Photoproducts of Porphyrins with a Focus on Protoporphyrin IX
Abstract
1. Introduction
2. Definitions of Photoreactions
- (1)
- Oxidation reactions:
- (a)
- Loss of one or more electrons from a chemical species as a result of the photoexcitation of this species;
- (b)
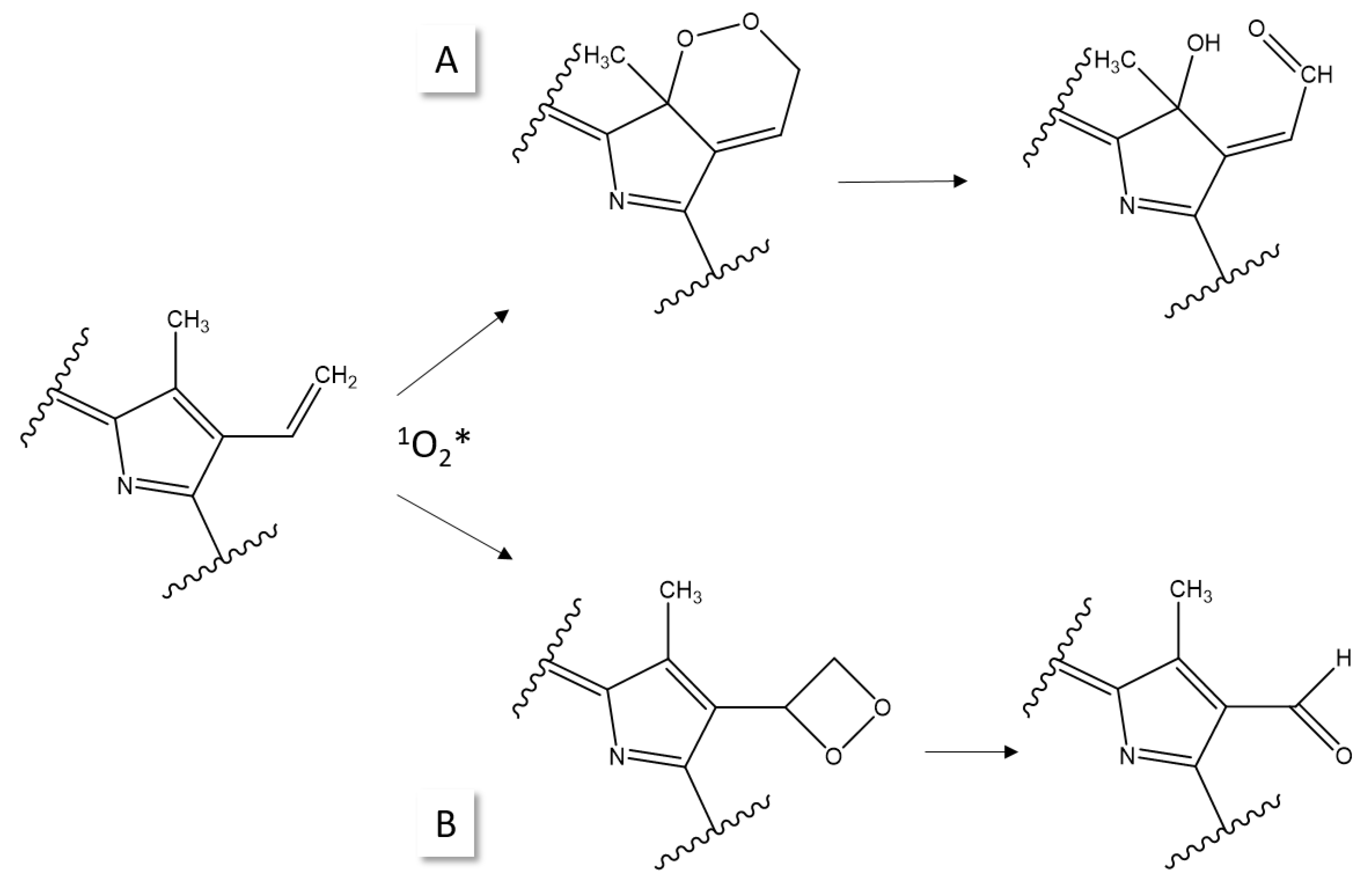
- Reaction of a substance with oxygen (called photo-oxygenation, when oxygen remains in the product).
- (2)
- Photoreduction:
- (a)
- Addition of one or more electrons to a photoexcited species;
- (b)
- Photochemical hydrogenation of a substance.
- (3)
- Photoinitiated reactions: neither the substrate nor the oxygen is electronically excited.
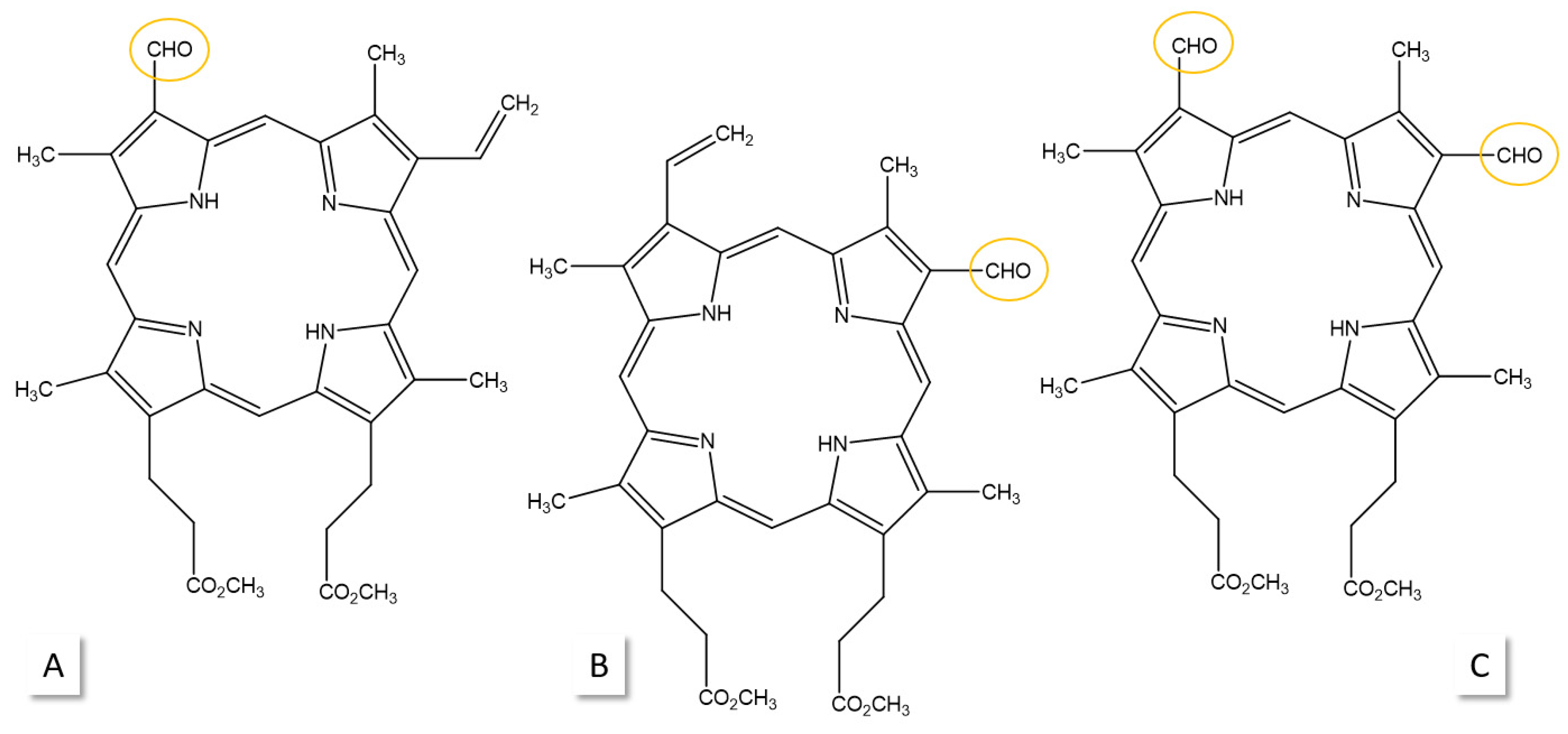
3. Photostability of PPIX
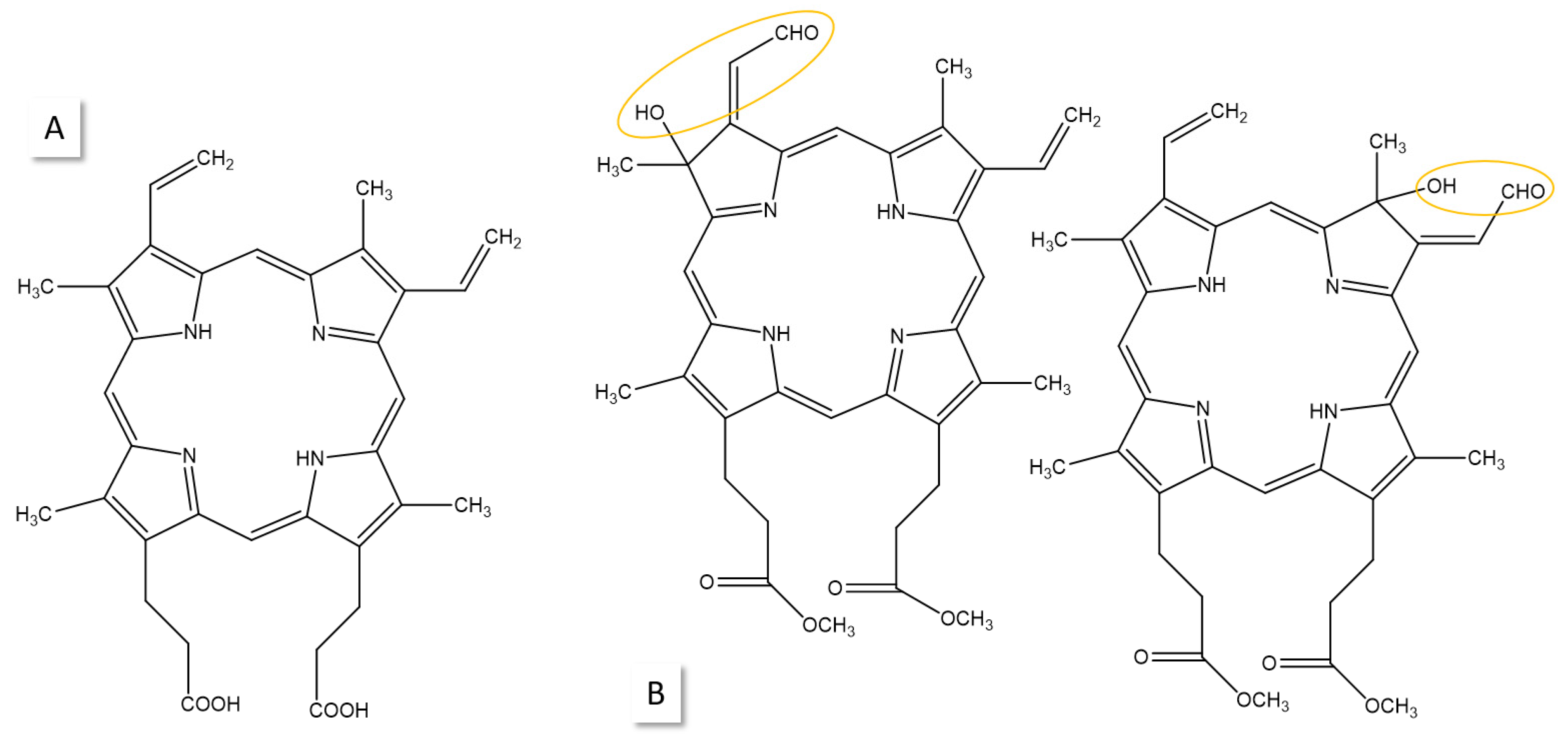
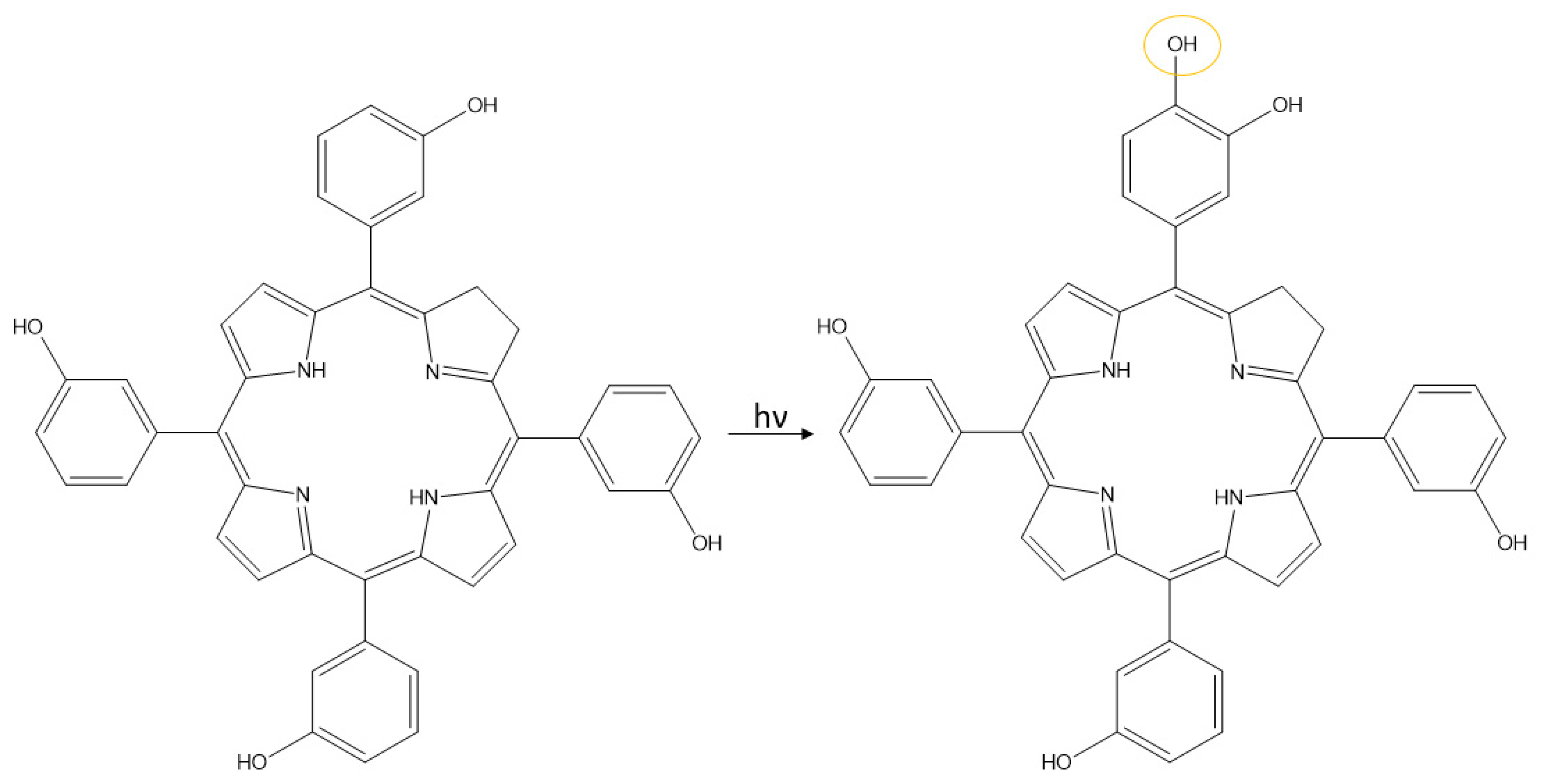
4. Photoreduction
5. Photobleaching of PDT Agents
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPIX | Protoporphyrin IX |
| PDT | Photodynamic therapy |
| MS | Mass spectrometry |
| FGR | Fluorescence-guided resection |
| 5-ALA | 5-Aminolevulinic acid |
| PPP | Photoprotoporphyrin |
| m-THPC | 5, 10, 15, 20 tetrakis (meso-hydroxyphenyl) clorin |
| LC | Liquid chromatography |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| NMR | Nuclear magnetic resonance |
| FTICR | Fourier-transform ion cyclotron resonance |
References
- Nikolaou, V.; Nikoloudakis, E.; Ladomenou, K.; Charalambidis, G.; Coutsolelos, A. Porphyrins—Valuable pigments of life. Front. Chem. Biol. 2024, 2, 1346465. [Google Scholar] [CrossRef]
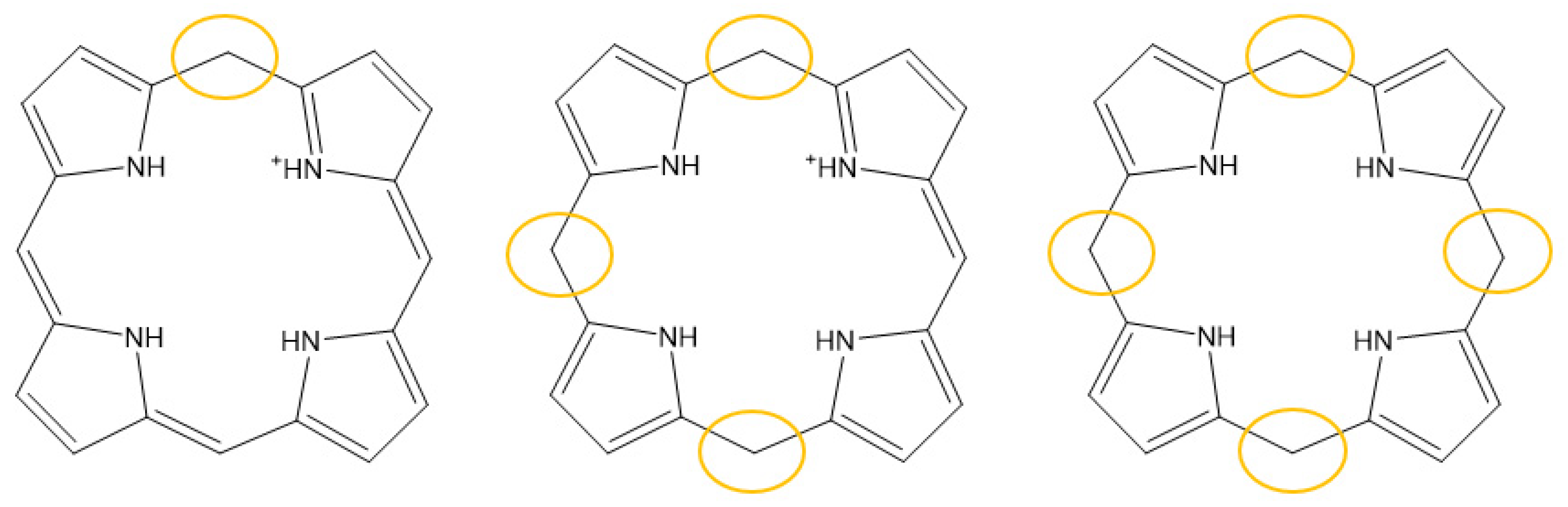
- Lash, T.D. Origin of aromatic character in porphyrinoid systems. J. Porphyr. Phthalocyanines 2011, 15, 1093–1115. [Google Scholar] [CrossRef]
- Senge, M.O.; MacGowan, S.A.; O′Brien, J.M. Conformational control of cofactors in nature—The influence of protein-induced macrocycle distortion on the biological function of tetrapyrroles. Chem. Commun. 2015, 51, 17031–17063. [Google Scholar] [CrossRef]
- Brothers, P.J.; Senge, M.O. Fundamentals of Porphyrin Chemistry; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Biesaga, M.; Pyrzynska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Marianov, A.N.; Lu, T.; Zhong, J. A review of the development of porphyrin-based catalysts for electrochemical CO2 reduction. Chem. Eng. J. 2023, 470, 144249. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Tomé, J.P.C.; Tomé, A.C.; Lourenço, L.M.O. Porphyrin-containing materials for photodegradation of organic pollutants in wastewaters: A review. Catal. Sci. Technol. 2024, 14, 2352–2389. [Google Scholar] [CrossRef]
- Song, H.; Liu, Q.; Xie, Y. Porphyrin-sensitized solar cells: Systematic molecular optimization, coadsorption and cosensitization. Chem. Commun. 2018, 54, 1811–1824. [Google Scholar] [CrossRef]
- Stummer, W.; Müther, M.; Spille, D. Beyond fluorescence-guided resection: 5-ALA-based glioblastoma therapies. Acta Neurochir. 2024, 166, 163. [Google Scholar] [CrossRef]
- Akbar, A.; Khan, S.; Chatterjee, T.; Ghosh, M. Unleashing the power of porphyrin photosensitizers: Illuminating breakthroughs in photodynamic therapy. J. Photochem. Photobiol. B 2023, 248, 112796. [Google Scholar] [CrossRef]
- Dar, U.A.; Shahnawaz, M.; Taneja, P.; Dar, M.A. Recent advances in main group coordination driven porphyrins: A comprehensive review. ChemistrySelect 2024, 9, e202304817. [Google Scholar] [CrossRef]
- Zanetti-Polzi, L.; Amadei, A.; Djemili, R.; Durot, S.; Schoepff, L.; Heitz, V.; Ventura, B.; Daidone, I. Interpretation of experimental Soret bands of porphyrins in flexible covalent cages and in their related Ag(I) fixed complexes. J. Phys. Chem. C 2019, 123, 13094–13103. [Google Scholar] [CrossRef]
- Pettersson, K.; Kilså, K.; Mårtensson, J.; Albinsson, B. Intersystem crossing versus electron transfer in porphyrin-based donor-bridge-acceptor systems: Influence of a paramagnetic species. J. Am. Chem. Soc. 2004, 126, 6710–6719. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Annamuradov, B.; Ateyeh, M.; Street, C.; Whipple, H.; Er, A.O. Photodynamic therapy review: Past, present, future, opportunities and challenges. Photochem 2024, 4, 434–461. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdés, P.A.; Montcel, B. 5-ALA induced PpIX fluorescence spectroscopy in neurosurgery: A review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef]
- Stummer, W.; Baumgartner, R. ALA-PDT and ALA-FD in the brain: New prospects for treating malignant gliomas. Compr. Ser. Photochem. Photobiol. Sci. 2006, 7, 155–175. [Google Scholar]
- Yien, Y.Y.; Perfetto, M. Regulation of heme synthesis by mitochondrial homeostasis proteins. Front. Cell Dev. Biol. 2022, 10, 895521. [Google Scholar] [CrossRef] [PubMed]
- Uehlinger, P.; Zellweger, M.; Wagnières, G.; Juillerat-Jeanneret, L.; van den Bergh, H.; Lange, N. 5-Aminolevulinic acid and its derivatives: Physical chemical properties and protoporphyrin IX formation in cultured cells. J. Photochem. Photobiol. B 2000, 54, 72–80. [Google Scholar] [CrossRef]
- Walke, A.; Black, D.; Valdes, P.A.; Stummer, W.; König, S.; Suero-Molina, E. Challenges in, and recommendations for, hyperspectral imaging in ex vivo malignant glioma biopsy measurements. Sci. Rep. 2023, 13, 3829. [Google Scholar] [CrossRef]
- Spikes, J.D. Quantum yields and kinetics of the photobleaching of hematoporphyrin, Photofrin II, tetra(4-sulfonatophenyl)-porphine and uroporphyrin. Photochem. Photobiol. 1992, 55, 797–808. [Google Scholar] [CrossRef]
- Fyrestam, J.; Bjurshammar, N.; Paulsson, E.; Johannsen, A.; Oestman, C. Determination of porphyrins in oral bacteria by liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 7013–7023. [Google Scholar] [CrossRef]
- Walke, A.; Krone, C.; Stummer, W.; König, S.; Suero Molina, E. Protoporphyrin IX in serum of high-grade glioma patients: A novel target for disease monitoring via liquid biopsy. Sci. Rep. 2024, 14, 4297. [Google Scholar] [CrossRef] [PubMed]
- Benton, C.M.; Lim, C.K.; Moniz, C.; Jones, D.J.L. Porphyrinogen fragmentation profiles by ultra-high-performance liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2011, 25, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Fateen, E.; Abd-Elfattah, A.; Gouda, A.; Ragab, L.; Nazim, W. Porphyrins profile by high performance liquid chromatography/electrospray ionization tandem mass spectrometry for the diagnosis of porphyria. Egypt. J. Med. Hum. Genet. 2011, 12, 49–58. [Google Scholar] [CrossRef][Green Version]
- Walke, A.; Molina, E.S.; Stummer, W.; Koenig, S. Protoporphyrin IX analysis from blood and serum in the context of neurosurgery of glioblastoma. In Mass Spectrometry in Life Sciences and Clinical Laboratory; IntechOpen Ltd.: London, UK, 2021; p. 1. [Google Scholar]
- Suero Molina, E.; Black, D.; Walke, A.; Azemi, G.; D′Alessandro, F.; König, S.; Stummer, W. Unraveling the blue shift in porphyrin fluorescence in glioma: The 620 nm peak and its potential significance in tumor biology. Front. Neurosci. 2023, 17, 1261679. [Google Scholar] [CrossRef]
- The International Union of Pure and Applied Chemistry. IUPAC—Photooxidation (P04640). Available online: https://goldbook.iupac.org/terms/view/P04640 (accessed on 29 January 2025).
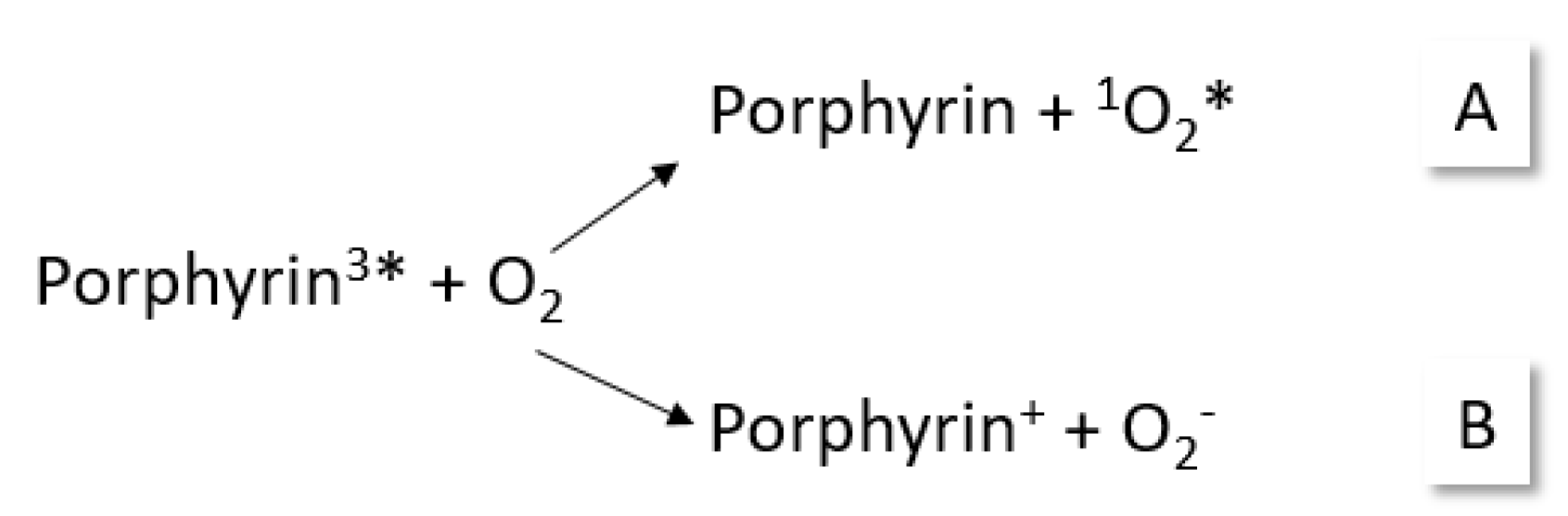
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Tang, H.; Zhang, P. Differentiation of Superoxide Radical Anion and Singlet Oxygen and Their Concurrent Quantifications by Nuclear Magnetic Resonance. Anal. Chem. 2023, 95, 5293–5299. [Google Scholar] [CrossRef]
- Klan, P.; Wirz, J. Photochemistry of Organic Compounds; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2009; ISBN 978-1-4051-6173-2. [Google Scholar]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, D.; Fagnoni, M. Wavelength dependence and wavelength selectivity in photochemical reactions. Photochem. Photobiol. Sci. 2019, 18, 2094–2101. [Google Scholar] [CrossRef]
- Rotomskis, R.; Bagdonas, S.; Streckyte, G. Spectroscopic studies of photobleaching and photoproduct formation of porphyrins used in tumor therapy. J. Photochem. Photobiol. B 1996, 33, 61. [Google Scholar] [CrossRef]
- Wessels, J.M.; Sroka, R.; Heil, P.; Seidlitz, H.K. Photodegradation of protoporphyrin-dimethylester in solution and in organized environments. Int. J. Radiat. Biol. 1993, 64, 475–484. [Google Scholar] [CrossRef]
- Fischer, H.; Herrle, K. Einwirkung von Licht auf Porphyrine. (I. Mitteilung.) Überführung von Ätio-porphyrin I in bilirubinoide Farbstoffe. Hoppe-Seyler´S Z. Physiol. Chem. 1938, 251, 85–96. [Google Scholar] [CrossRef]
- Inhoffen, H.H.; Bliesener, C.; Brockmann, H. Zur weiteren Kenntnis des Chlorophylls und des Hämins, VIII. Tetrahedron Lett. 1966, 7, 3779–3783. [Google Scholar] [CrossRef]
- Cox, G.S.; Whitten, D.G. Mechanisms for the photooxidation of protoporphyrin IX in solution. J. Am. Chem. Soc. 1982, 104, 516–521. [Google Scholar] [CrossRef]
- Cox, G.S.; Whitten, D.G. Excited state interactions of protoporphyrin IX and related porphyrins with molecular oxygen in solutions and in organized assemblies. Adv. Exp. Med. Biol. 1983, 160, 279–292. [Google Scholar] [CrossRef]
- Ericson, M.B.; Grapengiesser, S.; Gudmundson, F.; Wennberg, A.-M.; Larkö, O.; Moan, J.; Rosén, A. A spectroscopic study of the photobleaching of protoporphyrin IX in solution. Lasers Med. Sci. 2003, 18, 56–62. [Google Scholar] [CrossRef]
- Dysart, J.S.; Patterson, M.S. Photobleaching kinetics, photoproduct formation, and dose estimation during ALA induced PpIX PDT of MLL cells under well oxygenated and hypoxic conditions. Photochem. Photobiol. Sci. 2006, 5, 73–81. [Google Scholar] [CrossRef]
- Ogbonna, S.J.; Masuda, K.; Hazama, H. The effect of fluence rate and wavelength on the formation of protoporphyrin IX photoproducts. Photochem. Photobiol. Sci. 2024, 23, 1627–1639. [Google Scholar] [CrossRef]
- Reis, E.R.; Ferreira, L.P.; Nicola, E.M.D.; Borissevitch, I. Comparative study of phototoxicity of protoporphyrin IX synthetic and extracted from ssp Rattus novergicus albinus rats toward murine melanoma cells. Eur. Biophys. J. 2018, 47, 601–609. [Google Scholar] [CrossRef]
- Mauzerall, D. The photoreduction of porphyrins: Structure of the products. J. Am. Chem. Soc. 1962, 84, 2437–2445. [Google Scholar] [CrossRef]
- Rotomskis, R.; Streckyte, G.; Bagdonas, S. Phototransformations of sensitizers. 2. Photoproducts formed in aqueous solutions of porphyrins. J. Photochem. Photobiol. B 1997, 39, 172–175. [Google Scholar] [CrossRef]
- Jones, R.M.; Wang, Q.; Lamb, J.H.; Djelal, B.D.; Bonnett, R.; Lim, C.K. Identification of photochemical oxidation products of 5,10,15,20-tetra(m-hydroxyphenyl)chlorin by on-line high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. 1996, 722, 257–265. [Google Scholar] [CrossRef]
- Bonnett, R.; Martínez, G. Photobleaching of compounds of the 5,10,15,20-Tetrakis(m-hydroxyphenyl)porphyrin Series (m-THPP, m-THPC, and m-THPBC). Org. Lett. 2002, 4, 2013–2016. [Google Scholar] [CrossRef]
- Kasselouri, A.; Bourdon, O.; Demore, D.; Blais, J.C.; Prognon, P.; Bourg-Heckly, G.; Blais, J. Fluorescence and mass spectrometry studies of meta-tetra(hydroxyphenyl)chlorin photoproducts. Photochem. Photobiol. 1999, 70, 275–279. [Google Scholar] [CrossRef]
- Lassalle, H.-P.; Lourette, N.; Maunit, B.; Muller, J.-F.; Guillemin, F.; Bezdetnaya-Bolotine, L. MALDI-TOF mass spectrometric analysis for the characterization of the 5,10,15,20-tetrakis(m-hydroxyphenyl)bacteriochlorin (m-THPBC) photoproducts in biological environment. J. Mass Spectrom. 2005, 40, 1149–1156. [Google Scholar] [CrossRef]
- Golec, B.; Buczynska, J.; Nawara, K.; Gorski, A.; Waluk, J. Photodegradation of free base and zinc porphyrins in the presence and absence of oxygen. Photochem. Photobiol. Sci. 2023, 22, 2725–2734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, S. Photoproducts of Porphyrins with a Focus on Protoporphyrin IX. Photochem 2025, 5, 10. https://doi.org/10.3390/photochem5020010
König S. Photoproducts of Porphyrins with a Focus on Protoporphyrin IX. Photochem. 2025; 5(2):10. https://doi.org/10.3390/photochem5020010
Chicago/Turabian StyleKönig, Simone. 2025. "Photoproducts of Porphyrins with a Focus on Protoporphyrin IX" Photochem 5, no. 2: 10. https://doi.org/10.3390/photochem5020010
APA StyleKönig, S. (2025). Photoproducts of Porphyrins with a Focus on Protoporphyrin IX. Photochem, 5(2), 10. https://doi.org/10.3390/photochem5020010