Abstract
In the light reactions of photosynthesis, reactive oxygen species (ROS), such as superoxide anion radical (O2•−), hydrogen peroxide (H2O2), singlet oxygen (1O2*), and hydroxyl radical (OH•), are continuously generated at basal levels and are kept in homeostasis by the antioxidative enzymatic and non-enzymatic systems. Nevertheless, under abiotic or biotic stress conditions, this balance between the creation and elimination of ROS is disrupted, and the increased ROS production leads to oxidative stress, which is involved in the growth retardation of plants. However, ROS are also beneficial, since they trigger the plant’s defense mechanisms for handling oxidative stress and are fundamental signaling molecules for the regulation of a range of physiological functions under optimum growth conditions or environmental stress circumstances, activating a plethora of acclimation responses. Gaining insight into the relationship between ROS generation, ROS scavenging, and the protective role of ROS will contribute to improving agricultural sustainability in the face of global climate change.
1. Introduction
Photosynthesis is the process by which the solar energy is converted into chemical energy for the synthesis of all the essential organic molecules that maintain the life of all living organisms on earth [1,2,3]. Chlorophylls are the main pigments that absorb the light energy in the light-harvesting complexes (LHCs, antenna) of photosystem I (PSI) and photosystem II (PSII) and transfer it to the reaction centers (RCs), where charge separation occurs and the electron transport (ETR) is initiated from PSII to PSI to convert the light energy into chemical energy [4,5]. Light, through the process of photosynthesis, fuels with energy all life on earth [1,4,5]. However, excess light can lead to a decline in photosynthetic efficiency causing oxidative stress by creating reactive oxygen species (ROS) [5,6,7,8,9,10,11]. Moreover, almost all abiotic and biotic stresses result in increased ROS creation that leads to oxidative stress (Figure 1). Light-induced decrease in photosynthesis is called photoinhibition, which primarily affects the photosystem II (PSII) complex, which accomplishes the light-driven oxidation of water, although photosystem I (PSI), which executes the reduction of NADP, can also be damaged by environmental stress conditions [10]. Environmental stress conditions result in downregulation of the Calvin-Benson-Bassham cycle and in a diminished utilization of the reductive power generated in the electron transport chain, accelerating the production of ROS and oxidative stress but also photoinhibition [12,13,14].
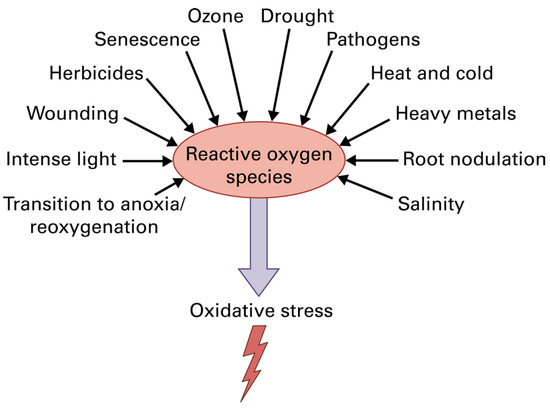
Figure 1.
Reactive oxygen species (ROS) production by various environmental stresses that have, as a result, the creation of oxidative stress in plant cells. Reprinted with permission from Ref. [15]. Copyright 2015, John Wiley & Sons.
Photoinhibition is described as an imbalance between PSII photodamage and PSII repair [16,17]. Thus, PSII photodamage further reduces the photochemical efficiency through photoinhibition [18,19]. PSII is one of the most susceptible components of the photosynthetic apparatus to environmental stress conditions, exhibiting high sensitivity to photoinhibition and thermal inactivation, which leads to decreased efficiency of the oxygen-evolving complex (OEC) [20,21]. To prevent ROS formation and photoinhibition, the absorbed light energy by the light-harvesting complexes (LHCs) must match the rate of electron transport from PSII to PSI [4,5,14,16,17,22,23,24].
2. ROS Generation, Scavenging, and Photoprotection
In the light reactions of photosynthesis, ROS, such as superoxide anion radical (O2•−), hydrogen peroxide (H2O2), and singlet oxygen (1O2*), are continuously generated at basal levels [5,22,23,24]. Under optimal growth conditions, ROS are kept in homeostasis by the antioxidative enzymatic and non-enzymatic systems, but under abiotic or biotic stress conditions, the balance between the creation and elimination of ROS is disrupted [8,14,24,25,26,27,28,29]. In a variety of environmental stress conditions, the increased ROS production leads to oxidative stress, which is involved in the growth retardation of plants [30,31]. However, PSII response reactions to stress depend on light intensity and leaf developmental stage [3,32], while whole-plant reactions to stress vary with developmental stage and fitness [33].
The absorbed light energy by chlorophylls and carotenoids initiates the electron transport in chloroplasts for the synthesis of ATP and NADPH [4,6]. When there is an excess of absorbed light energy by the photosynthetic pigments that cannot be de-excited by the process of photochemistry (photochemical quenching), or dissipated as heat (NPQ) or as fluorescence (FL), then photosystem II (PSII) is overexcited [4,6,9] (Figure 2).
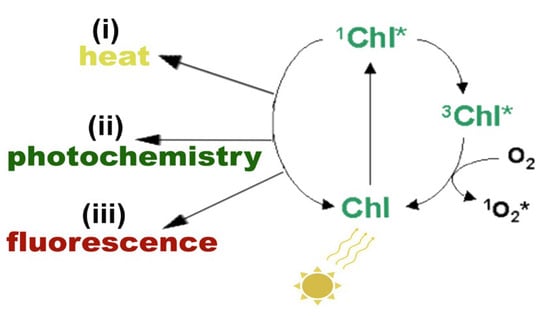
Figure 2.
Possible pathways of the singlet excited state chlorophyll molecule (1Chl*) de-excitation: (i) by losing energy as heat, (ii) by transferring the energy to an electron-acceptor molecule, called photochemistry, or (iii) by reemitting light through fluorescence. If 1Chl* is not quenched, it is converted to the triplet excited chlorophyll state (3Chl*) that can react with molecular O2, producing singlet excited oxygen (1O2*). Reprinted from Ref. [29].
This over-excitation of PSII increases the probability of a triplet excited chlorophyll state (3Chl*) formation from the singlet excited chlorophyll state (1Chl*). When the quenching of 1Chl* is not sufficient [4,34,35], then the singlet chlorophyll is converted to the lower-energy 3Chl*, through intersystem crossing (Figure 2). However, at this stage, 3Chl* can react with molecular O2, producing singlet excited oxygen (1O2*) at PSII [34,35,36] (Figure 2 and Figure 3). 1O2* is considered the major ROS involved in photooxidative damage of plants [37] and can be quenched by ß-carotene, α-tocopherol, or plastoquinol [36,37,38,39].
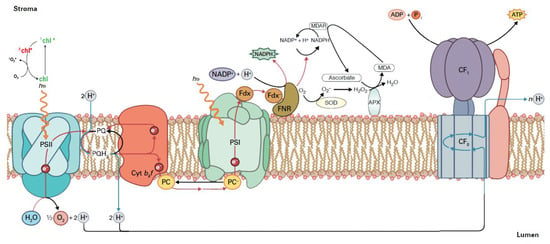
Figure 3.
Reactive oxygen species (ROS) production in the chloroplast electron transport chain. During the process of the electron transport chain for the production of NADPH and ATP, if there is a surplus of light energy, singlet excited oxygen (1O2*) is formed via the triplet state of chlorophyll (3chl*). When NADPH is not used in the Calvin-Benson-Bassham cycle for the synthesis of carbohydrates, then NADP+ is not available, and the electrons are transferred to O2 instead. As a consequence, the superoxide anion (O2•−) is formed. Sequentially, O2•− can be converted to hydrogen peroxide (H2O2) by the superoxide dismutase (SOD). The H2O2 can be reduced to H2O by the ascorbate peroxidase (APX). The oxidized ascorbate is reduced from NADPH, through monodehydroascorbate reductase (MDAR), and, as an outcome, NADP+ can be available. Reprinted from Ref. [40].
On the PSII electron acceptor side, electron leakage to molecular oxygen forms O2•−, which is converted to H2O2, and on the PSII electron donor side, incomplete water oxidation forms H2O2, which is reduced by Mn to hydroxyl radical (OH•) [41]. Electron leakage to O2 at PSI results in O2•−, which is converted via a disproportionation reaction catalyzed by the enzyme superoxide dismutase (SOD) to H2O2 and molecular oxygen [5,27,42,43,44] (Figure 3). Subsequently, H2O2 can be reduced to H2O by the ascorbate peroxidase (APX) [43,44]. The oxidized ascorbate is reduced from NADPH, through monodehydroascorbate reductase (MDAR), and, as an outcome, NADP+ can be available (Figure 3) [43,44].
The efficient dissipation of excess light is crucial to prevent overaccumulation of ROS in chloroplasts [4,45,46]. One mechanism for accomplishing this in PSII is through the photoprotective mechanism of nonphotochemical quenching (NPQ), which converts excess light energy into heat, thereby preventing increased ROS accumulation [9,22]. The photoprotective mechanism of NPQ is considered efficient under environmental stress conditions if it can maintain an equal percentage of open PSII reaction centers as under non-stress conditions [47,48,49]. Otherwise, an inconsistency between the absorbed light energy and the energy requirement occurs, indicating excess excitation energy under environmental stress conditions [22,48,50]. The vital strategy to increase the photosynthetic efficiency of crop plants lies in optimizing the light energy use efficiency [3]. An optimization of photosynthetic efficiency in crop plants is essential not only for improving the yield but also for all metabolic and growth processes and therefore results in better stress resilience and resource use efficiency.
In addition to the process of NPQ, which is considered the principal photoprotective mechanism to prevent overaccumulation of ROS and is typically estimated by chlorophyll fluorescence analysis, plants have efficient enzymatic and non-enzymatic antioxidant mechanisms [14,25,26,27]. Besides the antioxidant enzymes we already mentioned, SOD, APX, and MDAR, other enzymatic antioxidant mechanisms include glutathione peroxidase (GPX), glutathione reductase (GR), catalase (CAT), guaiacol peroxidase (GOPX), and the ascorbate-glutathione (AsA-GSH) cycle, which is an essential constituent of the enzymatic antioxidant defense system in plants [5,8,18,27,29,44,51,52,53,54]. As non-enzymatic antioxidants are considered a-tocopherol, glutathione (GSH), ascorbate (AsA), carotenoids, phenolic compounds, flavonoids, and proline, which also play serious roles in removing excessive ROS production [5,8,18,27,29,44,52,54].
Photoprotection can also be accomplished by photorespiration, which acts as a safety valve [55], counteracting the over-reduction of the electron transport chain and limiting ROS production [23,56,57,58]. However, photorespiration lowers net carbon fixation in the Calvin-Benson-Bassham cycle [59,60] and decreases photosynthetic income [58], being considered as an energy-expensive process [61]. Therefore, activation of photorespiration may result in lower yields. In addition to photorespiration, cyclic electron flow (CEF) is also employed for inhibiting the over-reduction of the plastoquinone pool and ROS creation [62], but it reduces photochemical efficiency [23]. The CEF involves PSI, plastoquinone, the cytochrome b6f complex, and plastocyanin (Figure 4). PSI reduces ferredoxin in the light, but the reduced ferredoxin, instead of transmitting electrons to NADP+ (Figure 3), transfers electrons to the plastoquinone (PQ) pool (Figure 4).
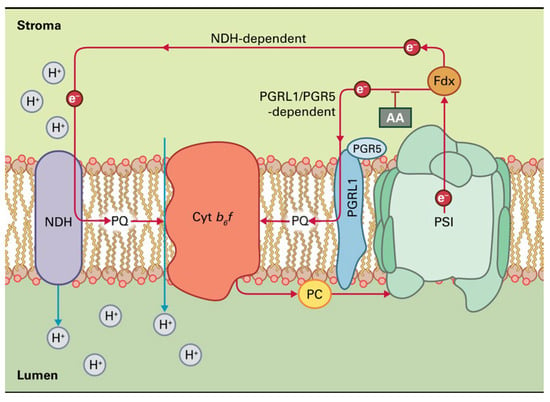
Figure 4.
The two pathways of cyclic electron transport around PSI in plants. The first pathway uses the NADH dehydrogenase-like (NDH) complex and is especially prominent during oxidative stress. The second pathway, which is sensitive to antimycin A (AA), implicates a ferredoxin-plastoquinone oxidoreductase activity involving the proteins PGRL1 and PGR5. Reprinted with permission from Ref. [15]. Copyright 2015, John Wiley & Sons.
Reduced plastoquinol can then be oxidized by the cytochrome b6f complex, permitting proton translocation across the membrane. The electrons then complete the cycle by returning to PSI via plastocyanin (Figure 4). Thus, cyclic electron transport creates only ATP as a product without the net production of NADPH. There are two pathways of CEF around PSI in plants (Figure 4). The first pathway uses the NADH dehydrogenase-like (NDH) complex and is especially prominent during oxidative stress, while, for the second pathway, an assumed ferredoxin-plastoquinone oxidoreductase activity involving the thylakoid proteins, PGRL1 and PGR5, is necessary [4,63,64]. This pathway is sensitive to antimycin A (AA) (Figure 4). CEF around PSI plays a key role in sustaining photosynthesis by balancing the ATP/NADPH ratio and protecting photosystems from photoinhibition [65,66,67].
3. ROS as Signaling Molecules
Light reactions of photosynthesis influence nuclear gene expression, a phenomenon referred to as retrograde signaling [68,69]. ROS created during the light reactions of photosynthesis play vital roles as regulatory molecules in retrograde signaling processes, activating the plant’s defense responses to environmental stressors and contributing to restoring the “oxidation-reduction” balance [11,14,43,70,71,72,73,74,75,76]. Oxidative damage under environmental stress conditions can be avoided by joint inhibition mechanisms and ROS detoxification [27,77,78,79]. Antioxidants (enzymatic and non-enzymatic) are not meant to eliminate ROS entirely, but rather to maintain a balanced interplay between ROS production and scavenging, supporting efficient photosynthetic process and enabling effective signal transmission to the nucleus [79,80,81], thus, underlining the crucial role of ROS in maintaining the cell homeostasis but also in fine-tuning the plant stress response. ROS regulate electron transport, preventing not only over-reduction and over-oxidation but also participating in the formation of redox regulatory networks, allowing plants to detect and respond to environmental stressors [71,82,83]. ROS information of the electron transport process acts as the chloroplast-to-nucleus retrograde signaling, indicating the role of chloroplasts as environmental sensors and affecting the entire plant, leading to stress-specific physiological changes [84,85]. The redox state of the plastoquinol pool (qp) is known to be important for retrograde signaling [86,87,88]. The redox state of the plastoquinol pool and ROS signaling connects the photosynthesizing chloroplast to the rest of the cell, influencing cytosolic and nuclear functions in response to light [87].
External H2O2 application revealed dose-dependent effects that stimulate or prevent growth [89]. Parallel or contrasting patterns of H2O2 with nitric oxide (NO) were found during plant responses to different environmental stressors [90]. NO is a signaling molecule employing both pro-oxidant and antioxidant effects depending on its concentration and the interaction with other molecules [91]. At low concentrations, NO inhibits lipid peroxidation, acting by scavenging as an antioxidant, thereby safeguarding cells from oxidative damage [91,92].
ROS were originally thought to be toxic by-products that must be removed to prevent oxidative damage to the cell, but subsequent studies revealed that ROS are used by most organisms as important signal transduction molecules [70,77,93,94]. A basal level of ROS is actually required to employ its beneficial function and support life. Disruption of this ROS homeostasis activates the plant’s defense mechanisms in order to cope with the oxidative stress and acts as signaling molecules for the regulation of a variety of physiological functions, including plant function and development [2,70,95]. However, excessive ROS levels can be harmful [2,80]. Since ROS at low levels exert beneficial action and are detrimental at high concentrations, they are considered as hormetic molecules [2,96,97]. Hormesis or hormetic response is a biphasic dose-response phenomenon of an organism responding to any disturbance of its homeostasis triggered by an abiotic or biotic stress factor that exerts a stimulatory beneficial effect at low doses and causes inhibition at high doses [2,98,99,100,101]. Thus, the term hormesis refers to an “overcompensation” response [102].
The shortest-lived ROS is the hydroxyl radical (OH•), but it is the most reactive of all, reacting with almost all molecules [103]. Longer lived than OH• is the superoxide anion radical (O2•−), which is shorter lived than 1O2* [5,103]. 1O2* is involved in photooxidative damage to plants [37]. The superoxide anion radical (O2•−) is formed by electron leakage to O2 at PSI, and it is rapidly converted to H2O2 (Figure 3) [75]. Hydrogen peroxide is the most stable and least reactive ROS with the longest lifetime, being able to easily diffuse through the membranes [27,104,105,106]. ROS levels in cells are controlled and balanced by the antioxidant systems, allowing only a basal ROS level to employ its beneficial function [5,77,93]. ROS can be formed either by energy transfer (1O2) or by electron transport (O2•−, H2O2), sometimes concurrently, with their signaling pathways to occasionally antagonize each other [5,27,70,79,80,107].
ROS signaling can confer tolerance to oxidative stress associated with different environmental stresses, and it is considered to be beneficial and necessary for acclimation [2,82,97]. Foliar spray of melatonin triggered the mechanism of non-photochemical quenching (NPQ), stimulating ROS creation and resulting in the hormetic stimulation of PSII functionality, thus enhancing the photosynthetic function [108]. Increased ROS generation in response to different environmental stressors can result in increased photosynthetic function, growth, and tolerance in diverse plant species [2,97,109,110,111,112,113]. ROS play essential roles in plant responses to environmental stress and, in coordination with the antioxidant network, are greatly involved in organizing the signaling pathways that support plant acclimation to environmental stress [114,115]. Salicylic acid application increased H2O2 concentration, conferring tolerance to abiotic stress [97,116], while exogenous application of H2O2 was also shown to increase stress tolerance [117]. Hydrogen peroxide has been frequently observed to diffuse through the leaf veins (Figure 5), acting as a molecule that triggers a long-distance stress defense response [27,80,81,107] or induces programmed cell death in plants [52,80,81,118,119]. H2O2 has long been recognized as a strategic signaling molecule that plays a prevailing function in facilitating plants to cope with biotic and abiotic stressors [120]. ROS are involved as signal molecules during cellular growth to control stomatal closure, in programmed cell death, and in biotic and abiotic stress responses in plants [28,121,122,123,124]. Moreover, ROS-antioxidant interactions provide essential information for the redox state that influences the gene expression associated with biotic and abiotic stress responses [52,80,83,118,125]. A fast transient creation of ROS, described as “oxidative burst”, is a mark of effective recognition of plant herbivory (Figure 6) [126,127].
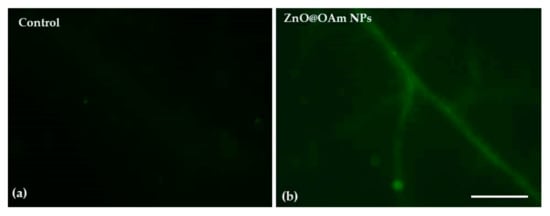
Figure 5.
Imaging of H2O2 generation in tomato leaflets 90 min after the spray with distilled water (control) (a) or with 15 mg L−1 zinc oxide nanorods (b). The light green color denotes the diffusion of H2O2 through the leaf veins. Scale bar 500 μm. Reprinted from Ref. [128].
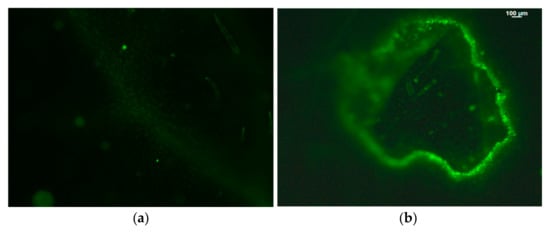
Figure 6.
Hydrogen peroxide detection in tomato leaflets before (a) and after (b) Tuta absoluta feeding. The whole area of a feeding zone is shown. Increased generation of H2O2 is visible by the light green color. Scale Bar: 100 μm. Reprinted from Ref. [81].
Furthermore, ROS have lately been linked as important signals that determine root hair formation and elongation [129]. ROS and multiple redox regulation signals in plants involve a high degree of synchronization and balance between metabolic pathways and signaling in different cellular parts [130]. ROS and antioxidant (enzymatic and non-enzymatic) defense systems arise as a paradigm that interlinks diverse aspects of plant metabolism, development, and stress acclimation.
ROS perform an essential role in sensing and recognition of different environmental stressors, in signal transduction, and in the activation of stress-response networks, thus contributing to plant defense mechanisms and plant resilience [131] (Figure 7). Therefore, they can also be used as an indicator of the initial plant response in stress studies. Currently, several mechanisms linked with ROS-induced signaling cascades have been recognized, but it is still challenging to realize how signals generated by ROS are influencing transcriptional regulation to control metabolic processes like growth and development, stress tolerance, or programmed cell death, highlighting the complexity and plasticity of these responses [91,131,132,133]. Additionally, understanding the interplay between the different pathways that amplify or reduce ROS production, cellular redox modifications, phytohormones, Ca2+ signaling, and other messenger molecules is also necessary [132,133]. Future studies on ROS-linked genes and signaling cascades can unravel the complexity of plant stress responses and thus improve our efforts towards sustainable food production and climate-resilient agriculture.
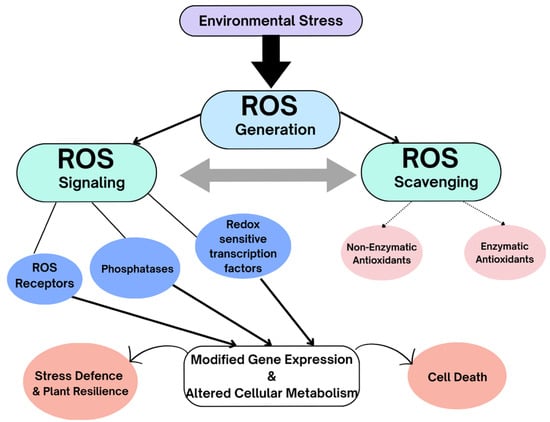
Figure 7.
Environmental stresses result in increased ROS generation. Antioxidants (enzymatic and non-enzymatic) are not meant to eliminate ROS entirely but rather maintain a balanced interplay between ROS production and scavenging, enabling signal transduction. ROS signals can be sensed by ROS sensors, such as two-component histidine kinase systems and redox-sensitive transcription factors and phosphatases. ROS eventually elicit changes in gene expression that modify cellular metabolism. ROS signals stimulate various ROS defense systems, such as changes in ROS-production/scavenging balance and production of ROS stress-protective proteins and compounds. The activation of stress-response networks contributes to plant defense mechanisms and plant resilience. Failure of ROS-scavenging mechanisms can result in cell death.
In the context of global climate change, it is increasingly important to understand the relationship between ROS generation, ROS scavenging, and ROS signaling. Gaining insights into this relationship can lead to pinpointing genes and unraveling pathways that are crucial in early stress responses. Therefore, it will contribute to improving agricultural sustainability in the face of changing environmental conditions [5,18]. Deepening our knowledge of the ROS-antioxidant defense system interaction is not only a scientific challenge but also a strategic priority for sustainable agriculture and food security. The manipulation of ROS generation to assist plants in coping with environmental stress is a promising challenge for practical agriculture.
Author Contributions
Conceptualization, J.M. and M.M.; validation, J.M. and M.M.; formal analysis, J.M. and M.M.; data curation, J.M. and M.M.; writing—original draft preparation, J.M. and M.M.; writing—review and editing, J.M. and M.M.; visualization, J.M. and M.M.; supervision, M.M.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ROS | Reactive oxygen species |
| 1O2* | Singlet oxygen |
| H2O2 | Hydrogen peroxide |
| O2•− | Superoxide anion radical |
| OH• | Hydroxyl radical |
| 3chl* | Triplet excited chlorophyll state |
| 1chl* | Singlet excited chlorophyll state |
| AA | Antimycin A |
| NDH | NADH dehydrogenase-like |
| MDAR | Monodehydroascorbate reductase |
| APX | Ascorbate peroxidase |
| SOD | Superoxide dismutase |
| LHCs | Light-harvesting complexes |
| RCs | Reaction centers |
| ETR | Electron transport |
| GR | Glutathione reductase |
| CAT | Catalase |
| GOPX | Quaiacol peroxidase |
| AsA | Ascorbate |
| GSH | Glutathione |
| GPH | Glutathione peroxidase |
| CEF | Cyclic electron flow |
| NPQ | Non-photochemical quenching (dissipation of excitation energy as heat) |
| FL | Fluorescence |
| OEC | Oxygen-evolving complex |
| NO | Nitric oxide |
| PSI | Photosystem I |
| PSII | Photosystem II |
| qp | Photochemical quenching (fraction of open PSII reaction centers, representing also the redox state of the plastoquinol pool |
References
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Moustaka, J.; Sperdouli, I.; Moustakas, M. Light energy use efficiency in photosystem II of tomato is related to leaf age and light intensity. Crops 2024, 4, 623–635. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Wolosiuk, R.A.; Malkin, R. Photosynthesis. In Biochemistry & Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 508–566. [Google Scholar]
- Moustakas, M. Plant Photochemistry, Reactive Oxygen Species, and Photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Ort, D.R. When there is too much light. Plant Physiol. 2001, 125, 29–32. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ruban, A.V. Light harvesting control in plants. FEBS Lett. 2018, 592, 3030–3039. [Google Scholar] [CrossRef]
- Vass, I.; Cser, K.; Cheregi, O. Molecular mechanisms of light stress of photosynthesis. Ann. New York Acad. Sci. 2007, 1113, 114–122. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S. Editorial: Reactive oxygen species in chloroplasts and chloroplast antioxidants under abiotic stress. Front. Plant Sci. 2023, 14, 1208247. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, S.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Lee, M. Plant Signal Transduction. In Biochemistry and Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 215–240. [Google Scholar]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition–A historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Aro, E.M.; Millar, A.H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Oguchi, R.; Terashima, I.; Kou, J.; Chow, W.S. Operation of dual mechanisms that both lead to photoinactivation of photosystem II in leaves by visible light. Physiol. Plant. 2011, 142, 47–55. [Google Scholar] [CrossRef]
- Terentyev, V.V.; Trubitsina, L.I.; Khoroshaeva, T.P.; Trubitsin, I.V. Protective effect of α-carbonic anhydrase CAH3 against photoinhibition and thermal inactivation of photosystem II in membrane preparations as compared with α-carbonic anhydrase CA4. Biochem. 2025, 90, 860–872. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Moustaka, J.; Sperdouli, I.; Panteris, E.; Adamakis, I.D.S.; Moustakas, M. Aspirin foliar spray-induced changes in light energy use efficiency, chloroplast ultrastructure, and ROS generation in tomato. Int. J. Mol. Sci. 2025, 26, 1368. [Google Scholar] [CrossRef]
- Moustakas, M. Molecular mechanisms of plant abiotic stress tolerance. Int. J. Mol. Sci. 2025, 26, 2731. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Tyystjarvi, E. Photoinhibition of photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [PubMed]
- Kasajima, I. Difference in oxidative stress tolerance between rice cultivars estimated with chlorophyll fluorescence analysis. BMC Res. Notes 2017, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Cui, H. Tender but tough: How light and developmental stage cooperate to protect young leaves from the cold. Plant Cell Environ. 2025, 48, 6962–6964. [Google Scholar] [CrossRef]
- Luklová, M.; Dubois, M.; Kameniarová, M.; Plačková, K.; Novák, J.; Kopecká, R.; Karady, M.; Pavlů, J.; Skalák, J.; Jindal, S.; et al. Light quantity impacts early response to cold and cold acclimation in young leaves of Arabidopsis. Plant Cell Environ. 2025, 48, 5030–5052. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol. Plant. 2011, 142, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Triantaphylidès, C.; Krischke, M.; Hoeberichts, F.A.; Ksas, B.; Gresser, G.; Havaux, M.; Van Breusegem, F.; Mueller, M.J. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008, 148, 960968. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Pospíšil, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 2012, 1817, 218–231. [Google Scholar] [CrossRef]
- Moustakas, M.; Bayçu, G.; Sperdouli, I.; Eroğlu, H.; Eleftheriou, E.P. Arbuscular mycorrhizal symbiosis enhances photosynthesis in the medicinal herb Salvia fruticosa by improving photosystem II photochemistry. Plants 2020, 9, 962. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Miyake, C. Molecular mechanism of oxidation of P700 and suppression of ROS production in photosystem I in response to electron-sink limitations in C3 plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Moustaka, J.; Adamakis, I.-D.S.; Apostolova, E. Photosystem II tolerance to excess zinc exposure and high light stress in Salvia sclarea L. Agronomy 2024, 14, 589. [Google Scholar]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 760–769. [Google Scholar]
- Moustakas, M.; Hanć, A.; Dobrikova, A.; Sperdouli, I.; Adamakis, I.D.S.; Apostolova, E. Spatial heterogeneity of cadmium effects on Salvia sclarea leaves revealed by chlorophyll fluorescence imaging analysis and laser ablation inductively coupled plasma mass spectrometry. Materials 2019, 12, 2953. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant defense system in plants: Reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Walker, B.; Schmiege, S.C.; Sharkey, T.D. Re-evaluating the energy balance of the many routes of carbon flow through and from photorespiration. Plant Cell Environ. 2024, 47, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Hagemann, M. Photorespiration-how is it regulated and how does it regulate overall plant metabolism? J. Exp. Bot. 2020, 71, 3955–3965. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Sun, H.; Timm, S.; Zhang, S.; Huang, W. Photorespiration alleviates photoinhibition of photosystem I under fluctuating light in tomato. Plants 2022, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Ogren, W.L. Photorespiration: Pathways, regulation, and modification. Annu. Rev. Plant Physiol. 1984, 35, 415–442. [Google Scholar]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef]
- Saint-Sorny, M.; Dimitriades, A.; Delrue, F.; Johnson, X. Proton Gradient Regulation 5 determines reserve partitioning between starch and lipids in C. reinhardtii. Physiol Plant. 2024, 176, e14539. [Google Scholar]
- Schuller, J.M.; Birrell, J.A.; Tanaka, H.; Konuma, T.; Wulfhorst, H.; Cox, N.; Schuller, S.K.; Thiemann, J.; Lubitz, W.; Sétif, P.; et al. Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science 2019, 363, 257–260. [Google Scholar] [CrossRef]
- Leister, D.; Marino, G.; Minagawa, J.; Dann, M. An ancient function of PGR5 in iron delivery? Trends Plant Sci. 2022, 27, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.M.; Aslam, S.M.; Madireddi, S.K.; Chouhan, N.; Subramanyam, R. Role of cyclic electron transport mutations pgrl1 and pgr5 in acclimation process to high light in Chlamydomonas reinhardtii. Photosynth. Res. 2020, 146, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Y.; Bai, C.; Yang, Y.; Sun, Z.; Liu, X.; Zhang, S.; Han, X.; Yong, J.W.H. The physiological functionality of pgr5/pgrl1-dependent cyclic electron transport in sustaining photosynthesis. Front. Plant Sci. 2021, 12, 702196. [Google Scholar] [CrossRef]
- Loudya, N.; Barkan, A.; López-Juez, E. Plastid retrograde signaling: A developmental perspective. Plant Cell 2024, 36, 3903–3913. [Google Scholar] [CrossRef]
- van Veen, E.; Küpers, J.J.; Gommers, C.M.M. Plastids in a pinch: Coordinating stress and developmental responses through retrograde signalling. Plant Cell Environ. 2025, 48, 6897–6911. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant andanimal cells. Free Radic. Biol. Med. 2018, 22, 21–27. [Google Scholar] [CrossRef]
- Janků, M.; Luhová, L.; Petřivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Molina-Moya, E.; Sandalio, L.M. An update on redox signals in plant responses to biotic and abiotic stress crosstalk: Insights from cadmium and fungal pathogen interactions. J. Exp. Bot. 2021, 72, 5857–5875. [Google Scholar] [CrossRef]
- Fedoreyeva, L.I. ROS as signaling molecules to initiate the process of plant acclimatization to abiotic stress. Int. J. Mol. Sci. 2024, 25, 11820. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.D.S.; Sperdouli, I.; Eleftheriou, E.P.; Moustakas, M. Hydrogen peroxide production by the spot-like mode action of bisphenol A. Front. Plant Sci. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Sohail; Zaman, S.; Li, G.; Fu, M. Adaptive responses of plants to light stress: Mechanisms of photoprotection and acclimation. A review. Front. Plant Sci. 2025, 16, 1550125. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, J.; Liebers, M.; Hirth, M.; Grübler, B.; Holtzegel, U.; Schröter, Y.; Dietzel, L.; Pfannschmidt, T. Environmental control of plant nuclear gene expression by chloroplast redox signals. Front. Plant Sci. 2012, 3, 257. [Google Scholar] [CrossRef]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Leverne, L.; Roach, T.; Perreau, F.; Maignan, F.; Krieger-Liszkay, A. Increased drought resistance in state transition mutants is linked to modified plastoquinone pool redox state. Plant Cell Environ. 2023, 46, 3737–3747. [Google Scholar] [CrossRef]
- Kock, C.; Helmig, J.; Gutsche, N.; Dierschke, T.; Müller-Schüssele, S.J.; Zachgo, S. Redox buffering and H2O2 orchestrate the vegetative development of Marchantia polymorpha. Plant J. 2025, 123, e70317. [Google Scholar] [CrossRef]
- Mohanty, D.; Peláez-Vico, M.Á.; Myers, R.J.; Sánchez-Vicente, M.I.; Lorenzo, O.; Mittler, R. Aboveground whole-plant live imaging method for nitric oxide (NO) reveals an intricate relationship between NO and H2O2. New Phytol. 2025, 247, 2473–2483. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Wink, D.A.; Miranda, K.M.; Espey, M.G.; Pluta, R.M.; Hewett, S.J.; Colton, C.; Vitek, M.; Feelisch, M.; Grisham, M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 2001, 3, 203–213. [Google Scholar] [CrossRef]
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, 5933–5943. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, C. Photosynthetic ROS and retrograde signaling pathways. New Phytol. 2024, 244, 1183–1198. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Hadacek, F.; Bachmann, G.; Engelmeier, D.; Chobot, V. Hormesis and a chemical raison d’ětre for secondary plant metabolites. Dose Response 2011, 9, 79–116. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.C.; Ozgur, R.; Uzilday, B. Reactive oxygen species: Connecting eustress, hormesis, and allostasis in plants. Plant Stress 2023, 8, 100164. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: The dose response for the 21st century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental hormesis: From cell to ecosystem. Curr. Opin. Environ. Sci. Health 2022, 29, 100378. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Editorial overview: Hormesis and dose-response. Curr. Opin. Toxicol. 2022, 30, 100343. [Google Scholar] [CrossRef]
- Calabrese, E.J. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef]
- Phua, S.Y.; De Smet, B.; Remacle, C.; Chan, K.X.; Van Breusegem, F. Reactive oxygen species and organellar signaling. J. Exp. Bot. 2021, 72, 5807–5824. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.J.; Ball, L.; Oxborough, K.; Karpiński, S.; Mullineaux, P.M.; Baker, N.R. Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 2003, 33, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signaling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Sousa, R.H.V.; Carvalho, F.E.L.; Ribeiro, C.W.; Passaia, G.; Cunha, J.R.; Lima-Melo, Y.; Margis-Pinheiro, M.; Silveira, J.A.G. Peroxisomal APX knockdown triggers antioxidant mechanisms favorable for coping with high photorespiratory H2O2 induced by CAT deficiency in rice. Plant Cell Environ. 2015, 38, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf age dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Şaş, B.; İşgören, S.; Moustaka, J.; Morales, F. Mechanistic approach on melatonin-induced hormesis of photosystem II function in the medicinal plant Mentha spicata. Plants 2023, 12, 4025. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.; Reis, A. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci. Total Environ. 2022, 804, 150059. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis in plants: Its common occurrence across stresses. Curr. Opin. Toxicol. 2022, 30, 100333. [Google Scholar] [CrossRef]
- Hendrix, S.; Vanbuel, I.; Colemont, J.; Bos Calderó, L.; Hamzaoui, M.A.; Kunnen, K.; Huybrechts, M.; Cuypers, A. Jacks of all trades: Reactive oxygen species in plant responses to stress combinations and priming-induced stress tolerance. J. Exp. Bot. 2025, 76, 3686–3705. [Google Scholar] [CrossRef] [PubMed]
- Boscari, A.; Frendo, P. Redox metabolism and signalling in plants. J. Exp. Bot. 2025, 76, 3629–3633. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.F.; Lopez-Delgado, H.; Foyer, C.H.; Scott, I.M. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998, 116, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Nguyen, H.M.; Seki, M. Advances in chemical priming to enhance abiotic stress tolerance in plants. Plant Cell Physiol. 2020, 61, 1995–2003. [Google Scholar] [CrossRef]
- Elena-Real, C.A.; González-Arzola, K.; Pérez-Mejías, G.; Díaz-Quintana, A.; Velázquez-Campoy, A.; Desvoyes, B.; Gutiérrez, C.; De la Rosa, M.A.; Díaz-Moreno, I. Proposed mechanism for regulation of H2O2-induced programmed cell death in plants by binding of cytochrome c to 14-3-3 proteins. Plant J. 2021, 106, 74–85. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Iqbal, H.; Yaning, C.; Raza, S.T.; Karim, S.; Shareef, M.; Waqas, M. From lab to field: Harnessing H2O2-mediated upregulation of plant capacities under abiotic stresses. Physiol. Plant. 2025, 177, e70488. [Google Scholar] [CrossRef]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klusener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef]
- Laloi, C.; Stachowiak, M.; Pers-Kamczyc, E.; Warzych, E.; Murgia, I.; Apel, K. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 672–677. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Nanda, A.K.; Andrio, E.; Marino, D.; Pauly, N.; Dunand, C. Reactive oxygen species during plant-microorganism early interactions. J. Integr. Plant Biol. 2010, 52, 195–204. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.-D.S.; Mourdikoudis, S.; Dendrinou-Samara, C.; Moustakas, M. Modification of tomato photosystem ΙΙ photochemistry with engineered zinc oxide nanorods. Plants 2023, 12, 3502. [Google Scholar] [CrossRef]
- Gerber, M.E.; White, M.G.; Muday, G.K. Reactive oxygen species act as signaling molecules to control root hair initiation and tip growth. New Phytol. 2025, 247, 2042–2048. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Wrzaczek, M. A negative feedback loop controls ROS production in plant immunity. Mol Plant. 2021, 14, 1221–1222. [Google Scholar] [CrossRef]
- Sood, M. Reactive oxygen species (ROS): Plant perspectives on oxidative signalling and biotic stress response. Discov. Plants 2025, 2, 187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).