Selective and Sensitive Dual Chromogenic Cyanide and Fluorescent Azide Probe
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of the Dye and Anion Stock Solutions
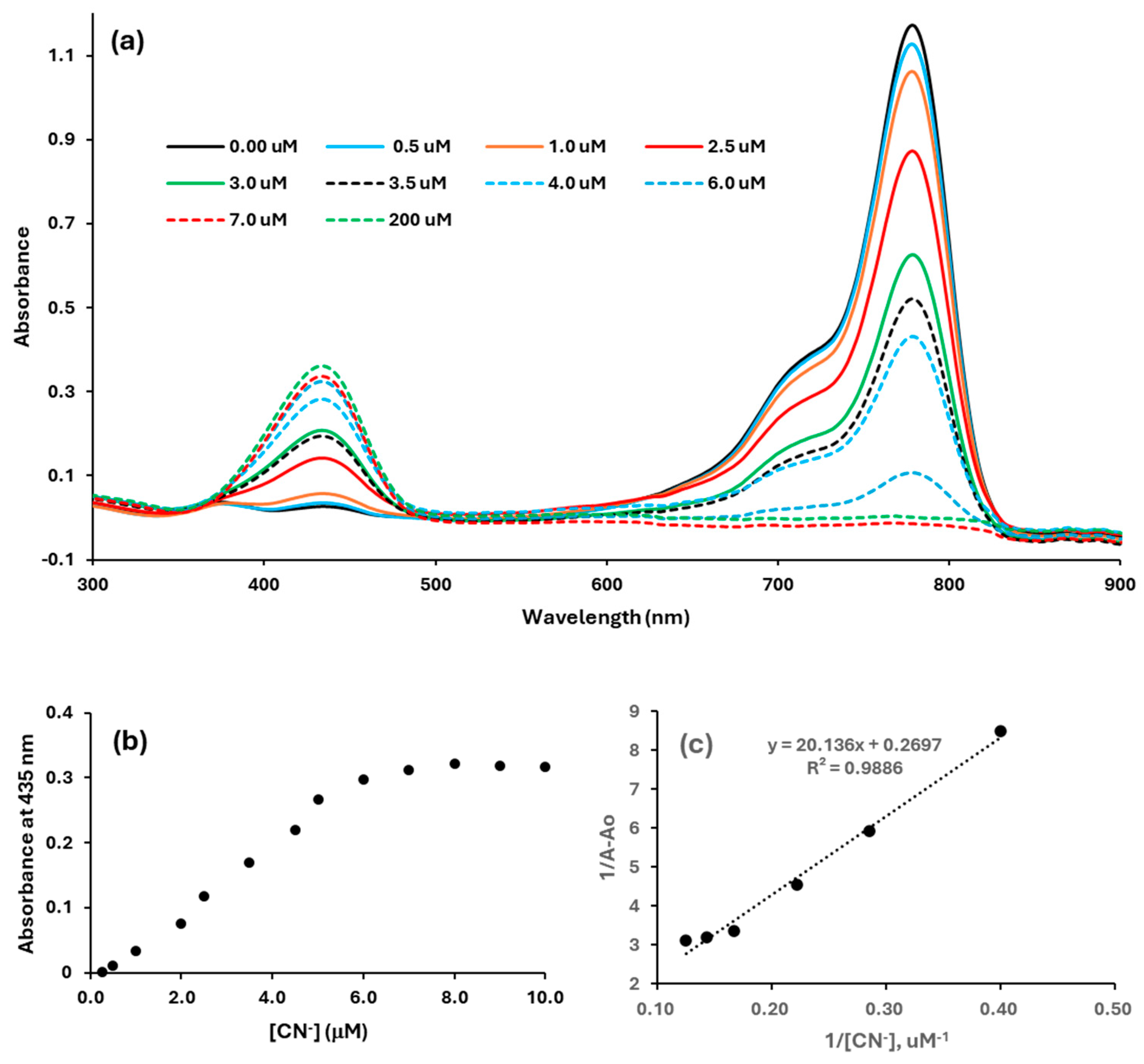
2.3. The Quantitative Response of the Dye to Various Anions by UV-Vis and Fluorescence Studies
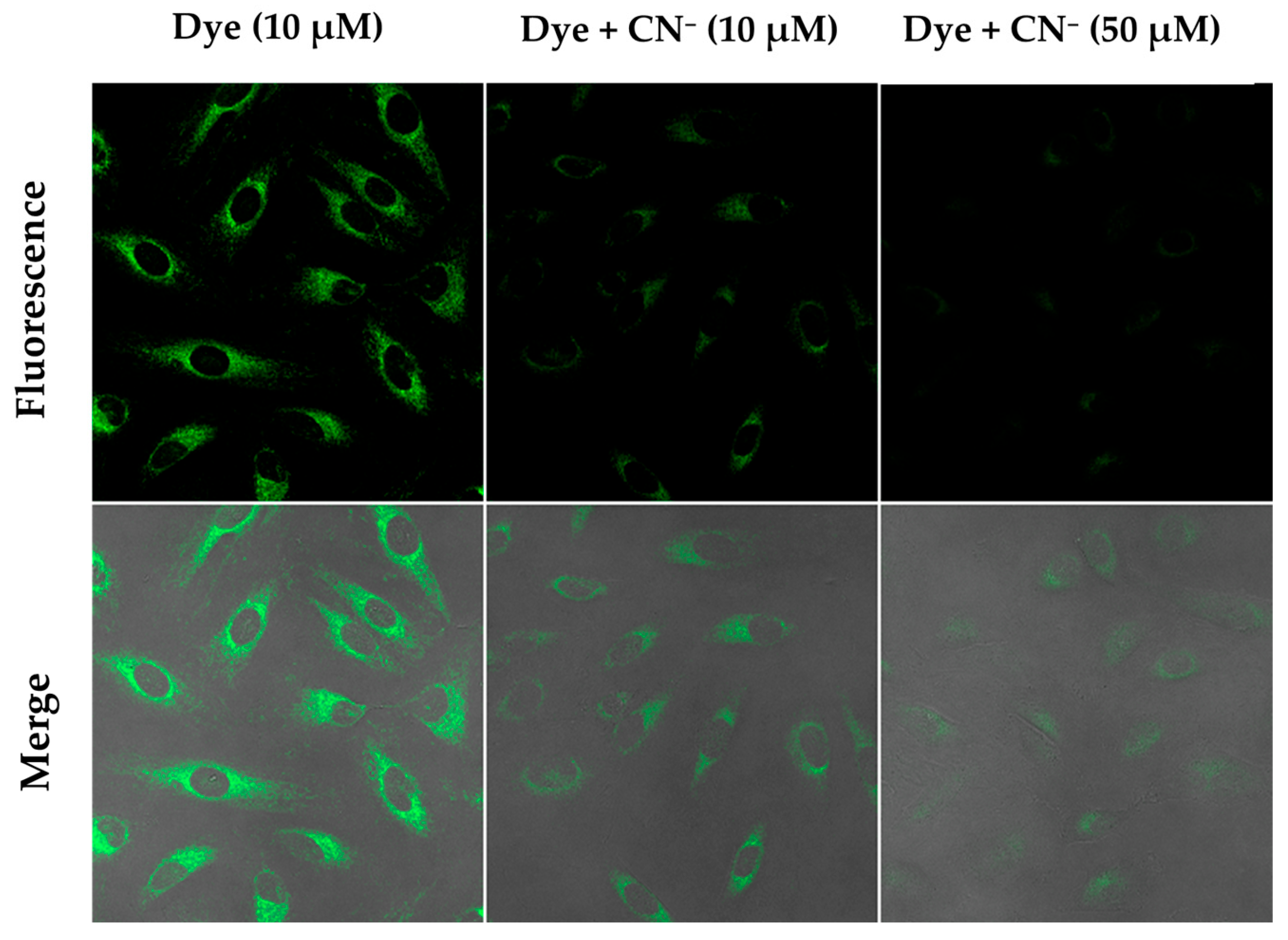
2.4. The Fluorescence Imaging of Live Cells with the Dye and the Quenching of Fluorescence in the Presence of Cyanide
3. Results and Discussions
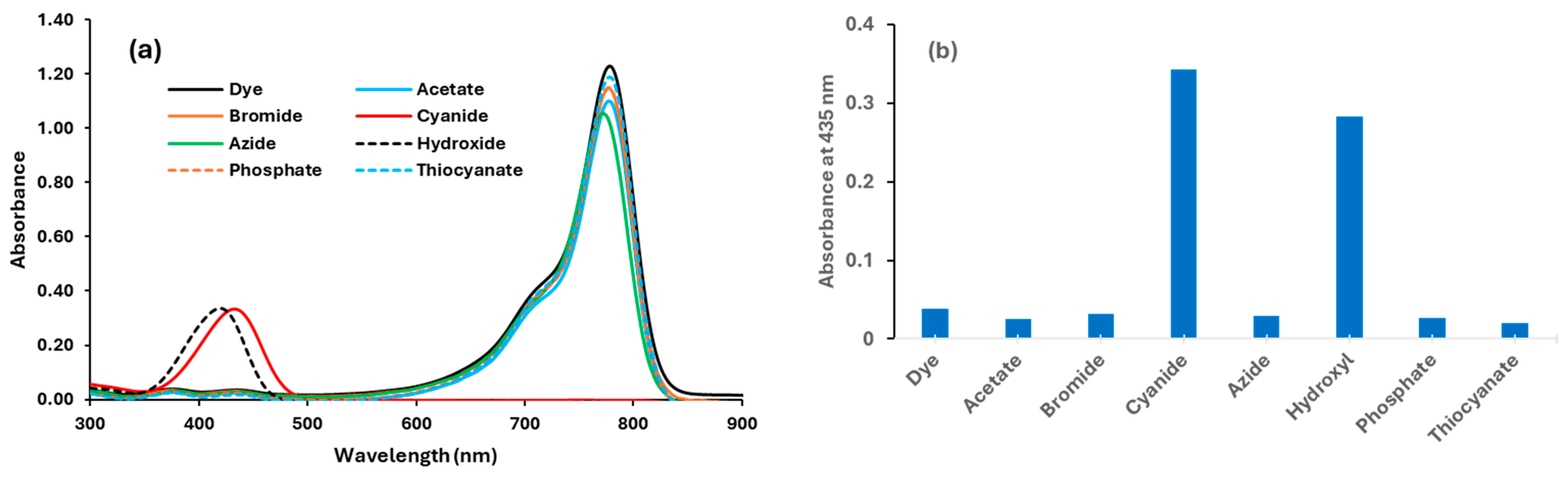
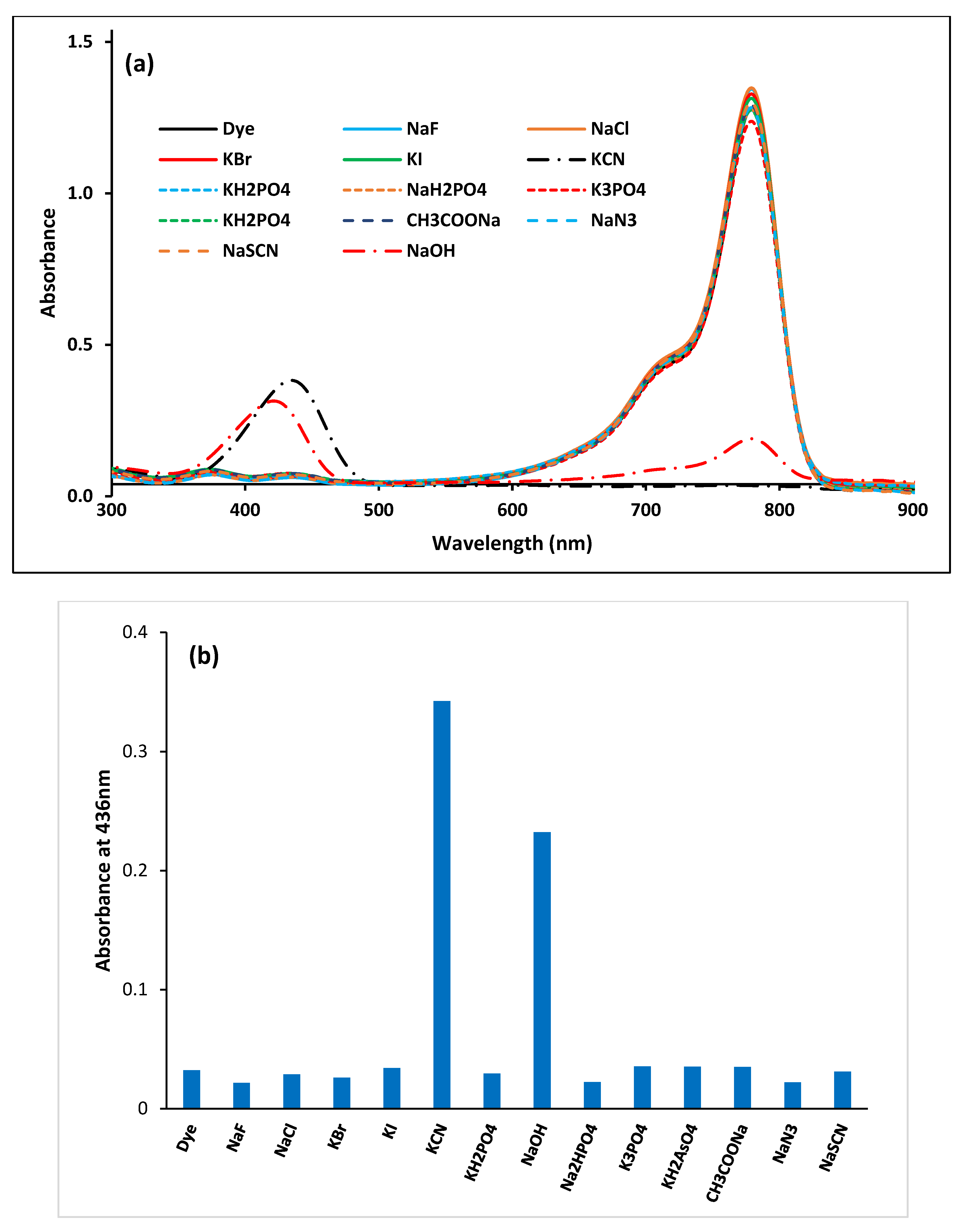
3.1. Investigation of the Dye’s Selectivity and Sensitivity Towards Anions in 100% Acetonitrile
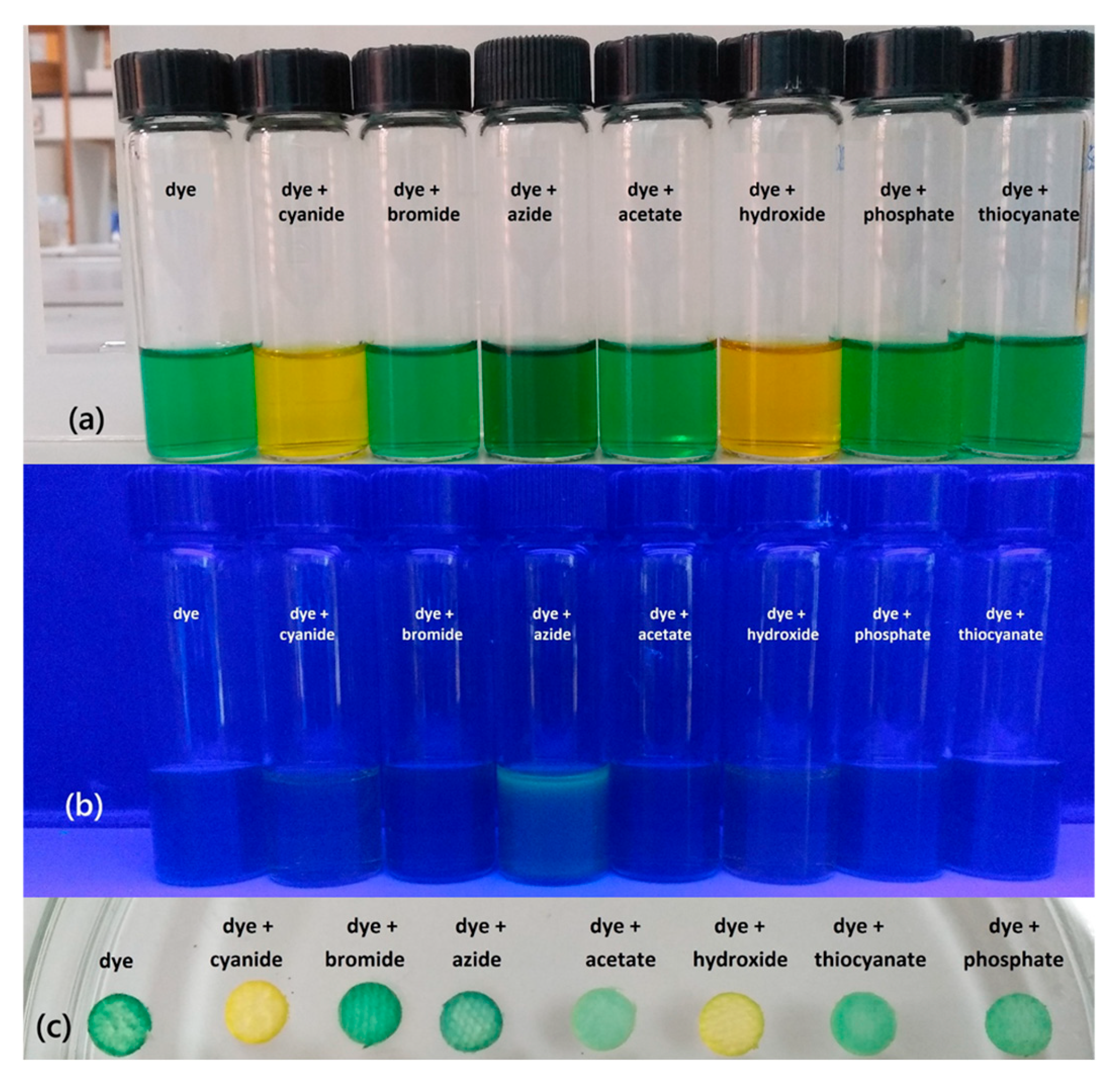
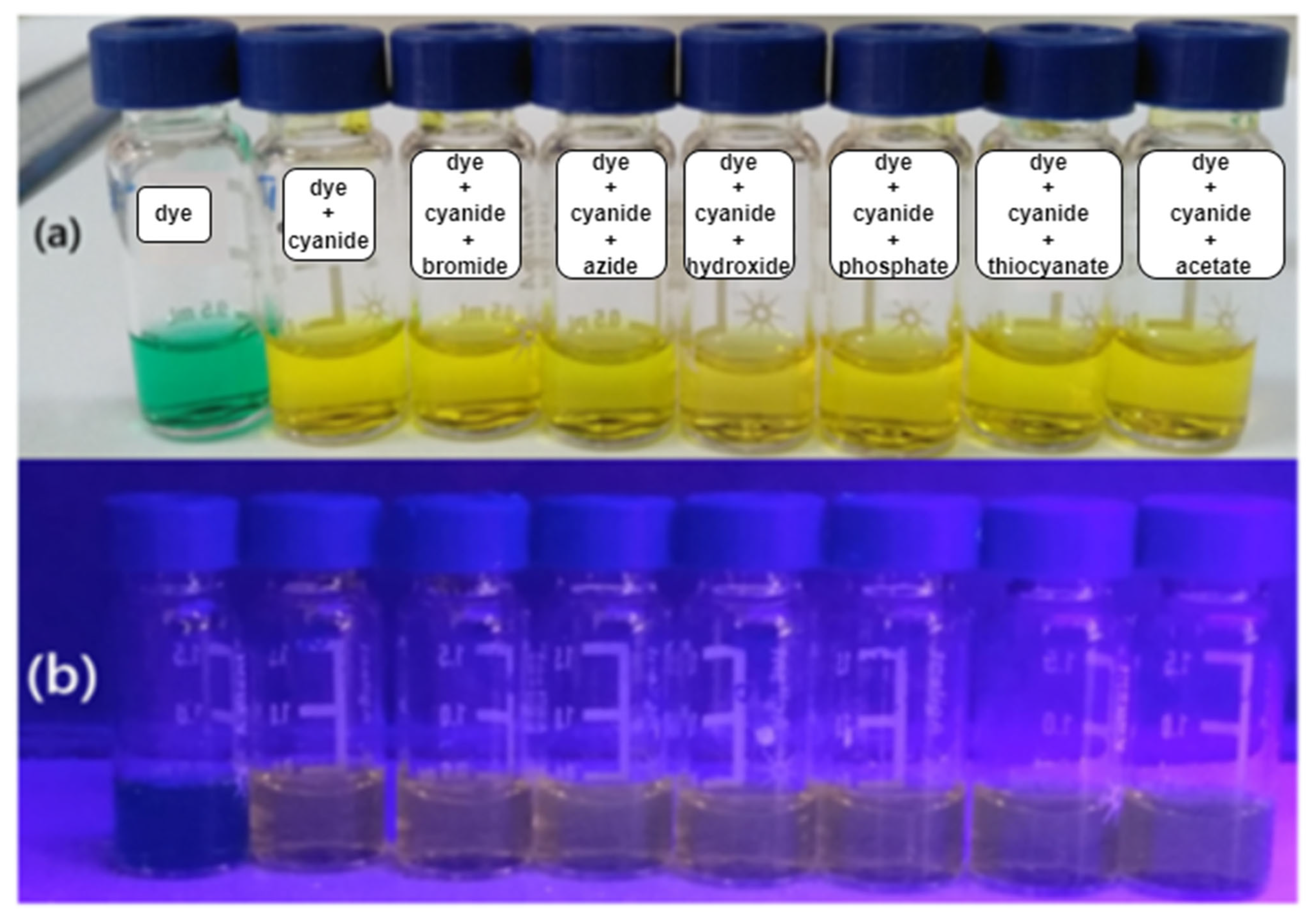
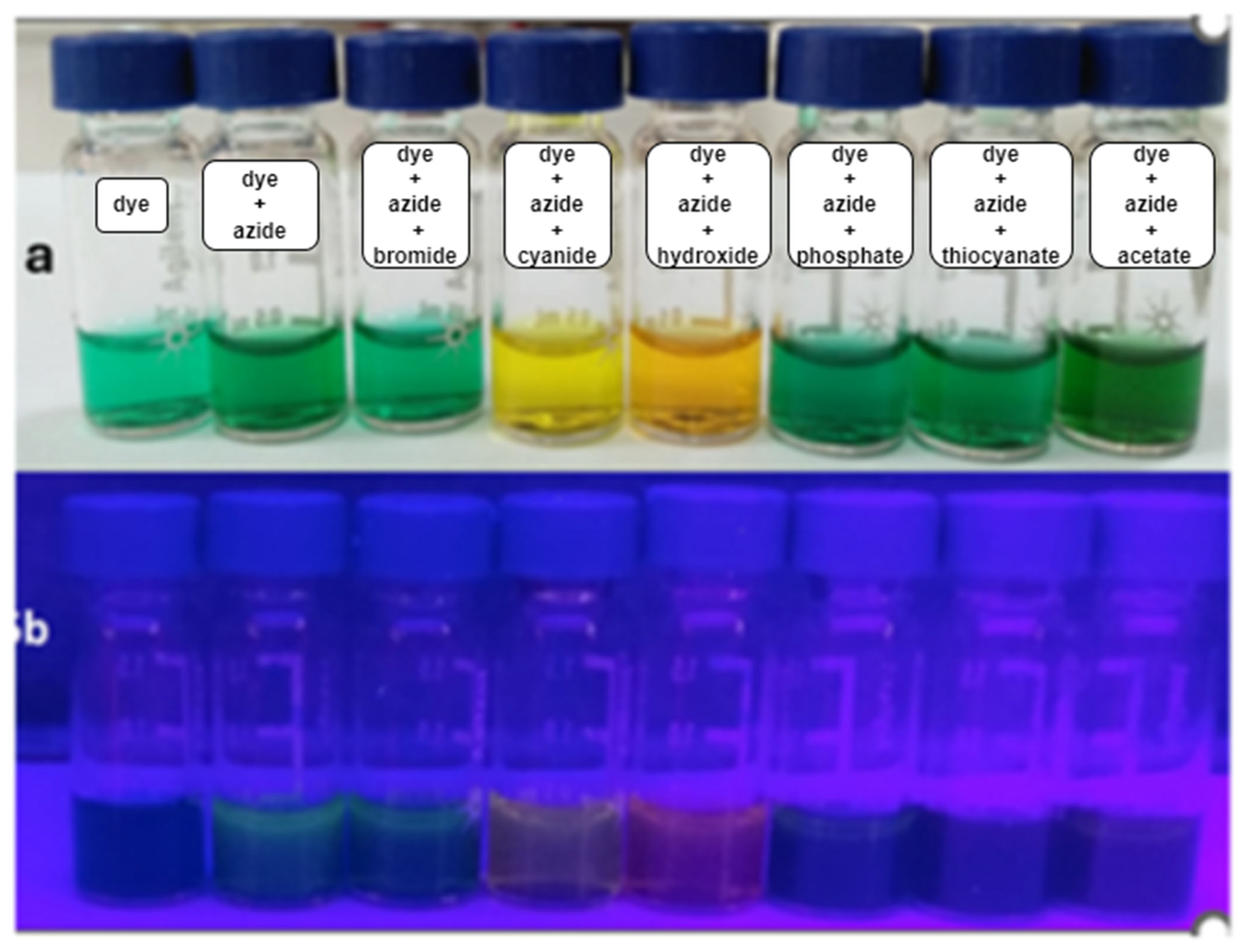
3.1.1. Visual Test
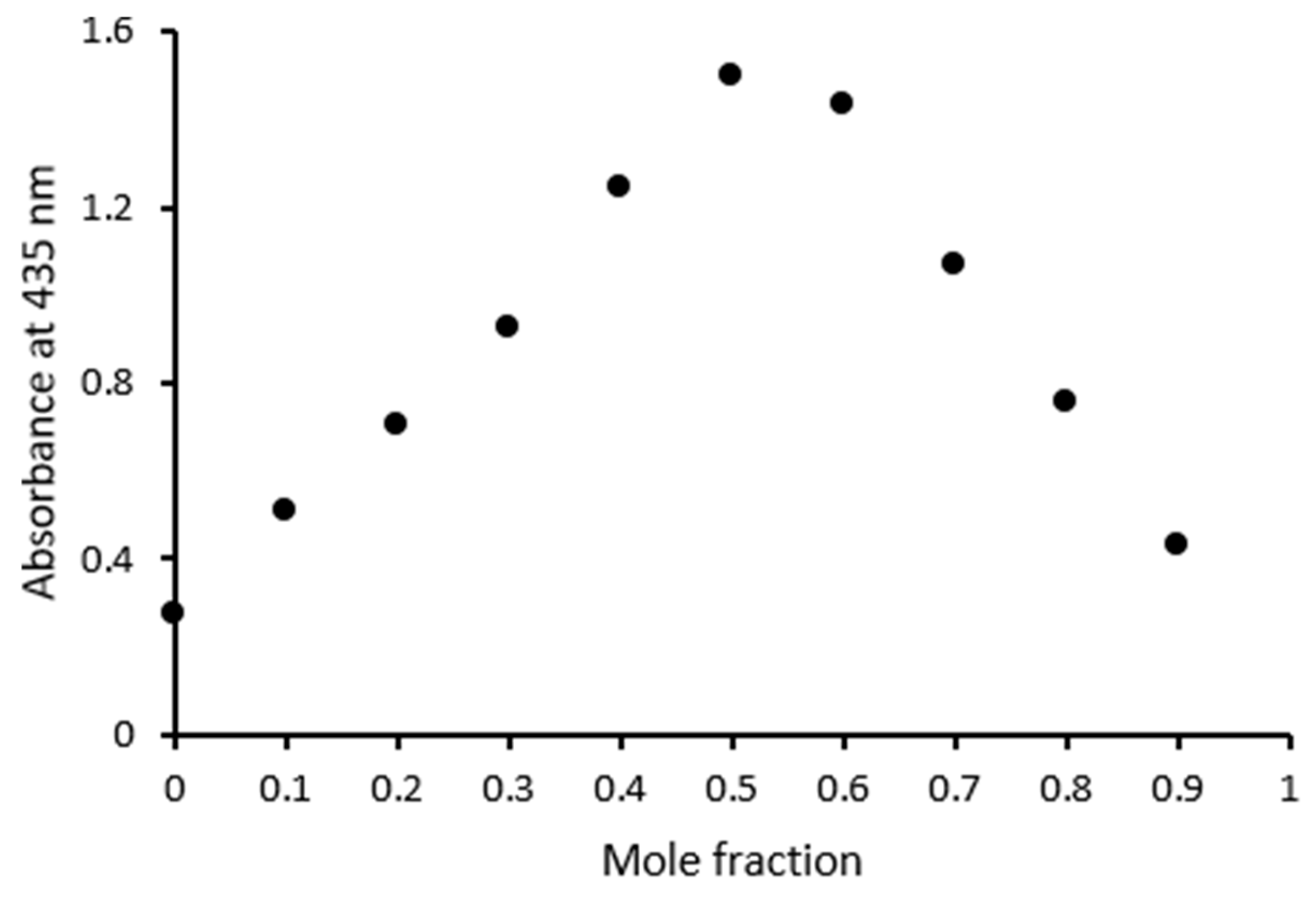
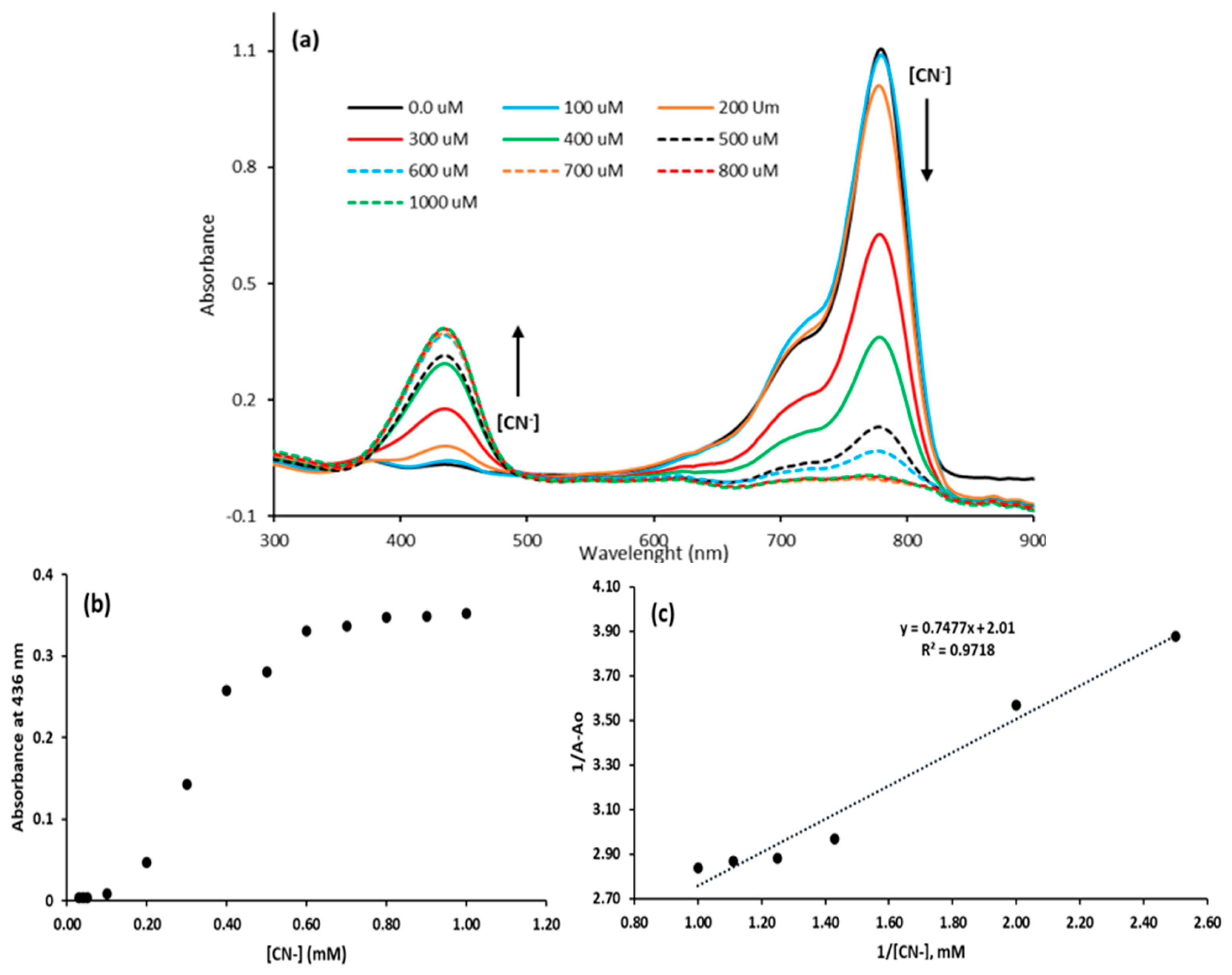
3.1.2. Stoichiometry and Binding Constants
3.1.3. UV-Vis Interaction of the Dye with Azide in Acetonitrile
3.1.4. Interference of Common Ions with Cyanide–Dye Interaction
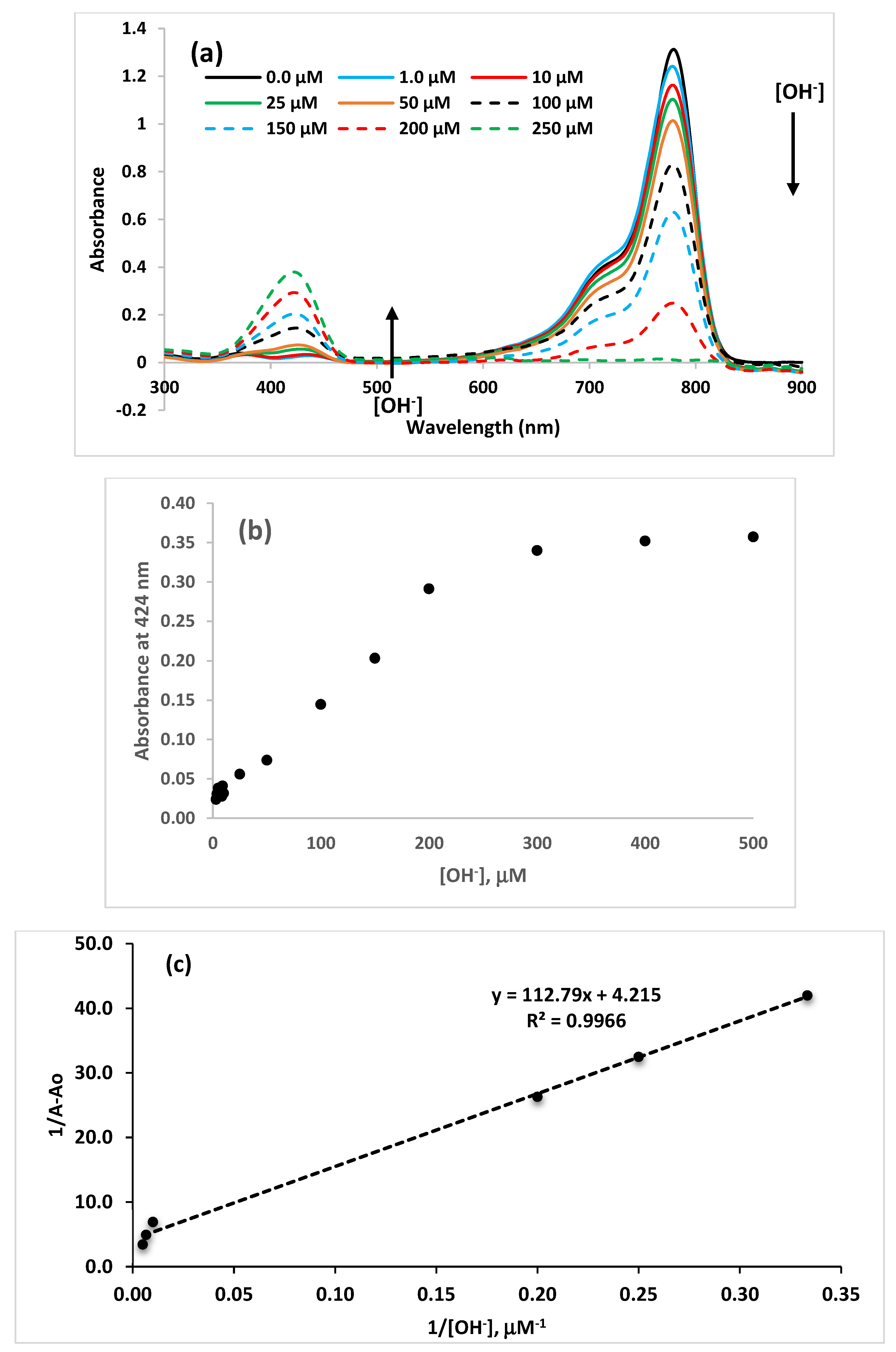
3.2. UV-Vis Selectivity and Sensitivity Studies in Acetonitrile and Water Mixture
3.2.1. Visual Tests
3.2.2. Binding Constants
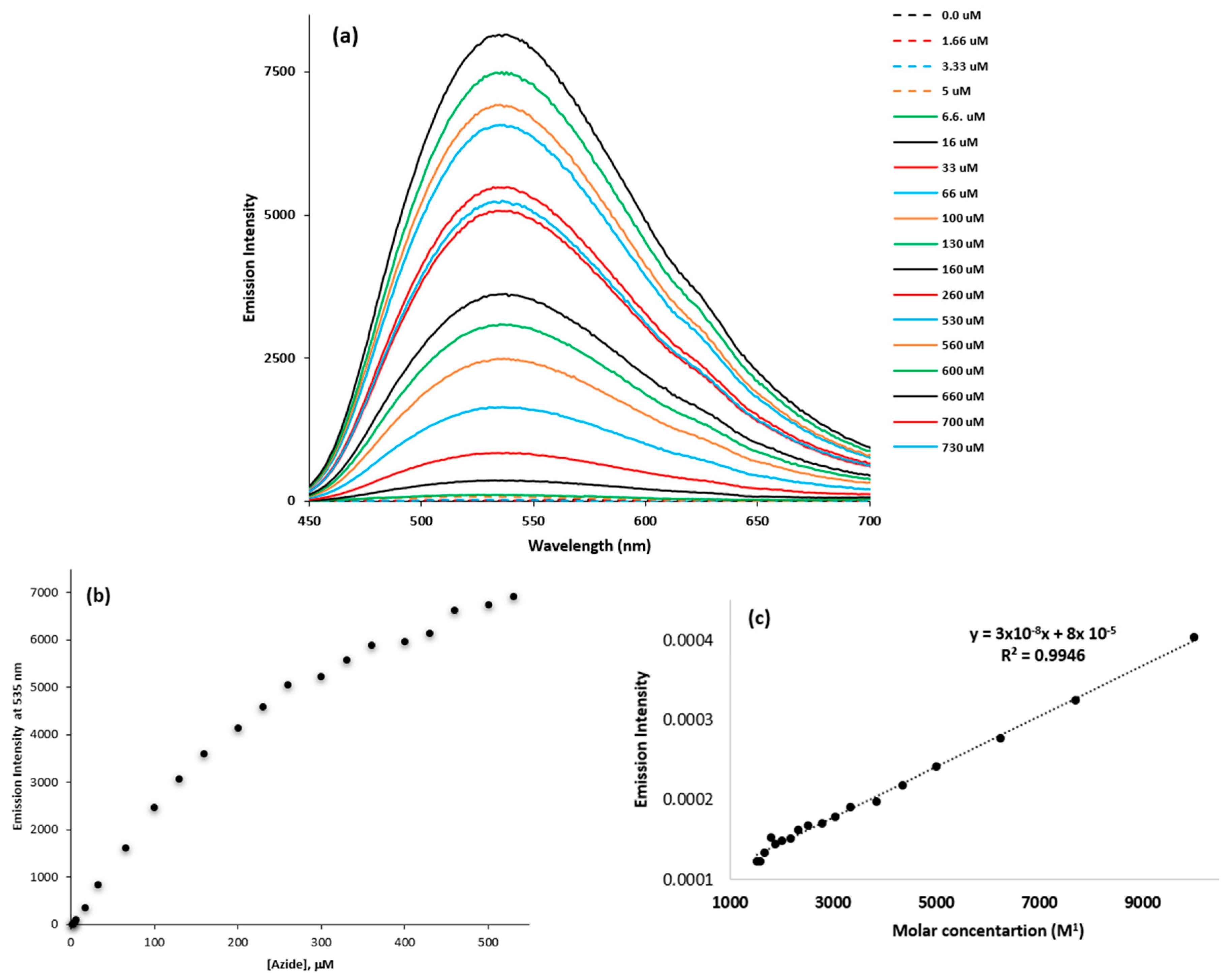
3.3. The Fluorescence Sensing Property of the Dye
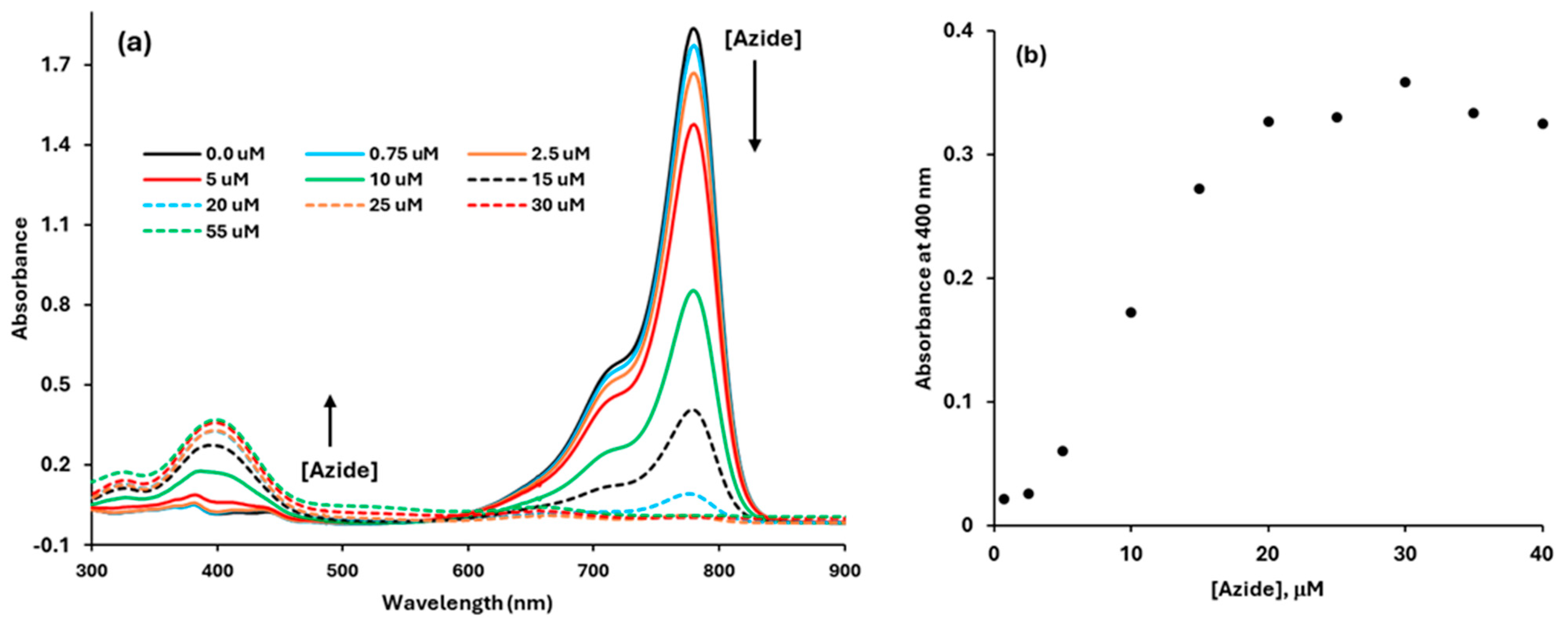
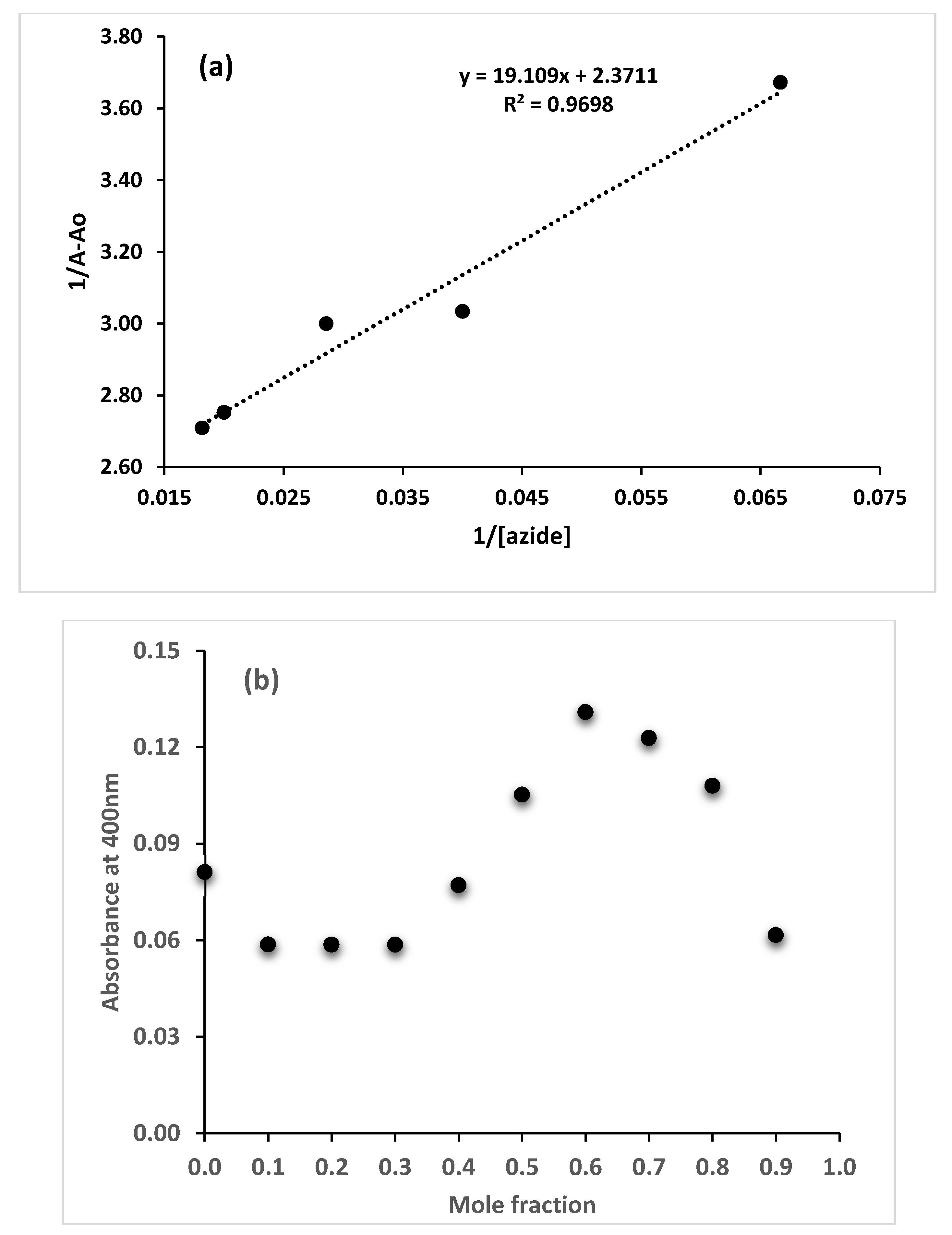
3.3.1. Fluorescence Titration of the Dye with Azide
3.3.2. The Competitive Signaling of Azide with the Dye in the Presence of Possibly Interfering Ions
3.4. Binding Mechanism
3.4.1. 1H NMR Analysis
3.4.2. 13C-NMR Analysis
3.4.3. Fluorescence Detection of CN⁻ in HeLa Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheng, X.; Ye, Z.; Liu, S.; He, W.; Ren, J.; Yin, J. Cyanine IR-780 for distinguishing 2-amino thiophenols from position isomers. Dye. Pigment. 2016, 131, 84–90. [Google Scholar] [CrossRef]
- Yi, X.; Yan, F.; Wang, F.; Qin, W.; Wu, G.; Yang, X.; Shao, C.; Chung, L.W.K.; Yuan, J. IR-780 dye for near-infrared fluorescence imaging in prostate cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 511–517. [Google Scholar]
- Thomas, R.G.; Jeong, Y.Y. NIRF Heptamethine Cyanine Dye Nanocomplexes for Multi Modal Theranosis of Tumors. Chonnam Med. J. 2017, 53, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Luo, S.; Tan, X.; Shi, C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials 2014, 35, 771–778. [Google Scholar] [CrossRef]
- Duan, L.; Wang, L.; Zhang, C.; Yu, L.; Guo, F.; Sun, Z.; Xu, Y.; Yan, F. Role of near-infrared heptamethine cyanine dye IR-783 in diagnosis of cervical cancer and its mechanism. Int. J. Clin. Exp. Pathol. 2019, 12, 2353–2362. [Google Scholar]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Suzuki, T.; Hioki, A.; Kurahashi, M. Development of a method for estimating an accurate equivalence point in nickel titration of cyanide ions. Anal. Chim. Acta 2003, 476, 159–165. [Google Scholar] [CrossRef]
- Safavi, A.; Maleki, N.; Shahbaazi, H.R. Indirect determination of cyanide ion and hydrogen cyanide by adsorptive stripping voltammetry at a mercury electrode. Anal. Chim. Acta 2004, 503, 213–221. [Google Scholar] [CrossRef]
- Rao, V.K.; Suresh, S.; Rao, N.B.S.N.; Rajaram, P. An electrochemical sensor for detection of hydrogen cyanide gas. Bull. Electrochem. 1997, 13, 327–329. [Google Scholar]
- Tomasulo, M.; Sortino, S.; White, A.J.P.; Raymo, F.M. Fast and Stable Photochromic Oxazines. J. Org. Chem. 2005, 70, 8180–8189. [Google Scholar] [CrossRef]
- Hudnall, T.W.; Gabbaï, F.P. Ammonium Boranes for the Selective Complexation of Cyanide or Fluoride Ions in Water. J. Am. Chem. Soc. 2007, 129, 11978–11986. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-K.; Tae, J. Acridinium Salt Based Fluorescent and Colorimetric Chemosensor for the Detection of Cyanide in Water. Org. Lett. 2006, 8, 5721–5723. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-G.; Kim, J.H.; Sessler, J.L. The Benzil−Cyanide Reaction and Its Application to the Development of a Selective Cyanide Anion Indicator. J. Am. Chem. Soc. 2008, 130, 12163–12167. [Google Scholar] [CrossRef]
- Jo, T.G.; Lee, J.J.; Nam, E.; Bok, K.H.; Lim, M.H.; Kim, C. A highly selective fluorescent sensor for the detection of Al3+ and CN− in aqueous solution: Biological applications and DFT calculations. New J. Chem. 2016, 40, 8918–8927. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Yang, Y.; Song, J.; Wang, J. Rational Design of a Highly Reactive Ratiometric Fluorescent Probe for Cyanide. Org. Lett. 2011, 13, 3730–3733. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Tong, H.; Wang, L. Highly Selective and Sensitive Detection of Cyanide by a Reaction-Based Conjugated Polymer Chemosensor. Macromolecules 2011, 44, 4241–4248. [Google Scholar] [CrossRef]
- Hong, S.-J.; Lee, C.-H. Nitrovinyl substituted calix [4]pyrrole as a unique, reaction-based chemosensor for cyanide anion. Tetrahedron Lett. 2012, 53, 3119–3122. [Google Scholar] [CrossRef]
- Afkhami, A.; Sarlak, N. A novel cyanide sensing phase based on immobilization of methyl violet on a triacetylcellulose membrane. Sens. Actuators B Chem. 2007, 122, 437–441. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Chen, Y.; Wang, X.; He, W.; Lu, Y. A new ratiometric and colorimetric chemosensor for cyanide anion based on Coumarin-hemicyanine hybrid. Org. Biomol. Chem. 2012, 10, 5073–5076. [Google Scholar] [CrossRef]
- Lv, X.; Liu, J.; Liu, Y.; Zhao, Y.; Chen, M.; Wang, P.; Guo, W. A ratiometric fluorescent probe for cyanide based on FRET. Org. Biomol. Chem. 2011, 9, 4954–4958. [Google Scholar] [CrossRef]
- Lee, H.; Chung, Y.M.; Ahn, K.H. Selective fluorescence sensing of cyanide with an o-(carboxamido)trifluoroacetophenone fused with a cyano-1,2-diphenylethylene fluorophore. Tetrahedron Lett. 2008, 49, 5544–5547. [Google Scholar] [CrossRef]
- Niu, H.-T.; Jiang, X.; He, J.; Cheng, J.-P. A highly selective and synthetically facile aqueous-phase cyanide probe. Tetrahedron Lett. 2008, 49, 6521–6524. [Google Scholar] [CrossRef]
- Ding, Y.; Li, T.; Zhu, W.; Xie, Y. Highly selective colorimetric sensing of cyanide based on formation of dipyrrin adducts. Org. Biomol. Chem. 2012, 10, 4201–4207. [Google Scholar] [CrossRef] [PubMed]
- Hijji, Y.M.; Barare, B.; Zhang, Y. Lawsone (2-hydroxy-1,4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sens. Actuators B Chem. 2012, 169, 106–112. [Google Scholar] [CrossRef]
- Hijji, Y.M.; Elsafy, A.G.; Al-Easa, H.S.; Attili, B.; Abdelrasoul, M.; Mohamed, N.; Nasrallah, G.K. Curcumin a colorimetric and fluorimetric cyanide probe in aqueous system and living cells. Anal. Methods 2019, 11, 5169–5176. [Google Scholar] [CrossRef]
- Betterton, E.A. Environmental Fate of Sodium Azide Derived from Automobile Airbags. Crit. Rev. Environ. Sci. Technol. 2003, 33, 423–458. [Google Scholar] [CrossRef]
- Paz-y-Miño, C.; Beaty, D.; López-Cortés, A.; Proaño, I. Frequency of GJB2 and del(GJB6-D13S1830) mutations among an Ecuadorian mestizo population. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1648–1654. [Google Scholar] [CrossRef]
- Lichstein, H.C. Studies of the Effect of Sodium Azide on Microbic Growth and Respiration: III. The Effect of Sodium Azide on the Gas Metabolism of B. subtilis and P. aeruginosa and the Influence of Pyocyanine on the Gas Exchange of a Pyocyanine-Free Strain of P. aeruginosa in the Presence of Sodium Azide. J. Bacteriol. 1944, 47, 239–251. [Google Scholar]
- Kim, H.W.; Choi, M.G.; Park, H.; Lee, J.W.; Chang, S.-K. Single molecular multianalyte signaling of sulfide and azide ions by a nitrobenzoxadiazole-based probe. RSC Adv. 2015, 5, 4623–4627. [Google Scholar] [CrossRef]
- Jeong, Y.A.; Chang, I.J.; Chang, S.-K. Discriminative sensing of sulfide and azide ions in solution by a nitrobenzoxadiazole-dansyl dyad by simply tuning the water content. Sens. Actuators B Chem. 2016, 224, 73–80. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef] [PubMed]
- Quang, D.T.; Kim, J.S. Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef] [PubMed]
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Fabbrizzi, L.; Poggi, A. Sensors and switches from supramolecular chemistry. Chem. Soc. Rev. 1995, 24, 197–202. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Shin, I.; Yoon, J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2011, 40, 4783–4804. [Google Scholar] [CrossRef]
- Xu, K.; Wang, L.; Qiang, M.; Wang, L.; Li, P.; Tang, B. A selective near-infrared fluorescent probe for singlet oxygen in living cells. Chem. Commun. 2011, 47, 7386–7388. [Google Scholar] [CrossRef]
- Wang, R.; Yu, F.; Chen, L.; Chen, H.; Wang, L.; Zhang, W. A highly selective turn-on near-infrared fluorescent probe for hydrogen sulfide detection and imaging in living cells. Chem. Commun. 2012, 48, 11757–11759. [Google Scholar] [CrossRef]
- Yin, J.; Kwon, Y.; Kim, D.; Lee, D.; Kim, G.; Hu, Y.; Ryu, J.-H.; Yoon, J. Cyanine-Based Fluorescent Probe for Highly Selective Detection of Glutathione in Cell Cultures and Live Mouse Tissues. J. Am. Chem. Soc. 2014, 136, 5351–5358. [Google Scholar] [CrossRef]
- Wang, X.; Lv, J.; Yao, X.; Li, Y.; Huang, F.; Li, M.; Yang, J.; Ruan, X.; Tang, B. Screening and investigation of a cyanine fluorescent probe for simultaneous sensing of glutathione and cysteine under single excitation. Chem. Commun. 2014, 50, 15439–15442. [Google Scholar] [CrossRef] [PubMed]
- Zhytniakivska, O.; Kurutos, A.; Tarabara, U.; Vus, K.; Trusova, V.; Gorbenko, G.; Gadjev, N.; Deligeorgiev, T. Probing the amyloid protein aggregates with unsymmetrical monocationic trimethine cyanine dyes. J. Mol. Liq. 2020, 311, 113287. [Google Scholar] [CrossRef]
- Tang, B.; Cui, L.J.; Xu, K.H.; Tong, L.L.; Yang, G.W.; An, L.G. A Sensitive and Selective Near-Infrared Fluorescent Probe for Mercuric Ions and Its Biological Imaging Applications. ChemBioChem 2008, 9, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Huang, H.; Xu, K.; Tong, L.; Yang, G.; Liu, X.; An, L. Highly sensitive and selective near-infrared fluorescent probe for zinc and its application to macrophage cells. Chem. Commun. 2006, 3, 3609–3611. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, T.; Zhu, W.; Xu, Y.; Qian, X. Highly Selective and Sensitive Near-Infrared Fluorescent Sensors for Cadmium in Aqueous Solution. Org. Lett. 2011, 13, 264–267. [Google Scholar] [CrossRef]
- Cao, X.; Lin, W.; Wan, W. Development of a near-infrared fluorescent probe for imaging of endogenous Cu+ in live cells. Chem. Commun. 2012, 48, 6247–6249. [Google Scholar] [CrossRef]
- Hirayama, T.; Van de Bittner, G.C.; Gray, L.W.; Lutsenko, S.; Chang, C.J. Near-infrared fluorescent sensor for in vivo copper imaging in a murine Wilson disease model. Biochemistry 2012, 109, 2228–2233. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Yu, Z.; Chen, B.; Han, Y. A highly sensitive hemicyanine-based fluorescent chemodosimeter for mercury ions in aqueous solution and living cells. RSC Adv. 2015, 5, 82531–82534. [Google Scholar] [CrossRef]
- Kar, C.; Shindo, Y.; Oka, K.; Nishiyama, S.; Suzuki, K.; Citterio, D. Spirolactam capped cyanine dyes for designing NIR probes to target multiple metal ions. RSC Adv. 2017, 7, 24970–24980. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Y.; Zhou, M.; Xiang, Y.; Tong, A. An in-situ, reversible fluorescent paper sensor for selective detection of ambient CO2. J. Mater. Chem. C 2023, 11, 299–306. [Google Scholar] [CrossRef]
- Abebe, F.; Sutton, T.; Perkins, P.; Shaw, R. Two colorimetric fluorescent turn-on chemosensors for detection of Al3+ and N3-: Synthesis, Photophysical and computational studies. Luminescence 2018, 33, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, W.; Chen, S.; Jia, J.; Ma, H.; Wolfbeis, O.S. A near-infrared fluorescent probe for monitoring tyrosinase activity. Chem. Commun. 2010, 46, 2560–2562. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, M.; Zhang, K.; Zhu, H.; Liu, Z.; Zhang, Y.; Zhao, J.; Wu, L.; Zhang, Z.; Wang, S. A reversible near-infrared pH probes for optical measurements of pH in complete water system and living cells. Sens. Actuators B Chem. 2015, 219, 294–300. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef]
- Li, X.; Yue, Y.; Wen, Y.; Yin, C.; Huo, F. Hemicyanine based fluorimetric and colorimetric pH probe and its application in bioimaging. Dye. Pigment. 2016, 134, 291–296. [Google Scholar] [CrossRef]
- Uota, S.; Hwang, B.J.; Butcher, R.; Mullins, R.; Wachira, J.; Hijji, Y.; Abebe, F. A simple benzothiazolium-based sensor for cyanide detection: Applications in environmental analysis and bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 326, 125155. [Google Scholar] [CrossRef]
- Udhayakumari, D. Chromogenic and fluorogenic chemosensors for lethal cyanide ion. A comprehensive review of the year 2016. Sens. Actuators B Chem. 2018, 259, 1022–1057. [Google Scholar] [CrossRef]
- Niu, H.-T.; Jiang, X.; He, J.; Cheng, J.-P. Cyanine dye-based chromofluorescent probe for highly sensitive and selective detection of cyanide in water. Tetrahedron Lett. 2009, 50, 6668–6671. [Google Scholar] [CrossRef]
- Barare, B.; Babahan, I.; Hijji, Y.M.; Bonyi, E.; Tadesse, S.; Aslan, K. A Highly Selective Sensor for Cyanide in Organic Media and on Solid Surfaces. Sensors 2016, 16, 271. [Google Scholar] [CrossRef]
- Ono, A.; Togashi, H. Highly Selective Oligonucleotide-Based Sensor for Mercury(II) in Aqueous Solutions. Angew. Chem. Int. Ed. 2004, 43, 4300–4302. [Google Scholar] [CrossRef]
- Tsui, Y.-K.; Devaraj, S.; Yen, Y.-P. Azo dyes featuring with nitrobenzoxadiazole (NBD) unit: A new selective chromogenic and fluorogenic sensor for cyanide ion. Sens. Actuators B Chem. 2012, 161, 510–519. [Google Scholar] [CrossRef]
- Wu, W.-N.; Wu, H.; Wang, Y.; Zhao, X.-L.; Xu, Z.-Q.; Xu, Z.-H.; Fan, Y.-C. A NIR sensor for cyanide detection and its application in cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Aduroja, O.; Shaw, R.; Uota, S.; Abiye, I.; Wachira, J.; Abebe, F. A Novel Fluorescent Chemosensor based on rhodamine Schiff base: Photophysical, computational, and bioimaging application in live cells. Inorganics 2025, 13, 5. [Google Scholar] [CrossRef]






| Dye | 1.68 (12H, s) | 4.21 (4H, t) | 8.72 (2H, d) | 6.35 (2H, d) | |
| Dye–CN− | 1.61 (6H, s) | 1.37 (3H, s) | 1.05 (3H, s) | 3.63 (2H, t) | 3.02(1H, dt) |
| 2.86 (1H, dt) | 7.36 (1H, d) | 7.31 (1H, d) | 5.77 (1H, d) | 5.47 (1H, d) | |
| Dye–OH− | 1.50 (6H, s) | 1.14 (3H, s) | 0.94 (3H, s) | 3.61 (2H, t) | 2.99(1H, dt) |
| 2.81 (1H, dt) | 7.26 (1H, d) | 6.47 (1H, d) | 5.88 (1H, d) | 5.49 (1H, d) | |
| Dye–N3− | 1.60 (6H, s) | 1.37 (3H, s) | 1.10 (3H, s) | 3.87 (2H, t) | 2.82(1H, dt) |
| 2.64 (1H, dt) | ---- | ---- | ---- | ---- | |
| complex spectra | 7.39 (1H, d) | 5.77 (1H, d) | 5.30 | ---- |
| Chiral Carbon (New Peaks) | Vinylic Carbon (New Peaks) | Other Peaks | ||
|---|---|---|---|---|
| Dye | 172 (quaternary C) | ------ | ----- | ----- |
| Dye–CN− | N/A | 80.79 | 92.96 | 119.20 for nitrile |
| Dye–OH− | N/A | 99.66 | 92.95 | ----- |
| Dye–N3− | N/A | 95.31 | 92.95 | complex spectra |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijji, Y.M.; Rajan, R.; Shraim, A.M.; Attili, B.; Uota, S.; Abebe, F. Selective and Sensitive Dual Chromogenic Cyanide and Fluorescent Azide Probe. Photochem 2025, 5, 12. https://doi.org/10.3390/photochem5020012
Hijji YM, Rajan R, Shraim AM, Attili B, Uota S, Abebe F. Selective and Sensitive Dual Chromogenic Cyanide and Fluorescent Azide Probe. Photochem. 2025; 5(2):12. https://doi.org/10.3390/photochem5020012
Chicago/Turabian StyleHijji, Yousef M., Rajeesha Rajan, Amjad M. Shraim, Bassam Attili, Sisay Uota, and Fasil Abebe. 2025. "Selective and Sensitive Dual Chromogenic Cyanide and Fluorescent Azide Probe" Photochem 5, no. 2: 12. https://doi.org/10.3390/photochem5020012
APA StyleHijji, Y. M., Rajan, R., Shraim, A. M., Attili, B., Uota, S., & Abebe, F. (2025). Selective and Sensitive Dual Chromogenic Cyanide and Fluorescent Azide Probe. Photochem, 5(2), 12. https://doi.org/10.3390/photochem5020012






