[2+2]-Photocycloadditions of 2-Acetoxy-1,4-naphthoquinone and Structure Determination of the Main Photoadducts
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. General Procedure for Photocycloadditions Under Batch Conditions
2.3. General Procedure for Photocycloadditions Under Continuous-Flow Conditions
2.4. Spectroscopic Details
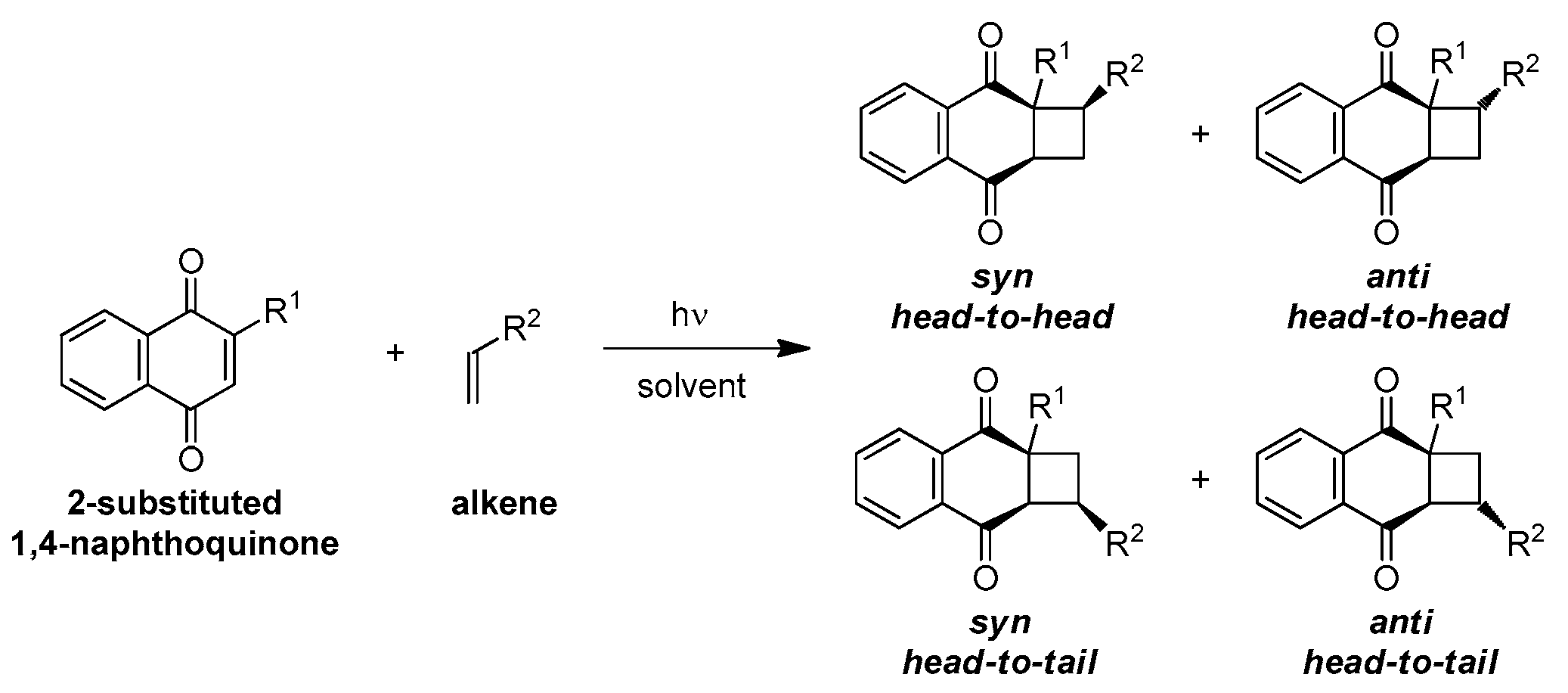
3. Results and Discussion
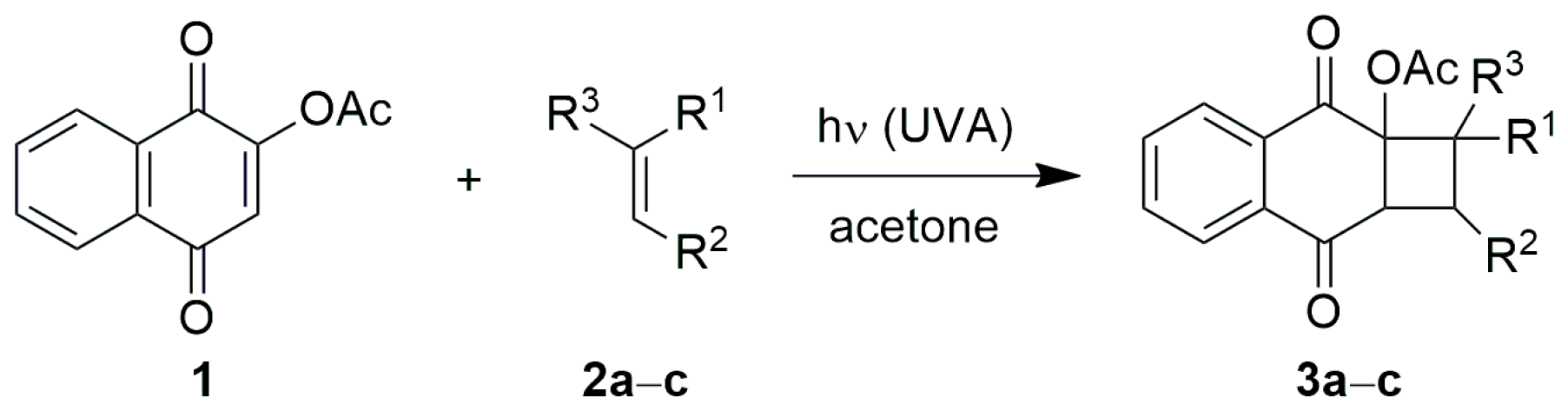
3.1. Synthesis of 2-Acetoxy-1,4-naphthoquinone
3.2. Photocycloadditions Under Batch Conditions
3.2.1. Optimization Studies
3.2.2. Preparative Photocycloadditions with Selected Alkenes
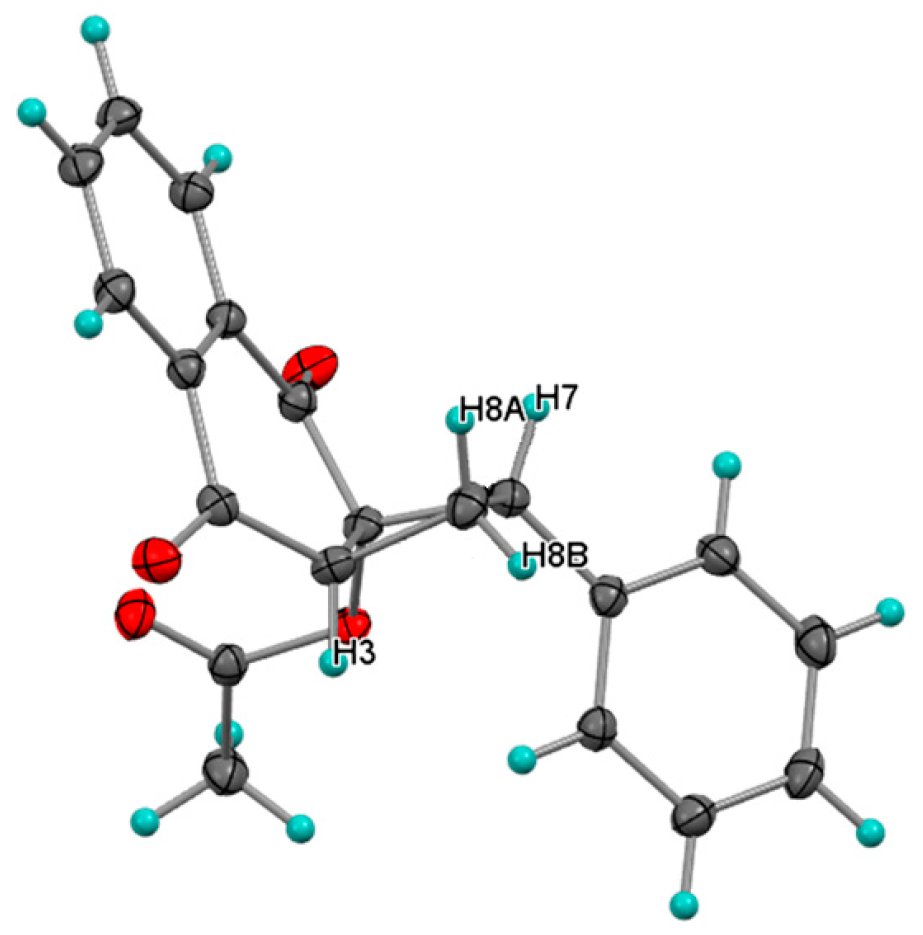
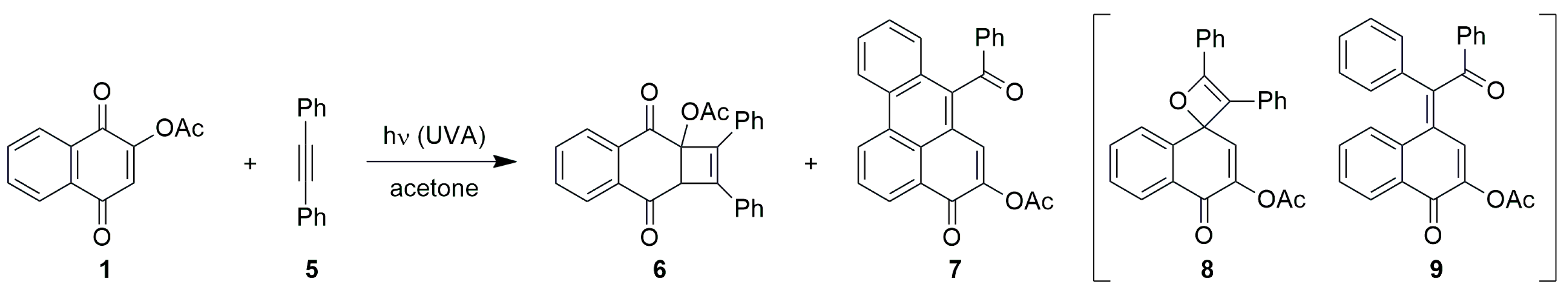
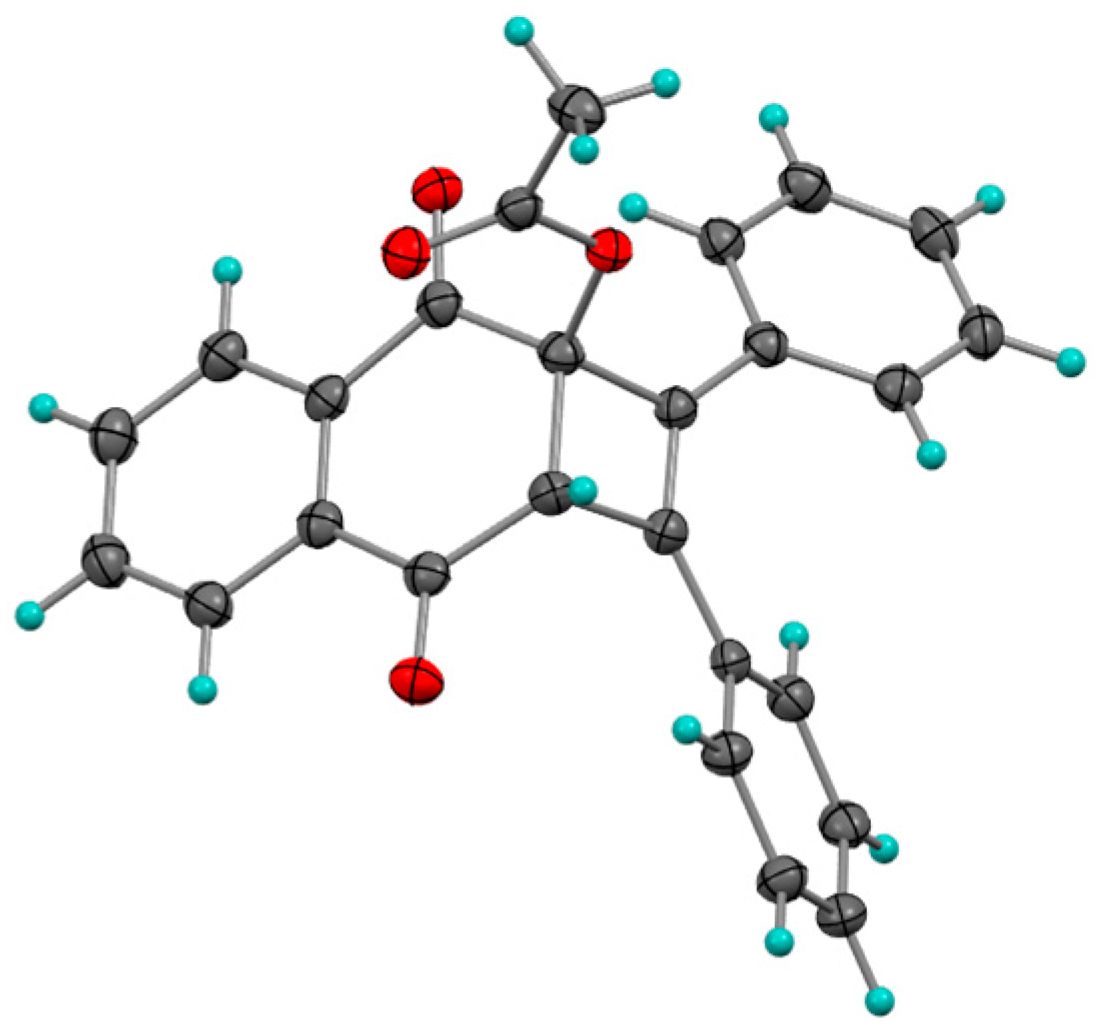
Photocycloaddition with 1,1-Diphenylethylene
Photocycloaddition with Styrene
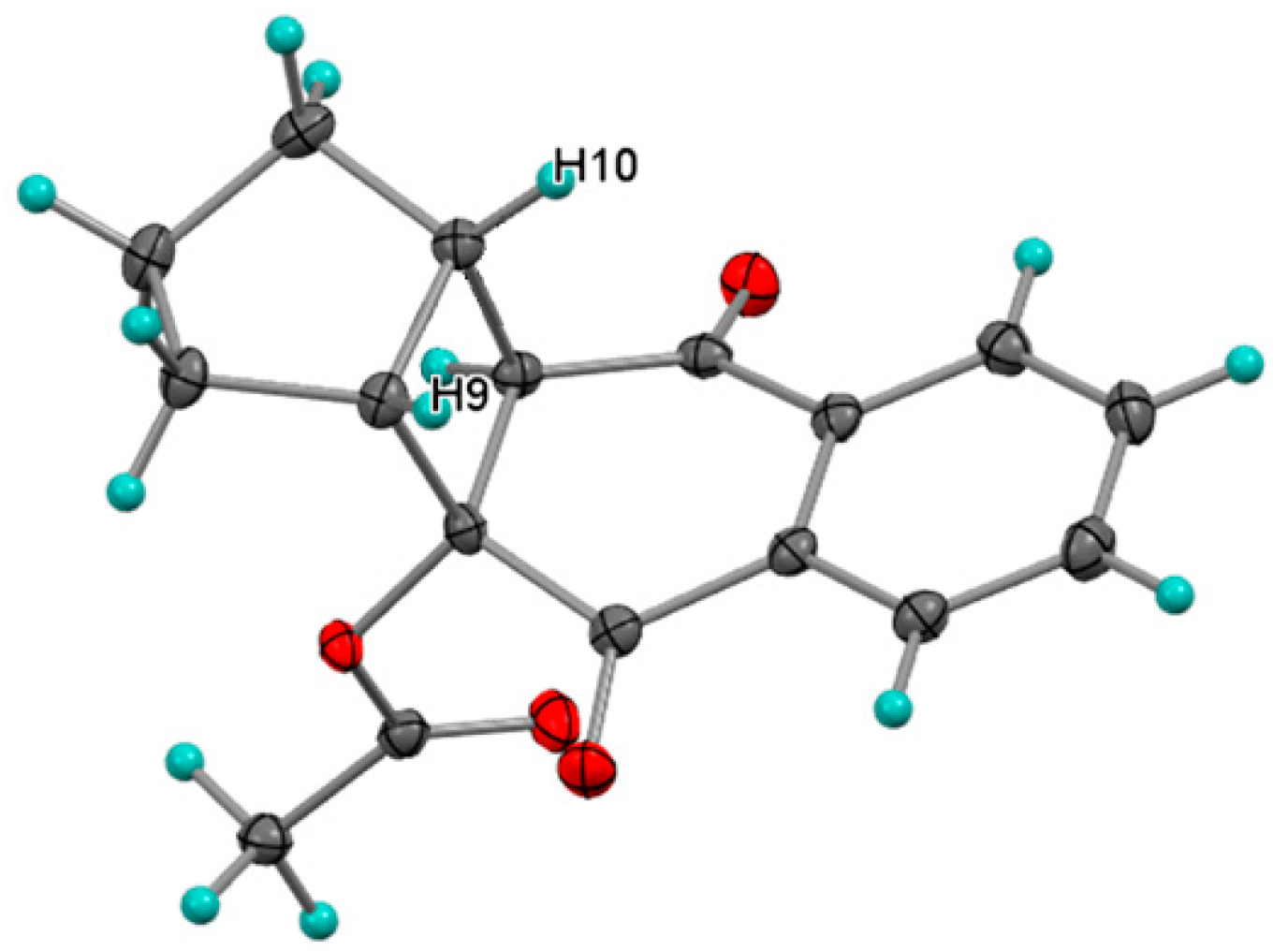
Photocycloaddition with Cyclopentene
3.2.3. Photocycloadditions with Diphenylacetylene
3.3. Photocycloadditions with Selected Alkenes Under Continuous-Flow Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jha, R.K.; Kumar, S. Direct Functionalization of para-Quinones: A Historical Review and New Perspectives. Eur. J. Org. Chem. 2024, 27, e202400535. [Google Scholar] [CrossRef]
- Angulo-Elizari, E.; Henriquez-Figuereo, A.; Morán-Serradilla, C.; Plano, D.; Sanmartín, C. Unlocking the Potential of 1,4-Naphthoquinones: A Comprehensive Review of Their Anticancer Properties. Eur. J. Med. Chem. 2024, 268, 116249. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tovar, G.; Vega-Rodríguez, S.; de Loera, D.; Leyva, E.; Loredo-Carrillo, S.; López-López, L.I. The Relevance and Insights on 1,4-Naphthoquinones as Antimicrobial and Antitumoral Molecules: A Systematic Review. Pharmaceuticals 2023, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Mone, N.S.; Bhagwat, S.A.; Sharma, D.; Chaskar, M.; Patil, R.H.; Zamboni, P.; Nawani, N.N.; Satpute, S.K. Naphthoquinones and Their Derivatives: Emerging Trends in Combating Microbial Pathogens. Coatings 2021, 11, 434. [Google Scholar] [CrossRef]
- Ahmadi, E.S.; Tajbakhsh, A.; Iranshahy, M.; Asili, J.; Kretschmer, N.; Shakeri, A.; Sahebkar, A. Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity. Mini-Rev. Med. Chem. 2020, 20, 2019–2035. [Google Scholar] [CrossRef] [PubMed]
- Perpète, E.A.; Lambert, C.; Wathelet, V.; Preat, J.; Jacquemin, D. Ab initio Studies of the λmax of Naphthoquinones Dyes. Spectrochim. Acta A 2007, 68, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lee, G.; Cho, E.J. Visible Light Induced Reactions of Quinones. Bull. Korean Chem. Soc. 2024, 45, 966–976. [Google Scholar] [CrossRef]
- de Lucas, N.C.; Ferreira, A.B.B.; Netto-Ferreira, J.C. Fotoquímica de Naftoquinonas. Rev. Virtual Quim. 2015, 7, 403–463. [Google Scholar] [CrossRef]
- Maruyama, K.; Osuka, A. Recent advances in the photochemistry of quinones. In The Chemistry of Quinonoid Compounds; Patai, S., Rappaport, Z., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 1988; Volume 2, Part 1, Chapter 13; pp. 759–878. [Google Scholar] [CrossRef]
- Gilbert, A. 1,4-Quinone Cycloaddition Reactions with Alkenes, Alkynes, and Related Compounds. In CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W.M., Lenci, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; Chapter 87; pp. 1–12. [Google Scholar] [CrossRef]
- Creed, D. 1,4-Quinone Cycloaddition Reactions with Alkene, Alkynes and Related Compounds. In CRC Handbook of Organic Photochemistry and Photobiology; Horspool, W.M., Song, P.-S., Eds.; CRC Press: Boca Raton, FL, USA, 1995; Chapter 59; pp. 737–747. [Google Scholar]
- Tan, S.-B.; Huang, C.; Chen, X.; Wu, Y.; Zhou, M.; Zhang, C.; Zhang, Y. Small Molecular Inhibitors of miR-1 Identified from Photocycloadducts of Acetylenes with 2-Methoxy-1,4-naphthalenequinone. Bioorg. Med. Chem. 2013, 21, 6124–6131. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; Zhang, W.; Wu, Y.; Chen, X.; Zhang, C.-Y.; Zhang, Y. A Universal Activator of micro RNAs Identified from Photoreaction Products. Chem. Comm. 2012, 48, 6432–6434. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, D.; Fagnoni, M. Introduction to Photochemistry for the Synthetic Chemist. In Enabling Tools and Techniques for Organic Synthesis: A Practical Guide to Experimentation, Automation, and Computation; Newman, S.G., Ed.; John Wiley & Sons Ltd.: New York, NY, USA, 2023; Chapter 2; pp. 37–72. [Google Scholar] [CrossRef]
- Douglas, P.; Evans, R.C.; Burrows, H.D. The Photochemical Laboratory. In Applied Photochemistry; Evans, R.C., Douglas, P., Burrows, H.D., Eds.; Springer: Dordrecht, The Netherlands, 2013; Chapter 14; pp. 467–531. [Google Scholar] [CrossRef]
- Bochet, C.G. On the Sustainability of Photochemical Reactions. Chimia 2019, 73, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.; Baumann, M. Continuous Flow Technology Enabling Photochemistry. Adv. Synth. Catal. 2025, 367, e202500133. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.P.; Sinha, S.; Singh, P.K.; Kumar, D. Continuous-Flow Photochemistry: The Synthesis of Marketed Pharmaceutical Compounds. ChemistrySelect 2024, 9, e202405020. [Google Scholar] [CrossRef]
- Zhang, M.; Roth, P. Flow photochemistry–From Microreactors to Large-scale Processing. Curr. Opin. Chem. Eng. 2023, 39, 100897. [Google Scholar] [CrossRef]
- Fukuyama, T.; Kasakado, T.; Hyodo, M.; Ryu, I. Improved Efficiency of Photo-induced Synthetic Reactions Enabled by Advanced Photo Flow Technologies. Photochem. Photobiol. Sci. 2022, 21, 761–775. [Google Scholar] [CrossRef]
- Rehm, T.H. Flow Photochemistry as a Tool in Organic Synthesis. Chem. Eur. J. 2020, 26, 16952–16974. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Hoffmann, N.; Shvydkiv, O. From ‘Lab & Light on a Chip’ to Parallel Microflow Photochemistry. Austr. J. Chem. 2014, 67, 337–342. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Oelgemöller, M. Photochemical Acylation of 1,4-Naphthoquinone with Aldehydes Under Continuous-Flow Conditions. Organics 2025, 6, 9. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Guo, Z.; Junk, P.J.; Oelgemöller, M. [2+2]-Photocycloadditions of 1,4-Naphthoquinone Under Batch and Continuous-Flow Conditions. Molecules 2024, 29, 5920. [Google Scholar] [CrossRef]
- Khan, H.; Rajesh, V.M.; Ravva, M.K.; Sen, S. Optimization of Blue LED Photo-Flow Synthesis in Continuous Flow Reactors Using Design of Experiments (DoE): Efficient Synthesis of Diverse Diaryl Ketones. Chem. Eng. J. 2024, 501, 157657. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Mumtaz, S.; Hunter, R.L.; Wall, D.; Belluau, V.; Robertson, M.J.; Oelgemöller, M. Continuous-Flow Photochemical Transformations of 1,4-Naphthoquinones and Phthalimides in a Concentrating Solar Trough Reactor. Austr. J. Chem. 2020, 73, 1149–1157. [Google Scholar] [CrossRef]
- Yaseen, M.A. Photochemical Transformations of Quinones under Batch and Continuous–Flow Conditions. Ph.D. Thesis, James Cook University, Townsville, QLD, Australia, 2019. [Google Scholar]
- Cleridou, S.; Covell, C.; Gadhia, A.; Gilbert, A.; Kamonnawin, P. Photocycloaddition of Arylethenes to 2-Substituted-1, 4-naphthoquinones and Reactions of the Cyclobutane Adduct Isomers. J. Chem. Soc. Perkin Trans. 1 2000, 1149–1155. [Google Scholar] [CrossRef]
- Covell, C.; Gilbert, A.; Richter, C. Sunlight-induced Regio-and Stereo-specific (2π + 2π) Cycloaddition of Arylethenes to 2-Substituted-1, 4-naphthoquinones. J. Chem. Res. (S) 1998, 316–317. [Google Scholar] [CrossRef]
- Senboku, H.; Kajizuka, Y.; Kobayashi, K.; Tokuda, M.; Suginome, H. Furan Annelation of 2-Hydroxynaphthoquinone Involving Photochemical Addition and Radical Fragmentation: Exclusion of the Intermediacy of [2+2] Cycloadduct in a One-pot Formation of Furanoquinones by the Regioselective 3+2 Photoaddition of Hydroxyquinones with Alkenes. Heterocycles 1997, 44, 341–348. [Google Scholar]
- Mumtaz, S.; Robertson, M.J.; Oelgemöller, M. Continuous Flow Photochemical and Thermal Multi-step Synthesis of Bioactive 3-Arylmethylene-2,3-dihydro-1H-isoindolin-1-ones. Molecules 2019, 24, 4527. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Cowieson, N.P.; Aragao, D.; Clift, M.; Ericsson, D.J.; Gee, C.; Harrop, S.J.; Mudie, N.; Panjikar, S.; Price, J.R.; Riboldi-Tunnicliffe, A.; et al. MX1: A Bending-magnet Crystallography Beamline Serving both Chemical and Macromolecular Crystallography Communities at the Australian Synchrotron. J. Synchrotron Radiat. 2015, 22, 187–190. [Google Scholar] [CrossRef]
- Clark, N.G. The Fungicidal Activity of Substituted 1, 4-Naphthoquinones. Part II: Alkoxy, Phenoxy and Acyloxy derivatives. Pesticide Sci. 1984, 15, 235–240. [Google Scholar] [CrossRef]
- Pappas, S.; Portnoy, N.A. Substituent Effects on the Photoaddition of Diphenylacetylene to 1, 4-Naphthoquinones. J. Org. Chem. 1968, 33, 2200–2203. [Google Scholar] [CrossRef]
- Maruyama, K.; Otsuki, T.; Takuwa, A.; Kako, S. Photochemical Reaction of 1, 4-Naphthoquinone with Olefins. Bull. Inst. Chem. Res. Kyoto Univ. 1972, 50, 344–347. [Google Scholar]
- Dekker, J.; van Vuuren, P.J.; Venter, D.P. Photodimerization. I. The syn and anti-Photodimers of 1,4-Naphthoquinone. J. Org. Chem. 1968, 33, 464–466. [Google Scholar] [CrossRef]
- Daglish, C. The Ultraviolet Absorption Spectra of Some Hydroxynaphthalenes. J. Am. Chem. Soc. 1950, 72, 4859–4864. [Google Scholar] [CrossRef]
- Hill, R.R.; Mitchell, G.H. The Ultraviolet and Visible Absorption Spectra of Some Polysubstituted 1,4-Naphthaquinones and 9,10-Anthraquinones. J. Chem. Soc. B 1969, 61–64. [Google Scholar] [CrossRef]
- Singh, I.; Ogata, R.T.; Moore, R.E.; Chang, C.W.J.; Scheuer, P.J. Electronic spectra of substituted naphthoquinones. Tetrahedron 1968, 24, 6053–6073. [Google Scholar] [CrossRef]
- Macbeth, A.K.; Price, J.R.; Winzor, F.L. The Colouring Matters of Drosera Whittakeri. Part I. The Absorption Spectra and Colour Reactions of Hydroxy-naphthaquinones. J. Chem. Soc. 1935, 325–333. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Reinhold, T.L. Enone Olefin [2+2] Photochemistry Cycloaddition. In Organic Reactions, Paquette, L.A., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1993; Volume 44, pp. 298–579. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Solvent Properties. In Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; Chapter 9; pp. 535–559. [Google Scholar] [CrossRef]
- Bunce, N.J.; Ridley, J.E.; Zerner, M.C. On the Excited States of p-Quinones and an Interpretation of the Photocycloaddition of p-Quinones to Alkenes. Theor. Chim. Acta 1977, 45, 283–300. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Wernerova, M.; Hudlicky, T. On the Practical Limits of Determining Isolated Product Yields and Ratios of Stereoisomers: Reflections, Analysis, and Redemption. Synlett 2010, 2701–2707. [Google Scholar] [CrossRef]
- Cotton, F.A.; Frenz, B.A. Conformations of Cyclobutane. Tetrahedron 1974, 30, 1587–1594. [Google Scholar] [CrossRef]
- Dragojlovic, V. Conformational Analysis of Cycloalkanes. ChemTexts 2015, 1, 14. [Google Scholar] [CrossRef]
- Bosch, E.; Hubig, S.; Kochi, J. Paterno-Büchi Coupling of (Diaryl) Acetylenes and Quinone via Photoinduced Electron Transfer. J. Am. Chem. Soc. 1998, 120, 386–395. [Google Scholar] [CrossRef]
- Farid, S.; Kothe, W.; Pfundt, G. Competitive Photoadditions of Acetylenes to the C=C and C=O Bonds of p-Quinones. Tetrahedron Lett. 1968, 9, 4147–4150. [Google Scholar] [CrossRef]
- Farid, S.; Kothe, W.; Pfundt, G. NMR-study of Cyclobutene Derivatives. Tetrahedron Lett. 1968, 9, 4151–4154. [Google Scholar] [CrossRef]
- Balo, C.; Fernández, F.; González, C.; Lopez, C. 1H NMR Spectra of Monosubstituted Phenanthrenes. Spectrochim. Acta 1994, 50A, 937–940. [Google Scholar] [CrossRef]
- Fadeev, A.A.; Kotora, M. Catalytic vs. Uncatalyzed [2+2] Photocycloadditions of Quinones with Alkynes. Org. Biomol. Chem. 2023, 21, 6174–6179. [Google Scholar] [CrossRef]
- Donnelly, K.; Baumann, M. Scalability of Photochemical Reactions in Continuous Flow Mode. J. Flow Chem. 2021, 11, 223–241. [Google Scholar] [CrossRef]
- Elliott, L.D.; Knowles, J.P.; Koovits, P.J.; Maskill, K.G.; Ralph, M.J.; Lejeune, G.; Edwards, L.J.; Robinson, R.I.; Clemens, I.R.; Cox, B.; et al. Batch versus Flow Photochemistry: A Revealing Comparison of Yield and Productivity. Chem. Eur. J. 2014, 20, 15226–15232. [Google Scholar] [CrossRef]
- Bonfield, H.E.; Mercer, K.; Diaz-Rodriguez, A.; Cook, G.C.; McKay, B.S.J.; Slade, P.; Taylor, G.M.; Ooi, W.X.; Williams, J.D.; Roberts, J.P.M.; et al. The Right Light: DeNovo Design of a Robust Modular Photochemical Reactor for Optimum Batch and Flow Chemistry. ChemPhotoChem 2020, 4, 45–51. [Google Scholar] [CrossRef]
- Loubière, K.; Oelgemöller, M.; Aillet, T.; Dechy-Cabaret, O.; Prat, L. Continuous-flow Photochemistry: A Need for Chemical Engineering. Chem. Eng. Process. 2016, 104, 120–132. [Google Scholar] [CrossRef]
- McPhillips, T.M.; McPhillips, S.E.; Chiu, H.J.; Cohen, A.E.; Deacon, A.M.; Ellis, P.J.; Garman, E.; Gonzalez, A.; Sauter, N.K.; Phizackerley, R.P.; et al. Blu-Ice and the Distributed Control System: Software for Data Acquisition and Instrument Control at Macromolecular Crystallography Beamlines. J. Synchrotron Radiat. 2002, 9, 401–406. [Google Scholar] [CrossRef]
- Kabsch, W. Automatic Processing of Rotation Diffraction Data from Crystals of Initially Unknown Symmetry and Cell Constants. J. Appl. Crystallogr. 1993, 26, 795–800. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Entry | Light | Solvent | Conversion (%) 1 | Composition 1 | |
|---|---|---|---|---|---|
| 3a (%) | 4a (%) | ||||
| 1 | 306 ± 20 nm | acetone | 88 | ≥95 | n.d. 2 |
| 2 | 306 ± 20 nm | acetonitrile | 82 | ≥95 | n.d. 2 |
| 3 | 352 ± 20 nm | acetone | 100 | ≥95 | n.d. 2 |
| 4 | 352 ± 20 nm | acetonitrile | 100 | ≥95 | n.d. 2 |
| 5 | 419 ± 25 nm | acetone | 78 | ≥95 | n.d. 2 |
| Entry | Alkene (2) | Time (h) | Conversion (%) 1 | Yield (%) 2 | ||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | ||||
| 1 | Ph | H | Ph | 20 | 100 | 60 (3a) |
| 2 | Ph | H | H | 10 | 95 | 69 (3b) |
| 3 | -(CH2)3- | H | 12 | 76 | 41 (3c) | |
| Entry | Alkene (2) | Residence Time (mins) | Conversion (%) 1 | Yield (%) 2 | ||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | ||||
| 1 | Ph | H | Ph | 300 | 100 | 55 (3a) |
| 2 | Ph | H | H | 120 | 97 | 91 (3b) |
| 3 | -(CH2)3- | H | 100 | 36 | 30 (3c)/83 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, M.A.; Guo, Z.; Junk, P.C.; Oelgemöller, M. [2+2]-Photocycloadditions of 2-Acetoxy-1,4-naphthoquinone and Structure Determination of the Main Photoadducts. Photochem 2025, 5, 31. https://doi.org/10.3390/photochem5040031
Yaseen MA, Guo Z, Junk PC, Oelgemöller M. [2+2]-Photocycloadditions of 2-Acetoxy-1,4-naphthoquinone and Structure Determination of the Main Photoadducts. Photochem. 2025; 5(4):31. https://doi.org/10.3390/photochem5040031
Chicago/Turabian StyleYaseen, Madyan A., Zhifang Guo, Peter C. Junk, and Michael Oelgemöller. 2025. "[2+2]-Photocycloadditions of 2-Acetoxy-1,4-naphthoquinone and Structure Determination of the Main Photoadducts" Photochem 5, no. 4: 31. https://doi.org/10.3390/photochem5040031
APA StyleYaseen, M. A., Guo, Z., Junk, P. C., & Oelgemöller, M. (2025). [2+2]-Photocycloadditions of 2-Acetoxy-1,4-naphthoquinone and Structure Determination of the Main Photoadducts. Photochem, 5(4), 31. https://doi.org/10.3390/photochem5040031







