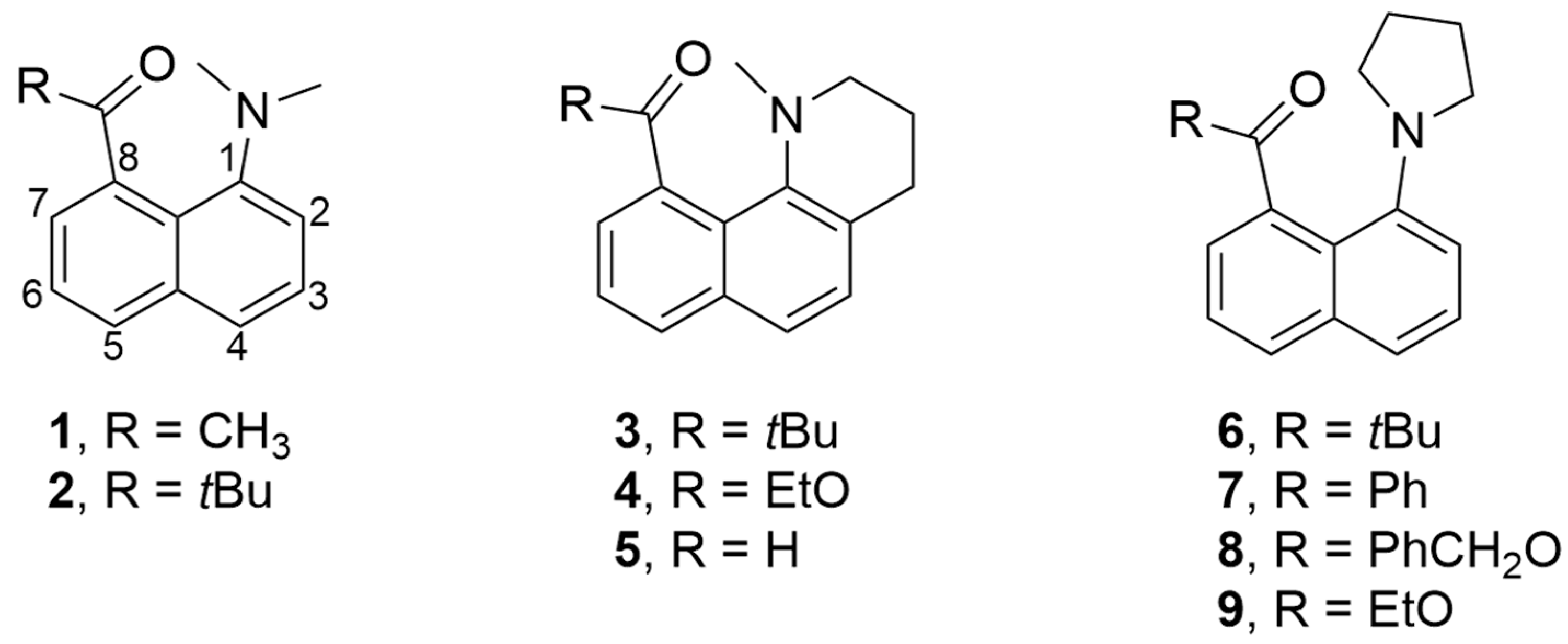
Fluorescence of 8-Acyl-1-Pyrrolidinylnaphthalenes
Abstract
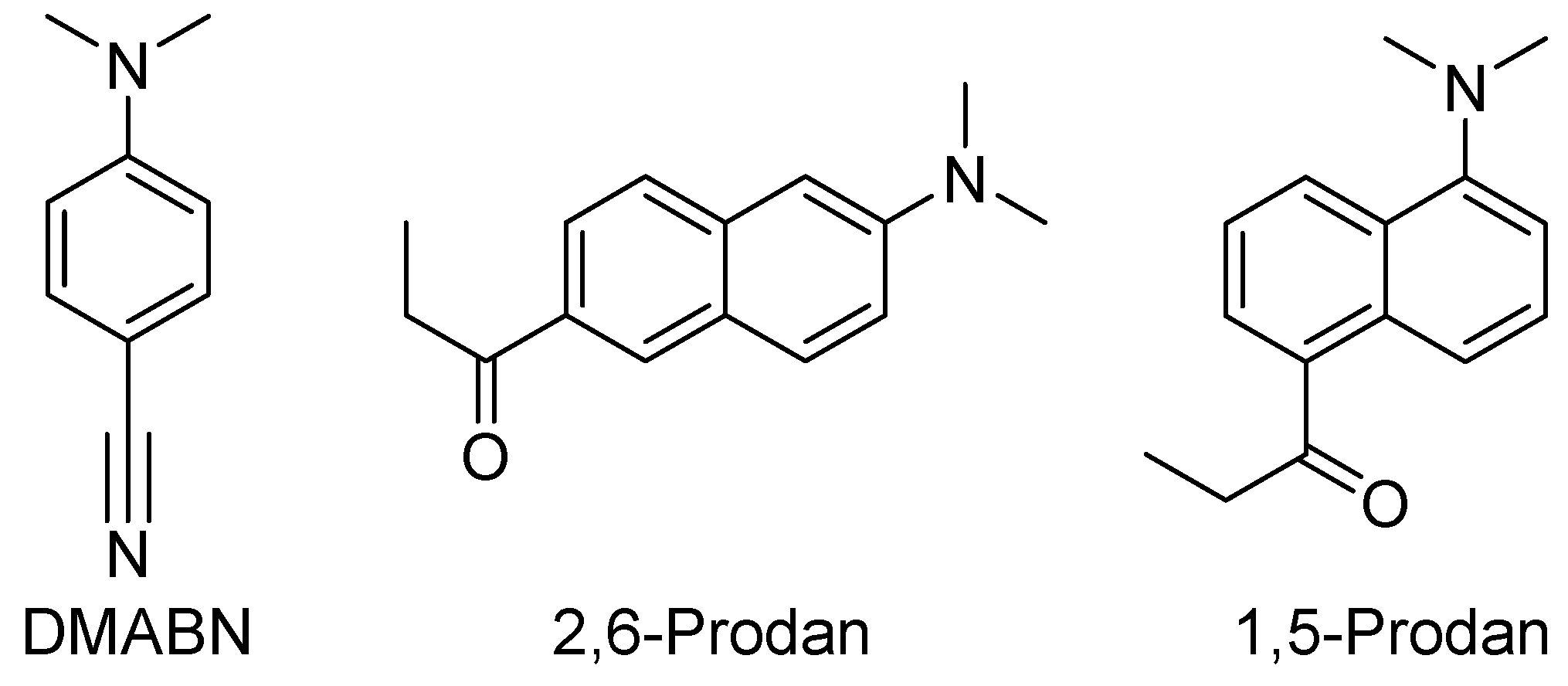
1. Introduction
2. Materials and Methods
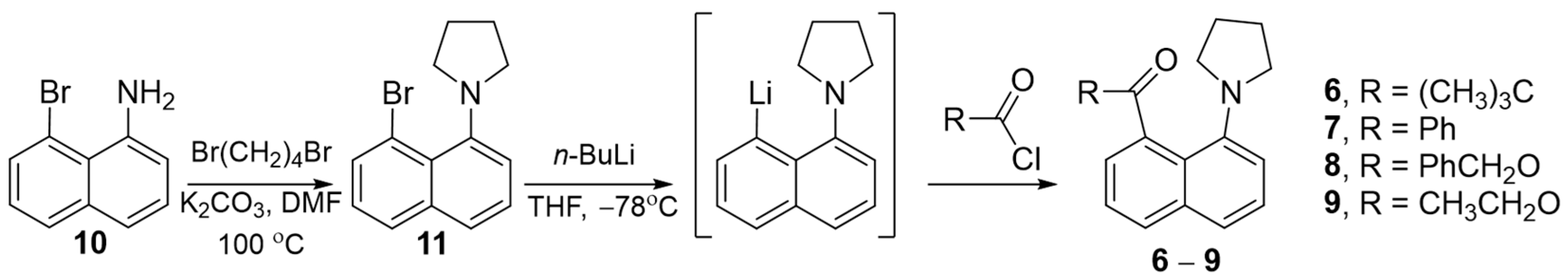
2.1. 1-(8-Bromonaphthalen-1-yl)pyrrolidine (11)
2.2. Acylation of (8-(Pyrrolidin-1-yl)naphthalen-1-yl)lithium
2.2.1. 2,2-Dimethyl-1-(8-(pyrrolidin-1-yl)naphthalen-1-yl)propan-1-one (6)
2.2.2. Phenyl(8-(pyrrolidin-1-yl)naphthalen-1-yl)methanone (7)
2.2.3. Benzyl 8-(Pyrrolidin-1-yl)-1-naphthoate (8)
2.2.4. Ethyl 8-(Pyrrolidin-1-yl)-1-naphthoate (9)
3. Results
3.1. Structural Studies
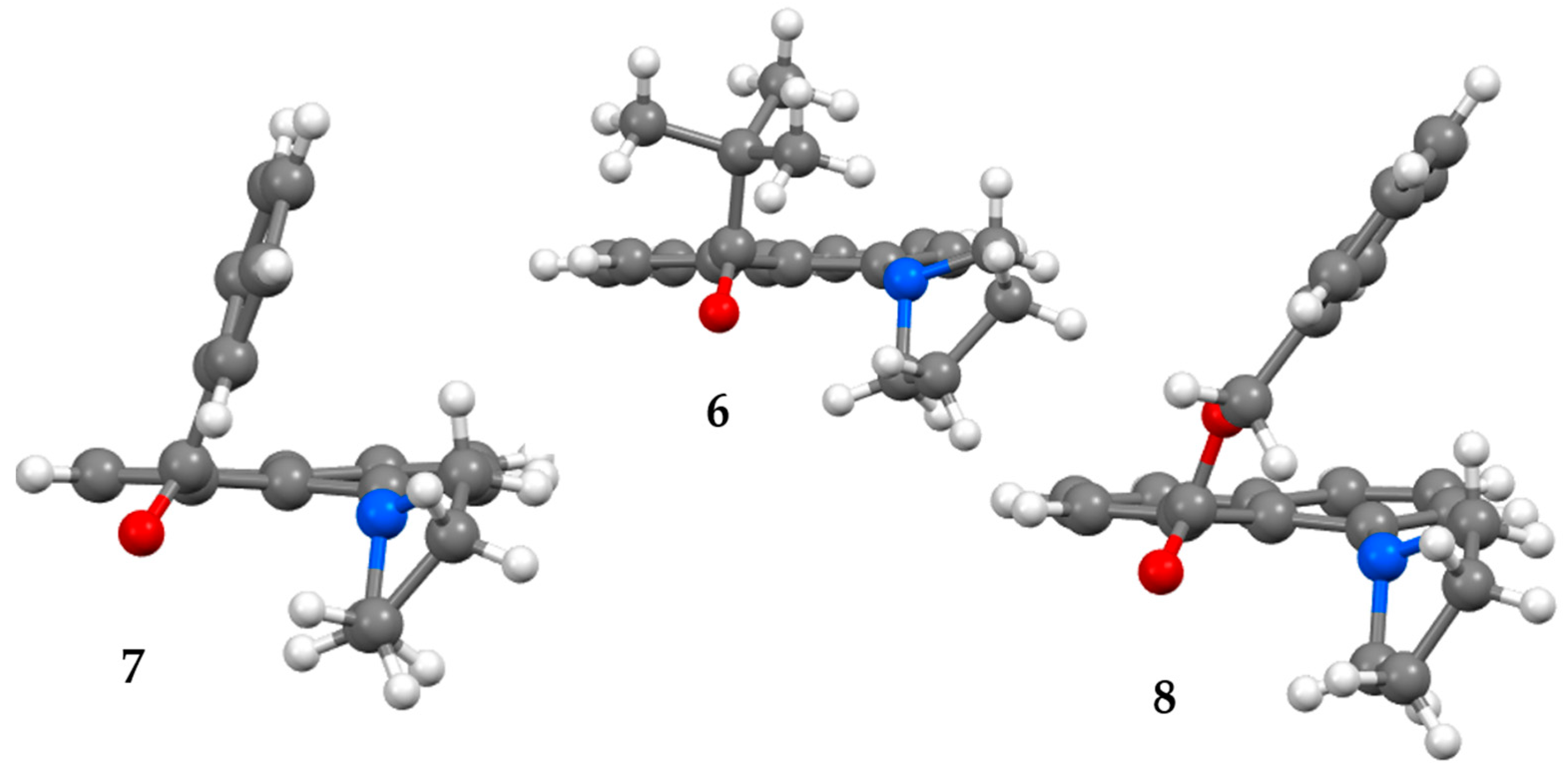
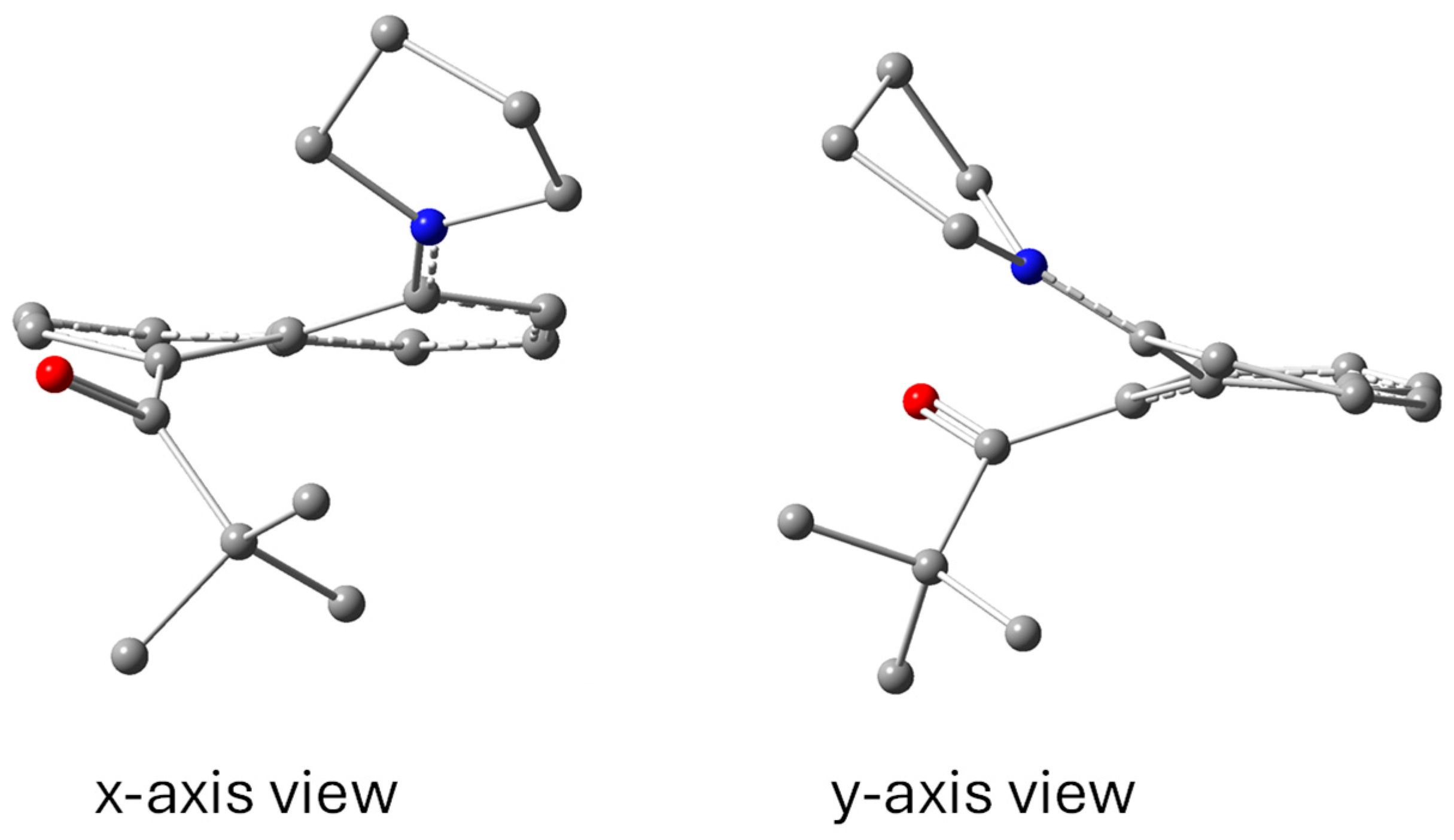
3.1.1. X-Ray Structures
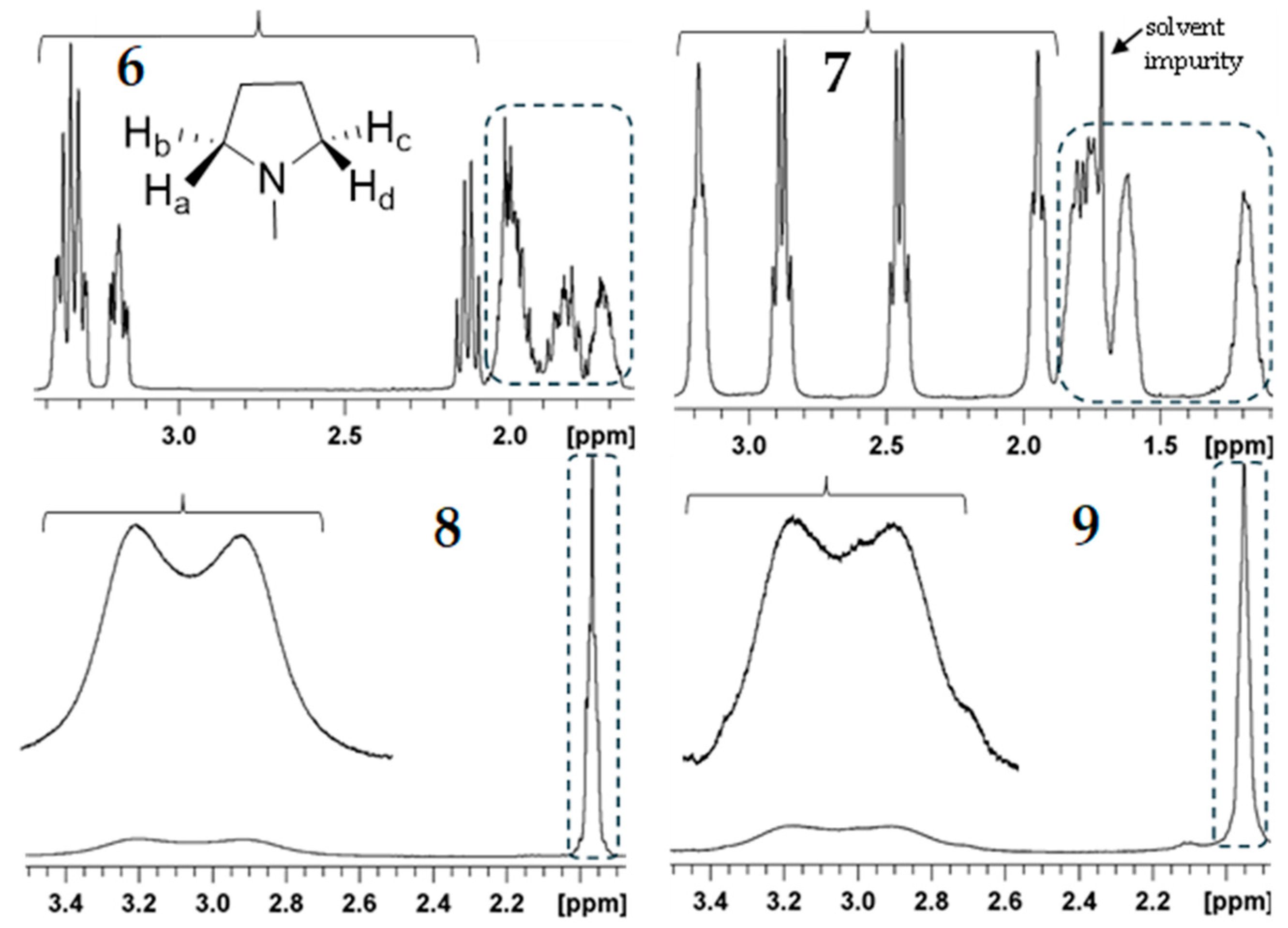
3.1.2. NMR Studies
3.2. Photophysical Studies
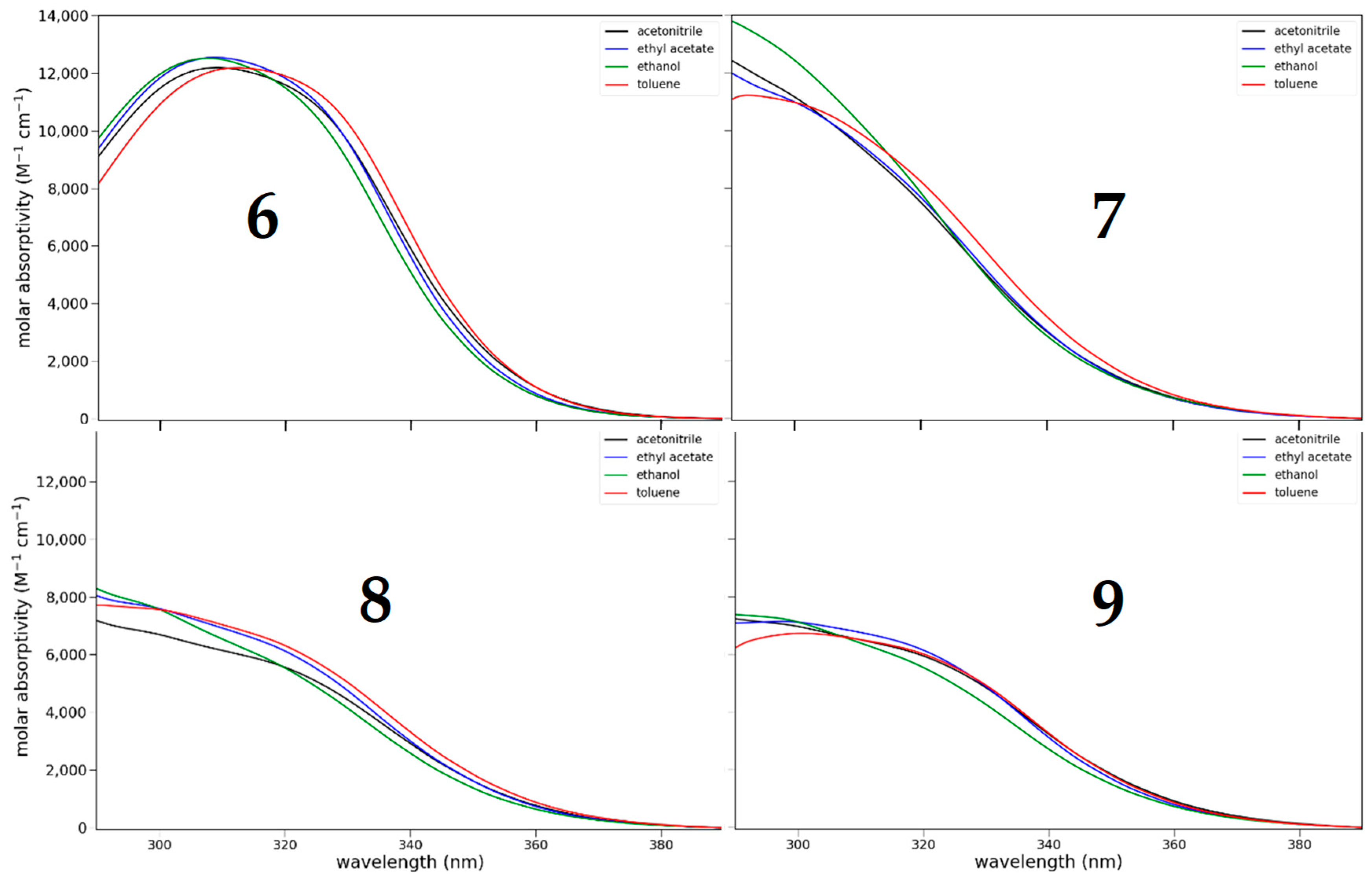
3.2.1. Absorption
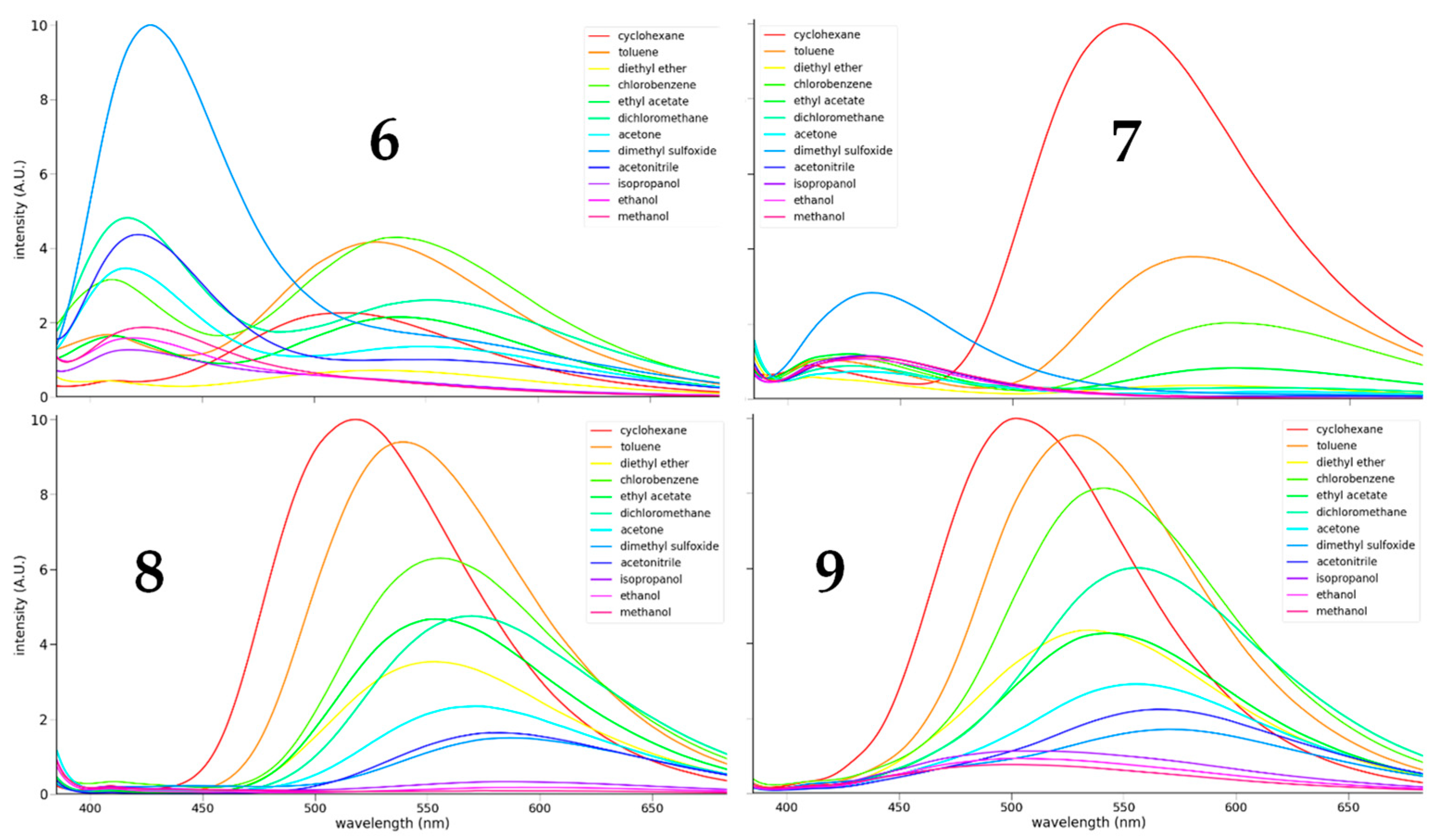
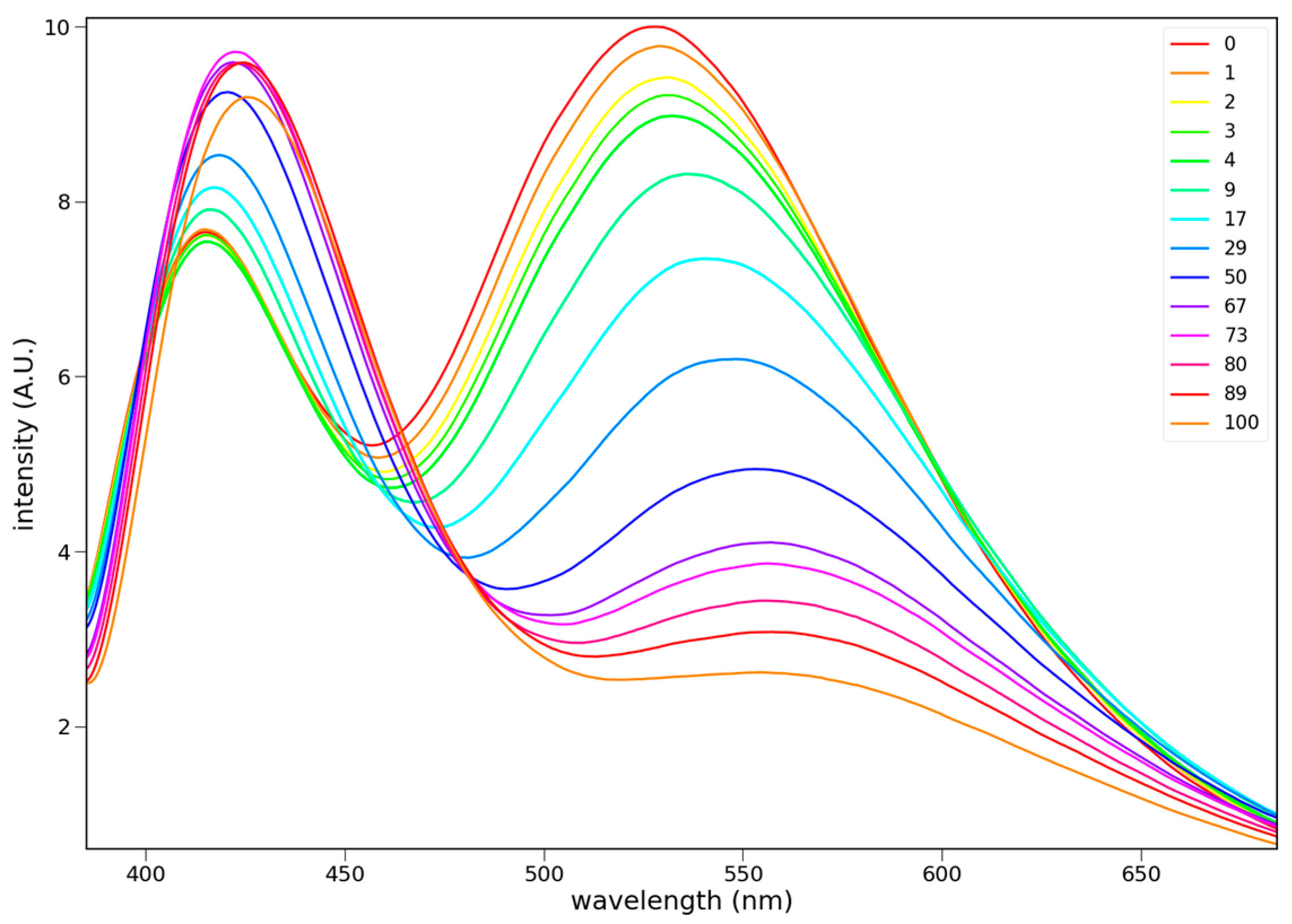
3.2.2. Fluorescence
3.3. Computational Studies
3.3.1. Ground State Structures
3.3.2. Excited State Structures
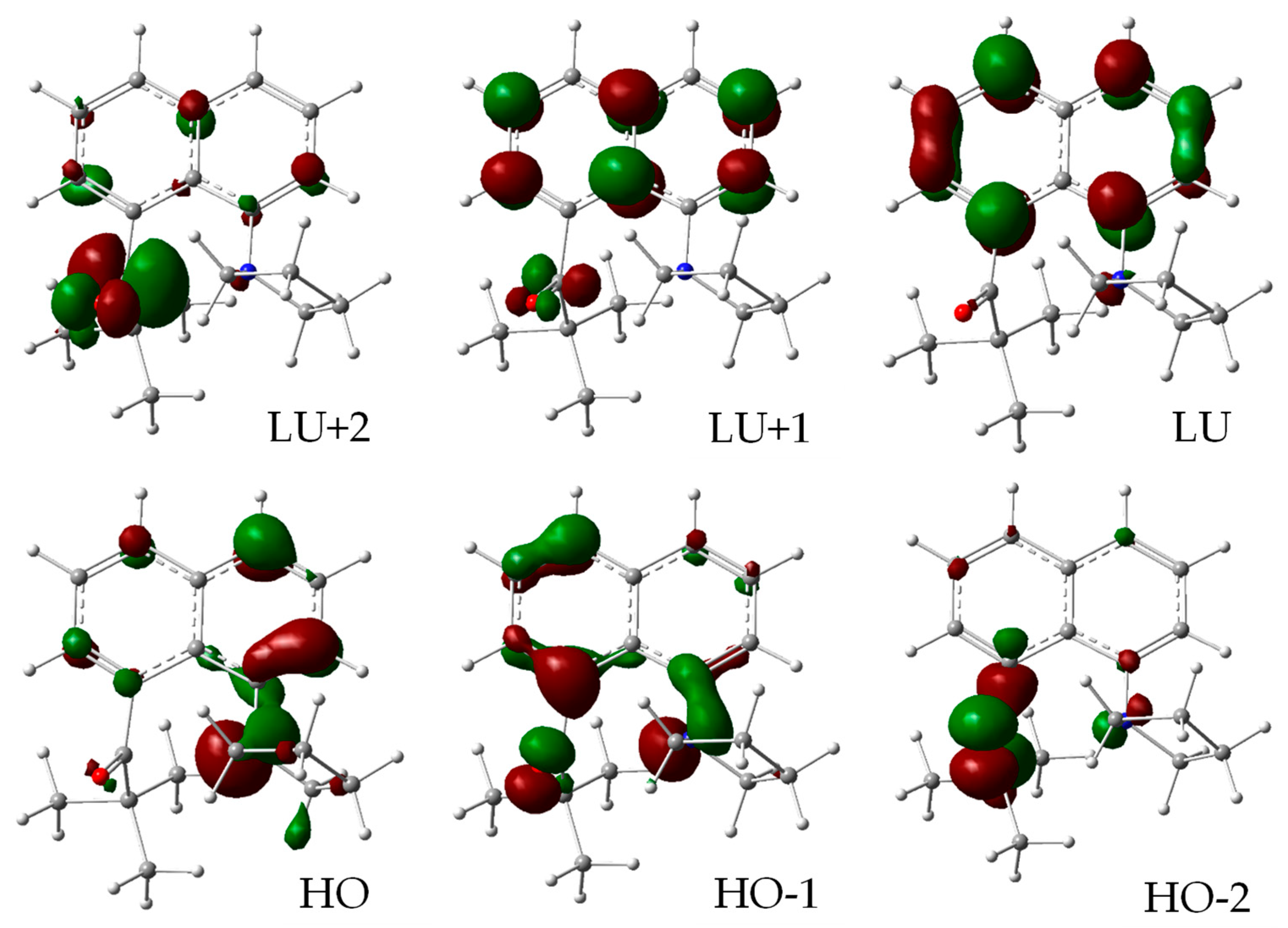
3.3.3. Molecular Orbitals and Electronic Transitions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMABN | Dimethylaminobenzonitrile |
| ICT | Intramolecular charge transfer |
| Prodan | Propionyldimethylaminonaphthalene |
| PICT | Planar intramolecular charge transfer |
| TICT | Twisted intramolecular charge transfer |
References
- Balasubramaniyan, V. Peri Interaction in Naphthalene Derivatives. Chem. Rev. 1966, 66, 567–641. [Google Scholar] [CrossRef]
- Reza, A.I.; Iwai, K.; Nishiwaki, N. A Study of the Correlation between the Bulkiness of Peri-Substituents and the Distortion of a Naphthalene Ring. Molecules 2023, 28, 5343. [Google Scholar] [CrossRef]
- Schiemenz, G.P.; Schiemenz, B.; Petersen, S.; Wolff, C. Hexacoordinated Carbon or Tetracovalent Silicon? Chirality 1998, 10, 180–189. [Google Scholar] [CrossRef]
- Einspahr, H.; Robert, J.B.; Marsh, R.E.; Roberts, J.D. Peri Interactions: An X-Ray Crystallographic Study of the Structure of 1,8-Bis(Dimethylaminonaphthalene). Acta Crystallogr. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 1611–1617. [Google Scholar] [CrossRef]
- Schweizer, W.B.; Procter, G.; Kaftory, M.; Dunitz, J.D. Structural Studies of 1,8-Disubstituted Naphthalenes as Probes for Nucleophile-electrophile Interactions. Helv. Chim. Acta 1978, 61, 2783–2808. [Google Scholar] [CrossRef]
- Clayden, J.; McCarthy, C.; Helliwell, M. Bonded Peri-Interactions Govern the Rate of Racemisation of Atropisomeric 8-Substituted 1-Naphthamides. Chem. Commun. 1999, 2059–2060. [Google Scholar] [CrossRef]
- Schiemenz, G.P. Peri-Interactions in Naphthalenes, 14 [1]. Pyramidalization versus Planarization at Nitrogen in 8-Dialkylamino-Naphth-1-Yl Compounds as a Measure of Peri Bond Formation. Z. Naturforsch. B 2006, 61, 535–554. [Google Scholar] [CrossRef]
- Lari, A.; Pitak, M.B.; Coles, S.J.; Rees, G.J.; Day, S.P.; Smith, M.E.; Hanna, J.V.; Wallis, J.D. Models for Incomplete Nucleophilic Attack on a Protonated Carbonyl Group and Electron-Deficient Alkenes: Salts and Zwitterions from 1-Dimethylamino-Naphthalene-8-Carbaldehyde. Org. Biomol. Chem. 2012, 10, 7763. [Google Scholar] [CrossRef]
- Mercadal, N.; Day, S.P.; Jarmyn, A.; Pitak, M.B.; Coles, S.J.; Wilson, C.; Rees, G.J.; Hanna, J.V.; Wallis, J.D. O- vs. N-Protonation of 1-Dimethylaminonaphthalene-8-Ketones: Formation of a Peri N-C Bond or a Hydrogen Bond to the Pi-Electron Density of a Carbonyl Group. CrystEngComm 2014, 16, 8363–8374. [Google Scholar] [CrossRef]
- Kiefl, C. Correlated Rotations and Unusual Fluorescence Properties of peri-Substituted, Axially Chiral Naphthyl Ketones. Eur. J. Org. Chem. 2000, 2000, 3279–3286. [Google Scholar] [CrossRef]
- Kiefl, C.; Zinner, H.; Burgemeister, T.; Mannschreck, A. Axially Chiral 2-(Dialkylamino)Benzamides. Barriers to Rotations, Determined by NMR and by Racemization (Neighboring Rotor Substituents on Arene Systems, Part 3). Recl. Trav. Chim. Pays-Bas 2010, 115, 125–132. [Google Scholar] [CrossRef]
- Rettig, W. Charge Separation in Excited States of Decoupled Systems: Twisted Intramolecular Charge Transfer (TICT) Compounds and Implications for the Development of New Laser Dyes and for the Primary Process of Vision and Photosynthesis. Angew. Chem. 1986, 98, 969–986. [Google Scholar] [CrossRef]
- Rettig, W.; Lapouyade, R. Fluorescence Probes Based on Twisted Intramolecular Charge Transfer (TICT) States and Other Adiabatic Photoreactions. In Topics in Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2002; pp. 109–149. [Google Scholar]
- Okada, T.; Uesugi, M.; Köhler, G.; Rechthaler, K.; Rotkiewicz, K.; Rettig, W.; Grabner, G. Time-Resolved Spectroscopy of DMABN and Its Cage Derivatives 6-Cyanobenzquinuclidine (CBQ) and Benzquinuclidine (BQ). Chem. Phys. 1999, 241, 327–337. [Google Scholar] [CrossRef]
- Köhler, G.; Rechthaler, K.; Grabner, G.; Luboradzki, R.; Suwińska, K.; Rotkiewicz, K. Structure of Cage Amines as Models for Twisted Intramolecular Charge-Transfer States. J. Phys. Chem. A 1997, 101, 8518–8525. [Google Scholar] [CrossRef]
- Wermuth, G.; Rettig, W. The Interaction of Close-Lying Excited States: Solvent Influence on Fluorescence Rate and Polarization in Substituted Indolines. J. Phys. Chem. 1984, 88, 2729–2735. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Siemiarczuk, A. Dual Fluorescence of Donor-Acceptor Molecules and the Twisted Intramolecular Charge Transfer (TICT) States. J. Lumin. 1979, 18, 420–424. [Google Scholar] [CrossRef]
- Kochman, M.A.; Durbeej, B. Simulating the Nonadiabatic Relaxation Dynamics of 4-(N, N-Dimethylamino) Benzonitrile (DMABN) in Polar Solution. J. Phys. Chem. A 2020, 124, 2193–2206. [Google Scholar] [CrossRef]
- Weber, G.; Farris, F.J. Synthesis and Spectral Properties of a Hydrophobic Fluorescent Probe: 6-Propionyl-2-(Dimethylamino) Naphthalene. Biochemistry 1979, 18, 3075–3078. [Google Scholar] [CrossRef] [PubMed]
- Parusel, A.B.J.; Nowak, W.; Grimme, S.; Kohler, G. Comparative Theoretical Study on Charge-Transfer Fluorescence Probes: 6-Propanoyl-2-(N, N-Dimethylamino) Naphthalene and Derivatives. J. Phys. Chem. A 1998, 102, 7149–7156. [Google Scholar] [CrossRef]
- Parusel, A.B.J.; Schneider, F.W.; Köhler, G. An Ab Initio Study on Excited and Ground State Properties of the Organic Fluorescence Probe PRODAN. J. Mol. Struct. THEOCHEM 1997, 398, 341–346. [Google Scholar] [CrossRef]
- Catalan, J.; Perez, P.; Laynez, J.; Blanco, F.G. Analysis of the Solvent Effect on the Photophysics Properties of 6-Propionyl-2-(Dimethylamino)Naphthalene (PRODAN). J. Fluoresc. 1991, 1, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Balter, A.; Nowak, W.; Pawekiewicz, W.; Kowalczyk, A. Some Remarks on the Interpretation of the Spectral Properties of Prodan. Chem. Phys. Lett. 1988, 143, 565–570. [Google Scholar] [CrossRef]
- Kawski, A. Ground-and Excited-State Dipole Moments of 6-Propionyl-2-(Dimethylamino) Naphthalene Determined from Solvatochromic Shifts. Z. Naturforsch. A 1999, 54, 379–381. [Google Scholar] [CrossRef]
- Lobo, B.C.; Abelt, C.J. Does PRODAN Possess a Planar or Twisted Charge-Transfer Excited State? Photophysical Properties of Two PRODAN Derivatives. J. Phys. Chem. A 2003, 107, 10938–10943. [Google Scholar] [CrossRef]
- Davis, B.N.; Abelt, C.J. Synthesis and Photophysical Properties of Models for Twisted PRODAN and Dimethylaminonaphthonitrile. J. Phys. Chem. A 2005, 109, 1295–1298. [Google Scholar] [CrossRef]
- Chen, T.; Lee, S.W.; Abelt, C.J. 1,5-Prodan Emits from a Planar Intramolecular Charge-Transfer Excited State. ACS Omega 2018, 3, 4816–4823. [Google Scholar] [CrossRef]
- Abelt, C.; Sweigart, K. Twisted 8-Acyl-1-Dialkyl-Amino-Naphthalenes Emit from a Planar Intramolecular Charge Transfer Excited State. Photochem 2024, 4, 1–13. [Google Scholar] [CrossRef]
- Benedetti, E.; Veliz, A.B.; Charpenay, M.; Kocsis, L.S.; Brummond, K.M. Attachable Solvatochromic Fluorophores and Bioconjugation Studies. Org. Lett. 2013, 15, 2578–2581. [Google Scholar] [CrossRef]
- Benedetti, E.; Kocsis, L.S.; Brummond, K.M. Synthesis and Photophysical Properties of a Series of Cyclopenta [b] Naphthalene Solvatochromic Fluorophores. J. Am. Chem. Soc. 2012, 134, 12418–12421. [Google Scholar] [CrossRef]
- Brummond, K.M.; Kocsis, L.S. Intramolecular Didehydro-Diels–Alder Reaction and Its Impact on the Structure–Function Properties of Environmentally Sensitive Fluorophores. Acc. Chem. Res. 2015, 48, 2320–2329. [Google Scholar] [CrossRef]
- Jockusch, S.; Zheng, Q.; He, G.S.; Pudavar, H.E.; Yee, D.J.; Balsanek, V.; Halim, M.; Sames, D.; Prasad, P.N.; Turro, N.J. Two-Photon Excitation of Fluorogenic Probes for Redox Metabolism: Dramatic Enhancement of Optical Contrast Ratio by Two-Photon Excitation. J. Phys. Chem. C 2007, 111, 8872–8877. [Google Scholar] [CrossRef]
- Abelt, C.; Day, I.; Zhao, J.; Pike, R. Fluorescence of Half-Twisted 10-Acyl-1-Methyltetrahydrobenzoquinolines. Molecules 2024, 29, 3016. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1999; ISBN 978-1-4757-3063-0. [Google Scholar]
- Mooney, J.; Kambhampati, P. Get the Basics Right: Jacobian Conversion of Wavelength and Energy Scales for Quantitative Analysis of Emission Spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Version 1.1; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Jurok, R.; Cibulka, R.; Dvořáková, H.; Hampl, F.; Hodačová, J. Planar Chiral Flavinium Salts—Prospective Catalysts for Enantioselective Sulfoxidation Reactions. Eur. J. Org. Chem. 2010, 2010, 5217–5224. [Google Scholar] [CrossRef]
- Knight, F.R.; Fuller, A.L.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Synthetic and Structural Studies of 1,8-Chalcogen Naphthalene Derivatives. Chem. Eur. J. 2010, 16, 7503–7516. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH: Hoboken, NJ, USA, 2011. [Google Scholar]
- El-Sayed, M.A. Triplet State. Its Radiative and Nonradiative Properties. Acc. Chem. Res. 1968, 1, 8–16. [Google Scholar] [CrossRef]


| Carbonyl | Pyrrolidine | Pyrrolidine’ | 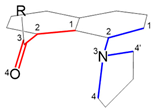 | |
| 6 | −108.1 | −98.1 | 23.2 | |
| 7 | −120.5 | −96.3 | 31.3 | |
| 8 | −117.3 | −94.5 | 31.1 |
| 1 | 2 | 6 | 7 | 8 |  | |
| C(O)····N (Å) | 2.56 | 2.67 | 2.69 | 2.60 | 2.65 | |
| C1····C8 (Å) | 2.49 | 2.52 | 2.51 | 2.50 | 2.51 | |
| Splay (°) | 3.8 | 8.3 | 8.2 | 4.5 | 5.5 | |
| θ1 (°) | 123.0 | 124.3 | 125.0 | 123.1 | 122.0 | |
| θ3 (°) | 116.1 | 117.7 | 116.8 | 116.6 | 117.4 | |
| %PL | 29.4 | 21.4 | 19.8 | 37.5 | 41.8 | |
| ΔC (Å) | 0.09 | 0.09 | 0.10 | 0.06 | 0.05 |
| 6 | 7 | 8 | 9 | |||||
|---|---|---|---|---|---|---|---|---|
| Solvent | λmax (nm) | Φ | λmax (nm) | Φ | λmax (nm) | Φ | λmax (nm) | Φ |
| (CH2)6 | 524 | 0.031 | 560 | 0.016 | 525 | 0.236 | 513 | 0.382 |
| PhCH3 | 539 | 0.062 | 591 | 0.007 | 548 | 0.242 | 536 | 0.390 |
| Et2O | 542 | 0.013 | 598 | 0.001 | 564 | 0.101 | 547 | 0.206 |
| PhCl | 548 | 0.076 | 613 | 0.005 | 564 | 0.179 | 550 | 0.360 |
| EtOAc | 550 | 0.039 | 613 | 0.003 | 563 | 0.130 | 554 | 0.198 |
| CH2Cl2 | 420 | 0.070 | 433 | 0.002 | 578 | 0.143 | 565 | 0.290 |
| (CH3)2CO | 420 | 0.042 | 440 | 0.001 | 578 | 0.072 | 566 | 0.149 |
| DMSO | 432 | 0.096 | 443 | 0.003 | 597 | 0.054 | 582 | 0.102 |
| CH3CN | 426 | 0.046 | 441 | 0.001 | 594 | 0.053 | 578 | 0.120 |
| iPrOH | 424 | 0.015 | 441 | 0.001 | 606 | 0.015 | 534 | 0.065 |
| EtOH | 424 | 0.016 | 439 | 0.001 | 615 | 0.009 | 528 | 0.051 |
| CH3OH | 430 | 0.019 | 443 | 0.001 | 625 | 0.006 | 522 | 0.039 |
| Carbonyl | Pyrrolidine | Pyrrolidine’ |  | |
| 6 | −107.5 | −97.8 | 23.3 | |
| (−108.1) | (−98.1) | (23.2) | ||
| 7 | −113.4 | −94.5 | 32.5 | |
| (−120.5) | (−96.3) | (31.3) | ||
| 8 | −106.3 | −93.8 | 29.6 | |
| (−117.3) | (−94.5) | (31.1) |
| red | blue | green |  | |
| 6 | −39.2 | −15.7 | 17.5 | |
| (7.8) | (13.7) | (5.6) | ||
| 7 | −30.9 | −15.4 | 16.4 | |
| (1.0) | (7.3) | (3.6) | ||
| 8 | −11.0 | −16.3 | 15.5 | |
| (7.0) | (4.8) | (4.0) | ||
| 9 | −12.0 | −14.7 | 15.7 | |
| (6.4) | (6.1) | (4.5) |
| 6 | 7 a | 8 | 9 | |
|---|---|---|---|---|
| S0→S1 | ||||
| λmax (nm) | 291 | 292 | 294 | 294 |
| f | 0.128 | 0.142 | 0.127 | 0.126 |
| Transition, | HO → LU, 68 | HO → LU + 1, 62 | HO → LU, 69 | HO → LU (0.69) |
| coeff. × 100 | HO-2 → LU + 1, −12 | HO → LU, 27 | HO-4 → LU + 1, 11 | HO-2 → LU + 1, 11 |
| HO-2 → LU + 2, 10 | ||||
| S0←S1 | ||||
| λmax (nm) | 465 | 490 | 480 | 472 |
| f | 0.080 | 0.052 | 0.082 | 0.084 |
| Transition, | HO → LU, 70 | HO → LU, 70 | HO → LU, 70 | HO → LU, 70 |
| coeff. × 100 |
| 6 | 7 | 8 | 9 | |
|---|---|---|---|---|
| n → π* Tn | T5 | T6 | T5 | T11 |
| E(Tn) (eV) a | 4.30 | 4.21 | 4.28 | 5.65 |
| (4.26) | (4.25) | (4.21) | (4.21) | |
| Transition, b | HO-2 → LU, 68 | HO-2 → LU, 33 | HO-4 → LU, 64 | HO-4 → LU + 6, 14 |
| coeff. × 100 | HO-2 → LU + 1, 19 | HO-2 → LU + 1, 41 | HO-4 → LU + 1, −13 | HO-4 → LU + 12, −27 |
| HO → LU + 1, 13 | HO-2 → LU + 2, 15 | HO-3 → LU, −12 | HO-4 → LU + 14, 11 | |
| HO → LU, 10 | HO-1 → LU, −16 | HO → LU + 1, −16 | HO-1 → LU + 1, −24 | |
| HO → LU, 34 | HO → LU + 6, 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, A.; Teuber, L.; Pike, R.; Abelt, C. Fluorescence of 8-Acyl-1-Pyrrolidinylnaphthalenes. Photochem 2025, 5, 27. https://doi.org/10.3390/photochem5030027
Liao A, Teuber L, Pike R, Abelt C. Fluorescence of 8-Acyl-1-Pyrrolidinylnaphthalenes. Photochem. 2025; 5(3):27. https://doi.org/10.3390/photochem5030027
Chicago/Turabian StyleLiao, Angela, Lucas Teuber, Robert Pike, and Christopher Abelt. 2025. "Fluorescence of 8-Acyl-1-Pyrrolidinylnaphthalenes" Photochem 5, no. 3: 27. https://doi.org/10.3390/photochem5030027
APA StyleLiao, A., Teuber, L., Pike, R., & Abelt, C. (2025). Fluorescence of 8-Acyl-1-Pyrrolidinylnaphthalenes. Photochem, 5(3), 27. https://doi.org/10.3390/photochem5030027







