Fluorescence Behavior of Fluorenone Derivative in Chlorinated Hydrocarbons: Verification of Solvent Proticity via Fluorescence Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stoye, D. Solvents. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 619–688. [Google Scholar]
- Wiezevich, P.J.; Vesterdal, H.G. Halogenated Hydrocarbon Solvents. Chem. Rev. 1936, 19, 101–117. [Google Scholar] [CrossRef]
- Llewellyn, O.P. Halogenated Hydrocarbons Used as Solvents. Ann. Occup. Hyg. 1966, 9, 199–208. [Google Scholar] [CrossRef]
- Durov, V.A.; Tereshin, O.G. Mixtures of halogenated hydrocarbons–organic solvent: Molecular interactions, structure and physicochemical properties. Fluid Phase Equilib. 2003, 210, 91–104. [Google Scholar] [CrossRef]
- Seppäläinen, A.M. Halogenated hydrocarbons. In Neurotoxicology; Blum, K., Manzo, L., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1985; pp. 459–472. [Google Scholar]
- Scharlin, P. Application of Acidity Function Method to the Determination of pKa Values of Trihalomethanes. I. Trichloromethane. Acta Chem. Scand. 1986, 40, 207–209. [Google Scholar] [CrossRef]
- Rossberg, M.; Lendle, W.; Pfleidere, G.; Tögel, A.; Drecher, E.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack, H.; Cook, R.; et al. Chlorinated Hydrocarbons. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006; pp. 1–184. [Google Scholar]
- Sherman, J.; Chin, B.; Huibers, P.D.; Garcia-Valls, R.; Hatton, T.A. Solvent replacement for green processing. Environ. Health Perspect. 1998, 106, 253–271. [Google Scholar]
- Durkee, J.B. Cleaning with Solvents. In Developments in Surface Contamination and Cleaning: Fundamentals and Applied Aspects; Kohli, R., Mittal, K.L., Eds.; William Andrew Publishing: Norwich, NY, USA, 2008; pp. 759–871. [Google Scholar]
- Troynikov, O.; Watson, C.; Jadhav, C.; Nawaz, N.; Kettlewell, R. Towards sustainable and safe apparel cleaning methods: A review. J. Environ. Manag. 2016, 182, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 1–694. [Google Scholar]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear Solvation Energy Relationship. 23. A comprehensive Collection of the Solvatochromic Parameters, π*, α, and β, and Some Methods for Simplifying the Generalized Solvatochromic Equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Kolling, O.W. Hydrogen Bond Donor Acidity Parameters for Kinetics Studies in Nonaqueous Solvents. J. Phys. Chem. 1992, 96, 1729–1733. [Google Scholar] [CrossRef]
- Thomas, J.R.; Gwinn, W.D. The Rotational Configuration and Dipole Moments of 1,1,2-Trichloroethane and 1,1,2,2-Tetrachloroethane. J. Am. Chem. Soc. 1949, 71, 2785–2790. [Google Scholar] [CrossRef]
- Smith, R.P.; Ree, T.; Magee, J.L.; Eyring, H. The Inductive Effect and Chemical Reactivity. I. General Theory of the Inductive Effect and Application to Electric Dipole Moments of Haloalkanes. J. Am. Chem. Soc. 1951, 73, 2263–2268. [Google Scholar] [CrossRef]
- Mark, J.E.; Sutton, C. Dipole Moments and Conformational Energies of the Chloroethanes. J. Am. Chem. Soc. 1972, 94, 1083–1090. [Google Scholar] [CrossRef]
- Lee, J.; Sakaguchi, T.; Kwak, G. Thermodynamic Fluorescence Behavior of a Fluorenone Derivative through Solvent- and Temperature-Controlled Hydrogen Bonding. ChemPhotoChem 2025, 9, e202400314. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Sakaguchi, T.; Kwak, G. Solvent-Dependent Fluorescence Behavior and Water Detection Sensor Application of Visible Light-Emitting Fluorenone Derivative. J. Fluoresc. 2025, 35, 437–443. [Google Scholar] [CrossRef] [PubMed]
- McGovern, E.W. Chlorohydrocarbon Solvents. Ind. Eng. Chem. 1943, 35, 1230–1239. [Google Scholar] [CrossRef]
- Liu, P.F.; Chen, W.T.; Kao, C.M. Chlorinated Solvents. In The Old and New Persistent Organic Pollutants; Naidu, R., Ed.; CRC Press: Boca Raton, FL, USA, 2025; pp. 163–181. [Google Scholar]
- Abraham, M.H.; Abraham, R.J.; Byrne, J.; Griffiths, L. NMR Method for the Determination of Solute Hydrogen Bond Acidity. J. Org. Chem. 2006, 71, 3389–3394. [Google Scholar] [CrossRef]
- Xu, J.; Takai, A.; Takeuchi, M. Multiple emissions from indenofluorenedione in solution and polymer films. RSC Adv. 2016, 6, 80867–80871. [Google Scholar] [CrossRef]
- Datta, S.; Kabir, M.P.; Maliha, S.R.; Liu, F.; Xu, J. Stimulus-Driven Tuning of Multipathway Emission in 9-Fluorenone Derivatives: Elucidating Charge Transfer Dynamics in Higher Excited Singlet and Triplet States. ACS Appl. Energy Mater. 2025, 8, 3973–3983. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wang, X.; Xu, L.; Dong, J. Hexafluoroisopropyl N-Fluorosulfonyl Carbamate: Synthesis and Its Facile Transformation to Sulfamoyl Ureas. Synthesis 2024, 56, 2136–2144. [Google Scholar] [CrossRef]
- Throup, A.; Zraikat, M.S.; Gordon, A.; Soumehsaraei, S.J.; Haase, K.D.; Patterson, L.H.; Cooper, P.A.; Hanlon, K.; Loadman, P.M.; Sutherland, M.; et al. Sidechain structure-activity relationships of cyclobutane-based small molecule αvβ3 antagonists. RSC Med. Chem. 2024, 15, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Leggio, A.; Belsito, E.L.; De Luca, G.; Di Gioia, M.L.; Leotta, V.; Romio, E.; Siciliano, C.; Liguori, A. One-pot synthesis of amides from carboxylic acids activated using thionyl chloride. RSC Adv. 2016, 6, 34468–34475. [Google Scholar] [CrossRef]
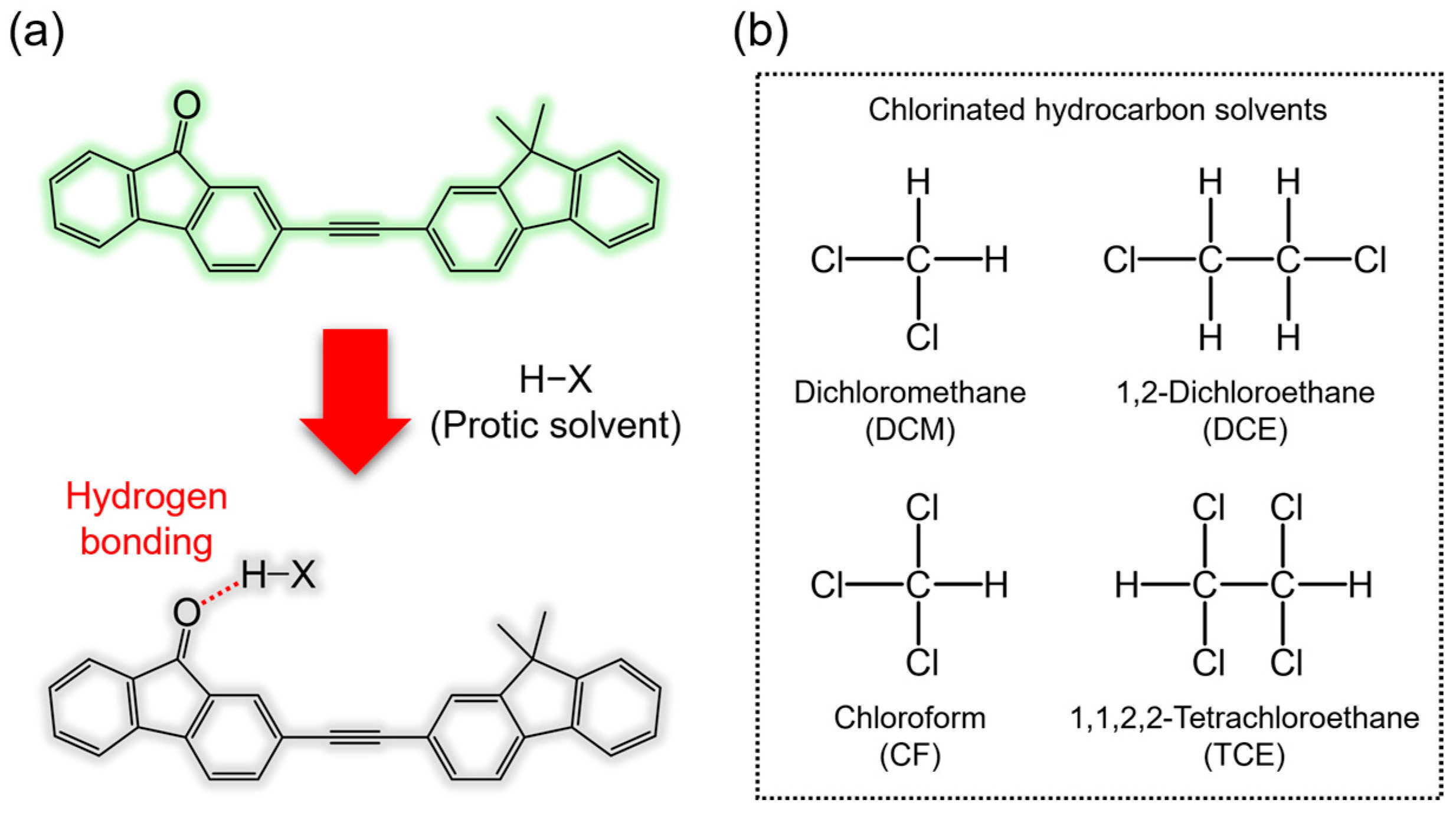
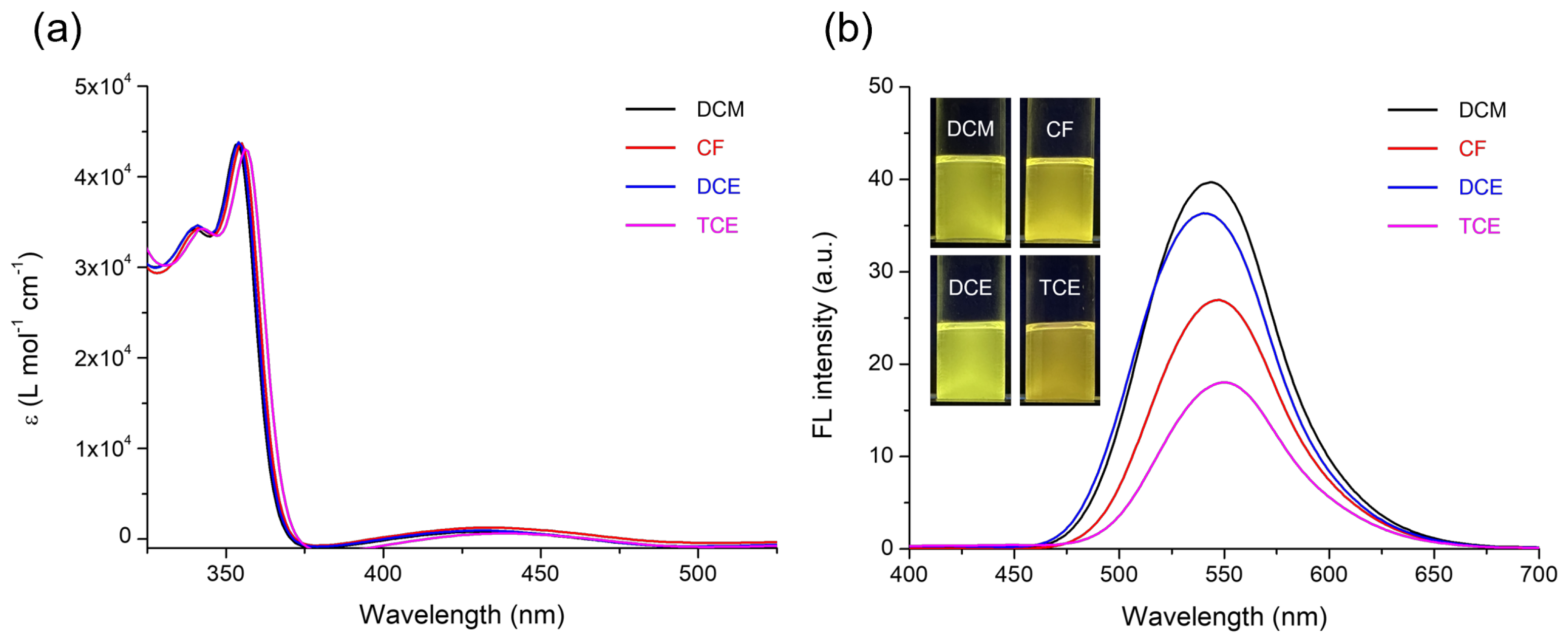
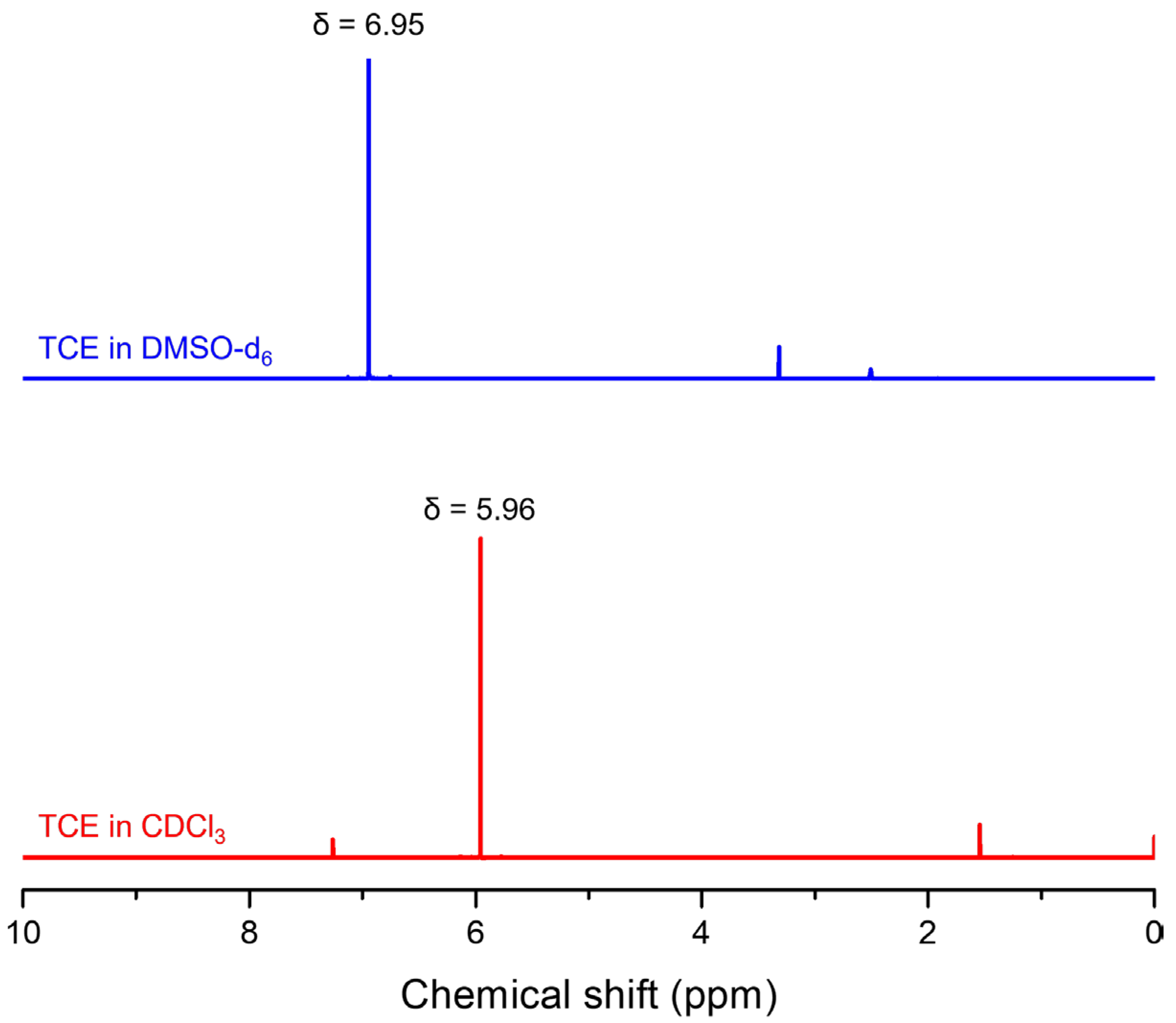
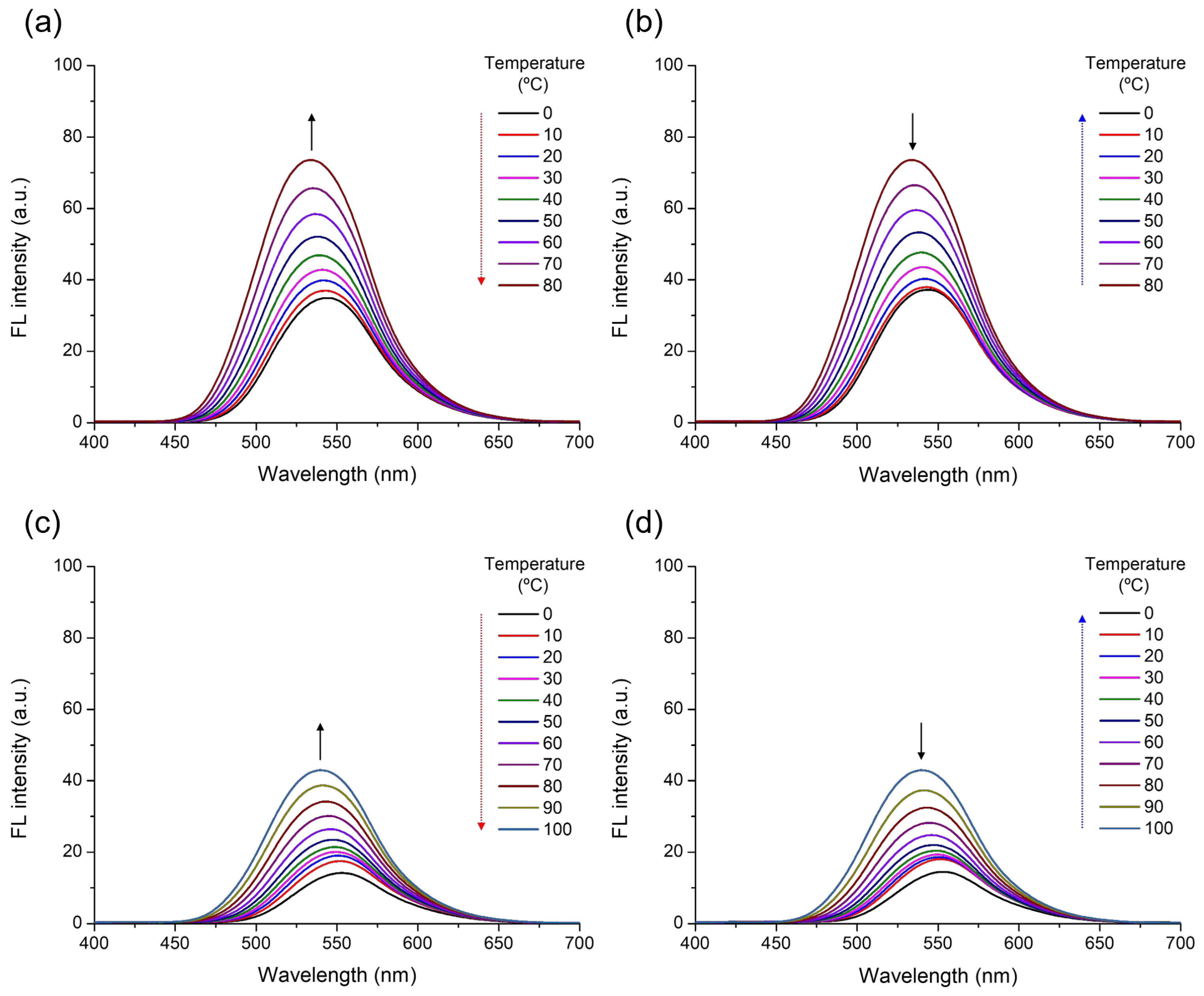
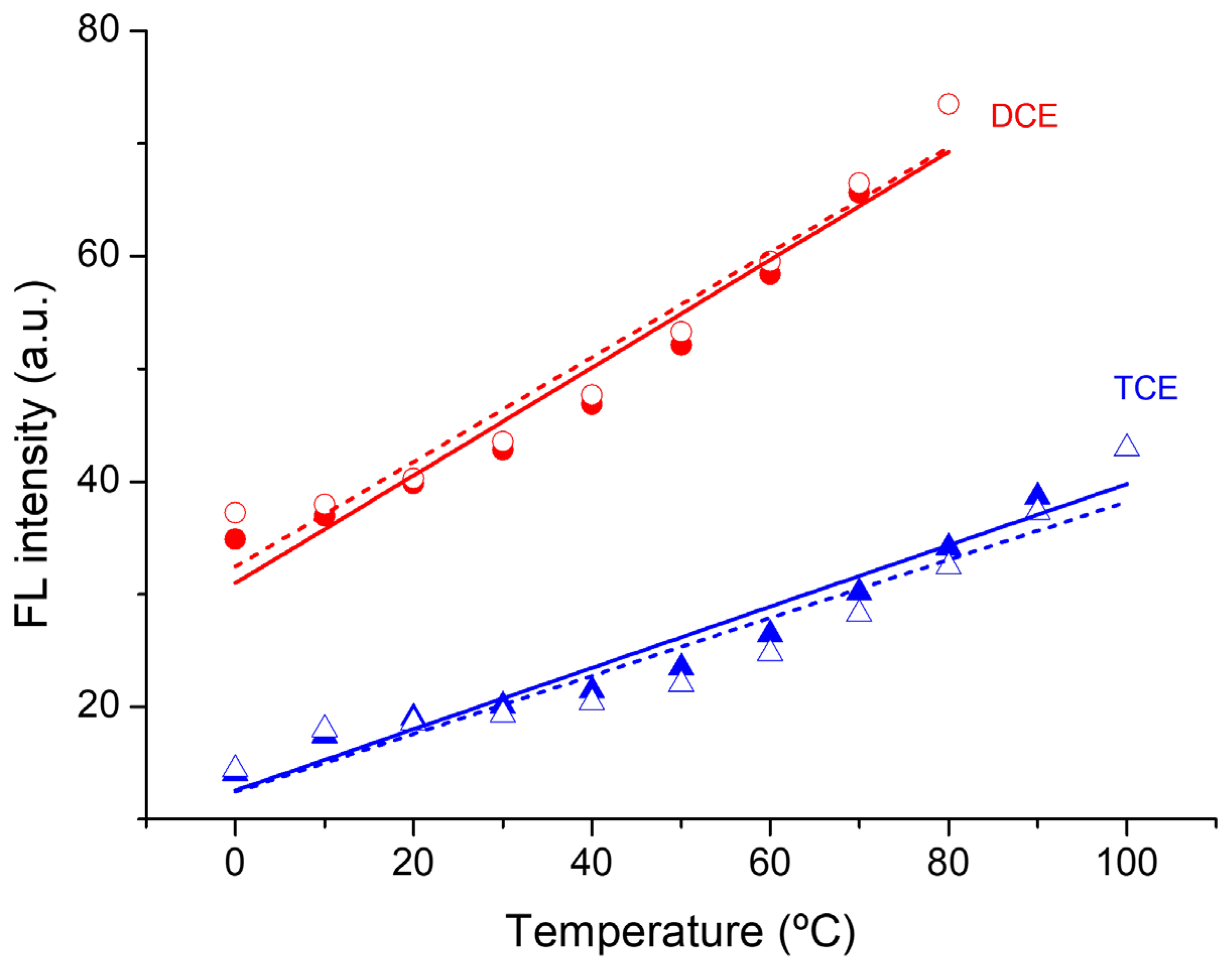
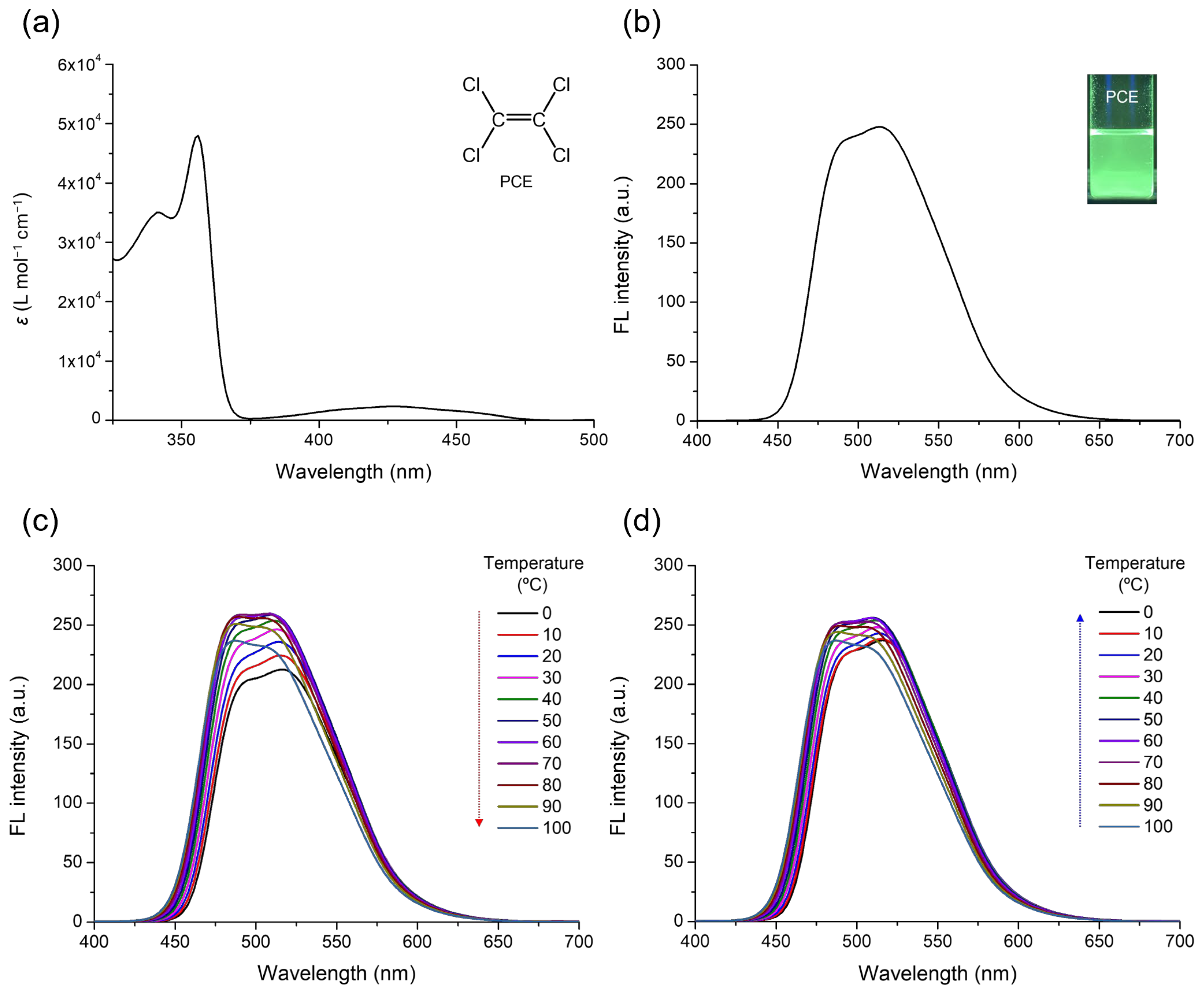
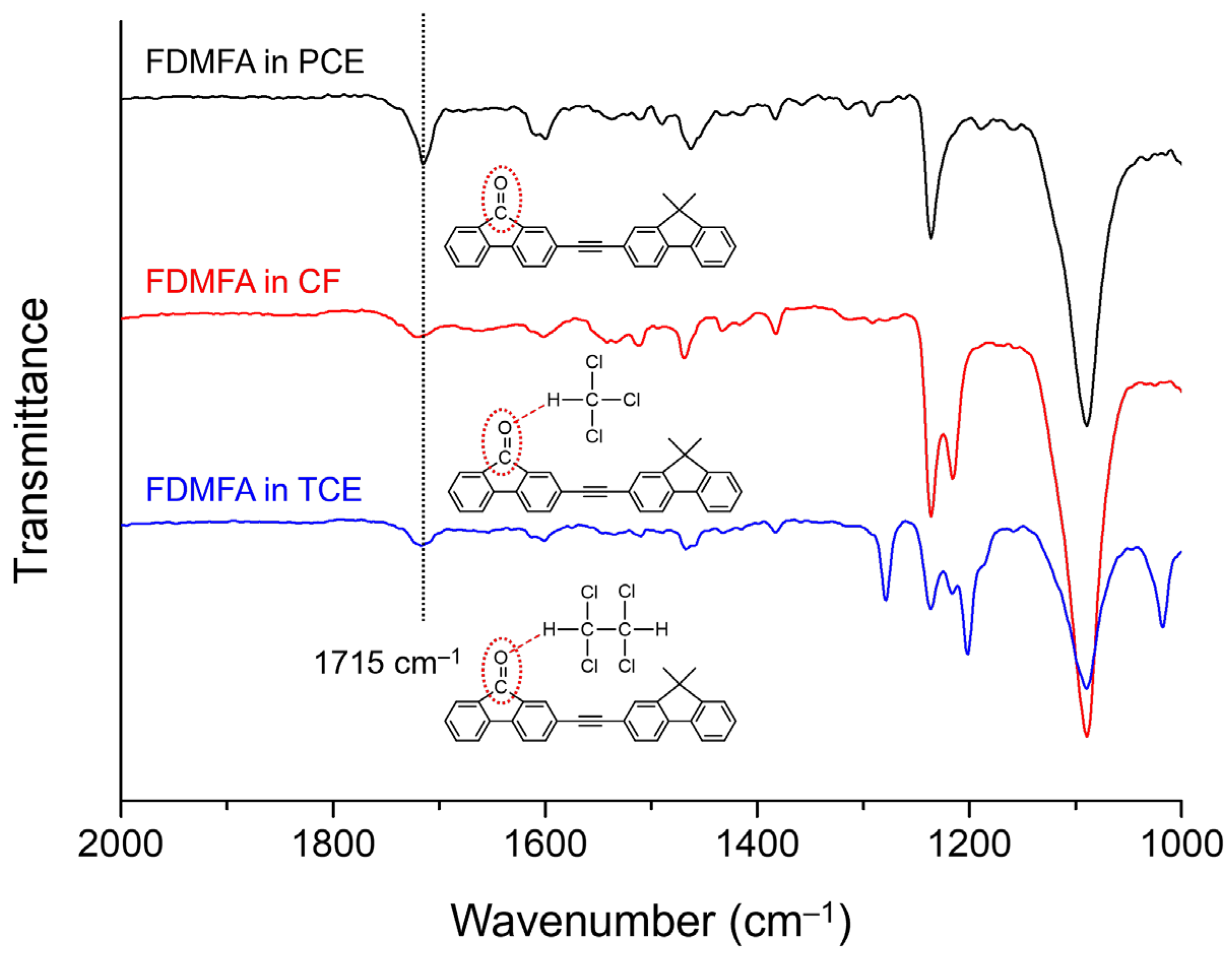
| Solvent | αA | β | π* | μ | ε (104 M−1·cm−1) | λabs, max (nm) | λFL, max (nm) | FLQE (%) |
|---|---|---|---|---|---|---|---|---|
| PCE | – | 0.00 | 0.28 | 0.00 | 4.55, 0.25 | 356, 428 | 492, 513 | 19.0 |
| DCM | 0.07 | 0.00 | 0.82 | 1.14 | 4.36, 0.08 | 354, 432 | 544 | 2.22 |
| CF | 0.15 | 0.00 | 0.58 | 1.15 | 4.37, 0.13 | 355, 433 | 547 | 1.61 |
| DCE | 0.03 | 0.00 | 0.81 | 1.83 | 4.38, 0.10 | 354, 430 | 540 | 2.17 |
| TCE | 0.14 | 0.00 | 0.95 | 1.31 | 4.30, 0.06 | 356, 438 | 550 | 1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Sakaguchi, T.; Kwak, G. Fluorescence Behavior of Fluorenone Derivative in Chlorinated Hydrocarbons: Verification of Solvent Proticity via Fluorescence Spectroscopy. Photochem 2025, 5, 37. https://doi.org/10.3390/photochem5040037
Lee J, Sakaguchi T, Kwak G. Fluorescence Behavior of Fluorenone Derivative in Chlorinated Hydrocarbons: Verification of Solvent Proticity via Fluorescence Spectroscopy. Photochem. 2025; 5(4):37. https://doi.org/10.3390/photochem5040037
Chicago/Turabian StyleLee, Jineun, Toshikazu Sakaguchi, and Giseop Kwak. 2025. "Fluorescence Behavior of Fluorenone Derivative in Chlorinated Hydrocarbons: Verification of Solvent Proticity via Fluorescence Spectroscopy" Photochem 5, no. 4: 37. https://doi.org/10.3390/photochem5040037
APA StyleLee, J., Sakaguchi, T., & Kwak, G. (2025). Fluorescence Behavior of Fluorenone Derivative in Chlorinated Hydrocarbons: Verification of Solvent Proticity via Fluorescence Spectroscopy. Photochem, 5(4), 37. https://doi.org/10.3390/photochem5040037






