Interactions of CdSe Nanocrystals with Cationic Proteins Extracted from Moringa oleifera Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used
2.2. Synthesis of CdSe Quantum Dots
2.3. Extraction and Purification of MSP
2.4. Synthesis of CdSe–MSP Complexes
2.5. Characterization of the Synthesized CdSe QDs and CdSe–MSP Complexes
2.6. Investigation of the Interactions between CdSe QDs and MSPn Complexes
3. Results and Discussion
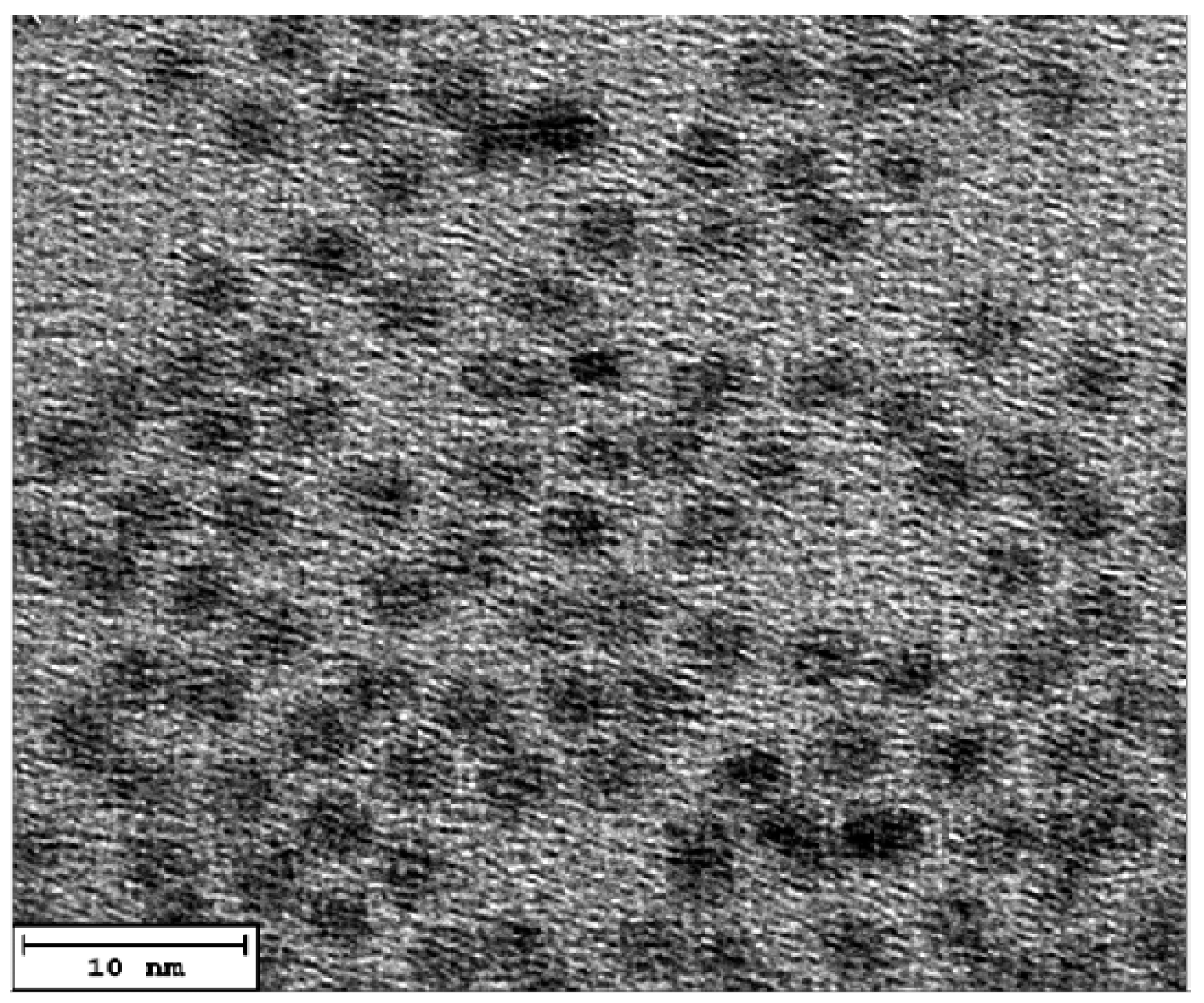
3.1. The Structural and Morphological Characterization of CdSe QDs
3.2. The Morphological Characterization of MSP and CdSe–MSP Complexes
3.3. The Structural and Morphological Characterization of CdSe–MSCP Complexes
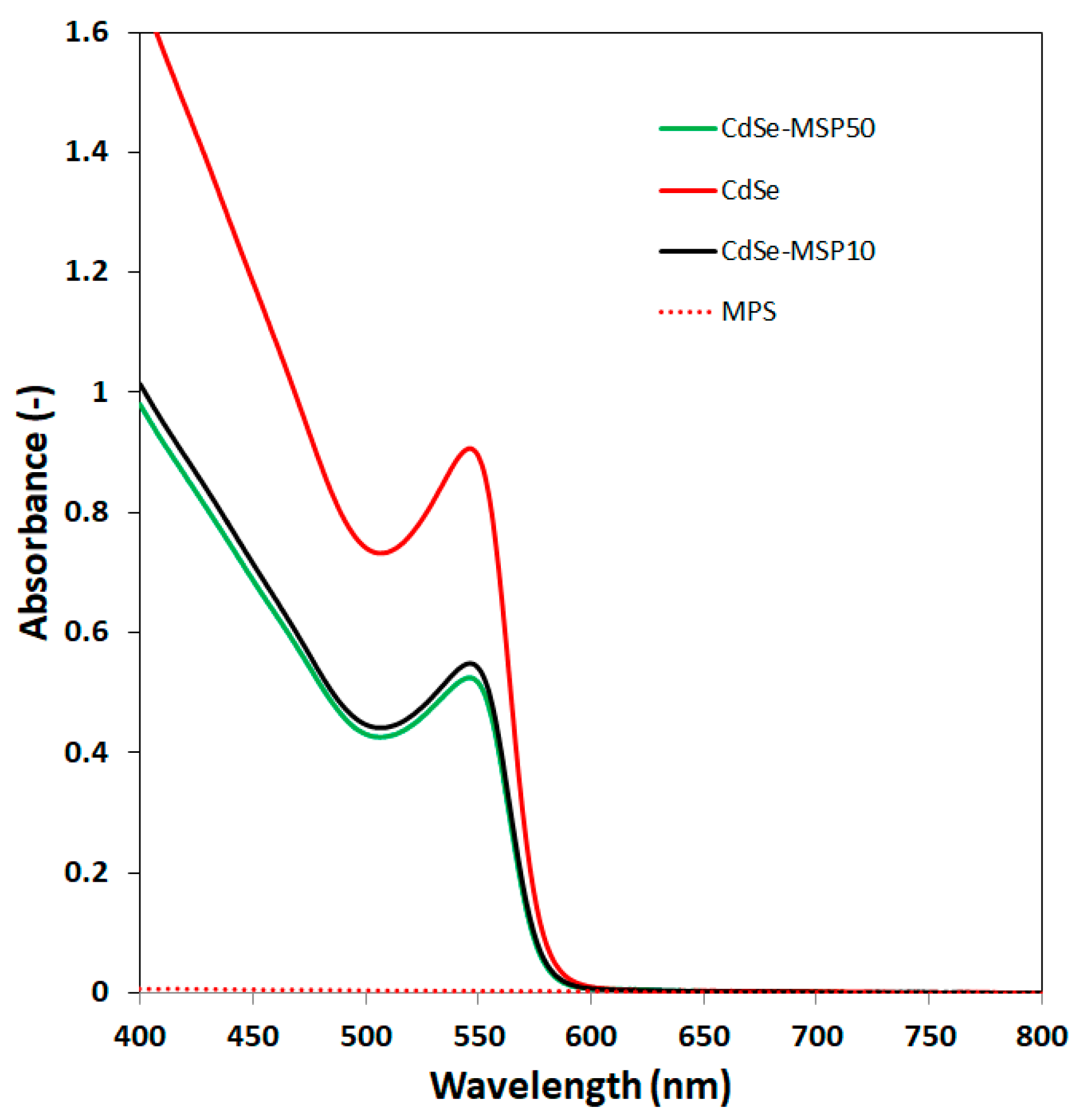
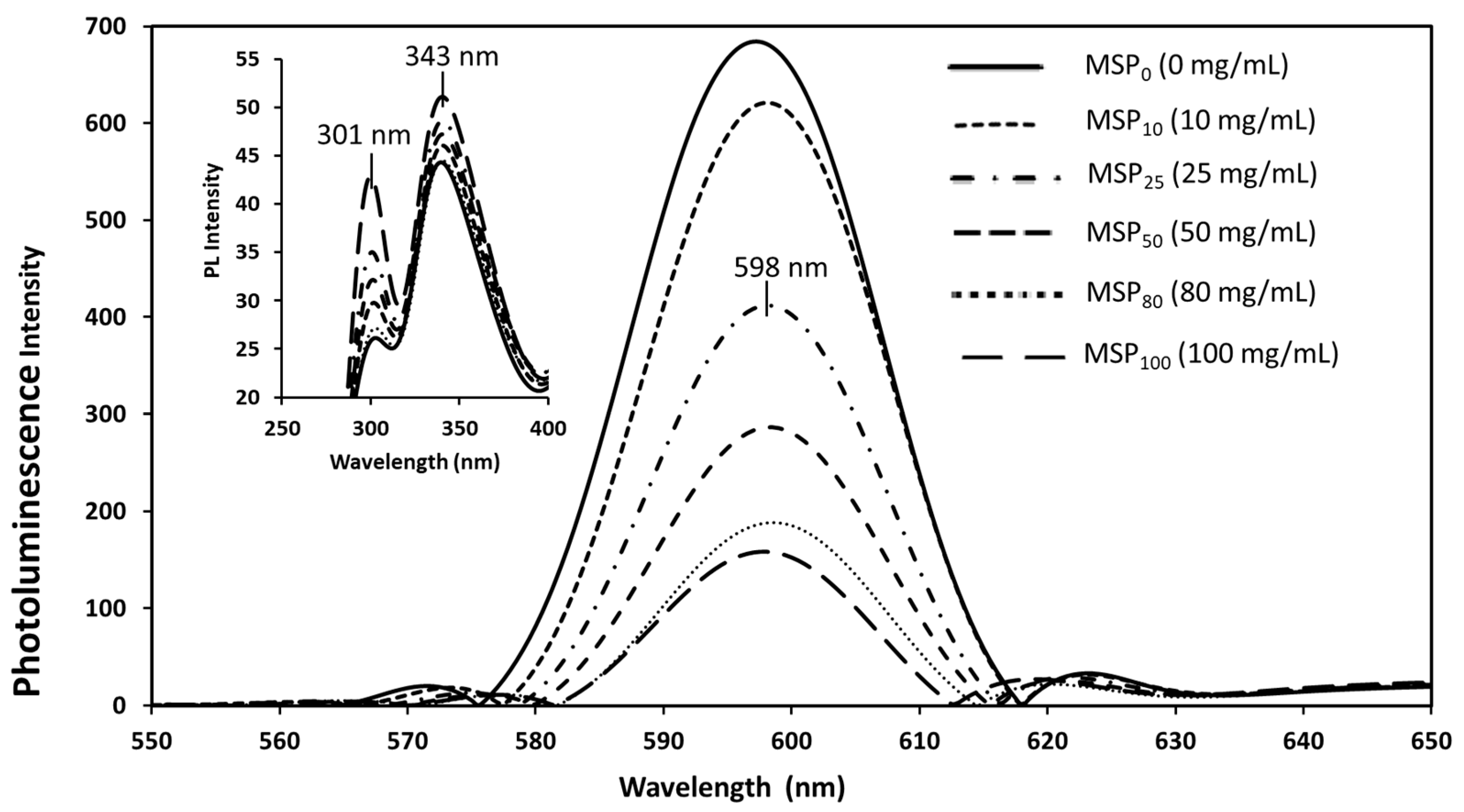
3.4. Interaction between CdSe QDs and MSP Using the UV–Vis Absorption and Fluorescence Spectra
3.5. Stern–Volmer Analysis of the CdSe QDs–MSP System Interactions
3.6. FTIR Analysis of the CdSe QDs–MSP System Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bera, D.; Qian, L.; Tseng, T.K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Poderys, V.; Matulionyte, M.; Selskis, A.; Rotomskis, R. Interaction of water-soluble CdTe quantum dots with bovine serum albumin. Nanoscale Res. Lett. 2011, 6, 9. Available online: http://www.nanoscalereslett.com/content/6/1/9 (accessed on 21 December 2023). [CrossRef]
- Joglekar, S.S.; Gholap, H.M.; Alegaonkar, P.S.; Kale, A.A. The interactions between CdTe quantum dots and proteins: Understanding nano-bio interface. AIMS Mater. Sci. 2017, 4, 209–222. [Google Scholar] [CrossRef]
- Shivaji, K.; Sridharan, K.; Kirubakaran, D.D.; Velusamy, J.; Emadian, S.S.; Krishnamurthy, S.; Devadoss, A.; Nagarajan, S.; Das, S.; Pitchaimuthu, S. Biofunctionalized CdS Quantum Dots: A Case Study on Nanomaterial Toxicity in the Photocatalytic Wastewater Treatment Process. ACS Omega 2023, 8, 19413–19424. Available online: https://pubs.acs.org/doi/10.1021/acsomega.3c00496 (accessed on 21 December 2023). [CrossRef] [PubMed]
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core–shell quantum dots: Properties and applications. J. Alloys Compd. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, M. The cytotoxicity of core-shell or non-shell structure quantum dots and reflection on environmental friendly: A review. Environ. Res. 2021, 194, 110593. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Zhang, M.; Kim, K. Quantum dots and their interaction with biological systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Ratnesh, R.K.; Mehata, M.S. Synthesis and optical properties of core-multi-shell CdSe/CdS/ZnS quantum dots: Surface modifications. Opt. Mater. 2017, 64, 250–256. [Google Scholar] [CrossRef]
- Liu, G.; Liang, W.; Xue, X.; Rosei, F.; Wang, Y. Atomic Identification of Interfaces in Individual Core@ shell Quantum Dots. Adv. Sci. 2021, 8, 2102784. [Google Scholar] [CrossRef]
- Tarantini, A.; Wegner, K.D.; Dussert, F.; Sarret, G.; Béal, D.; Mattera, L.; Lincheneau, C.; Proux, O.; Truffier-Boutry, D.; Moriscot, C.; et al. Physicochemical alterations and toxicity of InP alloyed quantum dots aged in environmental conditions: A safer by design evaluation. NanoImpact 2019, 14, 100168. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ndabigengesere, A.; Narasiah, K.S.; Talbot, B.G. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995, 29, 703–710. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Peres, J.A. Improvement of the flocculation process in water treatment by using Moringa oleifera seeds extract. Braz. J. Chem. Eng. 2012, 29, 495–502. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Libralato, G.; Tazart, Z.; Enaime, G.; Douma, M.; Ounas, A.; Yaacoubi, A.; Lofrano, G.; Carotenuto, M.; Saviano, L.; et al. Nature-based coagulants for drinking water treatment: An ecotoxicological overview. Water Environ. Res. 2022, 94, e10782. [Google Scholar] [CrossRef]
- Maikokera, R.; Kwaambwa, H.M. Interfacial properties and fluorescence of a coagulating protein extracted from Moringa oleifera seeds and its interaction with sodium dodecyl sulphate. Colloids Surf. B Biointerfaces 2007, 55, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kwaambwa, H.M.; Maikokera, R. A fluorescence spectroscopic study of a coagulating protein extracted from Moringa oleifera seeds. Colloids Surf. B Biointerfaces 2007, 60, 213–220. [Google Scholar] [CrossRef]
- Kwaambwa, H.M.; Maikokera, R. Infrared and circular dichroism spectroscopic characterisation of secondary structure components of a water treatment coagulant protein extracted from Moringa oleifera seeds. Colloids Surf. B Biointerfaces 2008, 64, 118–125. [Google Scholar] [CrossRef]
- Kwaambwa, H.M.; Rennie, A.R. Interactions of surfactants with a water treatment protein from Moringa oleifera seeds in solution studied by zeta-potential and light scattering measurements. Biopolymers 2012, 97, 209–218. [Google Scholar] [CrossRef]
- Kwaambwa, H.M.; Hellsing, M.; Rennie, A.R. Adsorption of a water treatment protein from Moringa oleifera seeds to a silicon oxide surface studied by neutron reflection. Langmuir 2010, 26, 3902–3910. [Google Scholar] [CrossRef]
- Jerri, H.A.; Adolfsen, K.J.; McCullough, L.R.; Velegol, D.; Velegol, S.B. Antimicrobial sand via adsorption of cationic Moringa oleifera protein. Langmuir 2012, 28, 2262–2268. [Google Scholar] [CrossRef]
- Kwaambwa, H.M.; Hellsing, M.S.; Rennie, A.R.; Barker, R. Interaction of Moringa oleifera seed protein with a mineral surface and the influence of surfactants. J. Colloid Interface Sci. 2015, 448, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.; Mossou, E.; Signor, L.; Kieffer-Jaquinod, S.; Kwaambwa, H.; Nermark, F.; Gutfreund, P.; Mitchell, E.; Haertlein, M.; Forsyth, V.; et al. Towards a molecular understanding of the water purification properties of Moringa seed proteins. J. Colloid Interface Sci. 2019, 554, 296–304. [Google Scholar] [CrossRef]
- Nordmark, B.A.; Bechtel, T.M.; Riley, J.K.; Velegol, D.; Velegol, S.B.; Przybycien, T.M.; Tilton, R.D. Moringa oleifera seed protein adsorption to silica: Effects of water hardness, fractionation, and fatty acid extraction. Langmuir 2018, 34, 4852–4860. [Google Scholar] [CrossRef] [PubMed]
- Nouhi, S.; Pascual, M.; Hellsing, M.S.; Kwaambwa, H.M.; Skoda, M.W.; Höök, F.; Rennie, A.R. Sticking particles to solid surfaces using Moringa oleifera proteins as a glue. Colloids Surf. B Biointerfaces 2018, 168, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Nouhi, S.; Kwaambwa, H.M.; Gutfreund, P.; Rennie, A.R. Comparative study of flocculation and adsorption behaviour of water treatment proteins from Moringa peregrina and Moringa oleifera seeds. Sci. Rep. 2019, 9, 17945. [Google Scholar] [CrossRef]
- Hellsing, M.S.; Kwaambwa, H.M.; Nermark, F.M.; Nkoane, B.B.; Jackson, A.J.; Wasbrough, M.J.; Berts, I.; Porcar, L.; Rennie, A.R. Structure of flocs of latex particles formed by addition of protein from Moringa seeds. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 460–467. [Google Scholar] [CrossRef]
- Amuanyena, M.O.; Kandawa-Schulz, M.; Kwaambwa, H.M. Magnetic iron oxide nanoparticles modified with Moringa seed proteins for recovery of precious metal ions. J. Biomater. Nanobiotechnol. 2019, 10, 142. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum dots—Characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Folkard, G.K.; Grant, W.D. Natural coagulants for appropriate water treatment: A novel approach. Waterlines 1990, 8, 30–32. [Google Scholar] [CrossRef]
- Thanki, A.; Padhiyar, H.; Singh, N.K.; Yadav, M.; Christian, J. Municipal Wastewater Treatment Using Moringa oleifera Seed and Press Cake Powder: A Comparative Analysis. CLEAN–Soil Air Water 2022, 51, 2100336. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible quantum dots for biological applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kapofi, S. Interactions of Cadmium Selenide (CdSe) Quantum Dots with Coagulant Proteins Extracted from Moringa oleifera Seeds. Doctoral Dissertation, University of Namibia, Windhoek, Namibia, 2019. Available online: https://repository.unam.edu.na/bitstream/handle/11070/2559/kapofi2019.pdf?sequence=1&isAllowed=y (accessed on 21 December 2023).
- Lohse, S.E.; Murphy, C.J. Applications of colloidal inorganic nanoparticles: From medicine to energy. J. Am. Chem. Soc. 2012, 134, 15607–15620. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, R.; Gopalakrishnan, S.; Kolandaivel, P. Interaction and bioconjugation of CdSe/ZnS core/shell quantum dots with maltose-binding protein. Comput. Theor. Chem. 2017, 1101, 96–101. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Zhang, Z.; Yuan, C.; Fu, D. CdTe nanocrystals as luminescent probes for detecting ATP, folic acid and l-cysteine in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 102–106. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, Y.; Han, H. Study on the interaction between bovine serum albumin and CdTe quantum dots with spectroscopic techniques. J. Mol. Struct. 2008, 892, 116–120. [Google Scholar] [CrossRef]
- Mekis, I.; Talapin, D.V.; Kornowski, A.; Haase, M.; Weller, H. One-pot synthesis of highly luminescent CdSe/CdS core-shell nanocrystals via organometallic and “Greener” chemical approaches. J. Phys. Chem. B 2003, 107, 7454–7462. [Google Scholar] [CrossRef]
- Gupta, D.K.; Verma, M.; Sharma, K.; Saxena, N.S. Synthesis, characterization and optical properties of CdSe/CdS and CdSe/ZnS core-shell nanoparticles. Indian J. Pure Appl. Phys. (IJPAP) 2017, 55, 113–121. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S. Use of Moringa oleifera seeds as a primary coagulant in wastewater treatment. Environ. Technol. 1998, 19, 789–800. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998, 32, 781–791. [Google Scholar] [CrossRef]
- Soni, U.; Arora, V.; Sapra, S. Wurtzite or zinc blende? Surface decides the crystal structure of nanocrystals. CrystEngComm 2013, 15, 5458–5463. [Google Scholar] [CrossRef]
- Kim, S.H.; Man, M.T.; Lee, J.W.; Park, K.D.; Lee, H.S. Influence of size and shape anisotropy on optical properties of CdSe quantum dots. Nanomaterials 2020, 10, 1589. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M. Surface area: Brauer–emmett–teller (BET). In Progress in Filtration and Separation; Academic Press: Cambridge, MA, USA, 2015; pp. 585–608. [Google Scholar] [CrossRef]
- Yu, M.; Saeed, M.H.; Zhang, S.; Wei, H.; Gao, Y.; Zou, C.; Zhang, L.; Yang, H. Luminescence Enhancement, Encapsulation, and Patterning of Quantum Dots Toward Display Applications. Adv. Funct. Mater. 2022, 32, 2109472. [Google Scholar] [CrossRef]
- Wang, H.; Nienhaus, K.; Shang, L.; Nienhaus, G.U. Highly Luminescent Positively Charged Quantum Dots Interacting with Proteins and Cells. Chin. J. Chem. 2022, 40, 2685–2693. [Google Scholar] [CrossRef]
- Nisha, R.R.; Jegathambal, P.; Parameswari, K.; Kirupa, K. Biocompatible water softening system using cationic protein from moringa oleifera extract. Appl. Water Sci. 2017, 7, 2933–2941. [Google Scholar] [CrossRef]
- Irfan, M.; Munir, H.; Ismail, H. Moringa oleifera gum based silver and zinc oxide nanoparticles: Green synthesis, characterization and their antibacterial potential against MRSA. Biomater. Res. 2021, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Gomez, D.; Kirkwood, N.; Mulvaney, P. Tuning single quantum dot emission with a micromirror. Nano Lett. 2018, 18, 1010–1017. [Google Scholar] [CrossRef]
- Dzagli, M.M.; Canpean, V.; Iosin, M.; Mohou, M.A.; Astilean, S. Study of the interaction between CdSe/ZnS core-shell quantum dots and bovine serum albumin by spectroscopic techniques. J. Photochem. Photobiol. A Chem. 2010, 215, 118–122. [Google Scholar] [CrossRef]
- Lai, L.; Lin, C.; Xu, Z.-Q.; Han, X.-L.; Tian, F.-F.; Mei, P.; Li, D.-W.; Ge, Y.-S.; Jiang, F.-L.; Zhang, Y.-Z.; et al. Spectroscopic studies on the interactions between CdTe quantum dots coated with different ligands and human serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 366–376. [Google Scholar] [CrossRef]
- Siddiqui, G.A.; Siddiqi, M.K.; Khan, R.H.; Naeem, A. Probing the binding of phenolic aldehyde vanillin with bovine serum albumin: Evidence from spectroscopic and docking approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 40–47. [Google Scholar] [CrossRef]
- Hu, Y.J.; Ou-Yang, Y.; Dai, C.M.; Liu, Y.; Xiao, X.H. Binding of berberine to bovine serum albumin: Spectroscopic approach. Mol. Biol. Rep. 2010, 37, 3827–3832. Available online: https://link.springer.com/article/10.1007/s11033-010-0038-x (accessed on 21 December 2023). [CrossRef]
- Chu, V.H.; Lien Nghiem, T.H.; Le, T.H.; Lam Vu, D.; Nhung Tran, H.; Lien Vu, T.K. Synthesis and optical properties of water-soluble CdSe/CdS quantum dots for biological applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025017. [Google Scholar] [CrossRef]
- Yan, H.; Wu, J.; Dai, G.; Zhong, A.; Chen, H.; Yang, J.; Han, D. Interaction mechanisms of ionic liquids [Cnmim] Br (n = 4, 6, 8, 10) with bovine serum albumin. J. Lumin. 2012, 132, 622–628. [Google Scholar] [CrossRef]
- Lima, C.N.; Cabral Filho, P.E.; Santos, B.S.; Moura, P.; Fontes, A. Interactions of mannose binding-lectin with red blood cells by employing cationic quantum dots. Int. J. Biol. Macromol. 2019, 125, 1168–1174. [Google Scholar] [CrossRef] [PubMed]

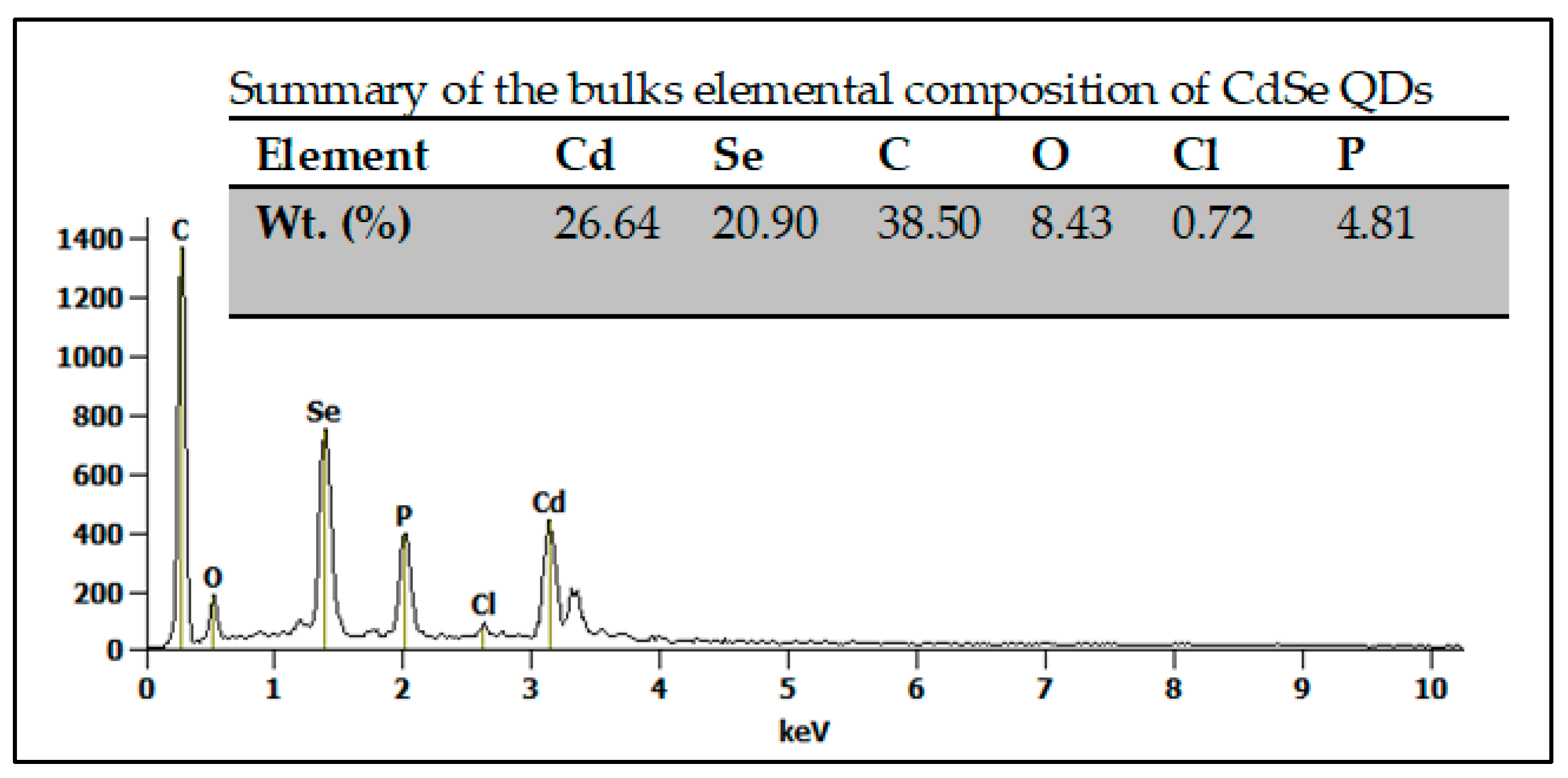



| Direct Band Gap | Indirect Band Gap | |
|---|---|---|
| CdSe QDs | 2.27 | 2.20 |
| CdSe–MSP10 | 2.19 | 2.12 |
| CdSe–MSP50 | 2.20 | 0.85 |
| CdSe–MSP100 | 2.21 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, L.S.; Kapofi, S.; Kandawa-Schulz, M.; Kwaambwa, H.M. Interactions of CdSe Nanocrystals with Cationic Proteins Extracted from Moringa oleifera Seeds. Photochem 2024, 4, 24-39. https://doi.org/10.3390/photochem4010003
Daniel LS, Kapofi S, Kandawa-Schulz M, Kwaambwa HM. Interactions of CdSe Nanocrystals with Cationic Proteins Extracted from Moringa oleifera Seeds. Photochem. 2024; 4(1):24-39. https://doi.org/10.3390/photochem4010003
Chicago/Turabian StyleDaniel, Likius Shipwiisho, Salatiel Kapofi, Martha Kandawa-Schulz, and Habauka Majority Kwaambwa. 2024. "Interactions of CdSe Nanocrystals with Cationic Proteins Extracted from Moringa oleifera Seeds" Photochem 4, no. 1: 24-39. https://doi.org/10.3390/photochem4010003
APA StyleDaniel, L. S., Kapofi, S., Kandawa-Schulz, M., & Kwaambwa, H. M. (2024). Interactions of CdSe Nanocrystals with Cationic Proteins Extracted from Moringa oleifera Seeds. Photochem, 4(1), 24-39. https://doi.org/10.3390/photochem4010003






