Measuring the Efficiency of Using Raman Photoexcitation to Generate Singlet Oxygen in Distilled Water
Abstract
1. Introduction
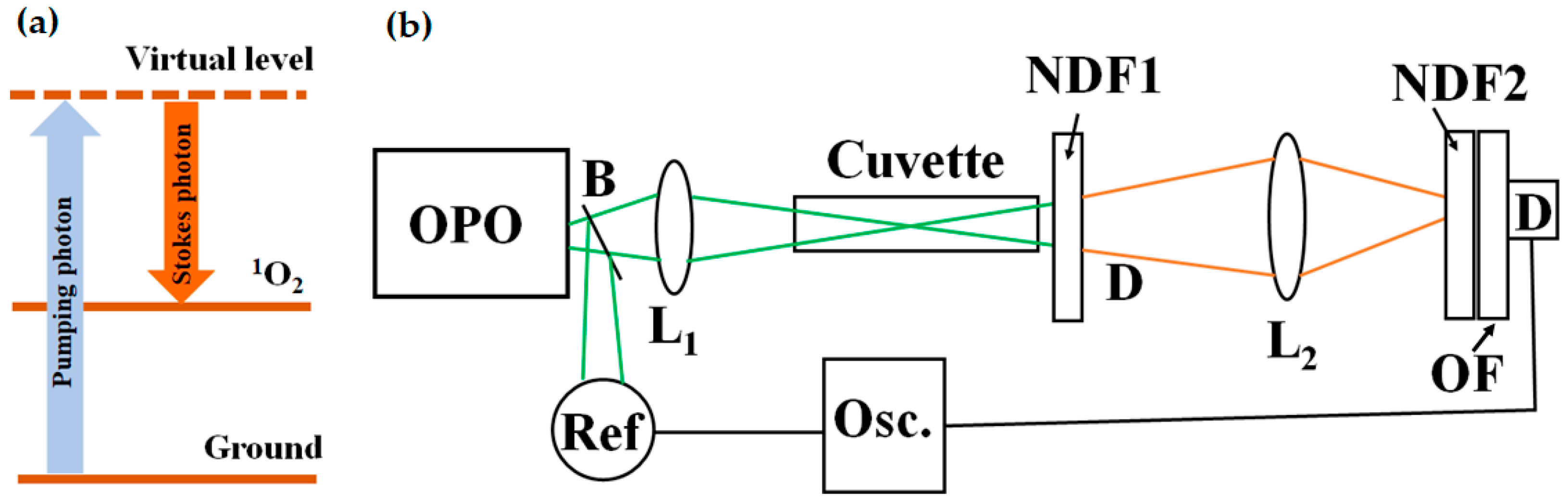
2. Theoretical Considerations
3. Materials and Methods
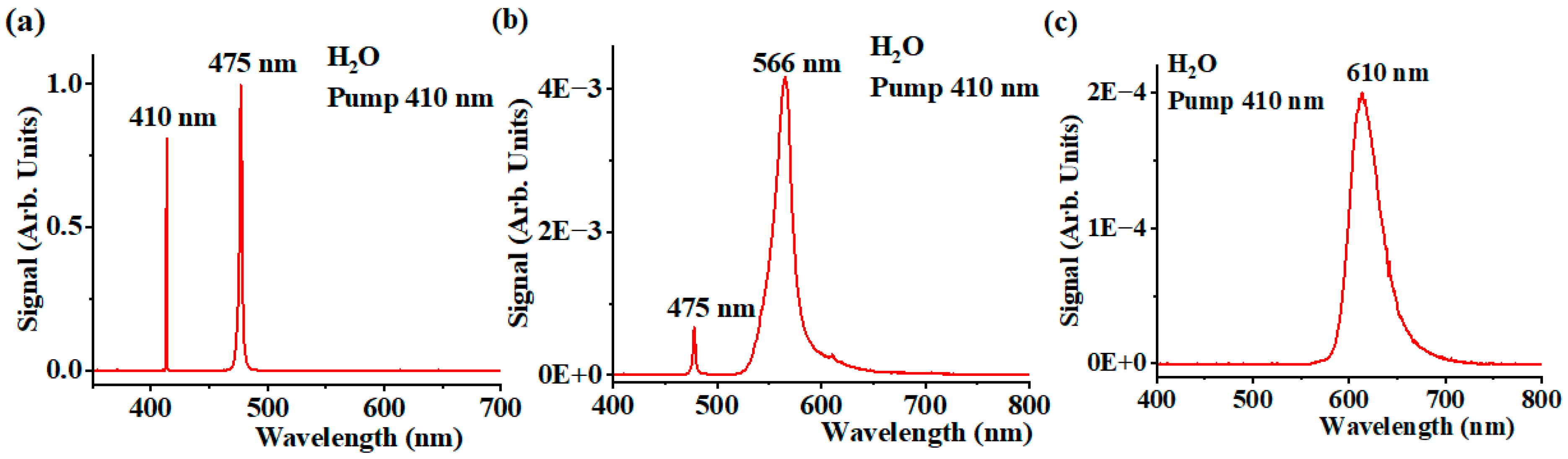
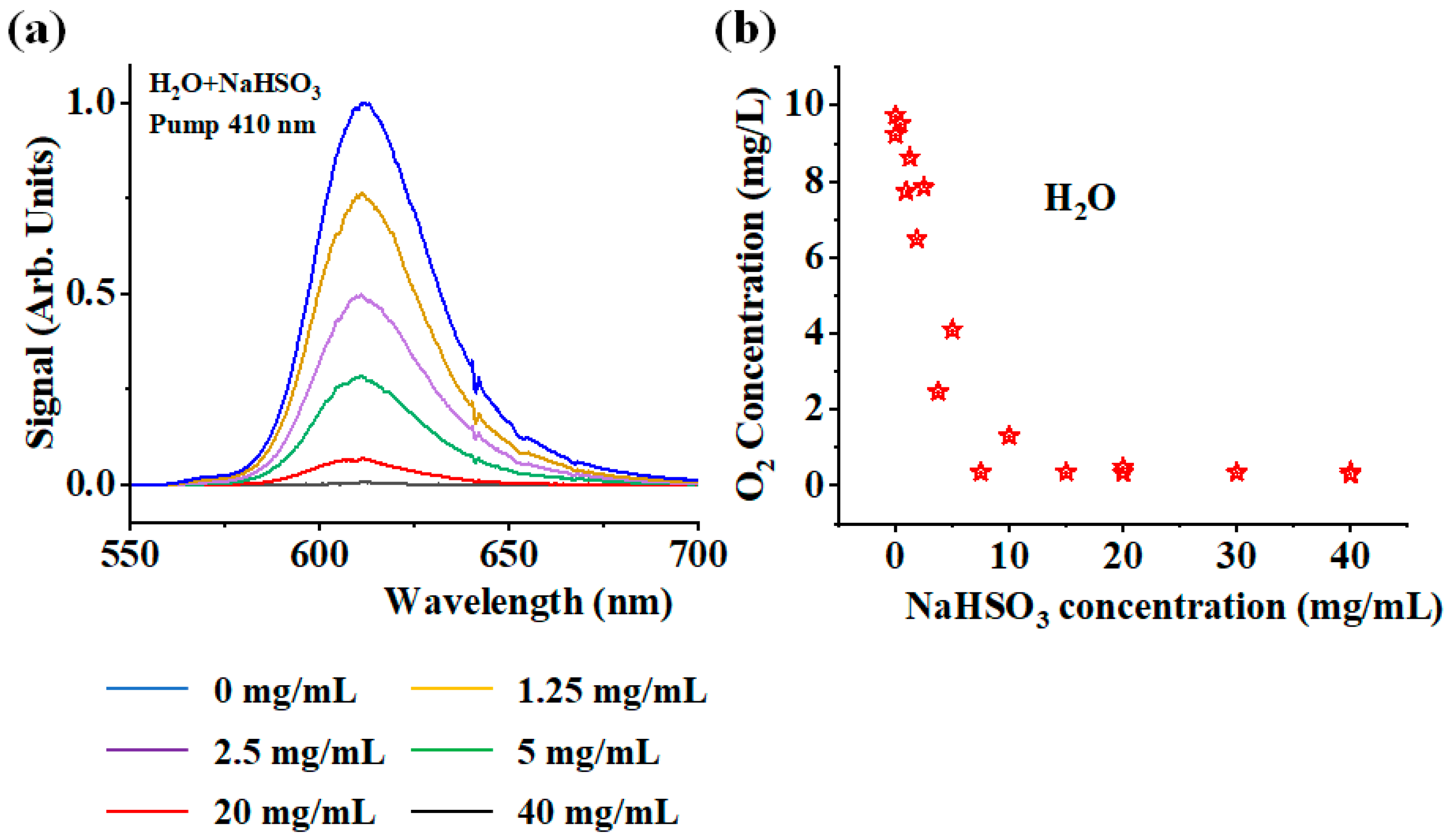
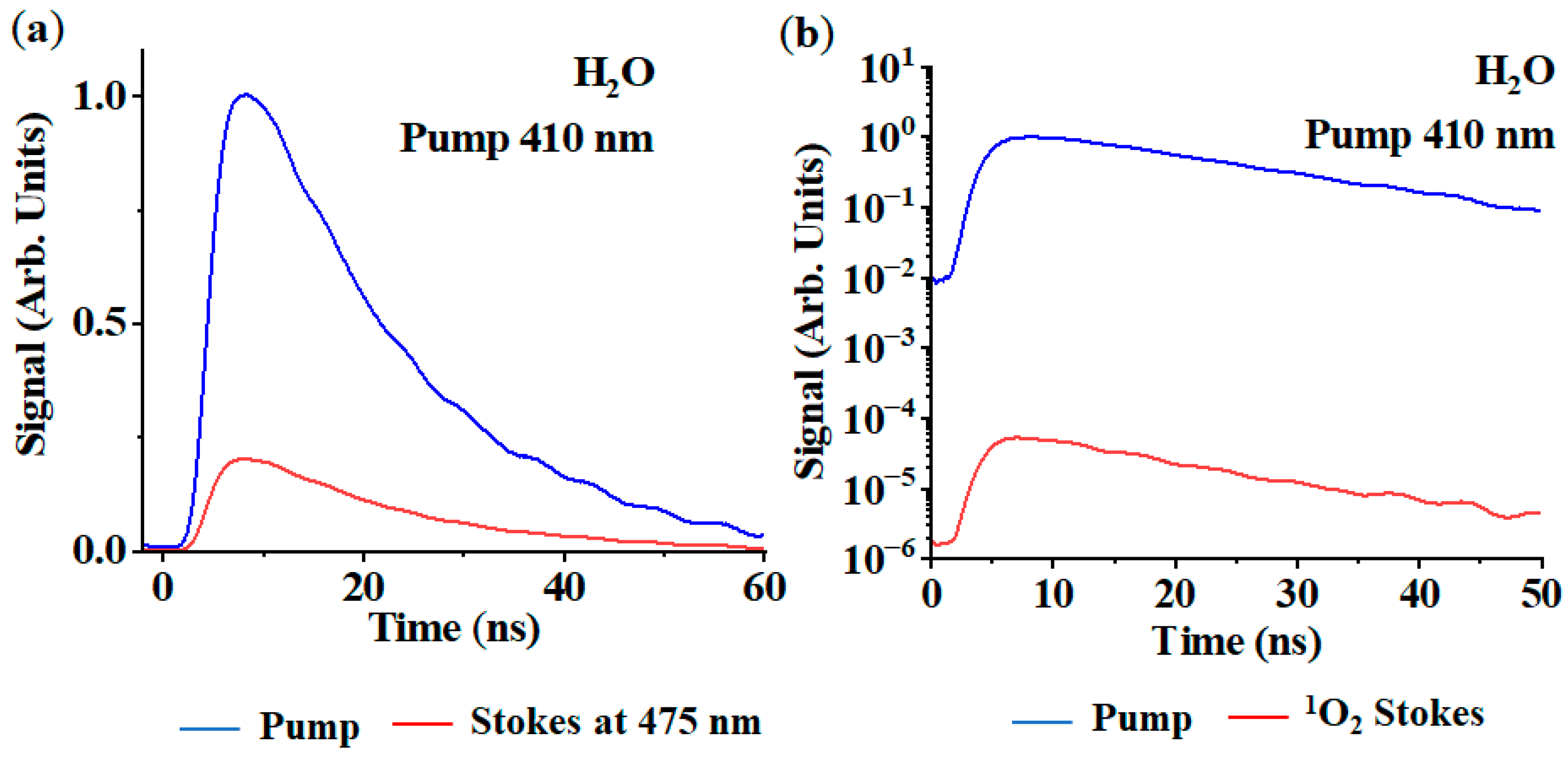
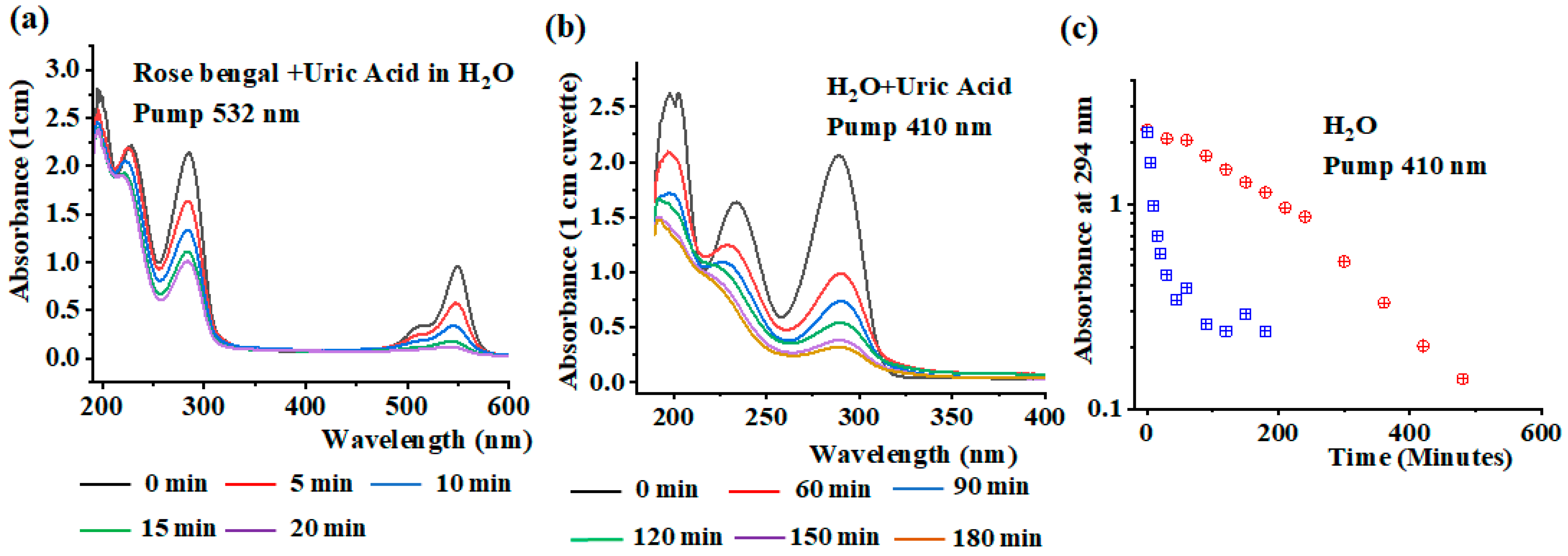
4. Results and Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nonell, S.; Flors, C. (Eds.) Singlet Oxygen: Applications in Biosciences and Nanosciences, 1st ed.; Volume 2—Comprehensive Series in Photochemical & Photobiological Sciences; Royal Society of Chemistry, Thomas Graham House: Cambridge, UK, 2016; Volume 14. [Google Scholar]
- Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A.; Pace, A. Photochemically Produced Singlet Oxygen: Applications and Perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized Singlet Oxygen and its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Mielcarek, J.; Goslinski, T.; Balzarini, J. Photosensitizers Mediated Photodynamic Inactivation Against Virus Particles. Mini Rev. Med. Chem. 2015, 15, 503–521. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.; Wang, J.Y.; Ozog, D.M.; Zeitouni, N.; Lim, H.W.; Jagdeo, J. Photodynamic Therapy: Overview and Mechanism of Action. J. Am. Acad. Dermatol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wu, X.; Li, Q.; Lu, C.; Carabineiro, S.A.C.; Zhao, Z.; Liu, Y.; Lv, K. Singlet Oxygen in Environmental Catalysis: Mechanism, Applications and Future Directions. Coord. Chem. Rev. 2025, 529, 216439. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, B.; Ding, J.; Sun, T.; Xie, Z. BODIPY Photosensitizers for Antibacterial Photodynamic Therapy. Chin. Chem. Lett. 2025, 36, 110645. [Google Scholar] [CrossRef]
- Quina, F.H.; Medeiros Silva, G.T. The Photophysics of Photosensitization: A brief overview. J. Photochem. Photobiol. 2021, 7, 100042. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photosensitization and Photocatalysis—Perspectives in Organic Chemistry. ACS Catal. 2018, 8, 12046–12055. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Jiang, L.; Huang, H. Photodynamic Therapy with Photodegradable Photosensitizers. Chem. Commun. 2025, 61, 2627–2635. [Google Scholar] [CrossRef]
- He, L.; Dong, J.; Yang, Y.; Huang, Z.; Ye, S.; Ke, X.; Zhou, Y.; Li, A.; Zhang, Z.; Wu, S.; et al. Accelerating the Discovery of Type II Photosensitizer: Experimentally Validated Machine Learning Models for Predicting the Singlet Oxygen Quantum Yield of Photosensitive Molecule. J. Mol. Struct. 2025, 1321, 139850. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Y.; Ding, S.; Huang, M.; Jiang, L. Recent Advances in Clinically Used and Trialed Photosensitizers for Antitumor Photodynamic Therapy. Mol. Pharm. 2025, 22, 3530–3541. [Google Scholar] [CrossRef]
- Marcano Olaizola, A.; Kingsley, D.; Kuis, R.; Johnson, A. Stimulated Raman Generation of Aqueous Singlet Oxygen Without Photosensitizers. J. Photochem. Photobiol. B Biol. 2022, 235, 112562. [Google Scholar] [CrossRef]
- Marcano Olaizola, A.; Zerrad, A.; Janneto, F.; Kingsley, D. Confirming the Stimulated Raman Origin of Singlet-Oxygen Photogeneration. J. Raman Spectrosc. 2024, 55, 58–64. [Google Scholar] [CrossRef]
- Marcano Olaizola, A. Near-Infrared Phosphorescence of Raman Photogenerated Singlet Oxygen. Photochem 2025, 5, 7. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Ossola, R.; Jönsson, O.M.; Moor, K.; McNeill, K. Singlet Oxygen quantum yields in environmental waters. Chem. Rev. 2021, 121, 4100–4146. [Google Scholar] [CrossRef] [PubMed]
- Lutkus, L.V.; Rickenbach, S.S.; McCormick, T.M. Singlet Oxygen Quantum Yields Determined by Oxygen Consumption. J. Photochem. Photobiol. A Chem. 2019, 378, 131–135. [Google Scholar] [CrossRef]
- Morgan, J.; Yun, Y.J.; Ayitou, A.J.L. Estimation of Singlet Oxygen Quantum Yield Using Novel Green-Absorbing Baird-Type Aromatic Photosensitizers. Photochem. Photobiol. 2022, 98, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Qin, F.; Wang, Y.; Peng, L.; Hu, Z.; Zhao, H.; Zhang, Z. Determination of Singlet Oxygen Quantum Yield Based on the Behaviour of Solvent Dimethyl Sulfoxide Oxidation by Singlet Oxygen. Anal. Chim. Acta 2024, 1329, 343222. [Google Scholar] [CrossRef]
- Szewczyk, G.; Mokrzynski, K. Concentration-Dependent Photoproduction of Singlet Oxygen by Common Photosensitizers. Molecules 2025, 30, 1130. [Google Scholar] [CrossRef]
- Murasecco-Suardi, P.; Gassmann, E.; Braun, A.M.; Oliveros, E. Determination of the Quantum Yield of Intersystem Crossing of Rose Bengal. Helvetica 1987, 70, 1760–1773. [Google Scholar] [CrossRef]
- Salasi, M.; Pojtanabuntoeng, T.; Wong, S.; Lehmann, M. Efficacy of bisulfite ions as an oxygen scavenger in monoethylene glycol (at least 20 wt%)/water mixtures. SPE J. 2017, 22, 1467–1477. [Google Scholar] [CrossRef]
- Bregnhøj, M.; Dichmann, L.; McLoughlin, C.K.; Westberg, M.; Ogilby, P. Uric Acid: A less-than-perfect probe for singlet oxygen. Photochem. Photobio. 2019, 95, 202–210. [Google Scholar] [CrossRef]
- Iida, S.; Ohkubo, Y.; Yamamoto, Y.; Fujisawa, A. Parabanic Acid is the singlet oxygen specific oxidation product of uric acid. J. Clin. Biochem. Nutr. 2017, 61, 169–175. [Google Scholar] [CrossRef]
- Trivedi, R.C.; Rebar, L.; Desai, K.; Stong, L.J. New ultraviolet (340 nm) method for assay of uric acid in serum or plasma. Clin. Chem. 1978, 24, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Graschew, G.; Sinn, H.J.; Maier-Borst, W.; Lorenz, W.J.; Schlag, P.M. A chemical dosimeter for the determination of photodynamic activity of photosensitizers. Clin. Chim. Acta 1998, 274, 89–104. [Google Scholar] [CrossRef]
- Gao, Y.; Gong, N.; Sun, C.; Fang, W.; Wang, S.; Men, Z. Stimulated Raman scattering investigation of isotopic substitution H2O/D2O system. J. Mol. Liq. 2020, 297, 11923. [Google Scholar] [CrossRef]
- Sun, Q. The Raman OH stretching bands of liquid water. Vib. Spectrosc. 2009, 51, 213–217. [Google Scholar] [CrossRef]
- Bertie, J.E.; Lan, Z. Infrared intensities of liquids XX: The intensity of the OH stretching band of liquid water revisited, and the best current values of the optical constants of H2O(I) at 25 °C Between 15,000 and 1 cm−1. Appl. Spectros. 1996, 50, 1047–1057. [Google Scholar] [CrossRef]
- Boyraz, O.; Jalali, B. Demonstration of a Silicon Raman Laser. Opt. Express 2004, 12, 5269–5273. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Jones, R.; Liu, A.; Cohen, O.; Hak, D.; Fang, A.; Paniccia, M. A Continuous-Wave Raman Silicon Laser. Nature 2005, 433, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, J.; Chen, Y.; Zhang, B.; Ji, M. Fiber-Enhanced Stimulated Raman Scattering and Sensitive Detection of Dilute Solutions. Biosensors 2022, 12, 243. [Google Scholar] [CrossRef]
- Blaskovits, J.T.; Corminboeuf, C.; Garner, M.H. Singlet—Triplet Inversions in Through-Bond Charge-Transfer States. J. Phys. Chem. Lett. 2024, 15, 10062–10067. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Kuis, R.; Perez, R.; Basaldua, I.; Burkins, P.; Marcano Olaizola, A.; Johnson, A. Oxygen-dependent laser inactivation of murine norovirus using visible light lasers. Virol. J. 2018, 15, 117. [Google Scholar] [CrossRef] [PubMed]
| Pump wavelength | 410 nm |
| Pulse width | 6 ns |
| Repetition rate | 10 Hz |
| Pulse energy | 13 mJ |
| Beam area at waist | 8.5 × 10−7 cm2 |
| Pulse intensity at waist | 2.5 × 1012 W/cm2 |
| Cuvette path length | 10 cm |
| Focusing lens focal length | 20 cm |
| Detector Type | DET100 A2 (Thorlabs) |
| Detector rise time | 35 ns |
| Stokes Signal | Signal Energy (mJ) | Efficiency | Relative Intensity |
|---|---|---|---|
| H2O mode at 475 nm | 3.2 ± 0.14 | 0.23 ± 0.01 | 1 |
| H2O overtone at 566 nm | (35 ± 1.4) × 10−3 | 0.0025 ± 0.0001 | 10−2 |
| 1O2 | (7 ± 2) × 10−4 | (8 ± 2) × 10−5 | 2.2 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcano Olaizola, A. Measuring the Efficiency of Using Raman Photoexcitation to Generate Singlet Oxygen in Distilled Water. Photochem 2025, 5, 24. https://doi.org/10.3390/photochem5030024
Marcano Olaizola A. Measuring the Efficiency of Using Raman Photoexcitation to Generate Singlet Oxygen in Distilled Water. Photochem. 2025; 5(3):24. https://doi.org/10.3390/photochem5030024
Chicago/Turabian StyleMarcano Olaizola, Aristides. 2025. "Measuring the Efficiency of Using Raman Photoexcitation to Generate Singlet Oxygen in Distilled Water" Photochem 5, no. 3: 24. https://doi.org/10.3390/photochem5030024
APA StyleMarcano Olaizola, A. (2025). Measuring the Efficiency of Using Raman Photoexcitation to Generate Singlet Oxygen in Distilled Water. Photochem, 5(3), 24. https://doi.org/10.3390/photochem5030024






