric formic and acetic acids: An overview. Rev. Geophys. 1999, 37, 227–248. [Google Scholar] [CrossRef]
- Vet, R.; Artz, R.S.; Carou, S.; Shaw, M.; Ro, C.-U.; Aas, W.; Baker, A.; Bowersox, V.C.; Dentener, F.; Galy-Lacaux, C.; et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014, 93, 3–100. [Google Scholar] [CrossRef]
- Zhang, N.; He, Y.; Cao, J.; Ho, K.; Shen, Z. Long-term trends in chemical composition of precipitation at Lijiang, southeast Tibetan Plateau, southwestern China. Atmos. Res. 2012, 106, 50–60. [Google Scholar] [CrossRef]
- Keene, W.C.; Galloway, J.N. Considerations regarding sources for formic and acetic acids in the troposphere. J. Geophys. Res. Atmos. 1986, 91, 14466–14474. [Google Scholar] [CrossRef]
- Andreae, M.O.; Talbot, R.W.; Andreae, T.W.; Harriss, R.C. Formic and acetic acid over the central Amazon region, Brazil: 1. Dry season. J. Geophys. Res. Atmos. 1988, 93, 1616–1624. [Google Scholar] [CrossRef]
- Yu, S. Role of organic acids (formic, acetic, pyruvic and oxalic) in the formation of cloud condensation nuclei (CCN): A review. Atmos. Res. 2000, 53, 185–217. [Google Scholar] [CrossRef]
- Myrén, C.; Hörnell, C.; Björnbom, E.; Sjöström, K. Catalytic tar decomposition of biomass pyrolysis gas with a combination of dolomite and silica. Biomass Bioenergy 2002, 23, 217–227. [Google Scholar] [CrossRef]
- Lauraguais, A.; El Zein, A.; Coeur, C.; Obeid, E.; Cassez, A.; Rayez, M.T.; Rayez, J.C. Kinetic Study of the Gas-Phase Reactions of Nitrate Radicals with Methoxyphenol Compounds: Experimental and Theoretical Approaches. J. Phys. Chem. A 2016, 120, 2691–2699. [Google Scholar] [CrossRef]
- Gour, N.K.; Deka, R.C.; Singh, H.J.; Mishra, B.K. A computational perspective on mechanism and kinetics of the reactions of CF3C(O)OCH2CF3 with OH radicals and Cl atoms at 298 K. J. Fluor. Chem. 2014, 160, 64–71. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Wang, W.; Ge, M. Gas-phase reaction of two unsaturated ketones with atomic Cl and O3: Kinetics and products. Phys. Chem. Chem. Phys. 2015, 17, 12000–12012. [Google Scholar] [CrossRef]
- Ye, J.T.; Bai, F.Y.; Pan, X.M. Computational study of H-abstraction reactions from CH3OCH2CH2Cl/CH3CH2OCH2CH2Cl by Cl atom and OH radical and fate of alkoxy radicals. Environ. Sci. Pollut. Res. 2016, 23, 23467–23484. [Google Scholar] [CrossRef]
- Sander, R.; Acree, W.E.; Visscher, A.D.; Schwartz, S.E.; Wallington, T.J. Henry’s law constants (IUPAC Recommendations 2021). Pure Appl. Chem. 2022, 94, 71–85. [Google Scholar] [CrossRef]
- Derwent, R.G.; Jenkin, M.E.; Saunders, S.M.; Pilling, M.J. Photochemical ozone creation potentials for organic compounds in northwest Europe calculated with a master chemical mechanism. Atmos. Environ. 1998, 32, 2429–2441. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Derwent, R.G.; Wallington, T.J. Photochemical ozone creation potentials for volatile organic compounds: Rationalization and estimation. Atmos. Environ. 2017, 163, 128–137. [Google Scholar] [CrossRef]
- NIST Chemical Kinetics Database, (n.d.). Available online: https://kinetics.nist.gov/kinetics/index.jsp (accessed on 1 October 2024).
- Derwent, R.G.; Jenkin, M.E.; Passant, N.R.; Pilling, M.J. Reactivity-based strategies for photochemical ozone control in Europe. Environ. Sci. Policy 2007, 10, 445–453. [Google Scholar] [CrossRef]
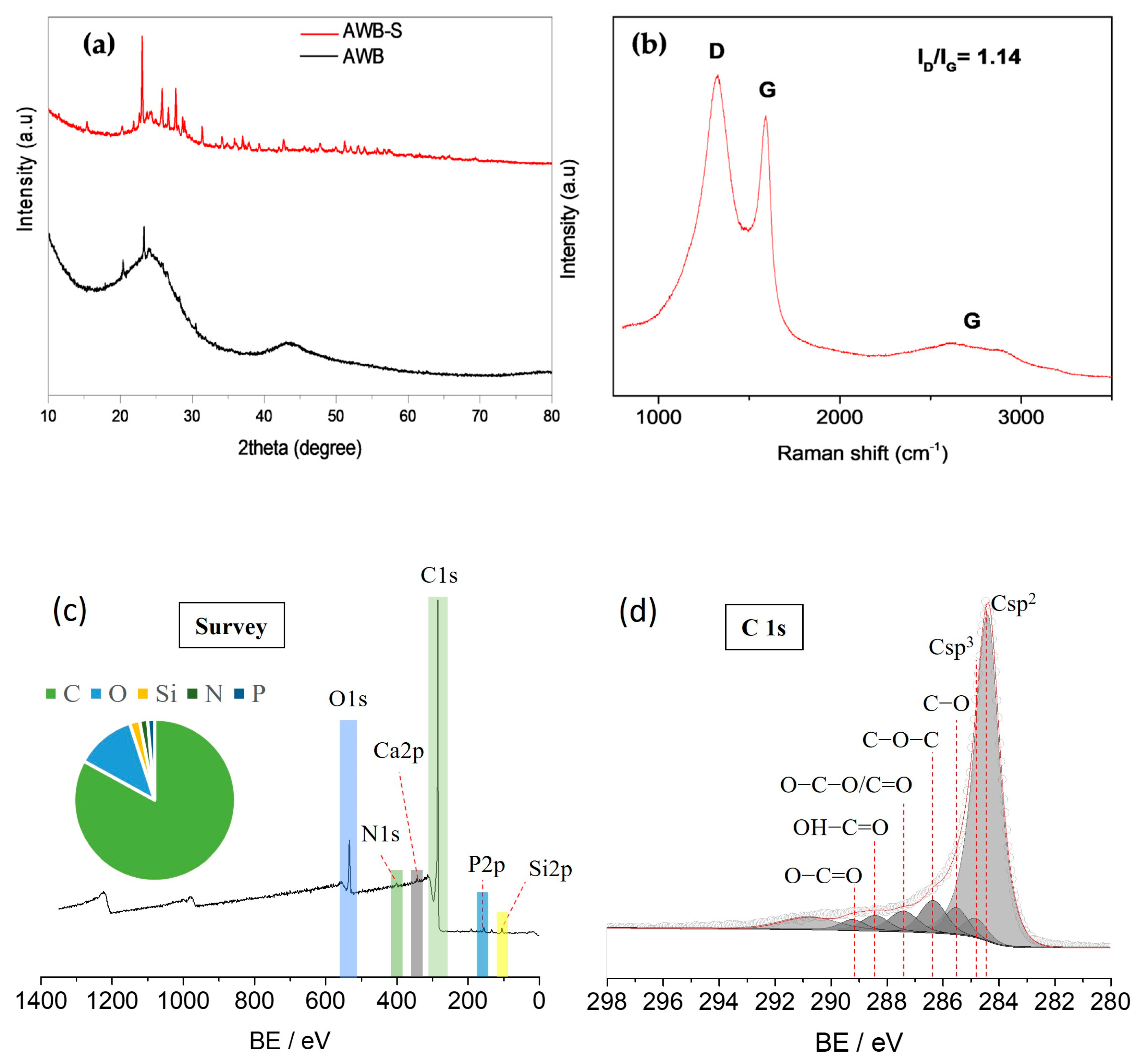

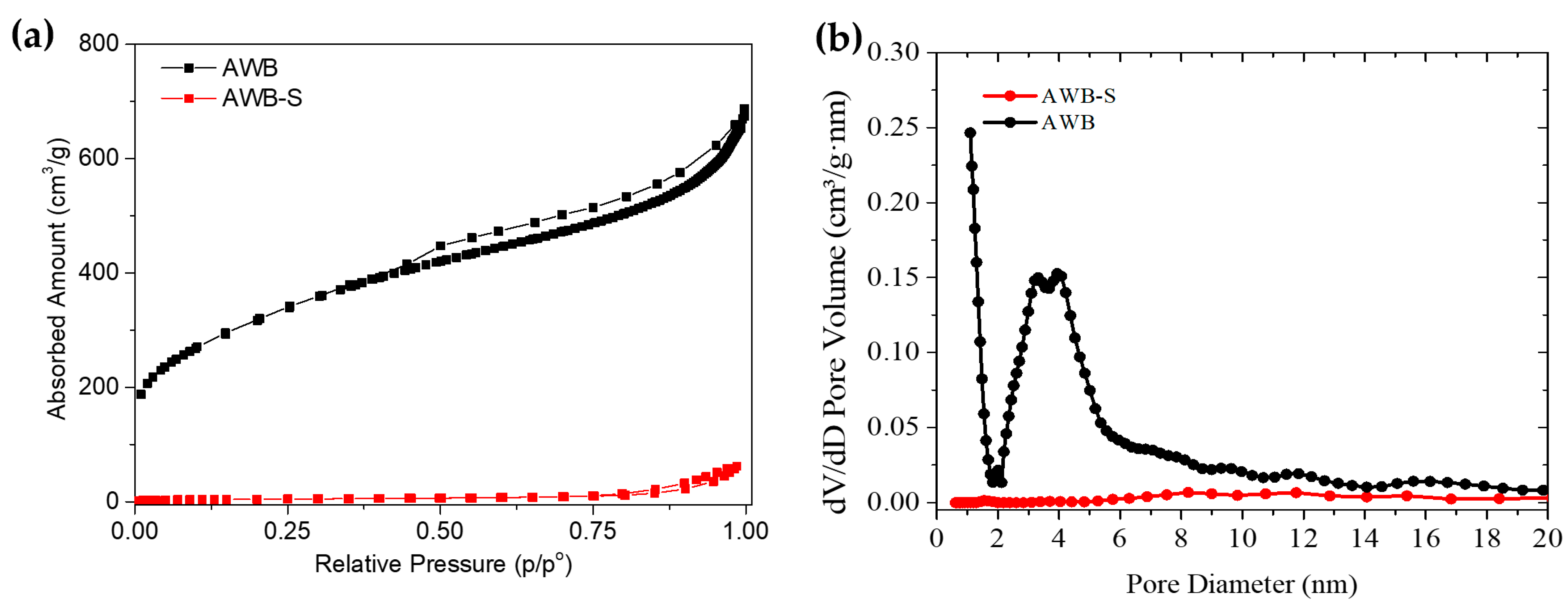
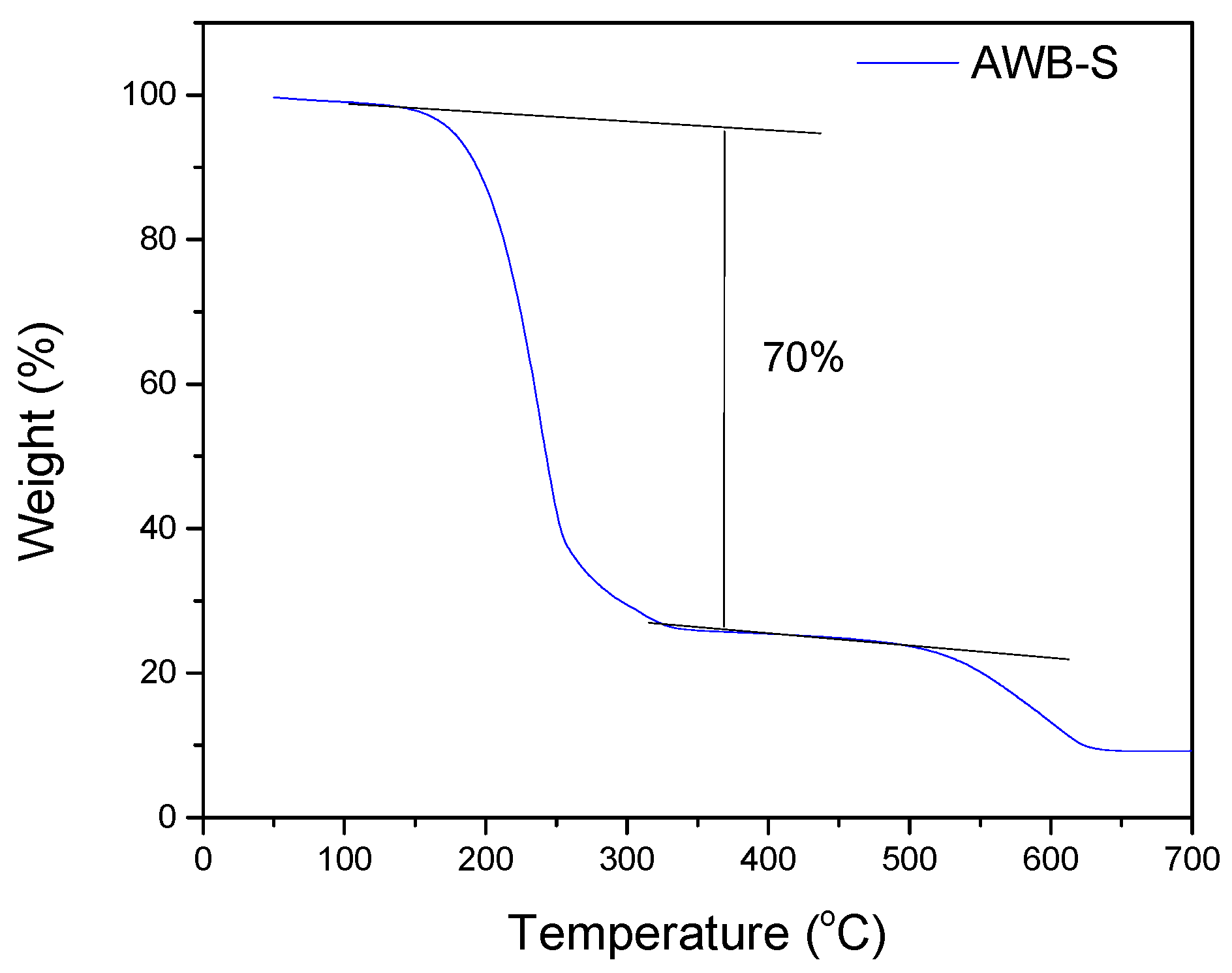
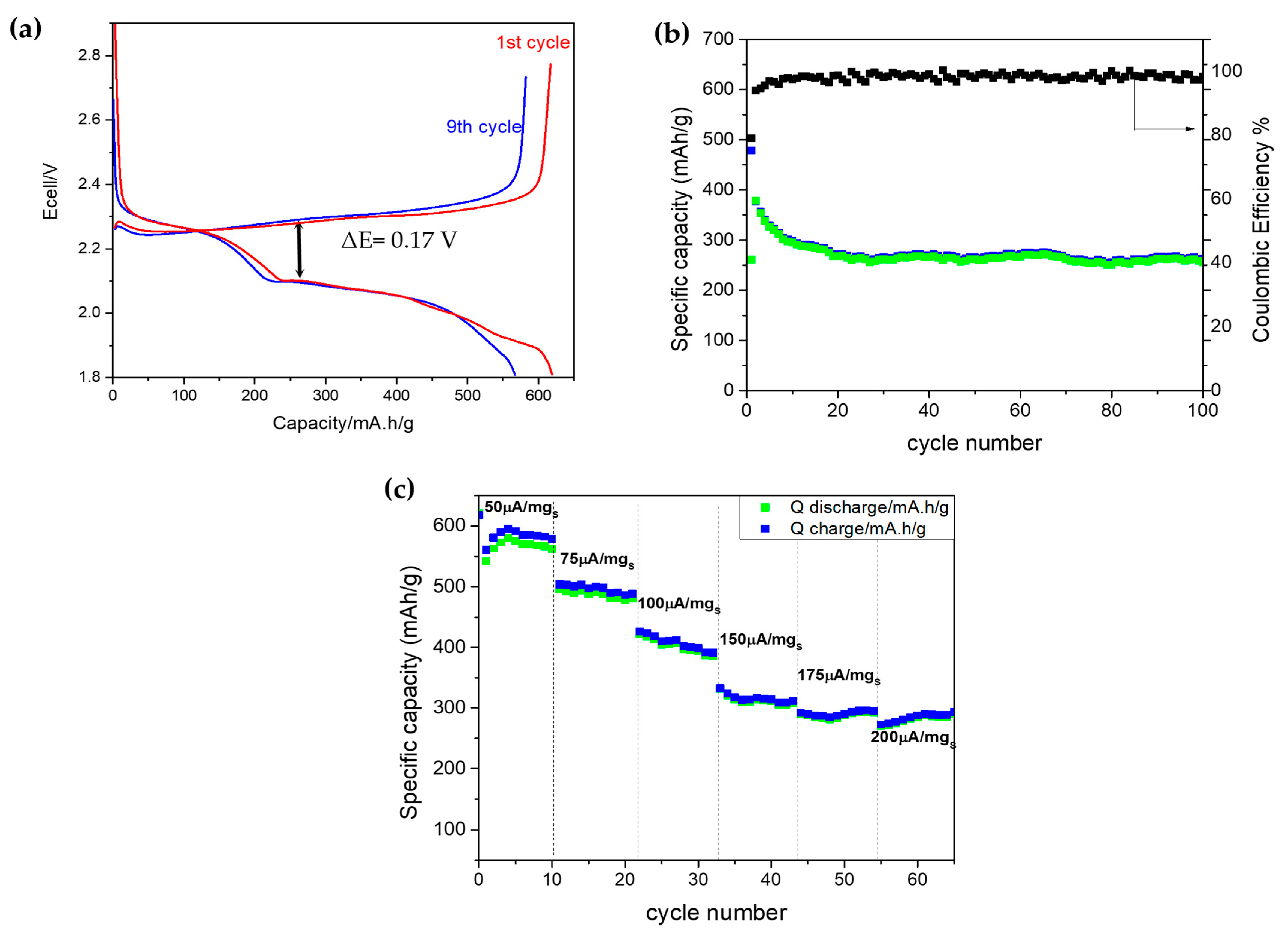
| Sample | SBET | Smeso | Smicro | VT P | Vmeso | Vmicro |
|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | |||||
| AWB | 959.00 | 521.00 | 438.00 | 0.907 | 0.703 | 0.204 |
| AWB-S | 14.00 | 13.40 | 0.60 | 0.082 | 0.082 | 0.001 |
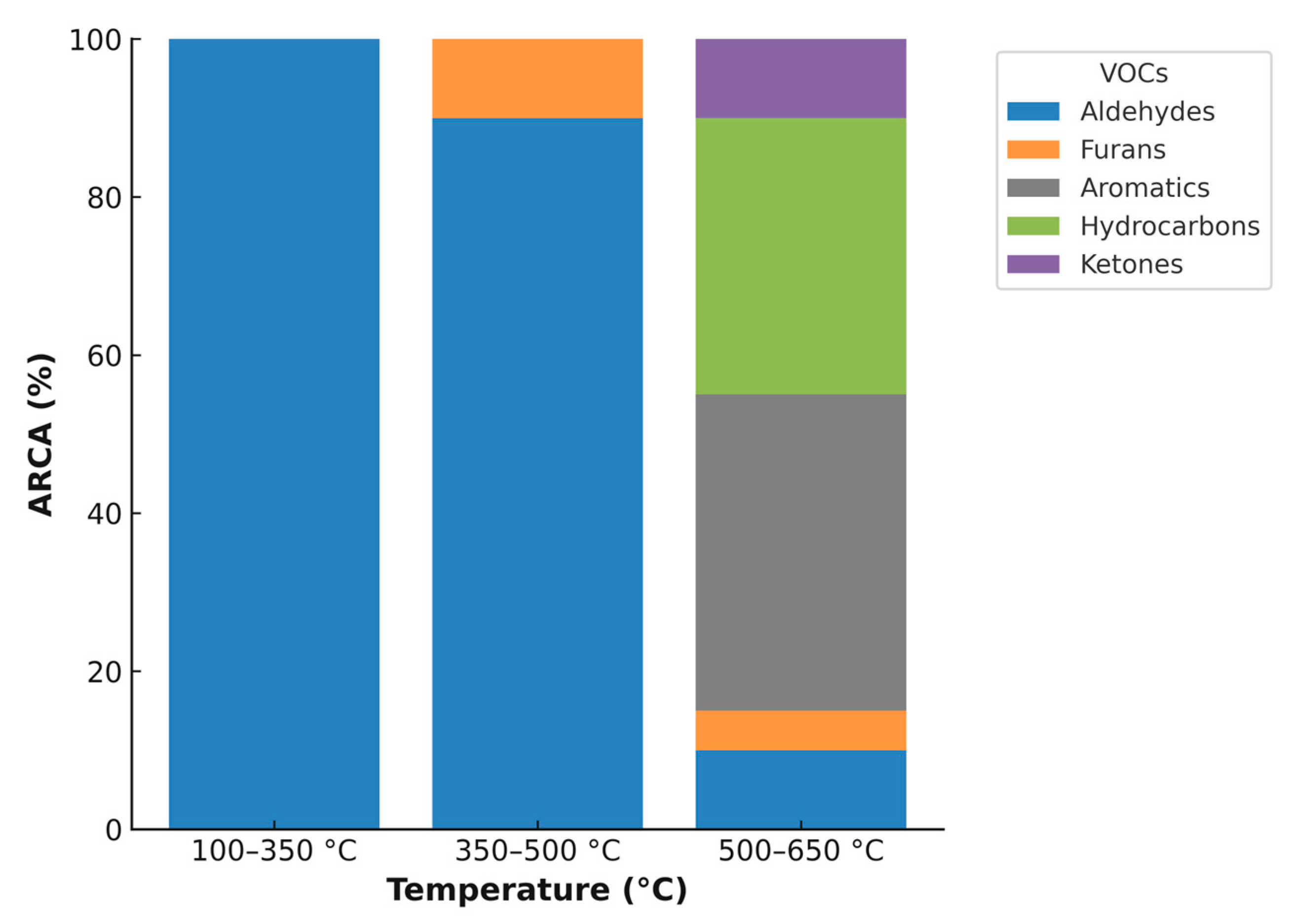
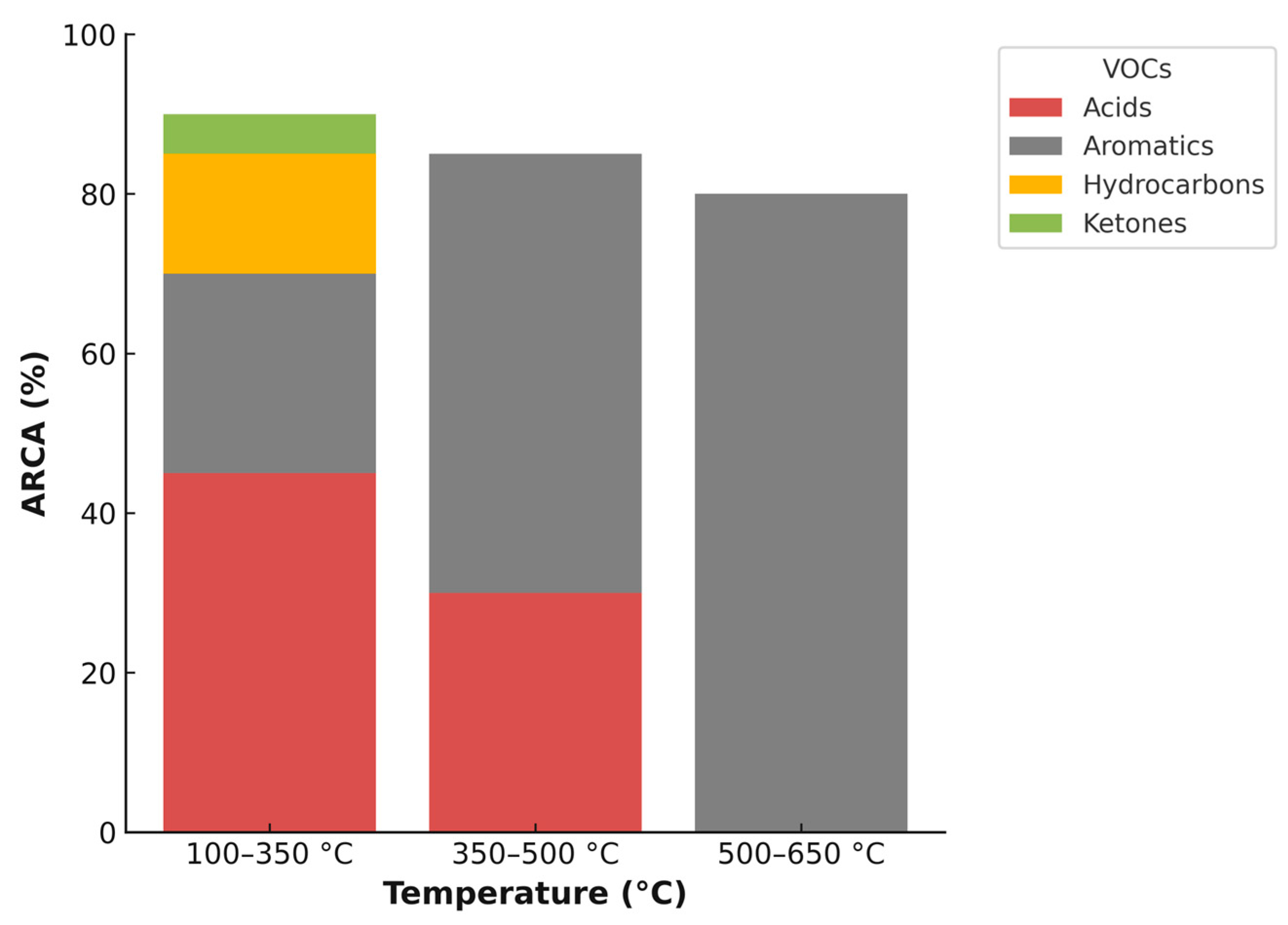
| Temperature (°C) | VOC Identified Without Activation/ARCA | VOC Identified with Activation/ARCA |
|---|---|---|
| 100–350 | Hexanal/95% | Acetic acid/45.08% Benzene 1,2-dimethyl/13.76% Benzene, 1-ethyl-3-methyl/4.46% Benzene, methyl/3.67% Benzene, ethyl/1.68% 1-heptene 2,4-dimethyl/15.02% 2-Pentanone, 4-hydroxyl-4-methyl/4.93% |
| 350–500 | Hexana/91.04% Furan, 2-methyl/8.96% | Acetic acid/29.25% Benzene, methyl/23.6% Benzene 1,2-dimethyl/19.09% Benzene, ethyl/12.33% |
| 500–650 | Hexanal/19.2% Benzene, methyl/27.8% Benzene 1,2-dimethy/l5.68% Benzene/4.91% Benzene, ethyl/3.83% Furan, 2-methyl/3.95% Furan, 2,5-dimethyl/2.19% Octane/6.36% 1,3,5,7-Cyclooctatetraene/6.03% Heptane/4.19% Nonane/3.19% 1-Octene/3.17% 1-Heptene/2.66% 2,3-Dimethyl-2-cyclopenten-1-one/3.91% | Benzene, methyl/29.08% Benzene, ethyl/19.55% Benzene 1,2-dimethyl/19.18% Benzene/11.8% Benzene, 1-ethyl-3-methyl/4.38% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre, F.; Luque, G.; Imwinkelried, G.; Cometto, F.; Saux, C.; Teruel, M.; Blanco, M.B. From Thermal Conversion to Cathode Performance: Acid-Activated Walnut Shell Biochar in Li–S Batteries and Its Impact on Air Quality. Thermo 2025, 5, 34. https://doi.org/10.3390/thermo5030034
Aguirre F, Luque G, Imwinkelried G, Cometto F, Saux C, Teruel M, Blanco MB. From Thermal Conversion to Cathode Performance: Acid-Activated Walnut Shell Biochar in Li–S Batteries and Its Impact on Air Quality. Thermo. 2025; 5(3):34. https://doi.org/10.3390/thermo5030034
Chicago/Turabian StyleAguirre, Fabricio, Guillermina Luque, Gabriel Imwinkelried, Fernando Cometto, Clara Saux, Mariano Teruel, and María Belén Blanco. 2025. "From Thermal Conversion to Cathode Performance: Acid-Activated Walnut Shell Biochar in Li–S Batteries and Its Impact on Air Quality" Thermo 5, no. 3: 34. https://doi.org/10.3390/thermo5030034
APA StyleAguirre, F., Luque, G., Imwinkelried, G., Cometto, F., Saux, C., Teruel, M., & Blanco, M. B. (2025). From Thermal Conversion to Cathode Performance: Acid-Activated Walnut Shell Biochar in Li–S Batteries and Its Impact on Air Quality. Thermo, 5(3), 34. https://doi.org/10.3390/thermo5030034








