Biomedical Applications of Raman Spectroscopy: A Review
Abstract
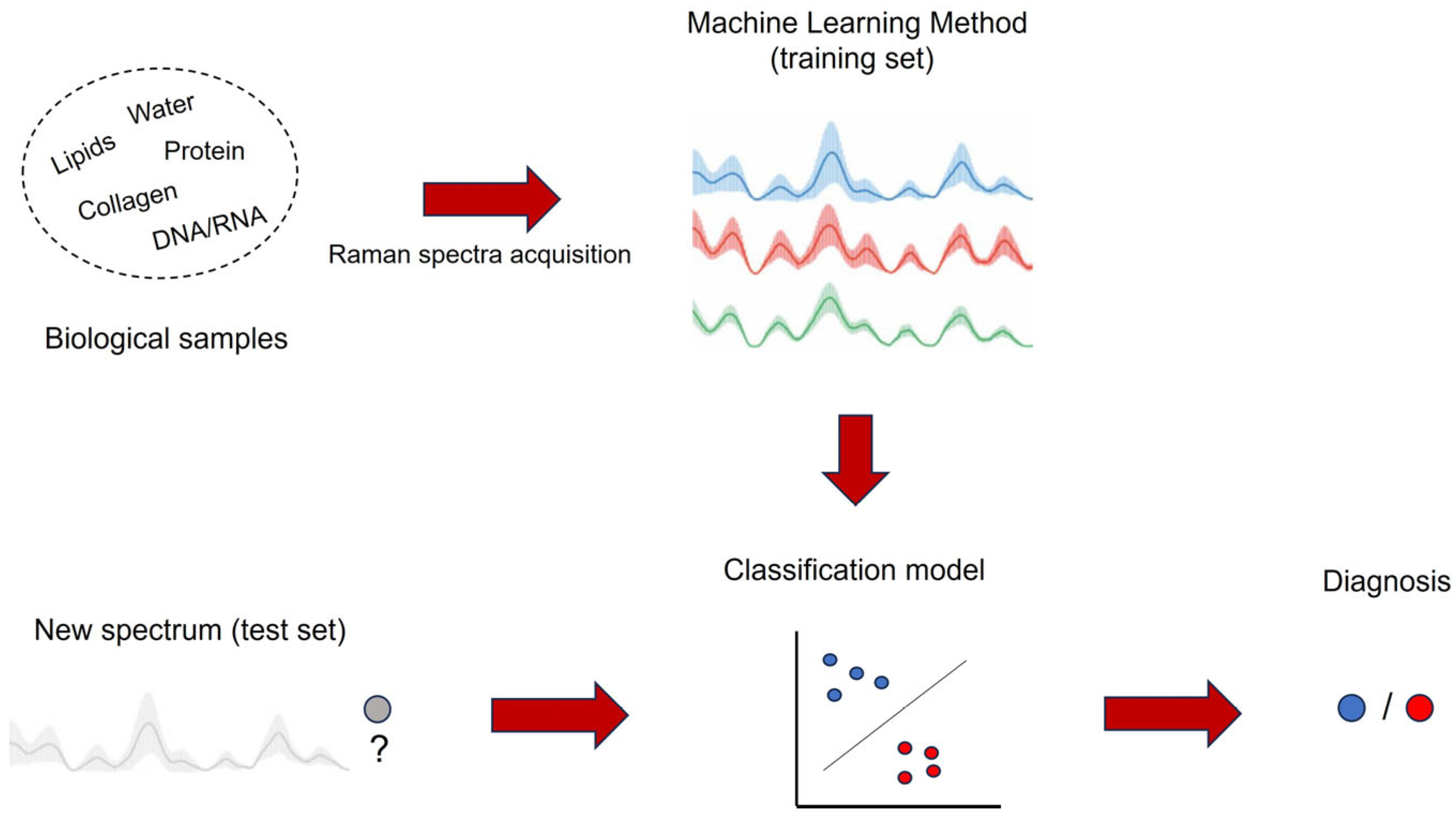
1. Introduction
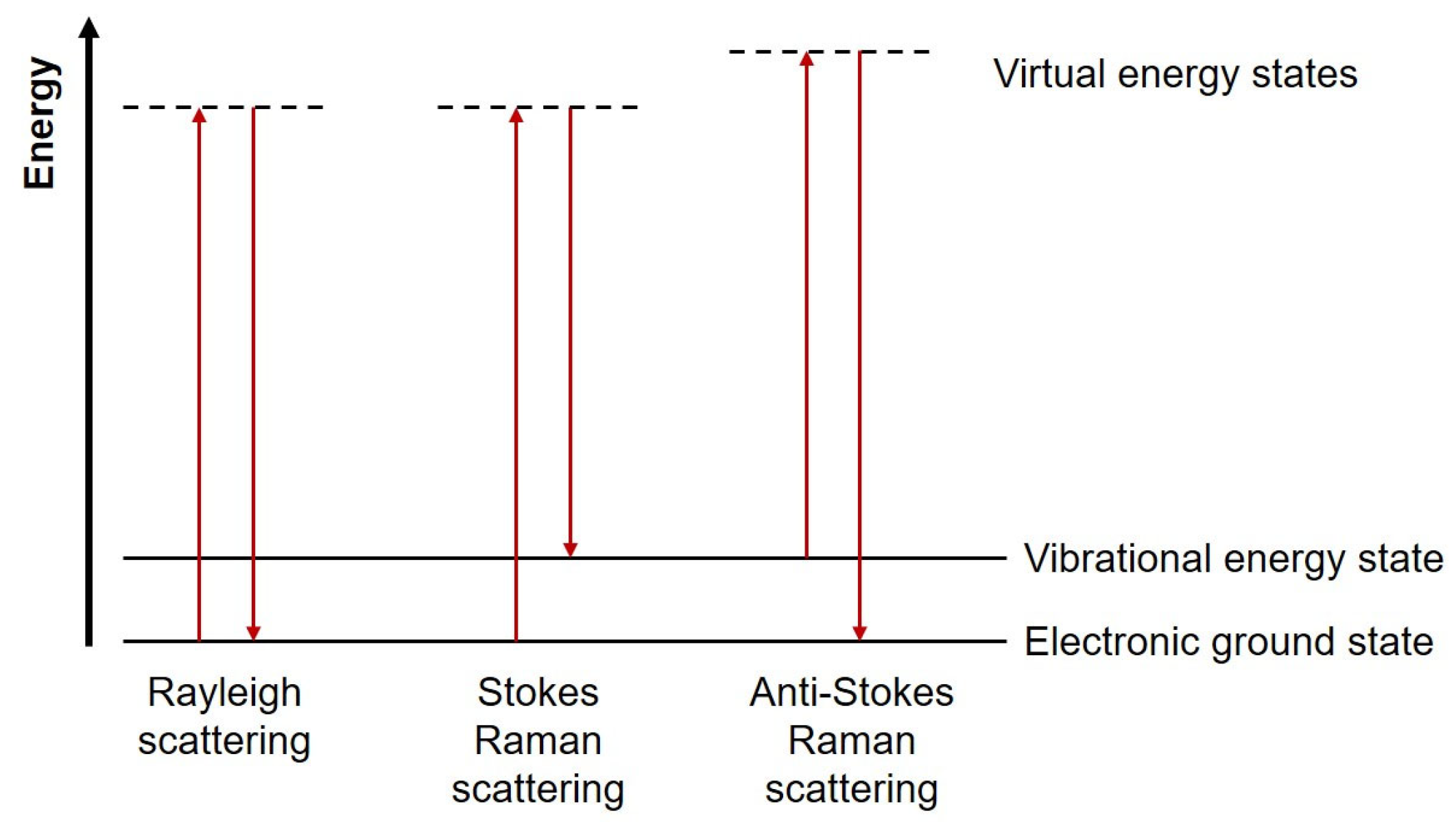
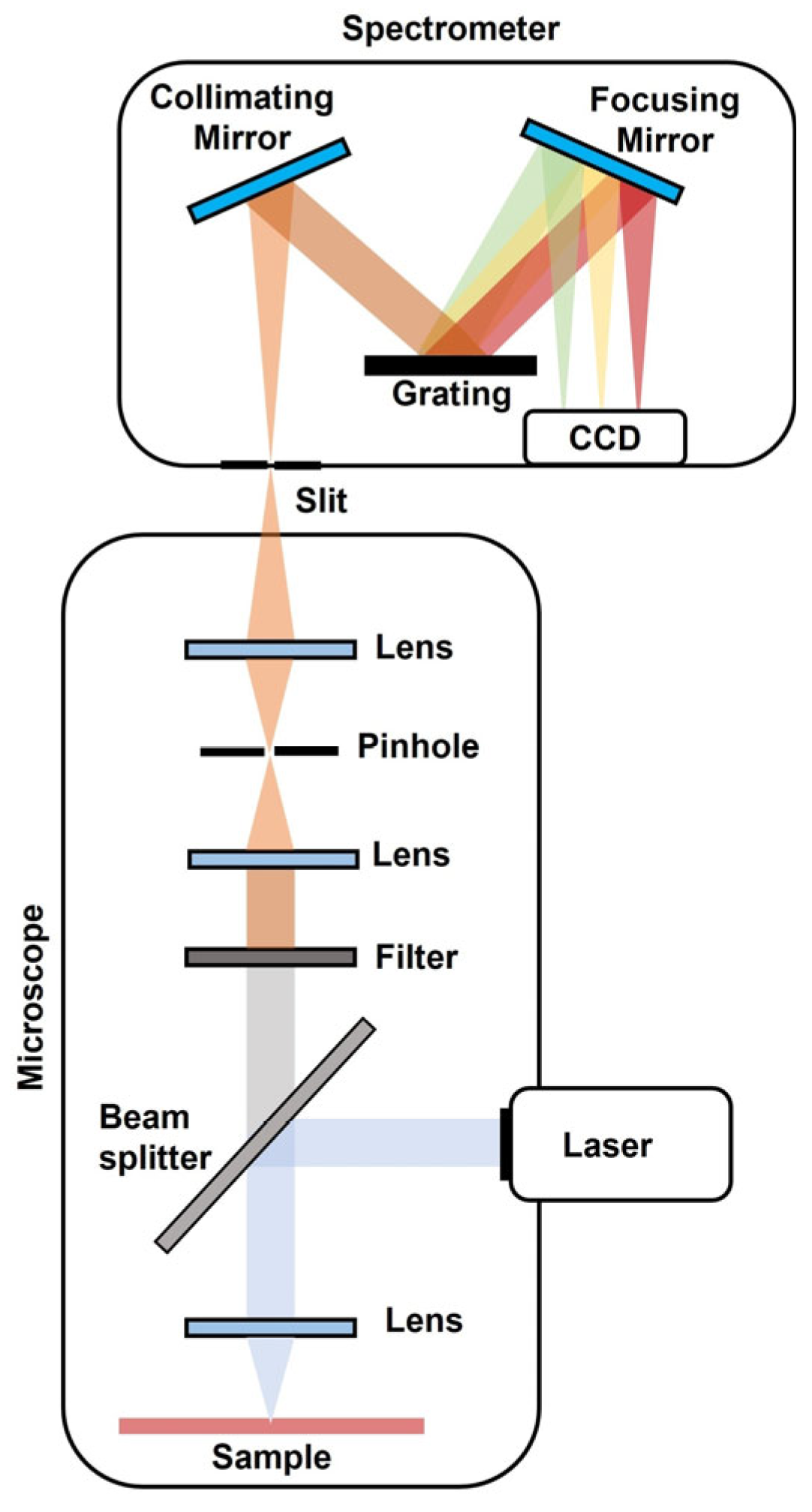
2. Principles of Raman Spectroscopy
3. Raman Biomedical Applications
3.1. Cancer
3.2. Neurodegenerative Diseases
3.3. Immunology
3.4. Other Applications
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khristoforova, Y.; Bratchenko, L.; Bratchenko, I. Raman-Based Techniques in Medical Applications for Diagnostic Tasks: A Review. Int. J. Mol. Sci. 2023, 24, 15605. [Google Scholar] [CrossRef]
- Chen, C.; Qi, J.; Li, Y.; Li, D.; Wu, L.; Li, R.; Chen, Q.; Sun, N. Applications of Raman Spectroscopy in the Diagnosis and Monitoring of Neurodegenerative Diseases. Front. Neurosci. 2024, 18, 1301107. [Google Scholar] [CrossRef]
- Chaudhary, N.; Wynne, C.; Meade, A.D. A Review of Applications of Raman Spectroscopy in Immunology. Biomed. Spectrosc. Imaging 2020, 9, 23–31. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, E.X.; Hu, D.; Yang, Y.; Wu, Z.; Zheng, M.; Sadi, M.A.; Jiang, Y.; Zhang, K.; Chen, Z.; et al. Applications of Raman Spectroscopy in Clinical Medicine. Food Front. 2024, 5, 392–419. [Google Scholar] [CrossRef]
- Ye, Q.; Spencer, P. Analyses of Material-Tissue Interfaces by Fourier Transform Infrared, Raman Spectroscopy, and Chemometrics. In Material-Tissue Interfacial Phenomena; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–251. [Google Scholar]
- Hernández, Y.; Galarreta, B.C. Noble Metal-Based Plasmonic Nanoparticles for SERS Imaging and Photothermal Therapy. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–109. [Google Scholar]
- Kim, Y.; Jung, S.U.; Choi, J. Application of Raman Spectroscopy in Breast Cancer Surgery. Kosin Med. J. 2023, 38, 176–183. [Google Scholar] [CrossRef]
- Bratchenko, I.A.; Bratchenko, L.A.; Khristoforova, Y.A.; Moryatov, A.A.; Kozlov, S.V.; Zakharov, V.P. Classification of Skin Cancer Using Convolutional Neural Networks Analysis of Raman Spectra. Comput. Methods Programs Biomed. 2022, 219, 106755. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Pan, X.; Yu, H.; Hua, S.; Wang, D.; Chen, D.Z.; Zhou, M.; Wu, J. A Deep Learning Approach for Detecting Colorectal Cancer via Raman Spectra. BME Front. 2022, 2022, 9872028. [Google Scholar] [CrossRef]
- Nargis, H.F.; Nawaz, H.; Bhatti, H.N.; Jilani, K.; Saleem, M. Comparison of Surface Enhanced Raman Spectroscopy and Raman Spectroscopy for the Detection of Breast Cancer Based on Serum Samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 246, 119034. [Google Scholar] [CrossRef]
- Li, Y.; Jia, M.; Zeng, X.; Huang, K.; Bai, Z. Pattern Recognition Methods Combined with Raman Spectra Applied to Distinguish Serums from Lung Cancer Patients and Healthy People. J. Biosci. Med. 2017, 5, 95–105. [Google Scholar] [CrossRef]
- Artemyev, D.N.; Kukushkin, V.I.; Avraamova, S.T.; Aleksandrov, N.S.; Kirillov, Y.A. Using the Method of “Optical Biopsy” of Prostatic Tissue to Diagnose Prostate Cancer. Molecules 2021, 26, 1961. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.-X.; Ma, C.-L.; Zheng, X.-X.; Lv, X.-Y.; Lv, G.-D.; Tang, J.; Wu, G.-H. Raman Spectroscopic Study of Cervical Precancerous Lesions and Cervical Cancer. Lasers Med. Sci. 2021, 36, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, R.; Khan, A.; Ashraf, R.; Ali, H.; Bilal, M.; Saleem, M. Analysis of Hepatitis B Virus Infection in Blood Sera Using Raman Spectroscopy and Machine Learning. Photodiagnosis Photodyn. Ther. 2018, 23, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Guevara, E.; Torres-Galván, J.C.; Ramírez-Elías, M.G.; Luevano-Contreras, C.; González, F.J. Use of Raman Spectroscopy to Screen Diabetes Mellitus with Machine Learning Tools. Biomed. Opt. Express 2018, 9, 4998. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.I.S.; Löbenberg, R.; Bou-Chacra, N.A. Raman Spectroscopy for Quantitative Analysis in the Pharmaceutical Industry. J. Pharm. Pharm. Sci. 2020, 23, 24–46. [Google Scholar] [CrossRef]
- Siraj, N.; Bwambok, D.K.; Brady, P.N.; Taylor, M.; Baker, G.A.; Bashiru, M.; Macchi, S.; Jalihal, A.; Denmark, I.; Le, T.; et al. Raman Spectroscopy and Multivariate Regression Analysis in Biomedical Research, Medical Diagnosis, and Clinical Analysis. Appl. Spectrosc. Rev. 2021, 56, 615–672. [Google Scholar] [CrossRef]
- Adamczyk, A.; Orzechowska, S.; Nowakowska, A.M.; Brzozowski, K.; Majzner, K.; Baranska, M. Stimulated Raman Scattering Microscopy in the Analysis of Cancer Cells—A Review and Own Research. TrAC Trends Anal. Chem. 2023, 169, 117366. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An Overview of Advanced Methods for the Characterization of Oxygen Vacancies in Materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Eberhardt, K.; Stiebing, C.; Matthäus, C.; Schmitt, M.; Popp, J. Advantages and Limitations of Raman Spectroscopy for Molecular Diagnostics: An Update. Expert. Rev. Mol. Diagn. 2015, 15, 773–787. [Google Scholar] [CrossRef]
- Kurouski, D.; Dazzi, A.; Zenobi, R.; Centrone, A. Infrared and Raman Chemical Imaging and Spectroscopy at the Nanoscale. Chem. Soc. Rev. 2020, 49, 3315–3347. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman Spectroscopy in Cancer Diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef]
- Schmid, T.; Dariz, P. Raman Microspectroscopic Imaging of Binder Remnants in Historical Mortars Reveals Processing Conditions. Heritage 2019, 2, 1662–1683. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, Q.; Li, B.; Zhao, X. Raman Spectroscopy: A Novel Technology for Gastric Cancer Diagnosis. Front. Bioeng. Biotechnol. 2022, 10, 856591. [Google Scholar] [CrossRef] [PubMed]
- Fousková, M.; Vališ, J.; Synytsya, A.; Habartová, L.; Petrtýl, J.; Petruželka, L.; Setnička, V. In Vivo Raman Spectroscopy in the Diagnostics of Colon Cancer. Analyst 2023, 148, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Esteves, B.; Pimenta, S.; Maciel, M.J.; Costa, M.; Baltazar, F.; Cerqueira, M.F.; Alpuim, P.; Silva, C.A.; Correia, J.H. Raman Spectroscopy for Classification of Neoplastic and Non-Neoplastic CAM Colon Tumors. Heliyon 2024, 10, e36981. [Google Scholar] [CrossRef]
- Hanna, K.; Krzoska, E.; Shaaban, A.M.; Muirhead, D.; Abu-Eid, R.; Speirs, V. Raman Spectroscopy: Current Applications in Breast Cancer Diagnosis, Challenges and Future Prospects. Br. J. Cancer 2022, 126, 1125–1139. [Google Scholar] [CrossRef]
- Lazaro-Pacheco, D.; Shaaban, A.M.; Titiloye, N.A.; Rehman, S.; ur Rehman, I. Elucidating the Chemical and Structural Composition of Breast Cancer Using Raman Micro-Spectroscopy. EXCLI J. 2021, 20, 1118–1132. [Google Scholar]
- Bhattacharjee, T.; Maru, G.; Ingle, A.; Murali Krishna, C. Transcutaneous in Vivo Raman Spectroscopy of Breast Tumors and Pretumors. J. Raman Spectrosc. 2015, 46, 1053–1061. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, R.; Oyaert, M.; De Bruyne, S.; Speeckaert, M.M. From Vibrations to Visions: Raman Spectroscopy’s Impact on Skin Cancer Diagnostics. J. Clin. Med. 2023, 12, 7428. [Google Scholar] [CrossRef]
- Hano, H.; Lawrie, C.H.; Suarez, B.; Paredes Lario, A.; Elejoste Echeverría, I.; Gómez Mediavilla, J.; Crespo Cruz, M.I.; Lopez, E.; Seifert, A. Power of Light: Raman Spectroscopy and Machine Learning for the Detection of Lung Cancer. ACS Omega 2024, 9, 14084–14091. [Google Scholar] [CrossRef]
- Fousková, M.; Habartová, L.; Vališ, J.; Nahodilová, M.; Vaňková, A.; Synytsya, A.; Šestáková, Z.; Votruba, J.; Setnička, V. Raman Spectroscopy in Lung Cancer Diagnostics: Can an in Vivo Setup Compete with Ex Vivo Applications? Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 322, 124770. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Yang, X.; Shao, X.; Li, T.; Chen, N.; Chen, Z.; Xue, W.; Pan, J.; Liu, S. Raman Spectroscopy Reveals Abnormal Changes in the Urine Composition of Prostate Cancer: An Application of an Intelligent Diagnostic Model with a Deep Learning Algorithm. Adv. Intell. Syst. 2021, 3, 2000090. [Google Scholar] [CrossRef]
- van Breugel, S.J.; Low, I.; Christie, M.L.; Pokorny, M.R.; Nagarajan, R.; Holtkamp, H.U.; Srinivasa, K.; Amirapu, S.; Nieuwoudt, M.K.; Simpson, M.C.; et al. Raman Spectroscopy System for Real-time Diagnosis of Clinically Significant Prostate Cancer Tissue. J. Biophotonics 2023, 16, e202200334. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Daniel, A.; Lyng, F.M. Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review. Molecules 2023, 28, 2502. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Prabitha, V.G.; Dora, T.K.; Chopra, S.; Maheshwari, A.; Deodhar, K.; Rekhi, B.; Sukumar, N.; Krishna, C.M.; Subhash, N. A Comparative Evaluation of Diffuse Reflectance and Raman Spectroscopy in the Detection of Cervical Cancer. J. Biophotonics 2017, 10, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Aggarwal, L.M.; Murali Krishna, C.; Pradhan, S.; Mishra, S.P.; Choudhary, S.; Patel, C.B.; Singla, S.; Singh, R.K. Diagnostic and Prognostic Application of Raman Spectroscopy in Carcinoma Cervix: A Biomolecular Approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119356. [Google Scholar] [CrossRef]
- Murugappan, S.; Tofail, S.A.M.; Thorat, N.D. Raman Spectroscopy: A Tool for Molecular Fingerprinting of Brain Cancer. ACS Omega 2023, 8, 27845–27861. [Google Scholar] [CrossRef]
- Stupak, E.V.; Glotov, V.M.; Askandaryan, A.S.; Clancy, S.E.; Hiana, J.C.; Cherkasova, O.P.; Stupak, V. V Raman Spectroscopy in the Diagnosis of Brain Gliomas: A Literature Review. Cureus 2025, 17, e79165. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; De Pasquale, D.; Fiaschi, P.; Ciofani, G. Discrimination of Glioma Patient-Derived Cells from Healthy Astrocytes by Exploiting Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 269, 120773. [Google Scholar] [CrossRef]
- Bukva, M.; Dobra, G.; Gomez-Perez, J.; Koos, K.; Harmati, M.; Gyukity-Sebestyen, E.; Biro, T.; Jenei, A.; Kormondi, S.; Horvath, P.; et al. Raman Spectral Signatures of Serum-Derived Extracellular Vesicle-Enriched Isolates May Support the Diagnosis of CNS Tumors. Cancers 2021, 13, 1407. [Google Scholar] [CrossRef]
- Kanmalar, M.; Abdul Sani, S.F.; Kamri, N.I.N.B.; Said, N.A.B.M.; Jamil, A.H.B.A.; Kuppusamy, S.; Mun, K.S.; Bradley, D.A. Raman Spectroscopy Biochemical Characterisation of Bladder Cancer Cisplatin Resistance Regulated by FDFT1: A Review. Cell Mol. Biol. Lett. 2022, 27, 9. [Google Scholar] [CrossRef]
- Bovenkamp, D.; Sentosa, R.; Rank, E.; Erkkilä, M.T.; Placzek, F.; Püls, J.; Drexler, W.; Leitgeb, R.A.; Garstka, N.; Shariat, S.F.; et al. Combination of High-Resolution Optical Coherence Tomography and Raman Spectroscopy for Improved Staging and Grading in Bladder Cancer. Appl. Sci. 2018, 8, 2371. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of Multidimensional Data Processing Approaches for Raman and Infrared Spectroscopy. EPJ Tech. Instrum. 2015, 2, 8. [Google Scholar] [CrossRef]
- Otel, I.; Silveira, J.; Vassilenko, V.; Mata, A.; Carvalho, M.L.; Santos, J.P.; Pessanha, S. Application of Unsupervised Multivariate Analysis Methods to Raman Spectroscopic Assessment of Human Dental Enamel. Computers 2021, 11, 5. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Morais, C.L.M.; Halliwell, D.E.; Mann, D.M.A.; Allsop, D.; Martin-Hirsch, P.L.; Martin, F.L. Raman Spectroscopy to Diagnose Alzheimer’s Disease and Dementia with Lewy Bodies in Blood. ACS Chem. Neurosci. 2018, 9, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Carota, A.G.; Campanella, B.; Del Carratore, R.; Bongioanni, P.; Giannelli, R.; Legnaioli, S. Raman Spectroscopy and Multivariate Analysis as Potential Tool to Follow Alzheimer’s Disease Progression. Anal. Bioanal. Chem. 2022, 414, 4667–4675. [Google Scholar] [CrossRef]
- Carlomagno, C.; Bertazioli, D.; Gualerzi, A.; Picciolini, S.; Andrico, M.; Rodà, F.; Meloni, M.; Banfi, P.I.; Verde, F.; Ticozzi, N.; et al. Identification of the Raman Salivary Fingerprint of Parkinson’s Disease Through the Spectroscopic– Computational Combinatory Approach. Front. Neurosci. 2021, 15, 704963. [Google Scholar] [CrossRef]
- Ryzhikova, E.; Ralbovsky, N.M.; Sikirzhytski, V.; Kazakov, O.; Halamkova, L.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Raman Spectroscopy and Machine Learning for Biomedical Applications: Alzheimer’s Disease Diagnosis Based on the Analysis of Cerebrospinal Fluid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119188. [Google Scholar] [CrossRef]
- Griffin, G.D.; Stratis-Cullum, D.N. Biosensors. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 88–103. [Google Scholar]
- Verma, P. Tip-Enhanced Raman Spectroscopy: Technique and Recent Advances. Chem. Rev. 2017, 117, 6447–6466. [Google Scholar] [CrossRef]
- Xu, J.; Morten, K.J. Raman Micro-Spectroscopy as a Tool to Study Immunometabolism. Biochem. Soc. Trans. 2024, 52, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Callery, E.L.; Morais, C.L.M.; Nugent, L.; Rowbottom, A.W. Classification of Systemic Lupus Erythematosus Using Raman Spectroscopy of Blood and Automated Computational Detection Methods: A Novel Tool for Future Diagnostic Testing. Diagnostics 2022, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Chen, C.; Luo, C.; Shi, Y.; Li, Z.; Lv, X.; Chen, C.; Su, J.; Wu, L. Raman Spectroscopy Combined with a Support Vector Machine Algorithm as a Diagnostic Technique for Primary Sjögren’s Syndrome. Sci. Rep. 2023, 13, 5137. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wu, X.; Chen, X.; Zhang, Z.; Wang, J.; Li, Z.; Chen, X.; Lei, X.; Li, Z.; Ma, M.; et al. Diagnosis and Activity Prediction of SLE Based on Serum Raman Spectroscopy Combined with a Two-Branch Bayesian Network. Front. Immunol. 2025, 16, 1467027. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, S.H.; Yu, L.; Zhang, Z.H.; Zhang, W. Analysis and Classification of Hepatitis Infections Using Raman Spectroscopy and Multiscale Convolutional Neural Networks. J. Appl. Spectrosc. 2021, 88, 441–451. [Google Scholar] [CrossRef]
- Wu, J.; Cui, X.; Kang, Z.; Wang, S.; Zhu, G.; Yang, S.; Wang, S.; Li, H.; Lu, C.; Lv, X. Rapid Diagnosis of Diabetes Based on ResNet and Raman Spectroscopy. Photodiagnosis Photodyn. Ther. 2022, 39, 103007. [Google Scholar] [CrossRef]
- Luo, R.; Popp, J.; Bocklitz, T. Deep Learning for Raman Spectroscopy: A Review. Analytica 2022, 3, 287–301. [Google Scholar] [CrossRef]
- Pathak, D.K.; Rani, C.; Sati, A.; Kumar, R. Developments in Raman Spectromicroscopy for Strengthening Materials and Natural Science Research: Shaping the Future of Physical Chemistry. ACS Phys. Chem. Au 2024, 4, 430–438. [Google Scholar] [CrossRef]
- Canetta, E. Current and Future Advancements of Raman Spectroscopy Techniques in Cancer Nanomedicine. Int. J. Mol. Sci. 2021, 22, 13141. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, L.; Wang, Y.; Xiong, Z. Current Trends of Raman Spectroscopy in Clinic Settings: Opportunities and Challenges. Adv. Sci. 2024, 11, 2300668. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Zhang, H.; Guo, Y.; Tang, M.; Wang, J.; Wu, N. Current Research Status of Raman Spectroscopy in Glioma Detection. Photodiagnosis Photodyn. Ther. 2024, 50, 104388. [Google Scholar] [CrossRef]

| Reference | Cancer | Experiment | Data Analysis |
|---|---|---|---|
| Cao et al. [9], 2022 | Colorectal | Ex vivo | Deep-learning approach |
| Fousková et al. [27], 2023 | In vivo | Supervised machine learning methods | |
| Esteves et al. [28], 2024 | Colon | CAM samples | Machine learning based algorithms |
| Lazaro-Pacheco et al. [30], 2021 | Breast | Ex vivo | PCA and LDA |
| Bhattacharjee et al. [31], 2015 | In vivo | PCA and PC-LDA | |
| Nargis et al. [10], 2021 | Serum sample | PCA and PLS-DA | |
| Bratchenko et al. [8], 2022 | Skin | In vivo | CNN analysis |
| Hano et al. [33], 2024 | Lung | Blood plasma samples | Machine learning models |
| Fousková et al. [34], 2024 | In vivo and ex vivo | PCA-SVM model | |
| Chen et al. [35], 2021 | Prostate | Urine samples | CNN algorithm |
| Breugel et al. [36], 2023 | Ex vivo | PCA and PLS-DA | |
| Wang et al. [13], 2021 | Cervical | Ex vivo | Independent t-test, SVM algorithm |
| Shaikh et al. [38], 2017 | In vivo | PCA-LDA | |
| Shrivastava et al. [39], 2021 | Serum samples | PCA-LDA | |
| Iturrioz-Rodríguez et al. [42], 2022 | Brain | Ex vivo | PCA-LDA |
| Bukva et al. [43], 2021 | Blood serum | PCA-SVM algorithm | |
| Bovenkamp et al. [45], 2018 | Bladder | Ex vivo | PCA and kNN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenta, S.; Correia, J.H. Biomedical Applications of Raman Spectroscopy: A Review. Photochem 2025, 5, 29. https://doi.org/10.3390/photochem5040029
Pimenta S, Correia JH. Biomedical Applications of Raman Spectroscopy: A Review. Photochem. 2025; 5(4):29. https://doi.org/10.3390/photochem5040029
Chicago/Turabian StylePimenta, Sara, and José H. Correia. 2025. "Biomedical Applications of Raman Spectroscopy: A Review" Photochem 5, no. 4: 29. https://doi.org/10.3390/photochem5040029
APA StylePimenta, S., & Correia, J. H. (2025). Biomedical Applications of Raman Spectroscopy: A Review. Photochem, 5(4), 29. https://doi.org/10.3390/photochem5040029






