Upcycled Carbon Dots as Multifunctional Boosters for Broad-Spectrum Photostable Sunscreens
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Methods
2.2.1. Synthesis of Carbon Dots
2.2.2. Nanoparticle Characterization
2.2.3. Cytotoxicity Assessment
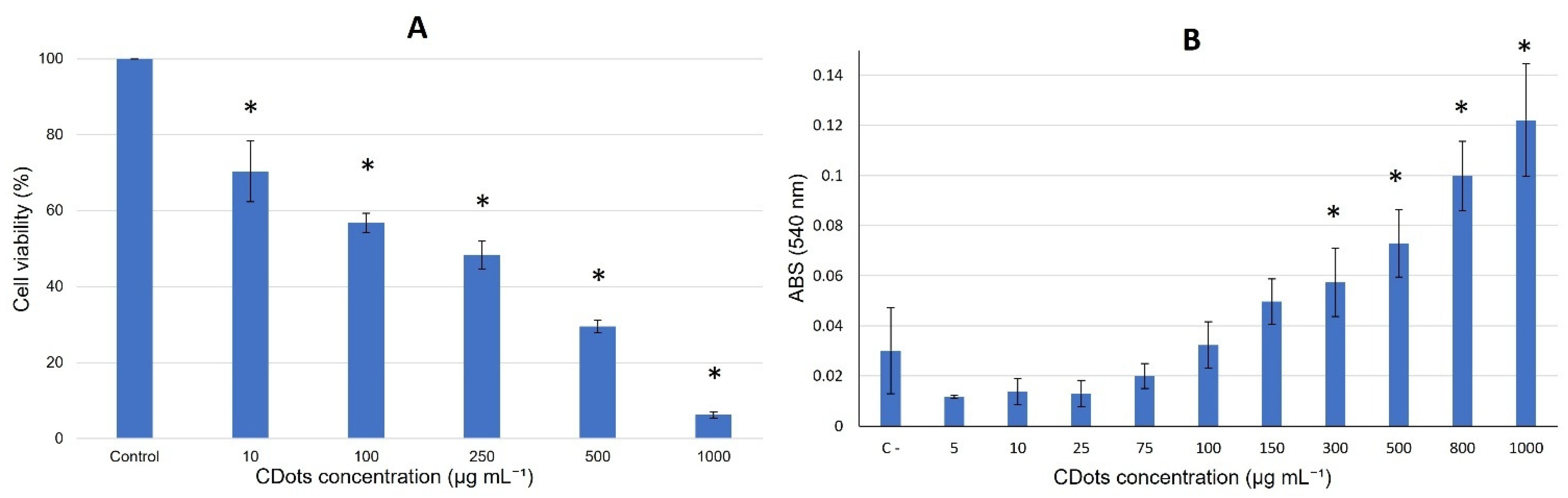
Cell Viability Assay
Membrane Stabilization Assay—Hemolysis Test
2.2.4. Evaluation of Antioxidant Activity
Free Radical Scavenging Capacity by the Copper Reduction Method
Antioxidant Activity by Electron Paramagnetic Resonance
2.2.5. Sunscreen Samples
2.2.6. In Vitro Photoprotective Efficacy and Photostability Assay
2.2.7. Statistical Treatment
3. Results and Discussion
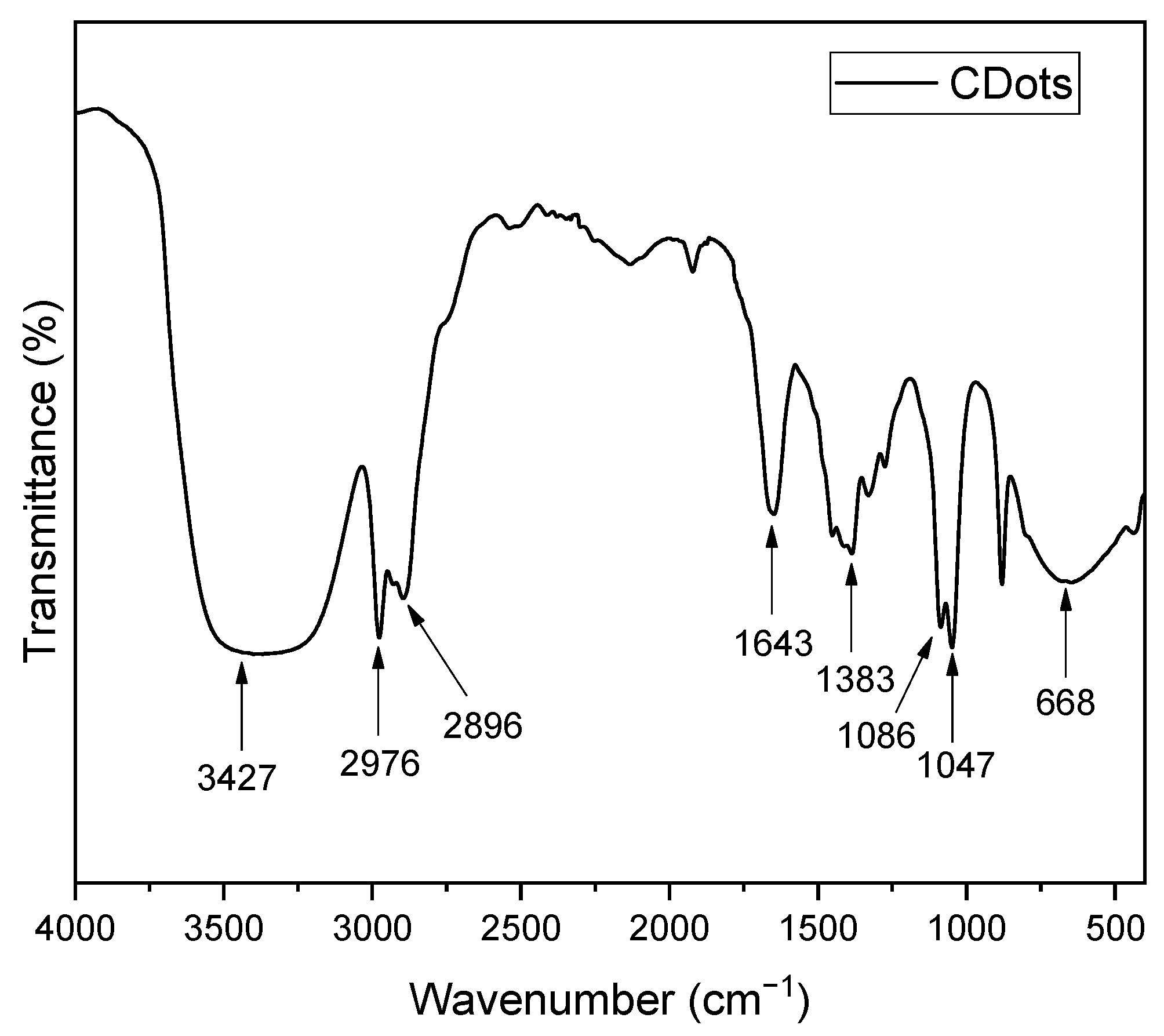
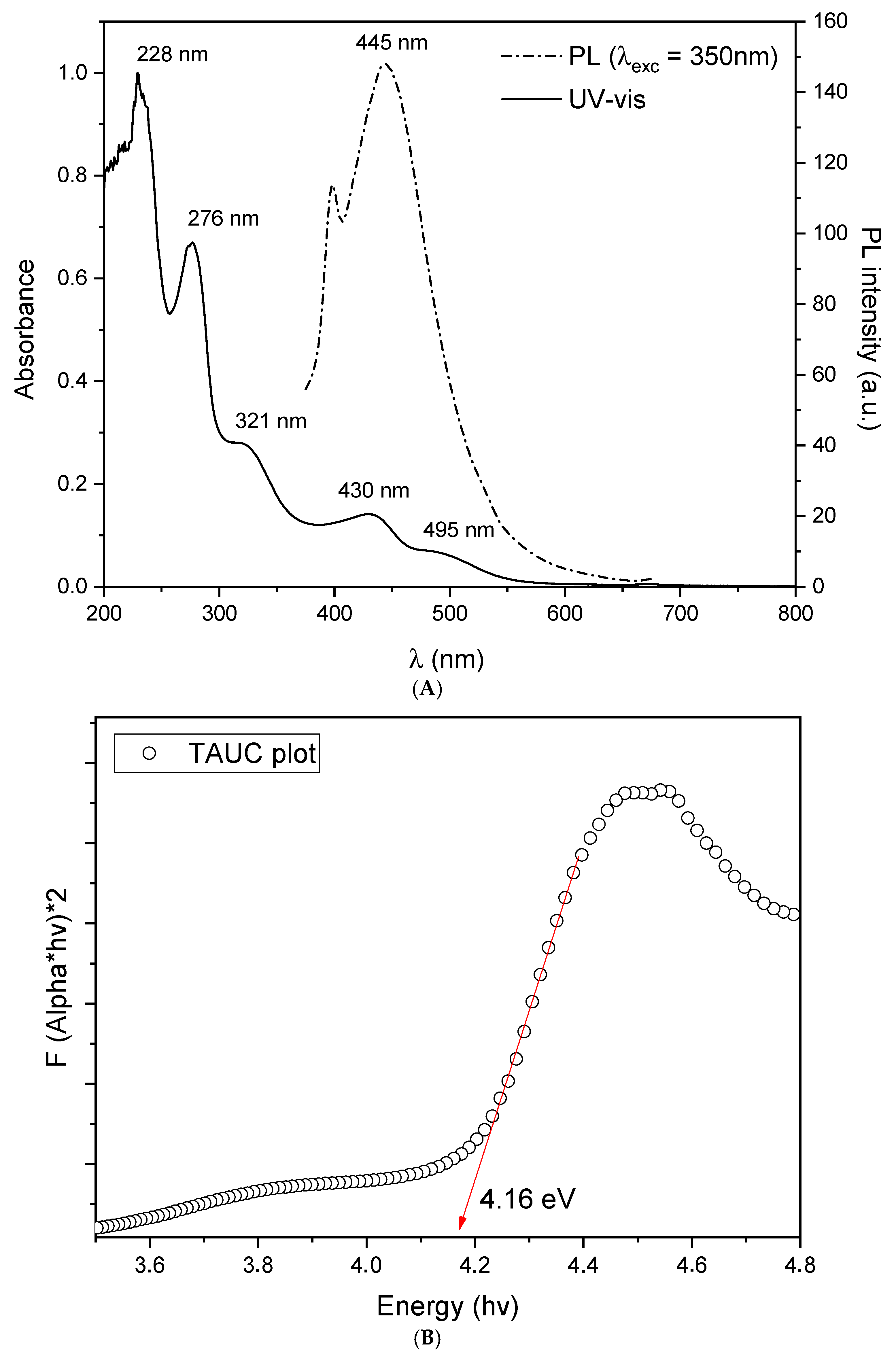
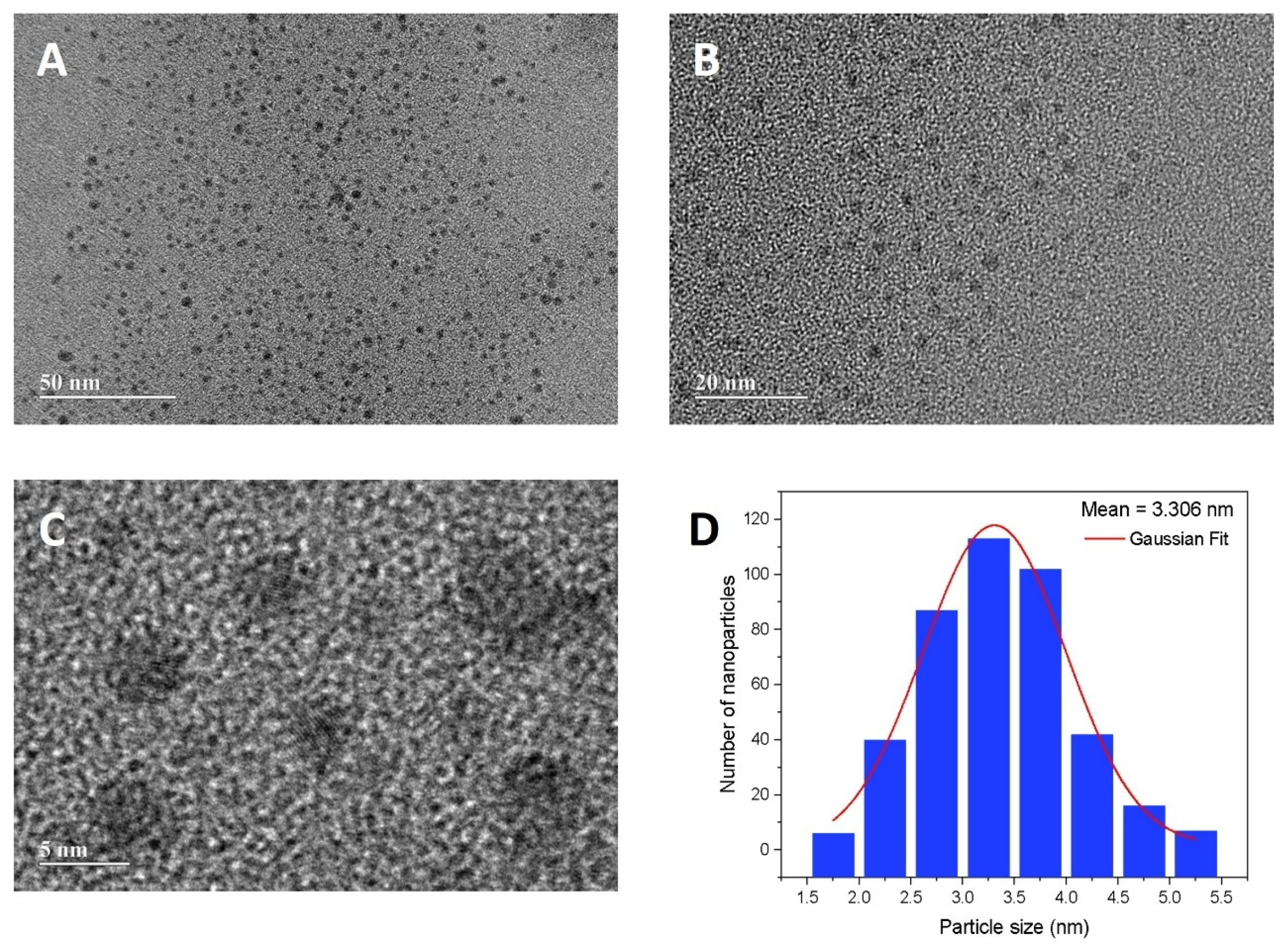
3.1. Synthesis and Characterization
3.2. Cytotoxicity Detection and Hemocompatibility
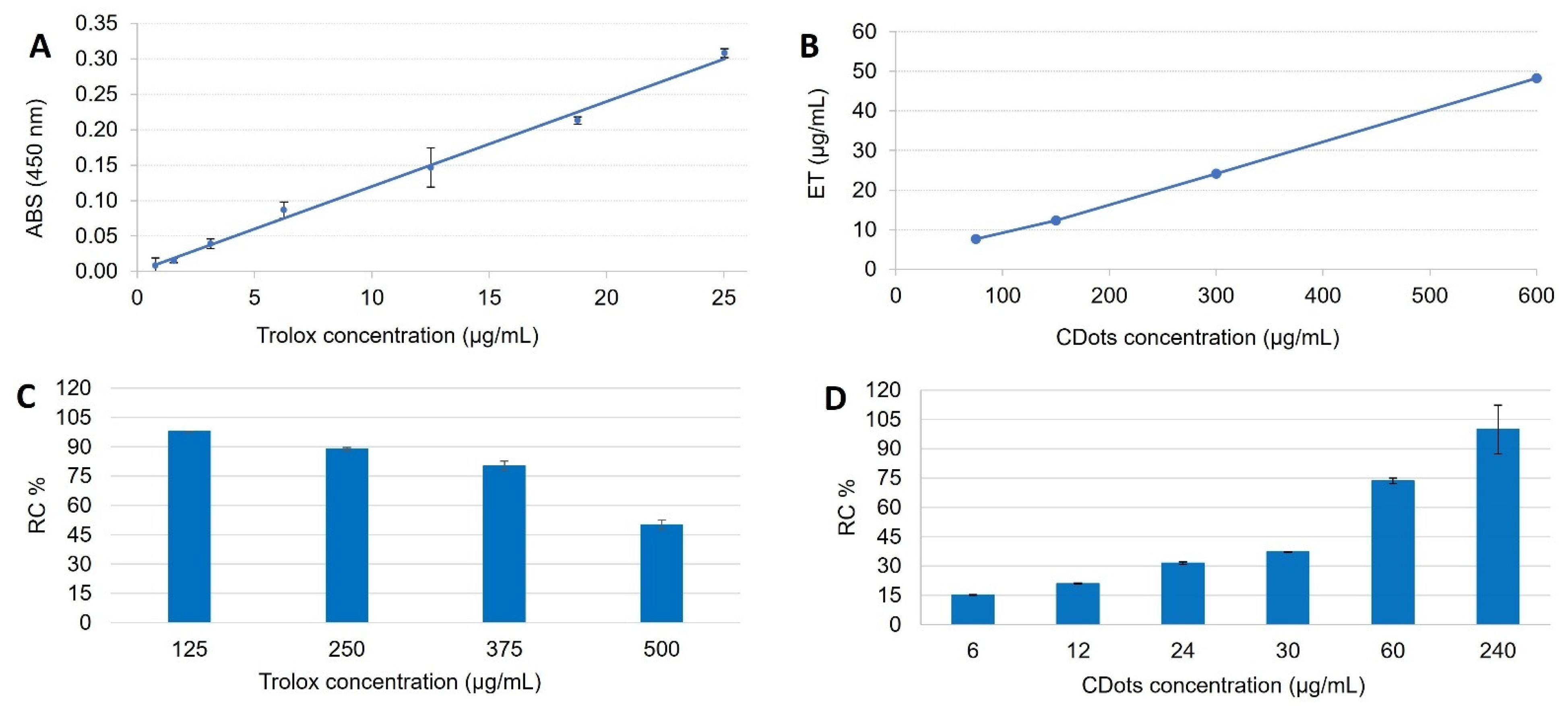
4. Antioxidant Activity
5. In Vitro SPF
6. Limitations and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marionnet, C.; Tran, C.; Bastien, P.; Bielicki, A.; Golebiewski, C.; Vieu, D.-L.; Suida-Batista, A.; Candau, D.; Bernerd, F. A broader filtration of UVA1 wavelengths improves skin photoprotection. J. Dermatol. Sci. 2018, 91, 337–340. [Google Scholar] [CrossRef]
- Chhabra, G.; Garvey, D.R.; Singh, C.K.; Mintie, C.A.; Ahmad, N. Effects and Mechanism of Nicotinamide Against UVA- and/or UVB-mediated DNA Damages in Normal Melanocytes. Photochem. Photobiol. 2019, 95, 331–337. [Google Scholar] [CrossRef]
- Cohen, G.; Jakus, J.; Portillo, M.; Gvirtz, R.; Ogen-Shtern, N.; Silberstein, E.; Ayzenberg, T.; Rozenblat, S. In vitro, ex vivo, and clinical evaluation of anti-aging gel containing EPA and CBD. J. Cosmet. Dermatol. 2023, 22, 3047–3057. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Fan, G.-B.; Tan, F.; Kong, H.-M.; Liu, Q.; Zou, Y.; Tan, Y.-M. The role and safety of UVA and UVB in UV-induced skin erythema. Front. Med. 2023, 10, 1163697. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively Generated Damage to Cellular DNA by UVB and UVA Radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef]
- Khan, M.A. Sun protection factor determination studies of some sunscreen formulations used in cosmetics for their selection. J. Drug Deliv. Ther. 2018, 8, 149–151. [Google Scholar] [CrossRef]
- Jallad, K.N. Chemical characterization of sunscreens composition and its related potential adverse health effects. J. Cosmet. Dermatol. 2017, 16, 353–357. [Google Scholar] [CrossRef]
- Nery, É.M.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. A short review of alternative ingredients and technologies of inorganic UV filters. J. Cosmet. Dermatol. 2021, 20, 1061–1065. [Google Scholar] [CrossRef]
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef]
- Downs, C.A.; Bishop, E.; Diaz-Cruz, M.S.; Haghshenas, S.A.; Stien, D.; Rodrigues, A.M.S.; Woodley, C.M.; Sunyer-Caldú, A.; Doust, S.N.; Espero, W.; et al. Oxybenzone contamination from sunscreen pollution and its ecological threat to Hanauma Bay, Oahu, Hawaii, U.S.A. Chemosphere 2022, 291, 132880. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802. [Google Scholar] [CrossRef]
- Rabe, D.I.A.; Awak, M.M.A.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.-P.; et al. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int. J. Nanomed. 2019, 14, 2655–2665. [Google Scholar] [CrossRef]
- Robein, Y.; Ambrusi, R.E.; Pronsato, M.E.; Di Nezio, M.S.; Brizuela, G. Brizuela A theoretical study of the functionalized carbon dots surfaces binding with silver nanostructures. Comput. Theor. Chem. 2023, 1223, 114087. [Google Scholar] [CrossRef]
- Fang, M.; Wang, B.; Qu, X.; Li, S.; Huang, J.; Li, J.; Lu, S.; Zhou, N. State-of-the-art of biomass-derived carbon dots: Preparation, properties, and applications. Chin. Chem. Lett. 2024, 35, 108423. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Jiang, Y.; Miao, M.; Cao, S.; Fang, J. Use of carbon dots to enhance UV-blocking of transparent nanocellulose films. Carbohydr. Polym. 2017, 161, 253–260. [Google Scholar] [CrossRef]
- DePaula, J.; Partelli, F.L.; Batista, A.M.; Calado, V.; Farah, A. Major Bioactive Compounds in Seeds, Husks, and Leaves of Selected Genotypes of Coffea canephora cv. Conilon from Three Consecutive Crops. Plants 2025, 14, 1040. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Plant Extract-Derived Carbon Dots as Cosmetic Ingredients. Nanomaterials 2023, 13, 2654. [Google Scholar] [CrossRef]
- Cheng, B.; Yang, Z.; Chen, F.; Yue, L.; Cao, X.; Li, J.; Qian, H.-L.; Yan, X.-P.; Wang, C.; Wang, Z. Biomass-derived carbon dots with light conversion and nutrient provisioning capabilities facilitate plant photosynthesis. Sci. Total Environ. 2023, 901, 165973. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kang, Y.; Yang, N.; Li, H.; Huang, S.; Liang, Z.; Zeng, G.; Huang, Y.; Li, W.; Zheng, M.; et al. Inhibition of UV-B stress in lettuce through enzyme-like Scutellaria baicalensis carbon dots. Ecotoxicol. Environ. Saf. 2022, 246, 114177. [Google Scholar] [CrossRef] [PubMed]
- de Paula Bernado, W.; Santos, A.R.; Vale, E.M.; Pireda, S.; Correia, L.Z.; Desouza, G.A.R.; de Abreu, D.P.; Carvalho, L.K.O.; Almeida, F.A.; Baroni, D.F. UV-B reduction and excess: Management strategies regarding Coffea sp. crop. Sci. Hortic. 2024, 323, 112499. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Z.; Shen, J.; Wang, J.; Lin, L. Green synthesis of carbon dots by microflow method and their application as sunscreen agent. Chin. J. Chem. Eng. 2025, 77, 135. [Google Scholar] [CrossRef]
- Campalani, C.; Causin, V.; Selva, M.; Perosa, A. Fish-Waste-Derived Gelatin and Carbon Dots for Biobased UV-Blocking Films. ACS Appl. Mater. Interfaces 2022, 14, 35148–35156. [Google Scholar] [CrossRef]
- Chen, C.; Yu, L.; Li, X.; Yu, Z.; Song, D.; Wang, S.; Li, F.; Jiang, S.; Chen, Y.; Xu, J.; et al. Reducing Oxidative Stress Levels and Inhibiting Aging by l-Cysteine-Derived Carbon Dots with Highly Efficient Broad-Spectrum UV Absorption. ACS Appl. Mater. Interfaces 2024, 16, 43189–43198. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Kasouni, A.; Troganis, A.; Leonardos, I.; Tzovenis, I.; Ntzouvaras, A.; Stalikas, C. Carbon Nanodots Synthesized from Dunaliella salina as Sun Protection Filters. C 2020, 6, 69. [Google Scholar] [CrossRef]
- Davi, L.B.O.; Silva, M.S.; Ferreira, R.L.; Muniz, W.; Ribeiro, A.S.; Lima, D.J.P.; de Oliveira, I.N.; Barbosa, C.D.A.E.S. Multifunctional carbon dots derived from dansyl chloride for ratiometric thermal sensor and reactive oxygen generation. Dye. Pigment. 2021, 194, 109549. [Google Scholar] [CrossRef]
- Kumawat, M.; Thakur, M.; Gurung, R.; Srivastava, R. Graphene Quantum Dots from Mangifera indica: Application in Near-Infrared Bioimaging and Intracellular Nano-thermometry. ACS Sustain. Chem. Eng. 2016, 5, 1382–1391. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA). Farmacopeia Brasileira, 6th ed.; Method 5.2.9 Loss on Drying; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2019; Volume 1.
- Flórido, A.; Saraiva, N.; Cerqueira, S.; Almeida, N.; Parsons, M.; Batinic-Haberle, I.; Miranda, J.P.; Costa, J.G.; Carrara, G.; Castro, M.; et al. The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells. Redox Biol. 2019, 20, 367–378. [Google Scholar] [CrossRef]
- Labu, Z.K.; Laboni, F.R.; Tarafdar, M.; Howlader, M.S.I.; Rashid, M.H. Membrane Stabilization as a Mechanism of Anti-Inflammatory and Thrombolytic Activities of Ethanolic Extract of Arial Parts of Spondiasis pinanata (Family: Anacardiaceae). Pharmacologyonline 2015, 2, 44–51. [Google Scholar]
- Sarveswaran, R.; Jayasuriya, W.J.A.B.; Thusharie, S.N.M.T.S.S. In vitro assays to investigate the anti-inflammatory activity of herbal extracts: A Review. World J. Pharm. Res. 2017, 6, 131–141. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Ferreira-Nunes, R.; da Silva, S.M.M.; de Souza, P.E.N.; de Magalhães, P.O.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Incorporation of Eugenia dysenterica extract in microemulsions preserves stability, antioxidant effect and provides enhanced cutaneous permeation. J. Mol. Liq. 2018, 265, 408–415. [Google Scholar] [CrossRef]
- Ordoñez Lozada, M.I.; Rodrigues Maldonade, I.; Bobrowski Rodrigues, D.; Silva Santos, D.; Ortega Sanchez, B.A.; Narcizo de Souza, P.E.; Longo, J.P.; Bernardo Amaro, G.; de Lacerda de Oliveira, L. Physicochemical characterization and nano-emulsification of three species of pumpkin seed oils with focus on their physical stability. Food Chem. 2021, 343, 128512. [Google Scholar] [CrossRef]
- Alves, T.E.P.; Kolodziej, C.; Burda, C.; Franco, A. Effect of particle shape and size on the morphology and optical properties of zinc oxide synthesized by the polyol method. Mater. Des. 2018, 146, 125–133. [Google Scholar] [CrossRef]
- Souza Neto, A.V.; Balla, D.Q.; Candido, T.M.; Rosado, C.; Baby, A.R.; Pessoa, F.V.L.S. Effect of an Emollient Emulsion Containing 15.0% of Caprylic/Capric Triglyceride on the Urocanic Acid of the Stratum Corneum. Life 2023, 13, 876. [Google Scholar] [CrossRef]
- Silva Marcelino, P.; Martinez, R.M.; Daneluti, A.L.M.; Morocho-Jácome, A.L.; Pessoa, F.V.L.S.; Rijo, P.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vitro Photoprotection and Functional Photostability of Sunscreen Lipsticks Containing Inorganic Active Compounds. Cosmetics 2023, 10, 46. [Google Scholar] [CrossRef]
- COLIPA. Method for the In Vitro Determination of UVA Protection Provided by Sunscreen Products; European Cosmetics Association: Brussels, Belgium, 2009. [Google Scholar]
- Matts, P.J.; Alard, V.; Brown, M.W.; Ferrero, L.; Gers-Barlag, H.; Issachar, N.; Moyal, D.; Wolber, R. The COLIPA in vitro UVA method: A standard and reproducible measure of sunscreen UVA protection. Int. J. Cosmet. Sci. 2010, 32, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Popiół, J.; Gunia-Krzyżak, A.; Piska, K.; Żelaszczyk, D.; Koczurkiewicz, P.; Słoczyńska, K.; Wójcik-Pszczoła, K.; Krupa, A.; Kryczyk-Poprawa, A.; Żesławska, E.; et al. Discovery of Novel UV-Filters with Favorable Safety Profiles in the 5-Arylideneimidazolidine-2,4-dione Derivatives Group. Molecules 2019, 24, 2321. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Mezzena, M. Photostabilization Effect of Quercetin on the UV Filter Combination, Butyl Methoxydibenzoylmethane–Octyl Methoxycinnamate. Photochem. Photobiol. 2010, 86, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Egorova, M.; Tomskaya, A.; Smagulova, S.A. Optical Properties of Carbon Dots Synthesized by the Hydrothermal Method. Materials 2023, 16, 4018. [Google Scholar] [CrossRef]
- Hijazi, S.; Ganie, A.S.; Rahman, M.M.; Shah, W.A. Facile synthesis of surface-functionalized fluorescent carbon quantum dots for the selective detection of ferric ions. Environ. Sci. Nano 2023, 10, 3281–3294. [Google Scholar] [CrossRef]
- Singaravelu, C.M.; Deschanels, X.; Rey, C.; Causse, J. Investigation on Fluorescence Origin and Spectral Heterogeneity in Carbon Dots: A Dynamic Perspective. ChemPhotoChem 2024, 8, e202400044. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Macedo, T.M.; Paulo, Í.S.; Dusi, R.G.; Magalhães, A.O.; Ferreira, E.S.; de Freitas Espeleta, A.; Lucchese, A.M. Innovations in dermocosmetics: Carbon Dot applications on human skin. MRS Commun. 2025; in press. [Google Scholar] [CrossRef]
- Dong, C.; Xu, M.; Wang, S.; Ma, M.; Akakuru, O.U.; Ding, H.; Wu, A.; Zha, Z.; Wang, X.; Bi, H. Fluorescent carbon dots with excellent moisture retention capability for moisturizing lipstick. J. Nanobiotechnol. 2021, 19, 299. [Google Scholar] [CrossRef]
- Li, Q.; Shen, X.; Xing, D. Carbon quantum dots as ROS-generator and -scavenger: A comprehensive review. Dye. Pigment. 2023, 208, 110784. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Wang, D.; Shen, Y.; Yang, L.; Zhang, T.; Ge, J. Facile synthesis of biomass waste-derived fluorescent N, S, P co-doped carbon dots for detection of Fe3+ ions in solutions and living cells. Anal. Methods 2021, 13, 789–795. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Cao, W.; Mao, B.; Liu, Y.; Kang, Z. Advances in carbon dots: From the perspective of traditional quantum dots. Mater. Chem. Front. 2020, 4, 1586–1613. [Google Scholar] [CrossRef]
- Piasek, A.; Pulit-Prociak, J.; Zielina, M.; Banach, M. Fluorescence of D-Glucose-Derived Carbon Dots: Effect of Process Parameters. J. Fluoresc. 2024, 34, 1693–1705. [Google Scholar] [CrossRef]
- Silva, L.E.; de Calado, O.L.L.; de Oliveira Silva, S.F.; da Silva, K.R.M.; Henrique Almeida, J.; de Oliveira Silva, M.; da Viana, R.S.; de Souza Ferro, J.N.; de Almeida Xavier, J.; Barbosa, C.D.A.E.S. Lemon-derived carbon dots as antioxidant and light emitter in fluorescent films applied to nanothermometry. J. Colloid Interface Sci. 2023, 651, 678–685. [Google Scholar] [CrossRef]
- Darwesh, A.H.A.; Mohammed, P.A.; Mamand, S.M.; Hussen, S.A.; Aziz, S.B.; Brza, M.A.; Abdullah, R.M.; Karim, W.O. Investigation of Structural and Optical Characteristics of Biopolymer Composites Based on Polyvinyl Alcohol Inserted with PbS Nanoparticles. Coatings 2023, 13, 578. [Google Scholar] [CrossRef]
- Al-Attafi, K.; Al-Keisy, A.; Alsherbiny, M.A.; Kim, J.H. Zn2SnO4 ternary metal oxide for ultraviolet radiation filter application: A comparative study with TiO2 and ZnO. Sci. Technol. Adv. Mater. 2023, 24, 2277678. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.R.F.; Alves, M.F.R.P.; Cunha, J.P.G.Q.; Costa, J.L.; Silva, C.A.; Fernandes, M.H.V.; Vilarinho, P.M.; Ferreira, P. Nanostructured transparent solutions for UV-shielding: Recent developments and future challenges. Mater. Today Phys. 2023, 35, 101131. [Google Scholar] [CrossRef]
- Lei, M.; Xie, Y.; Chen, L.; Liu, X.; Yang, Y.; Zheng, J.; Li, Q. Surface state modulation of blue-emitting carbon dots with high quantum yield and high product yield. RSC Adv. 2022, 12, 27431–27441. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef]
- Ozdemir, N.; Tan, G.; Tevlek, A.; Arslan, G.; Zengin, G.; Sargin, I. Dead Cell Discrimination with Red Emissive Carbon Quantum Dots from the Medicinal and Edible Herb Echinophora tenuifolia. J. Fluoresc. 2025; in press. [Google Scholar] [CrossRef]
- Jhonsi, M.A.; Ananth, D.A.; Nambirajan, G.; Sivasudha, T.; Yamini, R.; Bera, S.; Kathiravan, A. Antimicrobial activity, cytotoxicity and DNA binding studies of carbon dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, J.; Pu, J.; Cheng, G.; Liu, Y.; Xiao, S.; Cao, J. Epigynum auritum-Derived Near-Infrared Carbon Dots for Bioimaging and Antimicrobial Applications. Molecules 2025, 30, 422. [Google Scholar] [CrossRef]
- Yalshetti, S.; Thokchom, B.; Bhavi, S.M.; Singh, S.R.; Patil, S.R.; Harini, B.P.; Sillanpää, M.; Manjunatha, J.G.; Srinath, B.S.; Yarajarla, R.B. Microwave-assisted synthesis, characterization and in vitro biomedical applications of Hibiscus rosa-sinensis Linn.-mediated carbon quantum dots. Sci. Rep. 2024, 14, 9915. [Google Scholar] [CrossRef]
- Alghareeb, S.A.; Alsughayyir, J.; Alfhili, M.A. Stimulation of Hemolysis and Eryptosis by α-Mangostin through Rac1 GTPase and Oxidative Injury in Human Red Blood Cells. Molecules 2023, 28, 6495. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef] [PubMed]
- Kasif, M.; Alarifi, A.; Afzal, M.; Thirugnanasambandam, A. N, S-codoped carbon dots for antioxidants and their nanovehicle potential as molecular cargoes. RSC Adv. 2024, 14, 32041–32052. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Official Journal of the European Union, 1223/2009. 2009, pp. 59–209. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 10 August 2025).
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- FDA. Sunscreen Drug Products for Over-the-Counter Human Use. Fed. Regist. 2019, 84, 98–355. [Google Scholar]
- Berenbeim, J.A.; Wong, N.G.K.; Cockett, M.C.R.; Berden, G.; Oomens, J.; Rijs, A.M.; Dessent, C.E.H. Unravelling the Keto–Enol Tautomer Dependent Photochemistry and Degradation Pathways of the Protonated UVA Filter Avobenzone. J. Phys. Chem. A 2020, 124, 2919–2930. [Google Scholar] [CrossRef]
- Hu, G.; Lei, B.; Jiao, X.; Wu, S.; Zhang, X.; Zhuang, J.; Liu, X.; Hu, C.; Liu, Y. Synthesis of modified carbon dots with performance of ultraviolet absorption used in sunscreen. Opt. Express 2019, 27, 7629–7641. [Google Scholar] [CrossRef]
| Ingredients (INCI) | Function | Control (%) | CDots (%) |
|---|---|---|---|
| Phenoxyethanol (and) caprylyl glycol (Optiphen®) | Preservative | 1.0 | 1.0 |
| Cetearyl alcohol (and) dicetyl phosphate (and) ceteth—10 phosphate (Crodafos® CES) | Self-emulsifying wax | 6.0 | 6.0 |
| Butyl Methoxydibenzoymethane | UVA filter | 5.0 | 5.0 |
| Ethylhexyl Methoxycinnamate | UVB filter | 10.0 | 10.0 |
| CDots (dry-mass) | Active | - | 4.5 |
| Purified water | Vehicle | * | * |
| Before Irradiation | After Irradiation (Min) | |||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | ||||||
| SPF | cλ (nm) | SPF | cλ (nm) | SPF | cλ (nm) | SPF | cλ (nm) | |
| Control | 26 ± 13.0 | 381 ± 0.7 | 14.58 ± 2.2 | 379.2 ± 0.8 | _ | _ | _ | _ |
| CDots | 161 ± 7.8 A | 380 ± 0.6 | 145.22 ± 10.1 A | 378 ± 0.5 | 26.28 ± 5.2 B | 377 ± 1.0 | 14.26 ± 2.2 B | 375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, G.T.; Chiabai, C.R.; Orgino, I.d.S.; Ferraz, L.N.; França, F.D.; Partelli, F.L.; de Souza, P.E.N.; Fernandes, A.S.; Júlio, A.L.G.; Baby, A.R.; et al. Upcycled Carbon Dots as Multifunctional Boosters for Broad-Spectrum Photostable Sunscreens. Photochem 2025, 5, 32. https://doi.org/10.3390/photochem5040032
Machado GT, Chiabai CR, Orgino IdS, Ferraz LN, França FD, Partelli FL, de Souza PEN, Fernandes AS, Júlio ALG, Baby AR, et al. Upcycled Carbon Dots as Multifunctional Boosters for Broad-Spectrum Photostable Sunscreens. Photochem. 2025; 5(4):32. https://doi.org/10.3390/photochem5040032
Chicago/Turabian StyleMachado, Gustavo Teixeira, Caio Rui Chiabai, Isaac dos Santos Orgino, Leticia Neves Ferraz, Flavia Dayrell França, Fábio Luiz Partelli, Paulo Eduardo Narcizo de Souza, Ana Sofia Fernandes, Ana Luísa Gomes Júlio, André Rolim Baby, and et al. 2025. "Upcycled Carbon Dots as Multifunctional Boosters for Broad-Spectrum Photostable Sunscreens" Photochem 5, no. 4: 32. https://doi.org/10.3390/photochem5040032
APA StyleMachado, G. T., Chiabai, C. R., Orgino, I. d. S., Ferraz, L. N., França, F. D., Partelli, F. L., de Souza, P. E. N., Fernandes, A. S., Júlio, A. L. G., Baby, A. R., Andrade, G. R. S., & Pessoa, F. V. L. S. (2025). Upcycled Carbon Dots as Multifunctional Boosters for Broad-Spectrum Photostable Sunscreens. Photochem, 5(4), 32. https://doi.org/10.3390/photochem5040032









