Abstract
Here we present simple fluorophores based on the pyridine core, obtained with straightforward synthetic methodologies. These compounds present in solution absorption maxima in the UV region and fluorescence emission of between 300 and 450 nm, depending on the solvent and chemical structure of the fluorophore. The nature of the solvent was shown to play a fundamental role in their excite-state deactivation, which allowed successful exploration of these compounds as optical sensors for benzene and fuel adulteration in gasoline. In ethanolic solution, upon the addition of benzene, in general the fluorophores presented fluorescence quenching, where a linear correlation between the emission intensity and the amount of benzene (quencher) was observed. In addition, the application of an optical sensor for the detection of fuel adulteration in commercial standard and premium gasoline was successfully presented and discussed. Theoretical calculations were also applied to better understand the solvent–fluorophore interactions.
1. Introduction
Although renewable fuels have been widely used in our daily lives, petroleum-based fuels are still an energy source of global importance. In this context, the high dependence on these fuels makes their illegal adulteration a highly lucrative endeavor. The addition of industrial solvents as adulterants in petroleum-based fuels is stimulated by price disparities, usually caused by different taxation between them. However, illegal adulteration can have several impacts, such as increased toxic emissions and vehicle malfunctions [1,2,3,4].
Fuel adulteration, especially of commercial gasoline, involves the addition of organic solvents, methanol, or ethanol in concentrations higher than those established by current legislation. Thus, numerous analytical techniques have been used in the literature to evaluate and detect possible adulterations in fuels. Approaches based on simple physicochemical methods can be carried out, such as relative density measurements and evaporation/distillation methods. However, more complex analytical methods such as techniques based on the use of chemiresistors [5] and gas chromatography–mass spectrometry [6] (GC–MS) can be used to obtain more reliable results. These techniques usually require experienced professionals for the operation and data processing; in addition, they are dangerous techniques using flammable systems, expensive, and time-consuming. This makes it extremely important to develop new and easy-to-implement technologies to monitor fuel compliance. Other strategies employed are based on polymeric electronic gas sensors [7], polydiacetylene (PDA) paper-based colorimetric sensors [8,9,10], optical fiber sensors [11], membrane-type surface stress sensors [12], and quartz crystal microbalance (QCM) sensors [13,14,15]. In this way, a promising and easy-to-execute alternative is based on the use of organic dyes as optical sensors or marking materials. This technique is based on the absorption and/or emission properties obtained according to changes in dye concentration. Some fluorescent compounds have already been reported in the literature, such as rhodamine [16], polymethine [17,18], squaraine [19], anthraquinone [20], 4-dimethylamino-4-nitrostilbene [21], and 2,1,3-benzothiadiazole (BTD) derivatives [22].
Based on the photophysical properties of pyridine-based compounds, our proposal presents differences concerning the chemosensors presented in the literature for this application, such as the investigated fuel (diesel, kerosene, and jet fuel) or even the identification methodology (test strip) [23,24]. However, taking the adulteration in gasoline using ethanol into account, similar studies can be found, using flavonoid dyes [25], Rhodamine 800, and Atto 680 dyes [26] (Figure 1). In addition, UV-Vis studies have also been reported using pyridinium N-phenolate betaine dyes (Figure 1) [27,28,29].
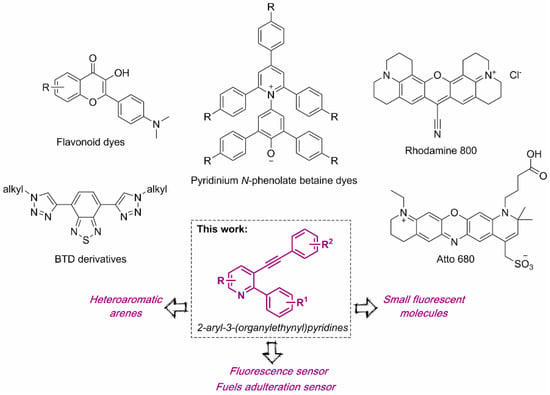
Figure 1.
Chemical structure of selected optical sensors for fuel adulteration.
In this context, recently described BTD derivatives (Figure 1) [22] were used as solvatochromic dyes in a similar way to the one presented here, being easy to perform and with high linearity, as well as showing low sensitivity to concentration by fluorescence spectroscopy. However, the fluorescent compounds used as fuel sensors still represent a small group of structures, usually formed by complex and/or expensive molecules, and obtained through multiple reaction steps. In this way, the use of small fluorescent molecules as chemosensors could become a cheap and easily obtainable alternative in the evaluation of possible fuel adulteration.
In general, the incorporation of a nitrogen atom in polycyclic aromatic structures can induce or improve their electromagnetic, physicochemical, optical, and structural properties [30,31,32]. Through chemical modification/doping of the π-conjugated system with nitrogen, it is possible to control the main characteristics of the electronic structure, including the band gap, optical absorption spectra, photoluminescence, and redox behavior. As these parameters can be easily adjusted, many heteroaromatic compounds have been applied as NIR-active dyes [33], two-photon absorbers [34], and fluorescence sensors [35]. In addition, nitrogen doping is employed as a means of band-gap adjustment in π-conjugated polymers [36] and graphene nanoribbons [37], and to obtain small-molecule semiconductors [38]. Finally, the literature also reports some interesting studies using push–pull systems based on pyridine groups in dye-sensitized solar cells [39], hole-transport material in perovskite solar cells [40,41,42], and as an optical sensor that can be used as a fluorescent probe for the detection of nitroaromatic explosives [43,44]. Recently, the electronic behavior of small molecules has also been studied in ITIC, a new generation of non-fullerene electron-accepting small molecules for organic photovoltaics (OPVs), due to nitrogen substitution/insertion in different positions of the molecule [45]. In addition, small nitrogen compounds from 2-N-aminoquinazolines were also evaluated as fluorophores in solution and solid state [46]. Our research group has focused attention on the influence of chalcogens on the reactivity and properties of organochalcogen compounds [47,48,49]. Finally, we demonstrate here the photophysical characterization of 2-aryl-3-(organylethynyl)pyridine derivatives (Figure 1), recently described by us as substrates in visible-light-promoted selenocyclization reactions for the formation of Se-functionalized benzo[h]quinolines [50]. In addition, to broaden the scope of application of these compounds, based on their electronic features, they were successfully employed as optical sensors for the detection of fuel adulteration in commercial standard and premium gasoline. It should be noted that the application of small molecules is often not explored in depth, leaving a barrier of knowledge behind molecules of “greater complexity”.
2. Materials and Methods
2.1. General Information
Unless otherwise stated, all reagents were purchased from commercial suppliers and used without further purification. Compounds 1,4-dioxane and Et3N (triethylamine) were purified and dried under classical methods [51]. Solvents used in liquid–liquid extraction and as eluents for chromatographic purification were distilled before use. Reactions were monitored by thin-layer chromatography (TLC) using silica gel 60 F254 aluminum sheets, and visualization of the spots was carried out under UV light (254 nm) and stained with iodine or with a mixture of 5% vanillin in 10% H2SO4 using heat as developing agent. Column chromatography was performed on silica gel (230–400 mesh). Some 1H NMR spectra were obtained on a Bruker Avance III HD 400 MHz (Billerica, MA, EUA) employing a direct broadband probe at 400 MHz. The spectra were recorded in CDCl3 solutions. The chemical shifts are reported in ppm and referenced to tetramethylsilane (TMS) as the internal reference. Coupling constants (J) are reported in Hertz. Abbreviations to denote the multiplicity of a particular signal are s (singlet), d (doublet), dd (doublet of doublet), t (triplet), quint (quintuplet), and m (multiplet). Some 13C{1H} NMR spectra were obtained on a Bruker Avance III HD 400 MHz employing a direct broadband probe at 100 MHz. The chemical shifts are reported in ppm, referenced to the solvent peak of CDCl3 (δ 77.0 ppm). Melting points were recorded on Buchi Melting Point M-560 equipment (Flawil, Switzerland). High-resolution mass spectra (HRMS) were recorded on a Micromass Q-TOF spectrometer (Milford, MA, EUA), using Atmospheric Pressure Chemical Ionization (APCI).
2.2. Synthesis
2.2.1. General Procedure for the Synthesis of Starting Materials 1a–e
The 2-chloro-3-(organylethynyl)pyridines 1a–e were prepared according to published procedures [52,53,54,55] (Scheme 1). PdCl2(PPh3)2 (5 mol%, 0.105 g) and triethylamine (8.0 mL) were added to a 50.0 mL two-mouthed flask equipped with magnetic stirring and reflux system under argon atmosphere. After that, the respective 3-bromo-2-chloropyridine 4 (3.0 mmol) was added, followed by the addition of organylacetylene 5 (3.3 mmol), and the mixture was stirred for 5 min at room temperature. Then CuI (2 mol%, 0.011 g) was added, and the temperature was increased to 65 °C (oil bath). The reaction remained under magnetic stirring for 24 h. After that, the reaction was extracted with ethyl acetate, and the organic phase was washed with aqueous HCl 1% solution until complete neutralization of the aqueous phase. The organic phase was separated, dried over MgSO4, filtered, and the solvent evaporated under reduced pressure. The crude material was further purified by column chromatography (hexanes/ethyl acetate) on silica gel.
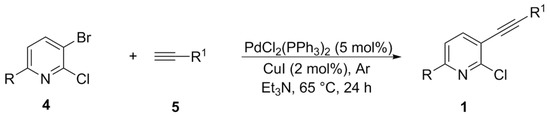
Scheme 1.
Synthesis of 2-chloro-3-(organylethynyl)pyridines 1a–e.
2.2.2. General Procedure for the Synthesis of 2-aryl-3-(organylethynyl)pyridines 3a–k
The 2-aryl-3-(organylethynyl)pyridines 3a–k were prepared according to a published procedure, with minor changes [56]. In a dried sealed Schlenk tube under argon atmosphere, the appropriate 2-chloro-3-(organylethynyl)pyridine 1 (2.0 mmol), aryl boronic acid 2 (2.0 equiv, 4.0 mmol), Pd(PPh3)4 (5 mol%, 0.115 g), K3PO4 (2.0 equiv, 2.0 mmol), and 1,4-dioxane (8.0 mL) were added (Scheme 2). The mixture remained under magnetic stirring for 24 h at 90 °C (oil bath). After that, the reaction was extracted with ethyl acetate, and the organic phase was washed with a saturated aqueous NaCl solution. The organic phase was separated, dried over MgSO4, filtered, and the solvent evaporated under reduced pressure. The crude material was further purified by column chromatography (hexanes/ethyl acetate) on silica gel. The yield was 51–91%.
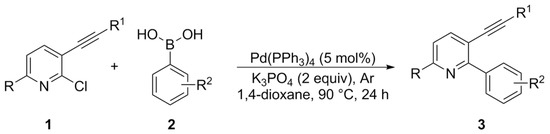
Scheme 2.
Synthesis of 2-aryl-3-(organylethynyl)pyridines 3a–k.
2-Phenyl-3-(phenylethynyl)pyridine (3a) [54,55]: purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.413 g (81%); brown solid, m.p: 69–71 °C; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.64 (dd, J = 4.8 and 1.7 Hz, 1H); 8.03–8.00 (m, 2H); 7.93 (dd, J = 7.8 and 1.7 Hz, 1H); 7.52–7.37 (m, 5H); 7.34–7.31 (m, 3H); 7.25 (dd, J = 7.8 and 4.8 Hz, 1H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.6, 148.5, 140.7, 139.3, 131.4, 129.3, 128.8, 128.6, 128.4, 127.9, 122.8, 121.4, 117.9, 94.5, 87.5.
3-(Phenylethynyl)-2-(4-tolyl)pyridine (3b) [54]: purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.458 g (85%); yellowish solid, m.p: 52–54 °C; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.62 (dd, J = 4.8 and 1.8 Hz, 1H); 7.95 (d, J = 8.2 Hz, 2H); 7.90 (dd, J = 7.8 and 1.8 Hz, 1H); 7.44–7.40 (m, 2H); 7.34–7.28 (m, 5H); 7.20 (dd, J = 7.8 and 4.8 Hz, 1H); 2.42 (s, 3H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.4, 148.4, 140.8, 138.8, 136.5, 131.3, 129.2, 128.6, 128.5, 128.3, 122.9, 121.1, 117.6, 94.4, 87.6, 21.4.
2-(4-Chlorophenyl)-3-(phenylethynyl)pyridine (3c) [54,55]: purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.364 g (63%); white solid, m.p: 121–123 °C; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.62 (dd, J = 4.8 and 1.7 Hz, 1H); 7.99 (d, J = 8.5 Hz, 2H); 7.92 (dd, J = 7.8 and 1.7 Hz, 1H); 7.46 (d, J = 8.5 Hz, 2H); 7.43–7.38 (m, 2H); 7.35–7.32 (m, 3H); 7.24 (dd, J = 7.8 and 4.8 Hz, 1H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 158.1, 148.5, 140.9, 137.7, 134.9, 131.3, 130.7, 128.8, 128.4, 128.0, 122.5, 121.6, 117.7, 94.9, 87.0.
2-(4-Methoxyphenyl)-3-(phenylethynyl)pyridine (3d) [54,55]: purified by column chromatography (hexane/ethyl acetate = 90:10); yield: 0.445 g (78%); yellowish oil; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.60 (dd, J = 4.8 and 1.5 Hz, 1H); 8.03 (d, J = 8.6 Hz, 2H); 7.89 (dd, J = 7.8 and 1.5 Hz, 1H); 7.45–7.41 (m, 2H); 7.34–7.30 (m, 3H); 7.18 (dd, J = 7.8 and 4.8 Hz, 1H); 7.01 (d, J = 8.6 Hz, 2H); 3.86 (s, 3H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 160.2, 159.0, 148.4, 140.8, 131.9, 131.3, 130.7, 128.5, 128.3, 122.9, 120.8, 117.2, 113.2, 94.3, 87.7, 55.3.
2-(Naphthalen-2-yl)-3-(phenylethynyl)pyridine (3e): purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.336 g (55%); yellowish solid, m.p: 76–78 °C; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.69 (dd, J = 4.8 and 1.7 Hz, 1H); 8.60 (s, 1H); 8.16 (dd, J = 8.6 and 1.7 Hz, 1H); 7.98–7.89 (m, 4H); 7.55–7.49 (m, 2H); 7.40–7.36 (m, 2H); 7.31–7.24 (m, 4H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.3, 148.6, 140.9, 136.7, 133.4, 133.0, 131.4, 129.1, 128.6, 128.4, 127.6, 127.4, 126.9, 126.6, 126.1, 122.7, 121.4, 118.1, 94.8, 87.6. HRMS (APCI-QTOF) calculated mass for C23H16N [M + H]+: 306.1283, found: 306.1272.
3-(Phenylethynyl)-2-(2-tolyl)pyridine (3f) [54,55]: purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.490 g (91%); yellowish oil; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.58 (dd, J = 4.9 and 1.7 Hz, 1H); 7.83 (dd, J = 7.8 and 1.7 Hz, 1H); 7.41 (d, J = 7.5 Hz, 1H); 7.33–7.24 (m, 3H); 7.21–7.14 (m, 6H); 2.26 (s, 3H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 161.8, 147.7, 139.4, 138.9, 135.9, 131.1, 129.8, 129.1, 128.3, 128.1, 128.0, 125.1, 122.4, 121.3, 119.4, 94.5, 86.5, 19.4.
2-(2-Chlorophenyl)-3-(phenylethynyl)pyridine (3g): purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.324 g (56%); yellowish oil; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.63 (dd, J = 4.8 and 1.7 Hz, 1H); 7.90 (dd, J = 7.8 and 1.7 Hz, 1H); 7.53–7.46 (m, 2H); 7.38–7.34 (m, 2H); 7.30–7.19 (m, 6H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.3, 148.0, 139.1, 138.8, 133.0, 131.3, 130.9, 129.6, 129.4, 128.5, 128.2, 126.4, 122.4, 122.2, 120.1, 95.0, 86.0. HRMS (APCI-QTOF) calculated mass for C19H13ClN [M + H]+: 290.0737, found: 290.0731.
2-Phenyl-3-(4-tolylethynyl)pyridine (3h): purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.484 g (90%); yellow solid, m.p: 65–67 °C; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.61 (dd, J = 4.8 and 1.7 Hz, 1H); 8.03–8.00 (m, 2H); 7.89 (dd, J = 7.8 and 1.7 Hz, 1H); 7.50–7.40 (m, 3H); 7.28 (d, J = 8.0 Hz, 2H); 7.20 (dd, J = 7.8 and 4.8 Hz, 1H); 7.11 (d, J = 8.0 Hz, 1H); 2.33 (s, 3H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.4, 148.2, 140.6, 139.2, 138.8, 131.2, 129.3, 129.1, 128.8, 127.8, 121.4, 119.7, 118.1, 94.8, 86.8, 21.5. HRMS (APCI-QTOF) calculated mass for C20H16N [M + H]+: 270.1283, found: 270.1272.
3-((4-Chlorophenyl)ethynyl)-2-phenylpyridine (3i): purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.399 g (69%); yellowish solid, m.p: 89–91 °C; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.66 (dd, J = 4.8 and 1.7 Hz, 1H); 8.00–7.98 (m, 2H); 7.92 (dd, J = 7.8 and 1.7 Hz, 1H); 7.51–7.44 (m, 3H); 7.32–7.25 (m, 5H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.8, 148.7, 140.7, 139.3, 134.7, 132.6, 129.3, 128.9, 128.8, 127.9, 121.4, 121.3, 117.6, 93.3, 88.4. HRMS (APCI-QTOF) calculated mass for C19H13ClN [M + H]+: 290.0737, found: 290.0723.
3-(Oct-1-yn-1-yl)-2-phenylpyridine (3j): purified by column chromatography (hexane/ethyl acetate = 97:3); yield: 0.268 g (51%); yellowish oil; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.56 (dd, J = 4.8 and 1.7 Hz, 1H); 7.96–7.93 (m, 2H); 7.77 (dd, J = 7.8 and 1.7 Hz, 1H); 7.45–7.36 (m, 3H); 7.13 (dd, J = 7.8 and 4.8 Hz, 1H); 2.33 (t, J = 7.0 Hz, 2H); 1.51 (quint, J = 7.0 Hz, 2H); 1.37–1.21 (m, 6H); 0.88 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.3, 147.7, 140.9, 139.4, 129.1, 128.4, 127.6, 121.1, 118.5, 96.1, 78.4, 31.2, 28.4, 28.1, 22.4, 19.5, 14.0. HRMS (APCI-QTOF) calculated mass for C18H22N [M + H]+: 264.1752, found: 264.1730.
6-Methyl-2-phenyl-3-(phenylethynyl)pyridine (3k): purified by column chromatography (hexane/ethyl acetate = 95:5); yield: 0.360 g (67%); yellowish solid, m.p: 101–103 °C; 1H NMR (CDCl3, 400 MHz) δ (ppm) = 8.01–7.98 (m, 2H); 7.78 (d, J = 7.9 Hz, 1H); 7.49–7.34 (m, 5H); 7.29–7.26 (m, 3H); 7.06 (d, J = 7.9 Hz, 1H); 2.61 (s, 3H). 13C{1H} NMR (CDCl3, 100 MHz) δ (ppm) = 159.0, 157.6, 140.7, 139.5, 131.2, 129.3, 128.6, 128.3, 128.2, 127.8, 123.0, 121.1, 114.7, 93.6, 87.7, 24.7. HRMS (APCI-QTOF) calculated mass for C20H16N [M + H]+: 270.1283, found: 270.1269.
2.3. Photophysical Characterization
Spectroscopic-grade solvents benzene, ethanol, dichloromethane, and hexane were used for photophysical characterization. UV-Vis absorption spectra in solution (10−5 mol·L−1) were acquired on a Shimadzu UV-2450 spectrophotometer (Kyoto, Japan) and steady-state fluorescence spectra were obtained on a Shimadzu spectrofluorometer model RF-5301PC. The maximum absorption wavelength was used as the excitation wavelength to acquire the respective fluorescence emission spectra. All measurements were performed at room temperature (25 °C). Based on the emission intensities in benzene and ethanol, additional exploratory fluorescence titrations were performed in ethanol at different amounts of benzene. In this way, ethanolic solutions of selected synthesized compounds at a concentration of ~10−5 M were prepared. To this solution, amounts of 10% benzene (v/v) were added up to 100% benzene (v/v). For these experiments, the absorption maxima in ethanol were used as excitation wavelengths.
2.4. Fuel Adulteration Sensing
Brazilian commercial gasoline (standard or premium) was previously treated as described in the literature [22]. This procedure is essential since commercial gasoline in Brazil presents 27% (standard) or 25% (premium) of anhydrous ethanol. Briefly, the ethanol content was removed by the salting-out methodology. A second treatment was necessary since commercial gasoline presents tagging dyes, which are added for fiscal and security purposes and must be removed before experiments. In this sense, the samples were passed in a column containing silica 60 (230–400 mesh), collected in an Pyrex® narrow-mouth graduated Erlenmeyer flask (Corning, EUA), and kept under stirring in presence of activated charcoal for 2 h. The mixture was filtered to produce colorless gasoline, which was stored at 4 °C. Solutions of selected synthesized compounds at a concentration of ~10−5 M were prepared using the treated standard and premium types of gasoline (4 mL). To this solution, amounts of 5% ethanol (v/v) were added up to 50% ethanol in gasoline (v/v). For these experiments, the absorption maxima in gasoline were used as excitation wavelengths.
2.5. Theoretical Calculations
All Density Functional Theory calculations used in this work were performed with the quantum chemistry package ORCA v5.0.3 [57,58,59]. The compounds 3a, 3e, 3f, and 3j were chosen as models for the calculations. The initial molecular geometries for the molecules were obtained by conformational sampling performed by the semiempirical CREST software [60] with the ALPB implicit solvation module [61] active to simulate benzene and ethanol environments. The most stable conformers were subsequently reoptimized using the ωB97X-D3 [62]/Def2-TZVP [63] level of theory with tight convergence criteria and the Conductor-like Polarizable Continuum Model (CPCM) [64] implicit solvation active. The resulting relaxed geometries have no imaginary vibrational modes (3a, 3e, and 3f), or the imaginary vibrational mode remaining (3f) is less than −20 cm−1, which is characteristic of numerical noise and not the formation of a saddle-point. Absorption spectra, from which the electronic density difference was obtained, used the same ωB97X-D3/Def2-TZVP level of theory for the first 80 electronic transitions with Tamm–Dancoff approximation active [65].
3. Results and Discussion
3.1. Synthesis
The synthesis of 2-aryl-3-(organylethynyl)pyridines 3 is little explored in the literature. We found two protocols starting from halopyridines (Scheme 3). The first, described by Shibata and coworkers [66], adopts two distinct Suzuki-type cross-coupling reactions using 3-organylethynyl-2-chloropyridines 1 (obtained by Sonogashira-type cross-coupling reactions) and aryl boronic acids 2 (1.2–1.5 equiv) (Scheme 3a). The second, described by Shestakov and coworkers [67], presents an inverse reaction proposal, initially obtaining 4 by a Suzuki-type cross-coupling reaction. Then, these 2-aryl-3-bromopyridines 4 were used together with organylacetylenes 5 in a Sonogashira-type cross-coupling reaction to give 3 (Scheme 3b).
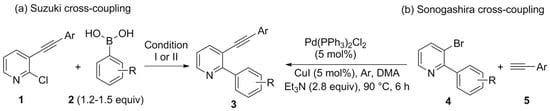
Scheme 3.
Synthetic routes by (a) Suzuki-type and (b) Sonogashira-type cross-couplings for the synthesis of 2-aryl-3-(organylethynyl)pyridines 3 described in the literature. Condition I: (AMPHOS)2PdCl2 (5 mol%), K2CO3 (2.0 equiv), toluene:H2O (10:1), 100 °C. Condition II: PEPSSI-IPr (5 mol%), tBuOK (1.3 equiv), iPrOH, 40–60 °C.
To begin our studies, we performed the synthesis of commercially unavailable 3-organylethynyl-2-chloropyridines 1. For this, based on a procedure described in the literature, a Sonogashira-type cross-coupling reaction was promoted using the respective organylacetylene 5 (1.1 equiv), PdCl2(PPh3)2 (5 mol%), CuI (2 mol%) in triethylamine under an argon atmosphere at 65 °C for 24 h to give the 3-(organylethynyl)-2-chloropyridines 1a–h (Scheme 1). The next synthesis step consists of a Suzuki-type cross-coupling reaction, based on the literature. Thus, the reaction was between 3-(organylethynyl)-2-chloropyridines 1 and arylboronic acids 2 (2.0 equiv), using Pd(PPh3)4 (5 mol%) as a catalyst and K3PO4 (2.0 equiv) as a base in 1,4-dioxane at 90 °C under an argon atmosphere in a sealed flask. By this method, eleven 2-aryl-3-(organylethynyl)pyridines 3 were obtained in 55–91% yield after 24 h of reaction (Scheme 2, Figure 2).
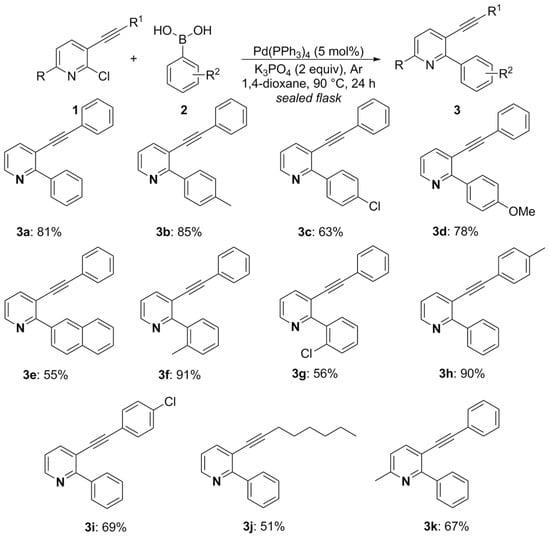
Figure 2.
Investigation on the scope of the substrates 2-aryl-3-(organylethynyl)pyridines 3a–k. 1 Reaction conditions: the mixture of 2-chloro-3-(organylethynyl)pyridines 1a–f (2.0 mmol), aryl boronic acids 2a–q (4.0 mmol), Pd(PPh3)4 (5 mol%), K3PO4 (2.0 equiv), 1,4-dioxane (8 mL) at 90 °C (oil bath) under an argon atmosphere was kept under magnetic stirring for 24 h in a sealed flask. 2 Isolated yields.
The Suzuki-type cross-coupling reaction was tolerant of a variety of neutral, electron-donating, and electron-withdrawing substituents at the aromatic ring of the 3-phenylethynyl and 2-aryl moieties of pyridines, allowing the synthesis of several 2-aryl-3-(organylethynyl)pyridines 3 in moderate to excellent yields (Figure 2, 3a–i). It should be noted that a bulky boronic acid (naphthalen-2-ylboronic acid) could also be used; in this case, 3e was obtained in 55% yield. We also prepared a derivative containing an aliphatic chain (oct-1-yne) in the 3-ethynyl portion of pyridine, giving 3j in 51% yield under standard reaction conditions (Figure 2, 3j). Finally, we evaluated the insertion of a methyl group attached to the pyridine core, giving 3k in 67% yield (Figure 2, 3k).
3.2. Photophysics and Optical Sensing
The photophysical characterization in solution was performed using benzene, ethanol, dichloromethane, and hexane. The choice of solvents was based on the possible interactions that could occur with the fluorophores, comprising aromatic, non-polar, polar protic, and aprotic solvents. Figure 3 and Figure 4 present the photophysical characterization of selected compounds, which were chosen based on their electronic properties. The relevant photophysical data are highlighted in Table 1. It can be observed that the main absorption bands are located between 250 and 350 nm, with maxima around 300 nm. These compounds presented different shapes and intensities based on their electronic structure. No significant solvatochromic effect was observed for these compounds, indicating an almost absent charge-transfer character in the ground state. A particular behavior was observed in compound 3j (Figure 3e), where there is a large variation in the absorption maximum position depending on the solvent, indicating that the phenyl group attached to the triple bond, absent in this derivative, plays an important role in the location of the absorption maxima of these compounds. Finally, the high molar absorptivity coefficient values (ε~104 cm−1·M−1) indicate that the observed electronic transitions are spin and symmetry-allowed 1π-π* transitions. In general, the other derivatives presented similar photophysical behavior (Figures S23–S28).
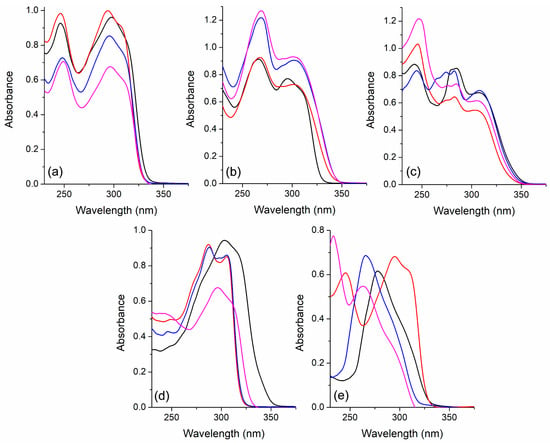
Figure 3.
UV-Vis absorption spectra of the pyridine-based fluorophores (a) 3a, (b) 3d, (c) 3e, (d) 3f, and (e) 3j in solution [~10−5 M] in benzene (black), ethanol (red), hexane (blue), and dichloromethane (magenta).
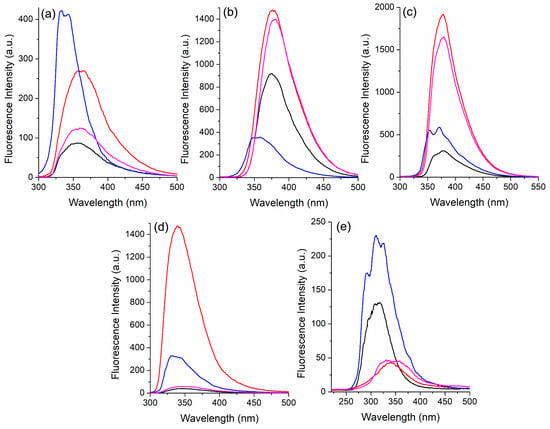
Figure 4.
Steady-state fluorescence emission spectra of the pyridine-based fluorophores (a) 3a, (b) 3d, (c) 3e, (d) 3f, and (e) 3j in solution [~10−5 M] in benzene (black), ethanol (red), hexane (blue), and dichloromethane (magenta).

Table 1.
Photophysical data of pyridine-based fluorophores 3a–3k in solution, where λabs and λem are the absorption and emission maxima (nm), respectively, ε is the molar extinction coefficient (M−1·cm−1), and ΔλST is the Stokes shift (nm/cm−1).
The fluorescence emission spectra of the selected compounds are presented in Figure 4. The relevant data are also summarized in Table 1. The spectra were acquired using the absorption maxima as excitation wavelengths. The compounds present fluorescence emission between 300 and 450 nm, with maxima depending on their electronic structure, as well as the environment polarity. Although the ground-state results do not present very significant photophysical differences between the fluorophores and studied solvents, these compounds in the excited state show a very interesting behavior. Once again, the other derivatives presented similar photophysical behavior (Figures S23–S28).
A significant variation in the maxima location could be observed as a function of the polarity of the medium, especially when comparing the same fluorophore. For instance, in hexane the emission maxima lie with higher energies. On the other hand, in highly polar solvents, the emission maxima shift to longer wavelengths (positive solvatochromism), suggesting that these compounds are more polar in the excited state. Moreover, in benzene and dichloromethane, these values tend to be similar, indicating that regardless of the lower polarity of benzene, more effective interaction with this solvent compensates for the higher polarity of dichloromethane. Finally, in ethanol, a solvent that exhibits more specific and strong interactions such as hydrogen bonding with fluorophores, the emission maxima values are also shifted towards the red, as expected. An exception to these observations is found in compound 3j, probably due to its lower π-conjugation provided by the absence of the phenyl linked to the double bond. In this case, no clear tendency is observed between the location of the emission maxima toward the solvent, indicating a diverse polarity of this derivative in comparison with its analogs. A relatively large Stokes shift indicates significant energy loss in the excited state. Based on these results, we can conclude that the electronic structure of these fluorophores, which is relatively simple, affects their photophysical properties in the excited state.
We would like to highlight that the most significant photophysical behavior in these compounds was the relative emission intensity as a function of the solvent. In ethanol, it was observed that the fluorescence intensity was 2-fold (3d), 3-fold (3a–3c, and 3k), 5-6-fold (3e, and 3g–3h), and even 39-fold (3f) higher if compared to the intensity in benzene. Similarly, the fluorescence intensities change depending on whether ethanol or hexane are used. Intensities of 2-fold (3g and 3h), 3-fold (3e and 3i), 4-fold (3d), and 5-fold (3f) higher were also observed in ethanol if compared to hexane. On the contrary, intensities of 2-fold (3a–3b, and 3k) and 5-fold (3j) higher were also observed in hexane if compared to ethanol. These observations allowed us to investigate some selected compounds for optical sensing. Benzene is recognized as one of the contaminants with the clearest evidence of carcinogenicity [68]. This compound is classified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer [69], and as highlighted by Lachenmeier et al., since the early 1990s, concerns about benzene contamination of food have been raised. In this way, since this compound can be found in processed foods and beverages [70,71], its detection is worth investigating.
Regarding the spectrophotometric titrations in ethanol, upon the addition of benzene, in general, the fluorophores presented fluorescence quenching (Figures S29–S34). However, as presented in Figure 4, three fluorophores (3c, 3e, and 3i) showed a linear correlation between the emission intensity and the amount of benzene (quencher). Fluorophores 3c and 3i presented linearity in the studied concentration range of benzene (0–80% benzene), with R2 values around 0.993 (3c) and 0.989 (3i). It is worth mentioning that compound 3e showed two linear correlations, the first in the range of 10 to 45% of added benzene (R2 = 0.991) and the second in the range of 30 to 80% of benzene (R2 = 0.985). Based on this behavior, the respective Stern–Volmer quenching constants (KSV) were calculated from the above titration experiments [72]. In this proposal, since the fluorophore concentration was constant in all mixtures, the benzene molecule was taken as the quencher. From the linear Stern–Volmer plots (Figure 5, bottom), the KSV constants were obtained as the slope of the linear plot with values around 0.05 M−1 (Table 2). In addition, the respective bimolecular quenching rate constants (kq = KSV/τ0) were obtained, using a fluorescence lifetime of benzene in ethanol of around 10 ns [73]. Values around 5 × 106 M−1·s−1 were obtained, which are three orders of magnitude smaller than the diffusion rate constants (kdiff ~109 M−1·s−1), according to the Smoluchowski–Stokes–Einstein theory [74], suggesting a dynamic mechanism (collisional quenching). Finally, the respective limits of detection (LOD) were also calculated.
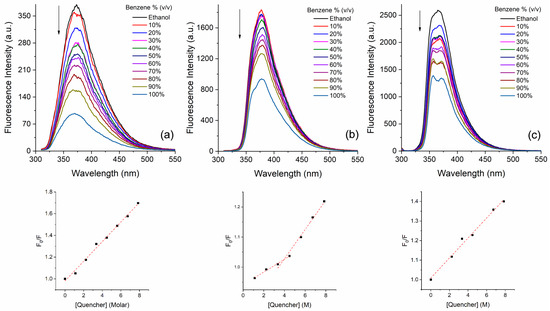
Figure 5.
Steady-state fluorescence emission titration of fluorophores (a) 3c, (b) 3e, and (c) 3i in ethanol upon different amounts of benzene (v/v). Below are presented the respective plots of F0/F vs. [quencher], according to Stern–Volmer Equation.

Table 2.
Quenching constants and detection limits of fluorophores 3c, 3e, and 3i.
On the other hand, fuel adulteration, particularly of commercial gasoline, is one of the major illegal practices nowadays involving the addition of organic solvents or alcohol in different amounts than those recommended by the legislation [22]. Again, in this specific issue, it is desirable to develop sensors for fuel-quality monitoring. As already observed in this investigation, the good solubility of the fluorophores in hexane, as well as their tailored intensity in this solvent and ethanol, allowed us to explore their ability to sense gasoline. This application is particularly interesting in our country since Brazilian gasoline presents 27% of anhydrous ethanol for standard and 25% for premium types of gasoline [76]. In this way, fluorophore 3f was chosen as a model, and its photophysical behavior was first investigated in real samples of commercial gasoline and anhydrous ethanol, as shown in Figure 6a. It can be observed that 3f presented a similar behavior if compared to hexane/ethanol, with increased emission in anhydrous ethanol (4-fold) if compared to commercial common gasoline (Figure 6a).
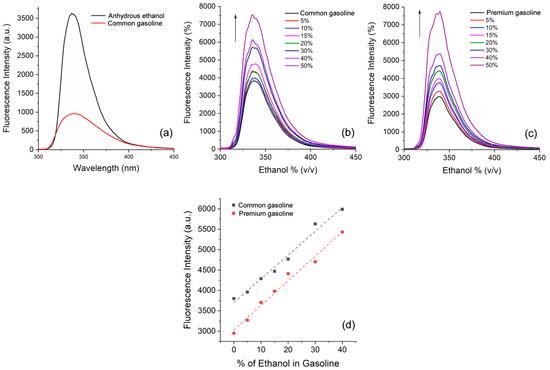
Figure 6.
(a) Steady-state fluorescence emission spectra of 3f in anhydrous ethanol and commercial standard gasoline [~10−5 M]. Fluorescence titration of 3f in commercial (b) standard and (c) premium types of gasoline upon different amounts of anhydrous ethanol. (d) Linear correlation between fluorescence intensity vs. (%) ethanol for 3f in commercial standard gasoline (R2 = 0.984) and premium gasoline (R2 = 0.985).
Fluorescence titrations of anhydrous ethanol in commercial standard (Figure 6b) and premium (Figure 6c) types of gasoline showed that by adding ethanol into the gasoline, the fluorescence emission intensity increases, as expected. Dilution effects were excluded since the addition of the respective gasoline into the initial samples (control experiment) led to different results, where there was no observed increase in fluorescence. These preliminary results corroborate the potential application of this fluorophore as fuel optical sensors for adulteration by the addition of ethanol. In addition, in both gasoline samples it was possible to observe a linear correlation in the range of 0–40% of added anhydrous ethanol with limits of detection (LOD) of ~4% for both commercial standard and premium gasoline. This latter seems to be an interesting result since adulterated gasoline presents in general a higher percentage of anhydrous ethanol than recommended by the Brazilian legislation.
3.3. Theoretical Calculations
As stated in the “computational details” section, the lowest conformers were obtained from a conformational sampling and were reoptimized using the density functional theory with implicit benzene and ethanol solvation. The change in the solvation environment does not significantly affect the resulting optimized geometries since the ground states obtained for the studied compounds have negligible differences under benzene and ethanol, with root-mean-square deviations (RMSD) of only ~0.04 Å. In addition, the resulting geometries have nearly identical frontier orbital features under benzene and ethanol, with immediately distinguishable π-conjugation spreading linearly through the triple bond over the molecule and a much smaller electronic probability density spreading to the lateral substituents (Figure 7). The HOMO–LUMO gaps under CPCM are also quite similar, with ethanol affording a slightly greater stabilization and, consequently, a larger H–L gap by about 0.03 eV when compared to benzene, as depicted in Figure 7.
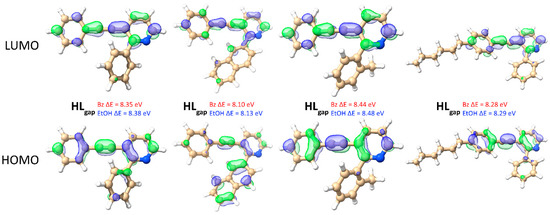
Figure 7.
Optimized geometries for 3a, 3e, 3f, and 3j. Since the geometries using CPCM to simulate benzene and ethanol are virtually identical, only the benzene results are shown. Isosurface = 0.05 e/Å3.
When optimized to the first excited state (S1), under CPCM, the geometric results remained mostly solvent-agnostic with benzene and ethanol generating compatible displacements. Compound 3a optimized to S1 experienced only a small displacement in benzene, showing RMSD = 0.27 Å (ethanol = 0.32 Å) compared to S0. Compound 3e afforded S1 in benzene with RMSD equal to 0.55 Å (ethanol = 0.49 Å). Additionally, for compound 3f an RMSD equal to 0.45 Å (ethanol = 0.52 Å) was found, while compound 3j showed the smallest displacements of the series with an RMSD equal to 0.26 Å (ethanol = 0.28 Å), as summarized in Figure 8.
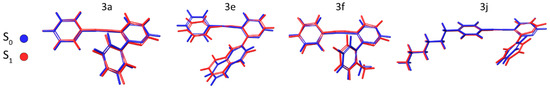
Figure 8.
Superposition of S0 (blue) and S1 (red) optimized geometries for 3a, 3e, 3f, and 3j. The results depicted are using implicit benzene solvation.
The first electronic transition, under CPCM, follows the same trend for all the studied molecules: a higher than 90% HOMO→LUMO (π→π*) participation that largely involves the electron-rich triple bond acting as a hole (electron donor) in a hole–particle system depicted by electronic density difference (Figure 9, top). The repetitive behavior described by implicit solvation with CPCM does not match the diversity of results observed in our experimental results. These discrepancies underline the shortcomings of using implicit solvation models [64] and the fact that the continuum scheme cannot correctly describe important non-covalent interactions such as π-stackings [77] that may be active in a real chemical environment.
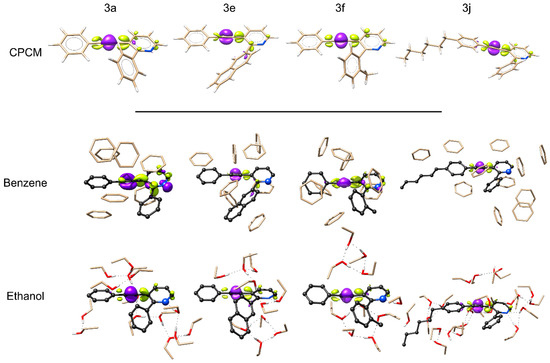
Figure 9.
Electronic density difference (EDD) for 3a, 3e, 3f, and 3j. Above, under implicit CPCM (benzene) solvation. Below, the target molecules are represented in dark gray with explicit solvent molecules. Purple density identifies electronic density lost and yellow electronic density gained.
Since the theoretical study of an emission spectra using explicit solvent molecules is computationally too demanding, even on a supercomputer, as the systems easily reach hundreds of atoms, a crude test simulating emission spectra for 3f and 3j using a simple vertical gradient approach [78] with the CPCM method is unable to reproduce, even qualitatively, the emission intensity experimentally observed. The test wrongly generates approximately the same intensity for 3f and 3j in both solvation schemes (Figures S35), an indication that the model is missing important components, probably from the absence of intermolecular interactions. Even though the simulation of emission spectra with explicit solvation is prohibitive due to computational costs, a nanocluster for absorption study with an arbitrary number of explicit solvent molecules was built using the CREST software and the procedure for optimization of non-covalent interactions. The new models, despite being quite large, were calculated using analytic TDDFT to identify the different effects using explicit solvation with intermolecular interactions on the first electronic transition. As depicted in Figure 8, the addition of intermolecular interactions shows an immediate impact on the electronic density difference for all systems, with a visually more remarkable change in molecule 3a with benzene, in which the charge transfer to the left side of the molecule appears inhibited while the right side sees an increase in electronic density.
4. Conclusions
In conclusion, a new and efficient protocol was successfully developed for the synthesis of 2-aryl-3-(organylethynyl)pyridines. The reaction showed high selectivity leading to the formation of eleven derivatives with good to moderate yields (55–91%); of these, six derivatives were unpublished in the literature. It is worth noting that these small molecules are easily prepared, expanding the scope of this class of N-derivatives of great synthetic relevance and wide photophysical potential. The photophysical characterization of these compounds showed that the solvent seems to affect their excite-state deactivation. Based on these results, the photophysical properties of these compounds were evaluated in ethanol with different amounts of benzene, where a linear correlation between the emission intensity and the amount of benzene (quencher) was obtained. In this investigation, the observed fluorescence quenching could be related to a dynamic mechanism (collisional quenching). These compounds were also investigated in real samples, using commercial standard and premium gasoline with different amounts of anhydrous ethanol. The studied compounds showed, in both gasoline samples, a linear correlation in the range of 0–40% of added ethanol. This latter seems to be an interesting result since adulterated gasoline presents in general a higher percentage of anhydrous ethanol than that recommended by the Brazilian legislation. Theoretical calculations using TDDFT showed that while the implicit conductor-like polarizable continuum model was unable to reproduce the experimental solvent effects, there is strong evidence that intermolecular interactions are preponderant factors that explain the experimental diversity on the emission spectra.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photochem3010008/s1, Figures S1–S35: Spectroscopic characterization of compounds 3a to 3k, additional photophysical data and additional theoretical data.
Author Contributions
Conceptualization, P.H.S. and F.S.R.; methodology, P.H.S. and F.S.R.; validation, P.H.S. and F.S.R.; investigation, T.J.P., M.M.V., N.B.P., B.T.D. and H.d.C.S.J.; resources, P.H.S. and F.S.R.; writing—original draft preparation, T.J.P., M.M.V., F.S.R., H.d.C.S.J. and P.H.S.; writing—review and editing, P.H.S. and F.S.R.; visualization, P.H.S.; supervision, P.H.S. and F.S.R.; project administration, P.H.S.; funding acquisition, P.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPERGS (PRONEX and RITEs-RS), CNPq (404503/2021-7, 308487/2021-4, and 163912/2020-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and INCT-CNM for the financial support.
Acknowledgments
Theoretical calculations were performed using the Lobo Carneiro supercomputer from Núcleo Avançado de Computação de Alto Desemprenho (NACAD), under the Project ID a20006 and the Sagarana Cluster from CEPAD—Centro de Processamento de Alto Desempenho ICB/UFMG. The authors would also like to thank the National Laboratory for Scientific Computing (LNCC/MCTI, Brazil) for providing HPC resources of the SDumont supercomputer, which have contributed to the research results reported in this paper. Available online: http://sdumont.lncc.br (accessed on 16 February 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalligeros, S.; Zannikos, F.; Stournas, S.; Lois, E. Fuel adulteration issues in Greece. Energy 2003, 28, 15–26. [Google Scholar] [CrossRef]
- Obeidat, S.M.; Al-Ktash, M.M.; Al-Momani, I.F. Study of Fuel Assessment and Adulteration Using EEMF and Multiway PCA. Energy Fuels 2014, 28, 4889–4894. [Google Scholar] [CrossRef]
- Mendes, G.; Barbeira, P.J.S. Detection and quantification of adulterants in gasoline using distillation curves and multivariate methods. Fuel 2013, 112, 163–171. [Google Scholar] [CrossRef]
- Barbeira, P.J.S.; Pereira, R.C.C.; Corgozinho, C.N.C. Identification of Gasoline Origin by Physical and Chemical Properties and Multivariate Analysis. Energy Fuels 2007, 21, 2212–2215. [Google Scholar] [CrossRef]
- Zanelli, A.; Bassini, S.; Giorgetti, M.; Li, Y.; Yang, M.J. Chemiresistors for ethanol detection in hydrocarbons. Sens. Actuators B Chem. 2010, 148, 147–152. [Google Scholar] [CrossRef]
- Wiziack, N.K.L.; Catini, A.; Santonico, M.; D’Amico, A.; Paolesse, R.; Paterno, L.G.; Fonseca, F.J.; Di Natale, C. A sensor array based on mass and capacitance transducers for the detection of adulterated gasolines. Sens. Actuators B Chem. 2009, 140, 508–513. [Google Scholar] [CrossRef]
- Benvenho, A.R.V.; Li, R.W.C.; Gruber, J. Polymeric electronic gas sensor for determining alcohol content in automotive fuels. Sens. Actuators B Chem. 2009, 136, 173–176. [Google Scholar] [CrossRef]
- Eaidkong, T.; Mungkarndee, R.; Phollookin, C.; Tumcharern, G.; Sukwattanasinitt, M.; Wacharasindhu, S. Polydiacetylene paper-based colorimetric sensor array for vapor phase detection and identification of volatile organic compounds. J. Mater. Chem. 2012, 22, 5970–5977. [Google Scholar] [CrossRef]
- Suklabaidya, S.; Chakraborty, S.; Sarkar, S.; Paul, R.; Banik, H.; Chakraborty, A.; Bhattacharjee, D.; Majumdar, S.; Hussain, S.A. Polydiacetylene-N-1-hexadecyl Imidazole Mixed Film and its Application Toward the Sensing of Volatile Organic Compounds, Gasoline, and Pollution Level in Car Exhaust. J. Phys. Chem. C 2021, 125, 15976–15986. [Google Scholar] [CrossRef]
- Lee, J.; Balakrishnan, S.; Cho, J.; Jeon, S.-H.; Kim, J.-M. Detection of adulterated gasoline using colorimetric organic microfibers. J. Mater. Chem. 2011, 21, 2648–2655. [Google Scholar] [CrossRef]
- Aristilde, S.; Cordeiro, C.M.B.; Osório, J.H. Gasoline Quality Sensor Based on Tilted Fiber Bragg Gratings. Photonics 2019, 6, 51. [Google Scholar] [CrossRef]
- Nishikawa, M.; Murata, T.; Ishihara, S.; Shiba, K.; Shrestha, L.K.; Yoshikawa, G.; Minami, K.; Ariga, K. Discrimination of Methanol from Ethanol in Gasoline Using a Membrane-type Surface Stress Sensor Coated with Copper(I) Complex. Bull. Chem. Soc. Jpn. 2021, 94, 648–654. [Google Scholar] [CrossRef]
- Regmi, B.P.; Adhikari, P.L.; Dangi, B.B. Ionic Liquid-Based Quartz Crystal Microbalance Sensors for Organic Vapors: A Tutorial Review. Chemosensors 2021, 9, 194. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; Vaughan, S.; Speller, L.N.; Warner, I.M. QCM virtual multisensor array for fuel discrimination and detection of gasoline adulteration. Fuel 2017, 199, 38–46. [Google Scholar] [CrossRef]
- Koshets, I.A.; Kazantseva, Z.I.; Shirshov, Y.M.; Cherenok, S.A.; Kalchenko, V.I. Calixarene films as sensitive coatings for QCM-based gas sensors. Sens. Actuators B Chem. 2005, 106, 177–181. [Google Scholar] [CrossRef]
- Nowak, A.V. Method for Determining Adulteration of Gasolines. U.S. Patent 5358873, 27 July 1992. [Google Scholar]
- Albert, B.; Kipper, J.; Vamvakaris, C.; Beck, K.H.; Wagenblast, G. Use of Compounds Which Absorb and/or Fluoresce in the IR Region as Markers for Liquids. U.S. Patent 5998211A, 23 May 2017. [Google Scholar]
- Rodembusch, F.S.; Duarte, R.C. Sensor Óptico Para a Detecção de Adulteração de Gasolina, Processo de Produção de Soluções Contendo um Sensor Óptico, Método de Detecção de Adulteração de Gasolina Automotiva Comum e Uso de um Corante Orgânico Derivado de Heptameteno Cianinas. Patent BR1020170107396, 31 May 2004. [Google Scholar]
- Krutak, J.J.; Cushman, M.R.; Weaver, M.A. Method for Tagging Petroleum Products. Patent WO1996010620A1, 30 September 1994. [Google Scholar]
- Baxter, D.R.; Cranmer, P.J.; Ho, K.S. Método Para Marcação de um Hidrocarboneto Líquido de Petróleo. Patent BR200305751, 31 May 2004. [Google Scholar]
- Gotor, R.; Tiebe, C.; Schlischka, J.; Bell, J.; Rurack, K. Detection of Adulterated Diesel Using Fluorescent Test Strips and Smartphone Readout. Energy Fuels 2017, 31, 11594–11600. [Google Scholar] [CrossRef]
- Isoppo, V.G.; Gil, E.S.; Gonçalves, P.F.B.; Rodembusch, F.S.; Moro, A.V. Highly fluorescent lipophilic 2,1,3-benzothiadiazole fluorophores as optical sensors for tagging material and gasoline adulteration with ethanol. Sens. Actuators B Chem. 2020, 309, 127701. [Google Scholar] [CrossRef]
- Gotor, R.; Bell, J.; Rurack, K. Tailored fluorescent solvatochromic test strips for quantitative on-site detection of gasoline fuel adulteration. J. Mater. Chem. C 2019, 7, 2250–2256. [Google Scholar] [CrossRef]
- Mineo, P.G.; Vento, F.; Abbadessa, A.; Scamporrino, E.; Nicosia, A. An optical sensor of acidity in fuels based on a porphyrin derivative. Dyes Pigment. 2019, 161, 147–154. [Google Scholar] [CrossRef]
- Huang, Y.; He, J.; Qin, T.; Xiang, X.; Liu, B.; Wang, L. Fluorescence Determination of Ethanol-Gasoline Blends without the Aid of Excitation-Emission Matrix Fluorescence. Chem. Lett. 2019, 48, 1383–1386. [Google Scholar] [CrossRef]
- Düwel, I.; Schorr, J.; Peuser, P.; Zeller, P.; Wolfrum, J.; Schulz, C. Spray Diagnostics Using an All-Solid-State Nd:YAlO3 Laser and Fluorescence Tracers in Commercial Gasoline and Diesel Fuels. Appl. Phys. B 2004, 79, 249–254. [Google Scholar] [CrossRef]
- Galgano, P.D.; Loffredo, C.; Sato, B.M.; Reichardt, C.; El Seoud, O.A. Introducing education for sustainable development in the undergraduate laboratory: Quantitative analysis of bioethanol fuel and its blends with gasoline by using solvatochromic dyes. Chem. Educ. Res. Pract. 2012, 13, 147–153. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, A.K. Quantification of ethanol in petrol–ethanol blends: Use of Reichardt’s ET(30) dye in introducing a petrol batch independent calibration procedure. Talanta 2012, 100, 414–418. [Google Scholar] [CrossRef]
- Budag, R.; Giusti, L.A.; Machado, V.G.; Machado, C. Quality analysis of automotive fuel using solvatochromic probes. Fuel 2006, 85, 1494–1497. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Maiti, U.N.; Lee, W.J.; Lee, J.M.; Oh, Y.; Kim, J.Y.; Kim, J.E.; Shim, J.; Han, T.H.; Kim, S.O. 25th Anniversary Article: Chemically Modified/Doped Carbon Nanotubes & Graphene for Optimized Nanostructures & Nanodevices. Adv. Mater. 2014, 26, 40–67. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Qian, G.; Wang, Z.Y. Near-Infrared Organic Compounds and Emerging Applications. Chem. Asian J. 2010, 5, 1006–1029. [Google Scholar] [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, J.; Li, S.; Wen, X.; Yu, T.; Lu, Y.; Xiong, X.; Liu, Y.; Xiong, X. Fluorescent difference between two rhodamine-PAHs polystyrene solid-phase sensors for Hg(II) detection based on crystal structure and density functional theory calculation. Spectrochim. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118277. [Google Scholar] [CrossRef]
- Roncali, J. Synthetic Principles for Bandgap Control in Linear π-Conjugated Systems. Chem. Rev. 1997, 97, 173–206. [Google Scholar] [CrossRef] [PubMed]
- Bronner, C.; Stremlau, S.; Gille, M.; Brauße, F.; Haase, A.; Hecht, S.; Tegeder, P. Aligning the Band Gap of Graphene Nanoribbons by Monomer Doping. Angew. Chem. Int. Ed. 2013, 52, 4422–4425. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q. Ten Years of N-Heteropentacenes as Semiconductors for Organic Thin-Film Transistors. Adv. Mater. 2014, 26, 5541–5549. [Google Scholar] [CrossRef] [PubMed]
- Verbitskiy, E.V.; Steparuk, A.S.; Zhilina, E.F.; Emets, V.V.; Grinberg, V.A.; Krivogina, E.V.; Kozyukhin, S.A.; Belova, E.V.; Lazarenko, P.I.; Rusinov, G.L.; et al. Pyrimidine-Based Push–Pull Systems with a New Anchoring Amide Group for Dye-Sensitized Solar Cells. Electron. Mater. 2021, 2, 142–153. [Google Scholar] [CrossRef]
- Ding, X.; Wang, H.; Chen, C.; Li, H.; Tian, Y.; Li, Q.; Wu, C.; Ding, L.; Yang, X.; Cheng, M. Passivation functionalized phenothiazine-based hole transport material for highly efficient perovskite solar cell with efficiency exceeding 22%. Chem. Eng. J. 2021, 410, 128328. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Azines as unconventional anchoring groups for dye-sensitized solar cells: The first decade of research advances and a future outlook. Dyes Pigment. 2021, 194, 109650. [Google Scholar] [CrossRef]
- Li, W.; Shen, C.; Wu, Z.; Wang, Y.; Ma, D.; Wu, Y. Pyridine functionalized phenothiazine derivatives as low-cost and stable hole-transporting material for perovskite solar cells. Mater. Today Energy 2022, 23, 100903. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of fluorescent sensors based on azaheterocyclic push-pull systems towards nitroaromatic explosives and related compounds: A review. Dyes Pigment. 2020, 180, 108414. [Google Scholar] [CrossRef]
- Dhiman, S.; Singla, N.; Ahmad, M.; Singh, P.; Kumar, S. Protonation- and electrostatic-interaction-based fluorescence probes for the selective detection of picric acid (2,4,6-trinitrophenol)–an explosive material. Mater. Adv. 2021, 2, 6466–6498. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A.; Wang, J.L. Developing Efficient Small Molecule Acceptors with sp2-Hybridized Nitrogen at Different Positions by Density Functional Theory Calculations, Molecular Dynamics Simulations and Machine Learning. Chem. Eur. J. 2022, 28, e202103712. [Google Scholar] [CrossRef]
- Motoyama, M.; Doan, T.-H.; Hibner-Kulicka, B.; Otake, R.; Lukarska, M.; Lohier, J.-F.; Ozawa, K.; Nanbu, S.; Alayrac, C.; Suzuki, Y.; et al. Synthesis and Structure-Photophysics Evaluation of 2-N-amino-quinazolines: Small Molecule Fluorophores for Solution and Solid State. Chem. Asian J. 2021, 16, 2087–2099. [Google Scholar] [CrossRef]
- De Salles, H.D.; Coelho, F.L.; Paixão, D.B.; Barboza, C.A.; Rampon, D.D.S.; Rodembusch, F.S.; Schneider, P.H. Evidence of a Photoinduced Electron-Transfer Mechanism in the Fluorescence Self-quenching of 2,5-Substituted Selenophenes Prepared through In Situ Reduction of Elemental Selenium in Superbasic Media. J. Org. Chem. 2021, 86, 10140–10153. [Google Scholar] [CrossRef] [PubMed]
- Radatz, C.S.; Coelho, F.L.; Gil, E.S.; Santos, F.D.S.; Schneider, J.M.F.M.; Gonçalves, P.F.B.; Rodembusch, F.; Schneider, P.H. Ground and excited-state properties of 1,3-benzoselenazole derivatives: A combined theoretical and experimental photophysical investigation. J. Mol. Struct. 2020, 1207, 127817. [Google Scholar] [CrossRef]
- Rampon, D.S.; Rodembusch, F.S.; Gonçalves, P.F.B.; Lourega, R.V.; Merlo, A.A.; Schneider, P.H. An evaluation of the chalcogen atom effect on the mesomorphic and electronic properties in a new homologous series of chalcogeno esters. J. Braz. Chem. Soc. 2010, 21, 2100–2107. [Google Scholar] [CrossRef]
- Peglow, T.J.; Martins, C.C.; da Motta, K.P.; Luchese, C.; Wilhelm, E.A.; Stieler, R.; Schneider, P.H. Synthesis and biological evaluation of 5-chalcogenyl-benzo[h]quinolines via photocyclization of arylethynylpyridine derivatives. New J. Chem. 2022, 46, 23030–23038. [Google Scholar] [CrossRef]
- Armarego, W.L.F. Purification of Laboratory Chemicals, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Peglow, T.J.; Bartz, R.H.; Martins, C.C.; Belladona, A.L.; Luchese, C.; Wilhelm, E.A.; Schumacher, R.F.; Perin, G. Synthesis of 2-Organylchalcogenopheno [2,3-b]pyridines from Elemental Chalcogen and NaBH4/PEG-400 as a Reducing System: Antioxidant and Antinociceptive Properties. ChemMedChem 2020, 15, 1741–1751. [Google Scholar] [CrossRef]
- Peglow, T.J.; Bartz, R.H.; Barcellos, T.; Schumacher, R.F.; Cargnelutti, R.; Perin, G. Synthesis of 2-Aryl-(3-organochalcogenyl)thieno [2,3-b] pyridines Promoted by Oxone®. Asian J. Org. Chem. 2021, 10, 1198–1206. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Begouin, A.; Peixoto, D. Regiocontrolled SNAr Reaction on 2,3-Dihalopyridines with NaSMe To Obtain Bromo(methylthio)pyridines as Key Precursors of 3-Halo-2-(hetero)arylthieno[2,3-b]pyridines and Thieno[3,2-b]pyridines. Synthesis 2013, 45, 1489–1496. [Google Scholar] [CrossRef]
- Peixoto, D.; Begouin, A.; Queiroz, M.-J.R. Synthesis of 2- arylthieno[2,3-b] [2,3-b] or [3,2-b]pyridines from 2,3-dihalopyridines, (hetero)arylalkynes, and Na2S. Further functionalizations. Tetrahedron 2012, 68, 7082–7094. [Google Scholar] [CrossRef]
- Molenda, R.; Boldt, S.; Villinger, A.; Ehlers, P.; Langer, P. Synthesis of 2-Azapyrenes and Their Photophysical and Electrochemical Properties. J. Org. Chem. 2020, 85, 12823–12842. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Li, G.-D.; Mao, S.-P.; Chai, J.-D. Long-Range Corrected Hybrid Density Functionals with Improved Dispersion Corrections. J. Chem. Theory Comput. 2013, 9, 263–272. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Takano, Y.; Houk, K.N. Benchmarking the Conductor-like Polarizable Continuum Model (CPCM) for Aqueous Solvation Free Energies of Neutral and Ionic Organic Molecules. J. Chem. Theory Comput. 2005, 1, 70–77. [Google Scholar] [CrossRef]
- Hirata, S.; Head-Gordon, M. Time-dependent density functional theory within the Tamm–Dancoff approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Shibata, T.; Takayasu, S.; Yuzawa, S.; Otani, T. Rh(III)-Catalyzed C-H Bond Activation Along with “Rollover” for the Synthesis of 4-Azafluorenes. Org. Lett. 2012, 14, 5106–5109. [Google Scholar] [CrossRef]
- Shestakov, A.N.; Pankova, A.S.; Kuznetsov, M.A. Cycloisomerization–a straightforward way to benzo[h]quinolines and benzo[c]acridines. Chem. Heterocycl. Compd. 2017, 53, 1103–1113. [Google Scholar] [CrossRef]
- Smith, M.T. Advances in Understanding Benzene Health Effects and Susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Reusch, H.; Sproll, C.; Schoeberl, K.; Kuballa, T. Occurrence of benzene as a heat-induced contaminant of carrot juice for babies in a general survey of beverages. Food Addit. Contam. Part A 2008, 25, 1216–1224. [Google Scholar] [CrossRef]
- Dos Santos, V.P.S.; Salgado, A.M.; Torres, A.G.; Pereira, K.S. Benzene as a Chemical Hazard in Processed Foods. Int. J. Food Sci. 2015, 2015, 545640. [Google Scholar] [CrossRef] [PubMed]
- Sadighara, P.; Pirhadi, M.; Sadighara, M.; Shavaly-Gilani, P.; Zirak, M.R.; Zeinali, T. Benzene food exposure and their prevent methods: A review. Nutr. Food Sci. 2022, 52, 971–979. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Luria, M.; Ofran, M.; Stein, G. Natural and experimental fluorescence lifetimes of benzene in various solvents. J. Phys. Chem. 1974, 78, 1904–1909. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- How to Determine the LOD Using the Calibration Curve? Available online: https://mpl.loesungsfabrik.de/en/english-blog/method-validation/calibration-line-procedure (accessed on 9 February 2023).
- Mistura Carburante (Etanol Anidro-Gasolina) Cronologia. Available online: http://www.agricultura.gov.br/assuntos/sustentabilidade/agroenergia/arquivos/cronologia-da-mistura-carburante-etanol-anidro-gasolina-no-brasil.pdf (accessed on 11 January 2023).
- Ho, J.; Ertem, M.Z. Calculating Free Energy Changes in Continuum Solvation Models. J. Phys. Chem. B 2016, 120, 1319–1329. [Google Scholar] [CrossRef]
- De Souza, B.; Neese, F.; Izsák, R. On the theoretical prediction of fluorescence rates from first principles using the path integral approach. J. Chem. Phys. 2018, 148, 034104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).