Distinct Piezochromic Properties of Cyanostilbene- and Cyanostyrene-Based Donor–Acceptor–Donor- and Donor–Acceptor-Structured Organic Luminogens
Abstract
1. Introduction
2. Materials and Methods
3. Results
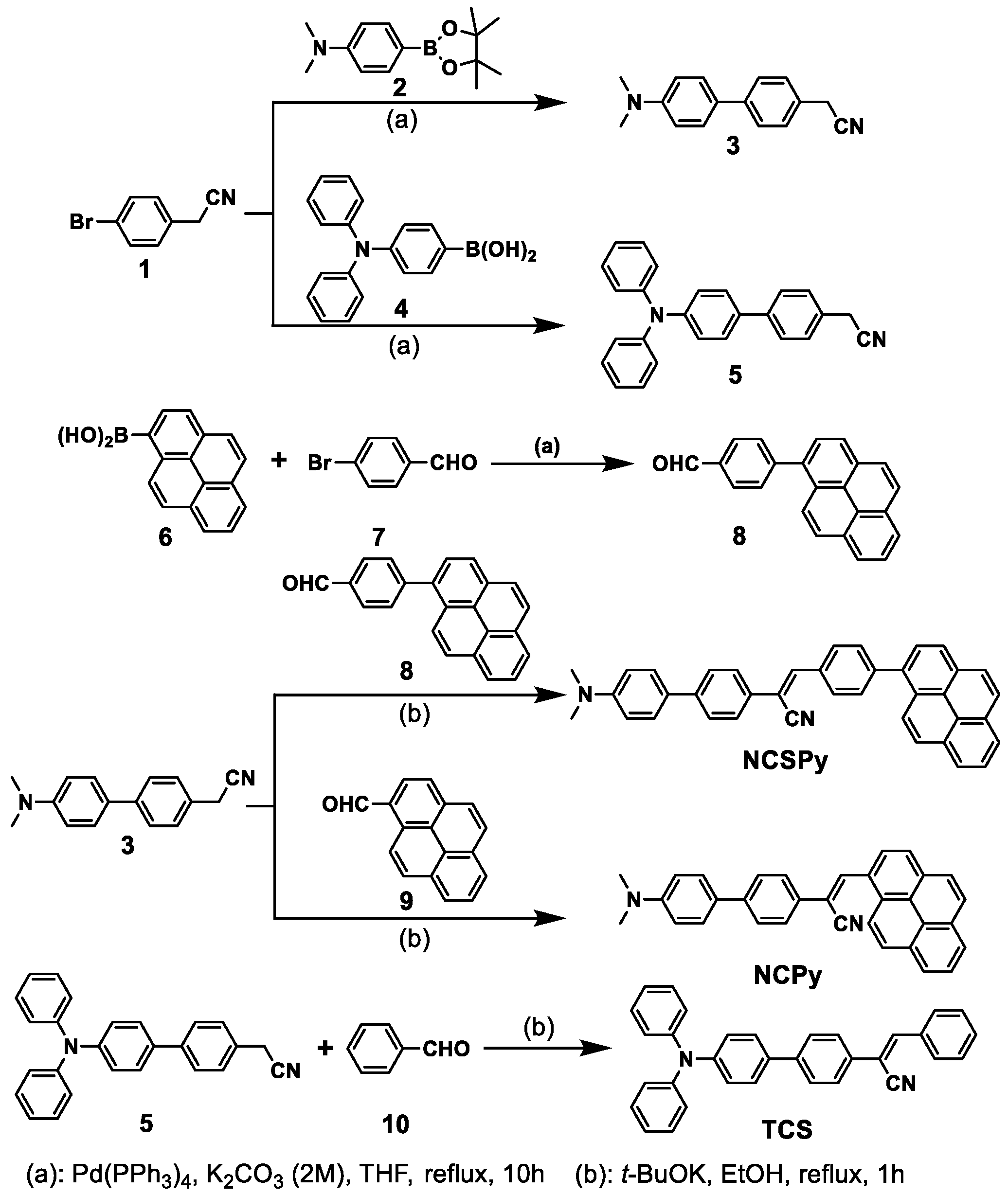
3.1. Synthetic Route to the Target Compounds
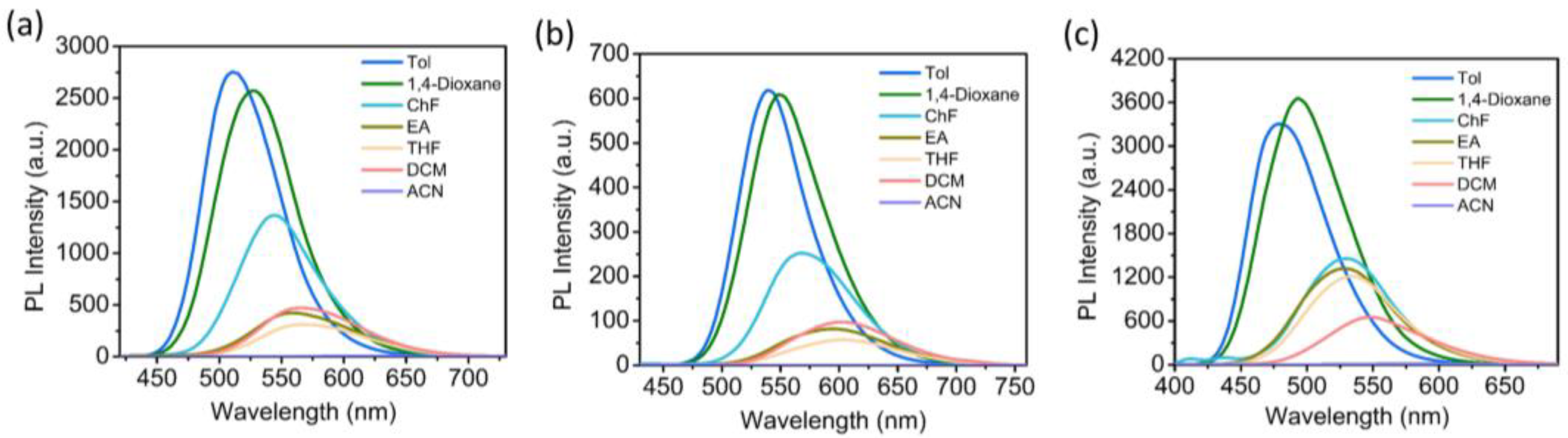
3.2. Photophysical Properties in Diluted Solution and the Aggregated State
3.3. Solvatochromic and Solvatokinetic Effects
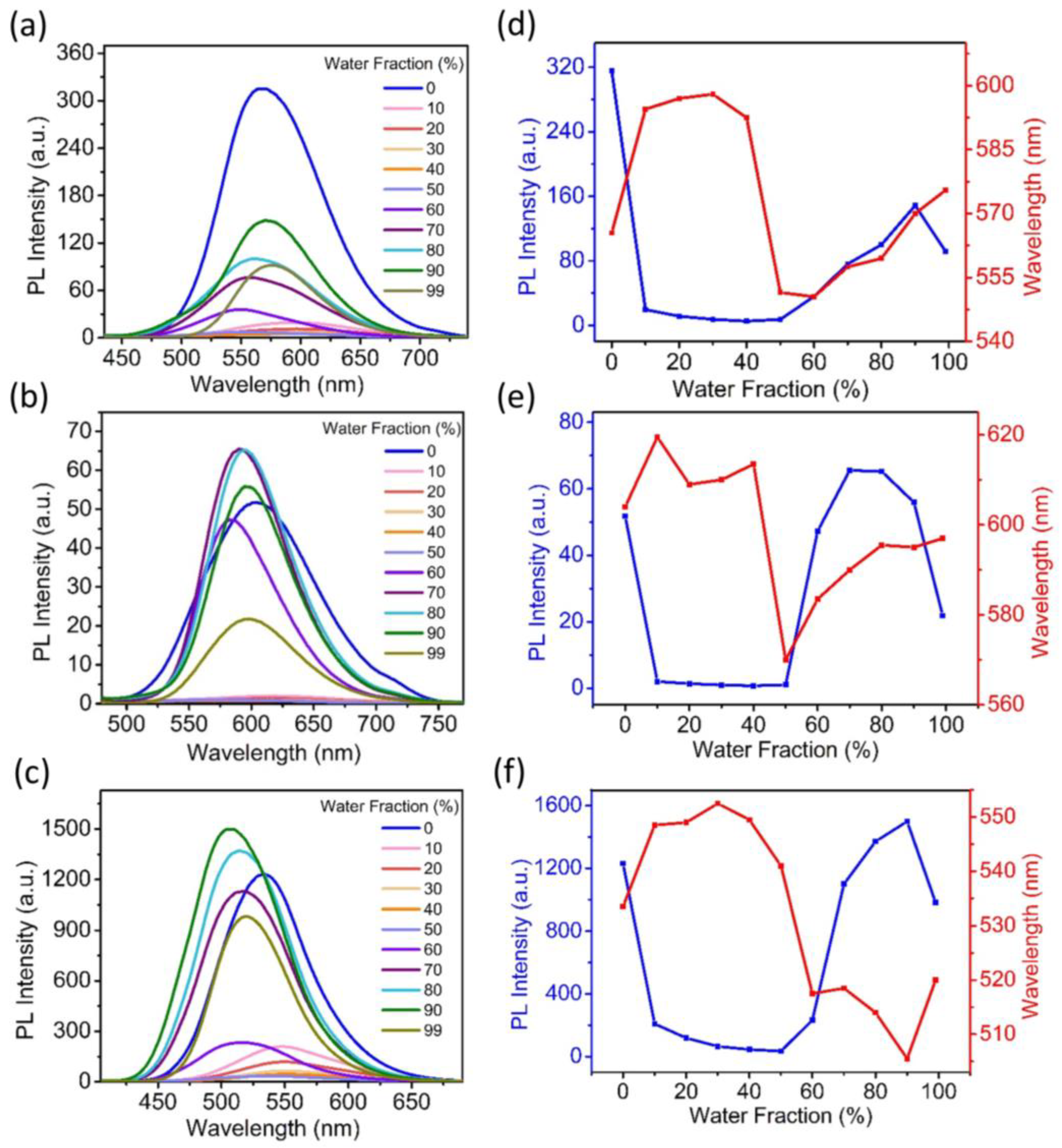
3.4. Aggregation-Induced Emission Properties
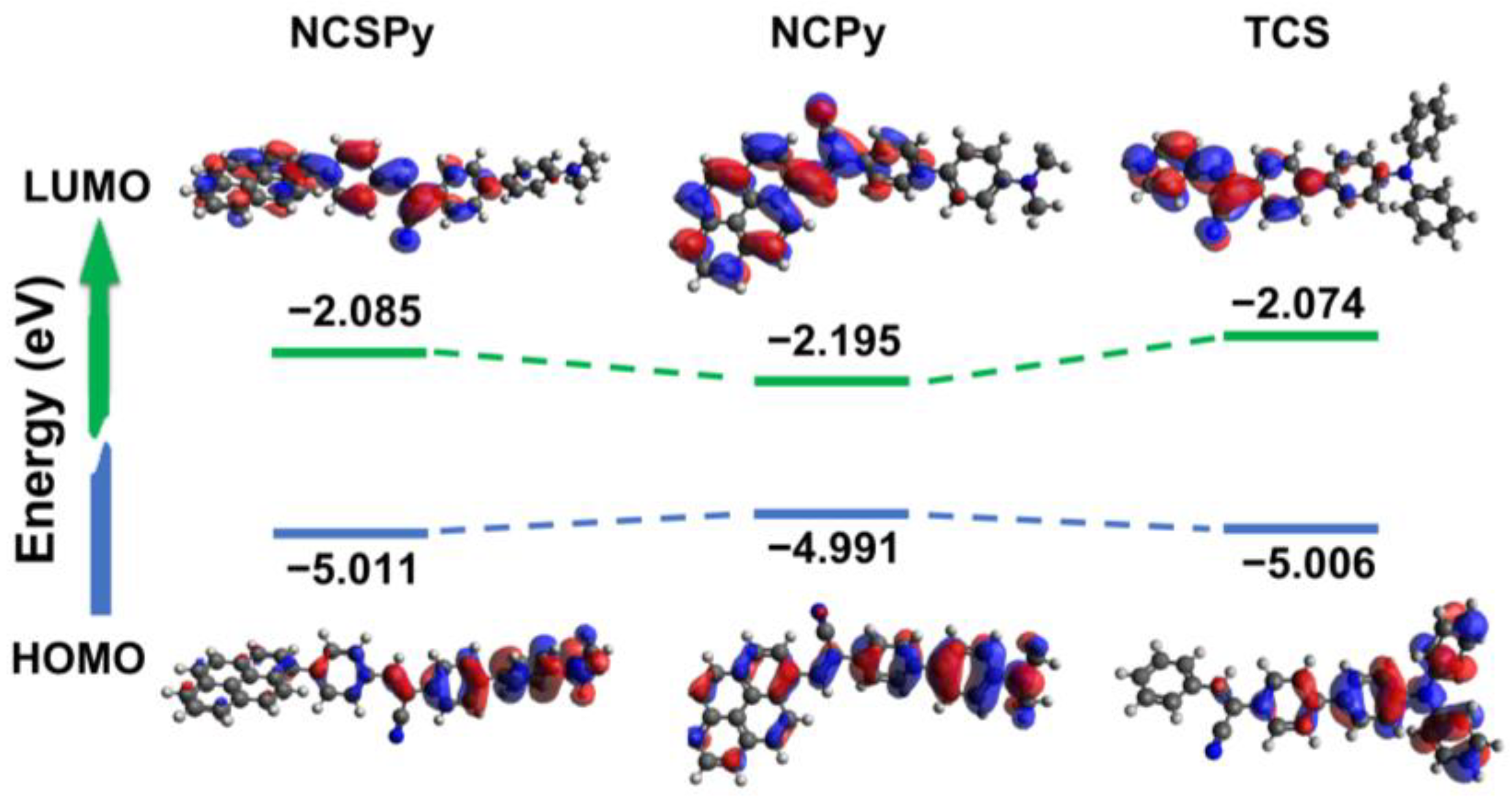
3.5. Theoretical Calculations
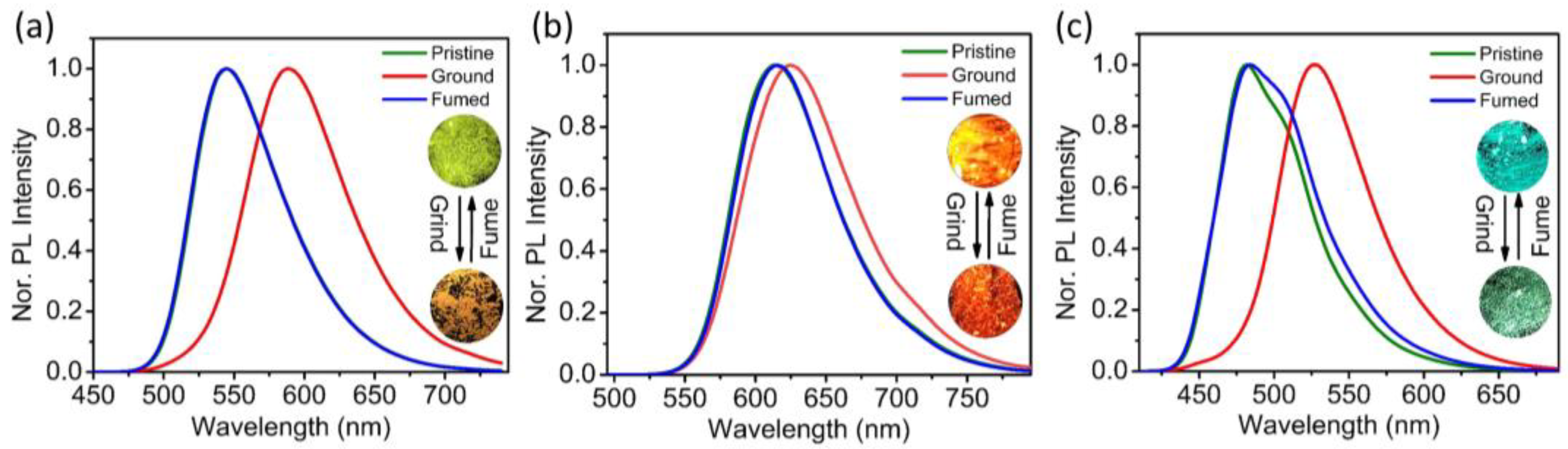
3.6. Piezochromic Properties upon Mechanical Grinding
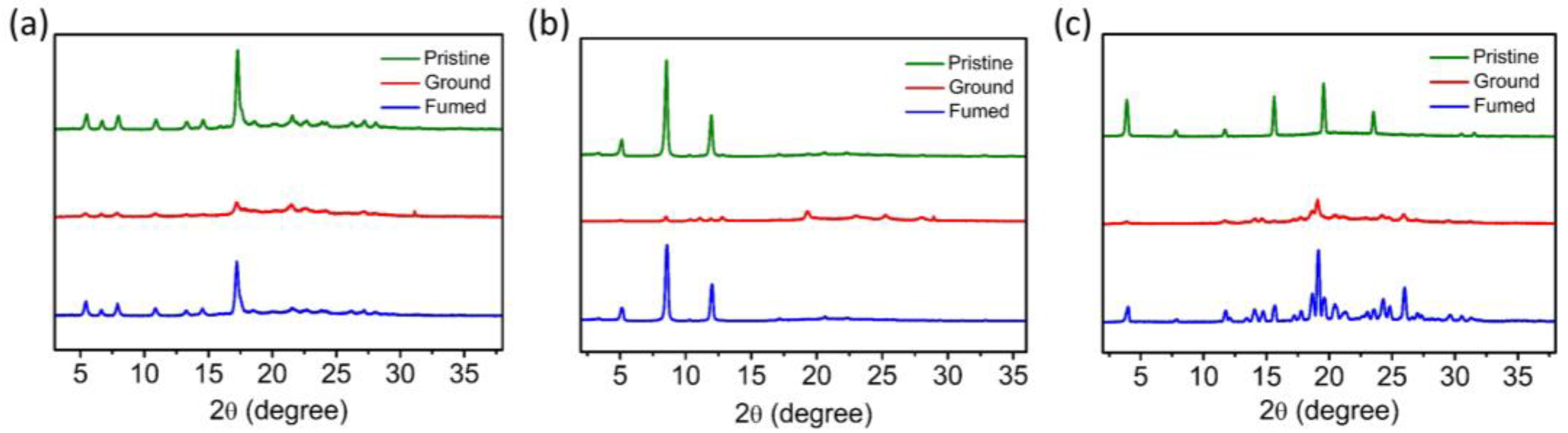
3.7. Mechanical Grinding-Induced Crystallinity Change
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Wang, H.; Ji, X.; Page, Z.A.; Sessler, J.L. Fluorescent materials-based information storage. Mater. Chem. Front. 2020, 4, 1024–1039. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Hu, W.; Stoddart, J.F. Color-Tunable Supramolecular Luminescent Materials. Adv. Mater. 2022, 34, 2105405. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Chong, K.C.; Liu, B. Reactivity-Based Organic Theranostic Bioprobes. Acc. Chem. Res. 2019, 52, 3051–3063. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, J.; Wang, D.; Liu, Z.; Han, G.; Liu, B.; Han, M.; Zhang, R.; Liu, G.; Zhang, Z. Real-time quantification of nuclear RNA export using an intracellular relocation probe. Chin. Chem. Lett. 2022, 33, 3865–3868. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef]
- Chung, K.; Kwon, M.S.; Leung, B.M.; Wong-Foy, A.G.; Kim, M.S.; Kim, J.; Takayama, S.; Gierschner, J.; Matzger, A.J.; Kim, J. Shear-Triggered Crystallization and Light Emission of a Thermally Stable Organic Supercooled Liquid. ACS Cent. Sci. 2015, 1, 94–102. [Google Scholar] [CrossRef]
- Li, A.; Xu, S.; Bi, C.; Geng, Y.; Cui, H.; Xu, W. Piezochromic mechanism of organic crystals under hydrostatic pressure. Mater. Chem. Front. 2021, 5, 2588–2606. [Google Scholar] [CrossRef]
- Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. Distinct Responses to Mechanical Grinding and Hydrostatic Pressure in Luminescent Chromism of Tetrathiazolylthiophene. J. Am. Chem. Soc. 2013, 135, 10322–10325. [Google Scholar] [CrossRef]
- Gong, Y.-B.; Zhang, P.; Gu, Y.-R.; Wang, J.-Q.; Han, M.-M.; Chen, C.; Zhan, X.-J.; Xie, Z.-L.; Zou, B.; Peng, Q.; et al. The Influence of Molecular Packing on the Emissive Behavior of Pyrene Derivatives: Mechanoluminescence and Mechanochromism. Adv. Opt. Mater. 2018, 6, 1800198. [Google Scholar] [CrossRef]
- Yang, J.; Fang, M.; Li, Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Meng, X.; Qi, G.; Li, X.; Wang, Z.; Wang, K.; Zou, B.; Ma, Y. Spiropyran-based multi-colored switching tuned by pressure and mechanical grinding. J. Mater. Chem. C 2016, 4, 7584–7588. [Google Scholar] [CrossRef]
- Yang, Z.; Chi, Z.; Mao, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Aldred, M.P.; Chi, Z. Recent advances in mechano-responsive luminescence of tetraphenylethylene derivatives with aggregation-induced emission properties. Mater. Chem. Front. 2018, 2, 861–890. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Gayathri, P.; Pannipara, M.; Al-Sehemi, A.G.; Anthony, S.P. Triphenylamine-based stimuli-responsive solid state fluorescent materials. New J. Chem. 2020, 44, 8680–8696. [Google Scholar] [CrossRef]
- Lee, J.; Yuk, S.; Namgoong, J.; Kim, J. Mechanofluorochromism of Triphenylamine-BODIPY: Effect of twisted intramolecular charge transfer and restriction in rotation on fluorescence. Dyes Pigments 2021, 185, 108864. [Google Scholar] [CrossRef]
- Fu, S.; Jia, H.; Meng, X.; Wang, C.; Li, Q.; Li, L.; Yang, J.; Niu, H. Fine-tuning the molecular conformation and packing structures of coumarin-based luminogens to achieve distinct piezochromic properties upon mechanical grinding and under hydrostatic pressures. Mater. Horiz. 2025, 12, 293–302. [Google Scholar] [CrossRef]
- Traven, V.F.; Cheptsov, D.A.; Svetlova, J.I.; Ivanov, I.V.; Cuerva, C.; Lodeiro, C.; Duarte, F.; Dunaev, S.F.; Chernyshev, V.V. The role of the intermolecular π···π interactions in the luminescence behavior of novel coumarin-based pyrazoline materials. Dyes Pigments 2021, 186, 108942. [Google Scholar] [CrossRef]
- Yang, M.; Guo, J.; Fu, S.; Zhang, Y.; Yang, L.; Li, Q.; Bao, Y.; Wang, C. Packing structures and piezochromic properties engineering through F functionalization in 4-position substituted coumarin based organic luminogens. Dyes Pigments 2025, 243, 113055. [Google Scholar] [CrossRef]
- An, B.-K.; Kwon, S.-K.; Jung, S.-D.; Park, S.Y. Enhanced Emission and Its Switching in Fluorescent Organic Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y. Cyanostilbene-based intelligent organic optoelectronic materials. J. Mater. Chem. C 2013, 1, 1059–1065. [Google Scholar] [CrossRef]
- Martínez-Abadía, M.; Giménez, R.; Ros, M.B. Self-Assembled α-Cyanostilbenes for Advanced Functional Materials. Adv. Mater. 2018, 30, 1704161. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Vázquez, J.L.; Amador-Sánchez, Y.A.; Rodríguez-Cortés, L.A.; Rodríguez-Molina, B. Dual-State Emission (DSE) in Organic Fluorophores: Design and Applications. Chem. Mater. 2021, 33, 7160–7184. [Google Scholar] [CrossRef]
- Qiu, Q.; Xu, P.; Zhu, Y.; Yu, J.; Wei, M.; Xi, W.; Feng, H.; Chen, J.; Qian, Z. Rational Design of Dual-State Emission Luminogens with Solvatochromism by Combining a Partially Shared Donor–Acceptor Pattern and Twisted Structures. Chem. Eur. J. 2019, 25, 15983–15987. [Google Scholar] [CrossRef] [PubMed]
- König, N.F.; Mutruc, D.; Hecht, S. Accelerated Discovery of α-Cyanodiarylethene Photoswitches. J. Am. Chem. Soc. 2021, 143, 9162–9168. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, F.; He, F.; Kong, L.; Huang, J.; Yang, J.; Ding, A. Pyrene and triphenylamine substituted cyanostyrene and cyanostilbene derivatives with dual-state emission for high-contrast mechanofluorochromism and cell imaging. Org. Chem. Front. 2022, 9, 5118–5124. [Google Scholar] [CrossRef]
- Shen, H.; Fu, X.; Wang, Y.; Shi, Q.; Xu, X.; Li, L.; Li, Q.; Yang, J.; Wang, C. Molecular conformation and cyano regio-isomerization effects to the piezochromic properties of cyanostilbene-bridged donor-acceptor-donor structured organic luminogens. J. Mol. Struct. 2025, 1327, 141198. [Google Scholar] [CrossRef]
- Ouyang, M.; Zhuo, C.; Cao, F.; Pan, G.; Lv, C.; Yang, S.; Li, C.; Zhang, C.; Sun, J.; Zhang, Y. Organogelator based on long alkyl chain attached excimer precursor: Two channels of TICT, highly efficient and switchable luminescence. Dyes Pigments 2020, 180, 108433. [Google Scholar] [CrossRef]
- Mayurachayakul, P.; Thianchai, J.; Attakul, N.; Pratumyot, K.; Sukwattanasinitt, M.; Srikittiwanna, K.; Niamnont, N. Novel pyrenylbenzylidene-malononitrile derivative mixed with cellulose acetate electrospun nanofibrous sheets for hydrazine detection. New J. Chem. 2024, 48, 16095–16106. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chi, W.; Qiao, Q.; Tan, D.; Xu, Z.; Liu, X. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: From mechanisms to rational designs of bright and sensitive fluorophores. Chem. Soc. Rev. 2021, 50, 12656–12678. [Google Scholar] [CrossRef] [PubMed]


| Compounds | λmax (Tol, nm) | λPL (Tol, nm) | λPL (Aggregated, nm) | Φ (Tol, nm) | Φ (THF, nm) |
|---|---|---|---|---|---|
| NCSPy | 397 | 511 | 544 | 0.34 | 0.10 |
| NCPy | 406 | 539 | 614 | 0.10 | 0.04 |
| TCS | 380 | 479 | 482 | 0.48 | 0.26 |
| Compounds | HOMO (eV) | LUMO (eV) | Bandgap (eV) |
|---|---|---|---|
| NCSPy | −5.011 | −2.085 | 2.926 |
| NCPy | −4.991 | −2.195 | 2.796 |
| TCS | −5.006 | −2.074 | 2.932 |
| Compounds | λPL (Original, nm) | λPL (Ground, nm) | λPL (Vapor Fumed, nm) |
|---|---|---|---|
| NCSPy | 545 | 589 | 544 |
| NCPy | 615 | 625 | 614 |
| TCS | 482 | 527 | 483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, M.; Chen, Y.; Yang, X.; Xie, B.; Duan, X.; Shen, H.; Wang, C. Distinct Piezochromic Properties of Cyanostilbene- and Cyanostyrene-Based Donor–Acceptor–Donor- and Donor–Acceptor-Structured Organic Luminogens. Photochem 2025, 5, 36. https://doi.org/10.3390/photochem5040036
Wang Z, Yang M, Chen Y, Yang X, Xie B, Duan X, Shen H, Wang C. Distinct Piezochromic Properties of Cyanostilbene- and Cyanostyrene-Based Donor–Acceptor–Donor- and Donor–Acceptor-Structured Organic Luminogens. Photochem. 2025; 5(4):36. https://doi.org/10.3390/photochem5040036
Chicago/Turabian StyleWang, Ziyang, Miao Yang, Yuxi Chen, Xinyue Yang, Bowen Xie, Xiaoke Duan, Haoyue Shen, and Chengyuan Wang. 2025. "Distinct Piezochromic Properties of Cyanostilbene- and Cyanostyrene-Based Donor–Acceptor–Donor- and Donor–Acceptor-Structured Organic Luminogens" Photochem 5, no. 4: 36. https://doi.org/10.3390/photochem5040036
APA StyleWang, Z., Yang, M., Chen, Y., Yang, X., Xie, B., Duan, X., Shen, H., & Wang, C. (2025). Distinct Piezochromic Properties of Cyanostilbene- and Cyanostyrene-Based Donor–Acceptor–Donor- and Donor–Acceptor-Structured Organic Luminogens. Photochem, 5(4), 36. https://doi.org/10.3390/photochem5040036








