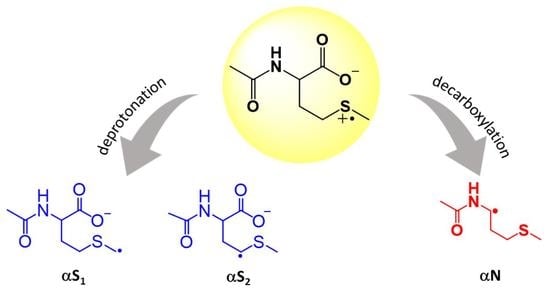
The Fate of Sulfur Radical Cation of N-Acetyl-Methionine: Deprotonation vs. Decarboxylation
Abstract
1. Introduction
2. Experimental Section
2.1. Laser Flash Photolysis (LFP)
2.2. Steady-State Photolysis
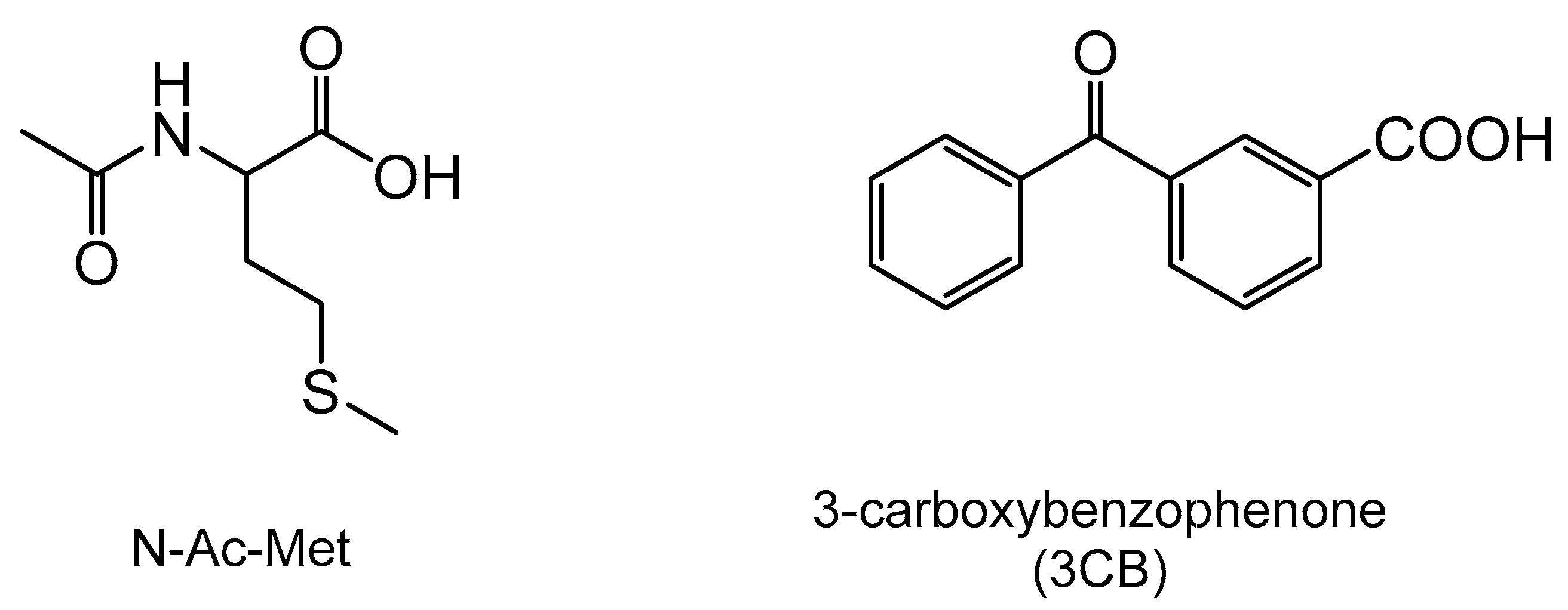
2.3. Chemicals and Sample Preparation
2.4. High Performance Liquid Chromatography (HPLC)
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS)
3. Results
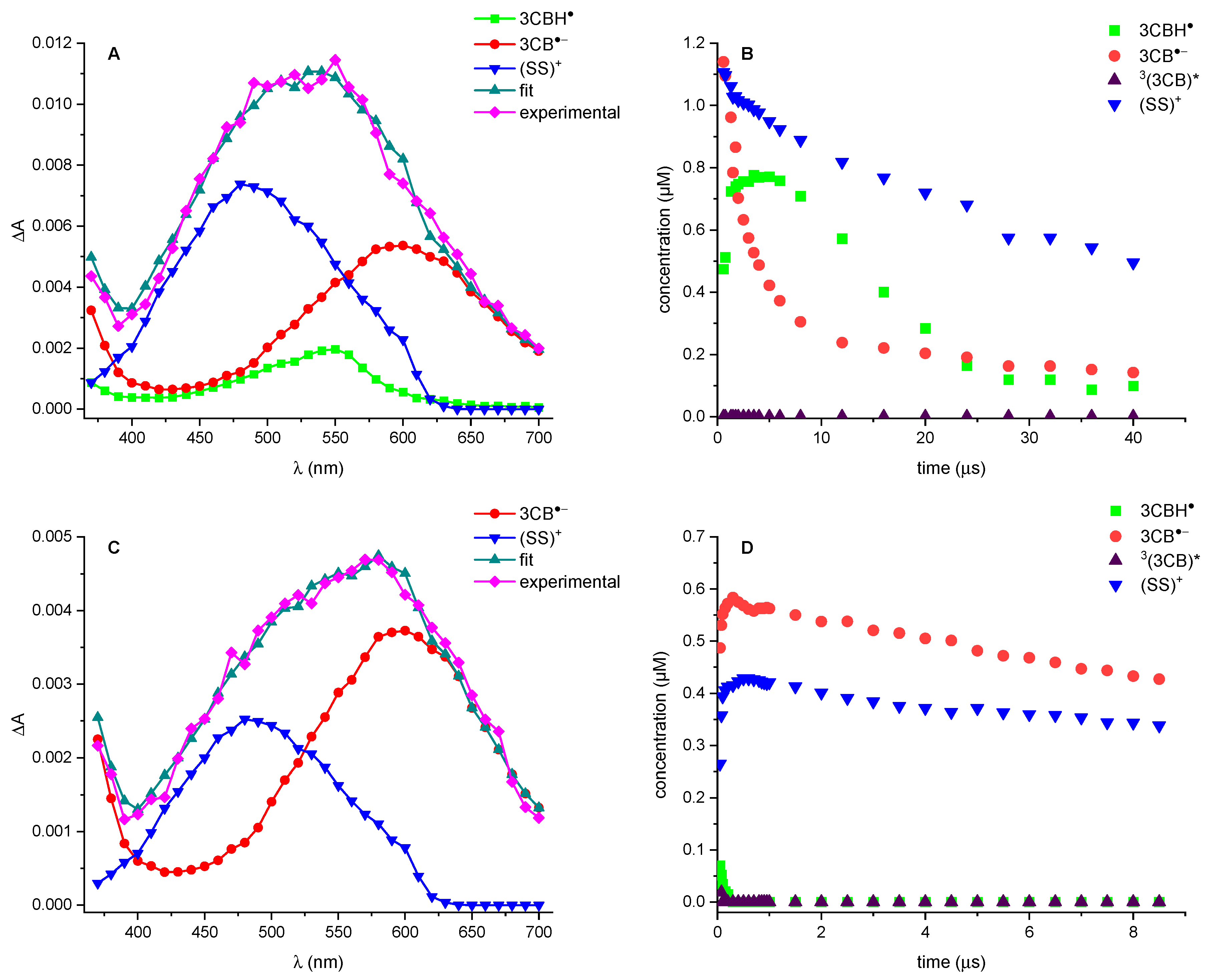
3.1. Laser Flash Photolysis
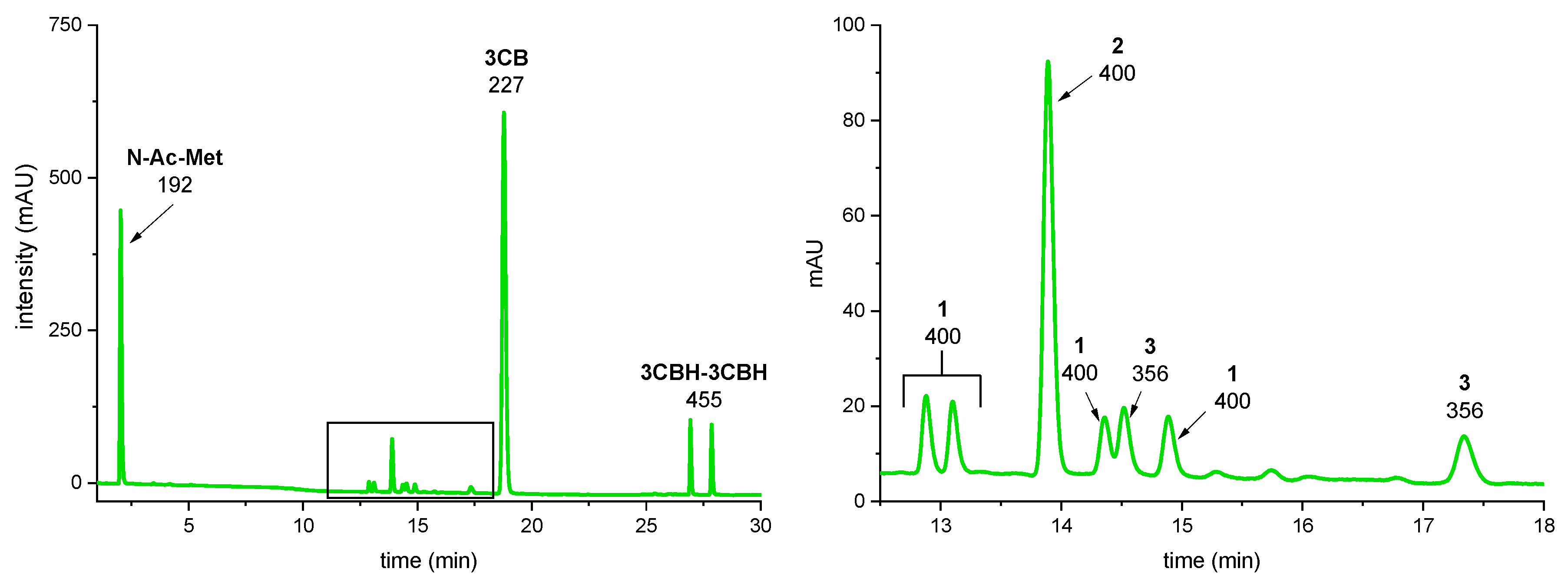
3.2. LC-MS/MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berges, J.; Trouillas, P.; Houée-Levin, C. Oxidation of protein tyrosine or methionine residues: From the amino acid to the peptide. J. Phys. Conf. Ser. 2011, 261, 012003. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Shimonaga, M.; Muraoka, Y.; Kobayashi, M.; Nozawa, T. Nozawa, Methionine oxidation and its effect on the stability of a reconstituted subunit of the light-harvesting complex from Rhodospirillum rubrum: Methionine oxidation and its effect on LH1 subunit. JBIC J. Biol. Inorg. Chem. 2001, 268, 3375–3382. [Google Scholar] [CrossRef]
- Schöneich, C.; Bobrowski, K.; Holcman, J.; Asmus, K.-D. Oxidation mechanisms of methionine containing peptides by hydroxyl and peroxyl radicals. In Oxidative Damage Repair; Elsevier: Amsterdam, The Netherlands, 1991; pp. 380–385. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Fändrich, M. Protein aggregation in Alzheimer’s disease: Aβ and τ and their potential roles in the pathogenesis of AD. Acta Neuropathol. 2015, 129, 163–165. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Sultana, R. Methionine-35 of Aβ(1–42): Importance for Oxidative Stress in Alzheimer Disease. J. Amino Acids 2011, 2011, 198430. [Google Scholar] [CrossRef]
- Schöneich, C. Redox Processes of Methionine Relevant to β-Amyloid Oxidation and Alzheimer’s Disease. Arch. Biochem. Biophys. 2002, 397, 370–376. [Google Scholar] [CrossRef]
- Ignasiak, M.T.; Marciniak, B.; Houée-Levin, C. A Long Story of Sensitized One-Electron Photo-oxidation of Methionine. Isr. J. Chem. 2014, 54, 248–253. [Google Scholar] [CrossRef]
- Bobrowski, K.; Marciniak, B.; Hug, G.L. 4-Carboxybenzophenone-sensitized photooxidation of sulfur-containing amino acids. Nanosecond laser flash photolysis and pulse radiolysis studies. J. Am. Chem. Soc. 1992, 114, 10279–10288. [Google Scholar] [CrossRef]
- Bobrowski, K.; Houée-Levin, C.; Marciniak, B. Stabilization and Reactions of Sulfur Radical Cations: Relevance to One-Electron Oxidation of Methionine in Peptides and Proteins. Chimia 2008, 62, 728. [Google Scholar] [CrossRef]
- Pedzinski, T.; Bobrowski, K.; Ignasiak, M.; Kciuk, G.; Hug, G.L.; Lewandowska-Andralojc, A.; Marciniak, B. 3-Carboxybenzophenone (3-CB) as an efficient sensitizer in the photooxidation of methionyl-leucine in aqueous solutions: Spectral, kinetic and acid–base properties of 3-CB derived transients. J. Photochem. Photobiol. A Chem. 2014, 287, 1–7. [Google Scholar] [CrossRef]
- Bobrowski, K.; Hug, G.L.; Pogocki, D.; Marciniak, B.; Schöneich, C. Stabilization of Sulfide Radical Cations through Complexation with the Peptide Bond: Mechanisms Relevant to Oxidation of Proteins Containing Multiple Methionine Residues. J. Phys. Chem. B 2007, 111, 9608–9620. [Google Scholar] [CrossRef] [PubMed]
- Pedzinski, T.; Markiewicz, A.; Marciniak, B. Photosensitized oxidation of methionine derivatives. Laser flash photolysis studies. Res. Chem. Intermed. 2009, 35, 497–506. [Google Scholar] [CrossRef]
- Bobrowski, K.; Hug, G.L.; Pogocki, D.; Marciniak, B.; Schöneich, C. Sulfur Radical Cation−Peptide Bond Complex in the One-Electron Oxidation of S-Methylglutathione. J. Am. Chem. Soc. 2007, 129, 9236–9245. [Google Scholar] [CrossRef]
- Goez, M.; Rozwadowski, J.; Marciniak, B. CIDNP Spectroscopic Observation of (S:.+N) Radical Cations with a Two-Center Three-Electron Bond During the Photooxidation of Methionine. Angew. Chem. Int. Ed. 1998, 37, 628–630. [Google Scholar] [CrossRef]
- Glass, R.S. Neighboring Group Participation: General Principles and Application to Sulfur-Centered Reactive Species. In Sulfur-Centered Reactive Intermediates in Chemistry and Biology; Chatgilialoglu, C., Asmus, K.-D., Eds.; Springer: Boston, MA, USA, 1990; pp. 213–226. [Google Scholar] [CrossRef]
- Hiller, K.-O.; Asmus, K.-D. Oxidation of Methionine by X in Aqueous Solution and Characterization of Some Three-electron Bonded Intermediates. A Pulse Radiolysis Study. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1981, 40, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Asmus, K.-D. Sulfur-Centered Three-Electron Bonded Radical Species. In Sulfur-Centered Reactive Intermediates in Chemistry and Biology; Chatgilialoglu, C., Asmus, K.-D., Eds.; Springer: Boston, MA, USA, 1990; pp. 155–172. [Google Scholar] [CrossRef]
- Hug, G.L.; Bobrowski, K.; Kozubek, H.; Marciniak, B. Photooxidation of Methionine Derivatives by the 4-Carboxybenzophenone Triplet State in Aqueous Solution. Intracomplex Proton Transfer Involving the Amino Group. Photochem. Photobiol. 1998, 68, 785–796. [Google Scholar] [CrossRef]
- Pedzinski, T.; Kazmierczak, F.; Filipiak, P.; Marciniak, B. Oxidation studies of a novel peptide model N-acetyl-3-(methylthio)propylamine. J. Photochem. Photobiol. A Chem. 2017, 336, 98–104. [Google Scholar] [CrossRef]
- Hashimoto, M.; Eda, Y.; Osanai, Y.; Iwai, T.; Aoki, S. A novel decarboxylation of α-amino acids. a facile method of decarboxylation by the use of 2-cyclohexen-1-one as a catalyst. Chem. Lett. 1986, 15, 893–896. [Google Scholar] [CrossRef]
- Pedzinski, T.; Grzyb, K.; Kaźmierczak, F.; Frański, R.; Filipiak, P.; Marciniak, B. Early Events of Photosensitized Oxidation of Sulfur-Containing Amino Acids Studied by Laser Flash Photolysis and Mass Spectrometry. J. Phys. Chem. B 2020, 124, 7564–7573. [Google Scholar] [CrossRef]
- Pędzinski, T.; Grzyb, K.; Skotnicki, K.; Filipiak, P.; Bobrowski, K.; Chatgilialoglu, C.; Marciniak, B. Radiation- and Photo-Induced Oxidation Pathways of Methionine in Model Peptide Backbone under Anoxic Conditions. Int. J. Mol. Sci. 2021, 22, 4773. [Google Scholar] [CrossRef]
- Marciniak, B.; Hug, G.L.; Bobrowski, K.; Kozubek, H. Mechanism of 4-carboxybenzophenone-sensitized photooxidation of methionine-containing dipeptides and tripeptides in aqueous solution. J. Phys. Chem. 1995, 99, 13560–13568. [Google Scholar] [CrossRef]
- Filipiak, P.; Bobrowski, K.; Hug, G.L.; Schöneich, C.; Marciniak, B. N-Terminal Decarboxylation as a Probe for Intramolecular Contact Formation in γ-Glu-(Pro)n-Met Peptides. J. Phys. Chem. B 2020, 124, 8082–8098. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, K.; Frański, R.; Pedzinski, T. Sensitized photoreduction of selected benzophenones. Mass spectrometry studies of radical cross-coupling reactions. J. Photochem. Photobiol. B Biol. 2022, 234, 112536. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pavlov, J.; Attygalle, A.B. Collision-induced dissociation processes of protonated benzoic acid and related compounds: Competitive generation of protonated carbon dioxide or protonated benzene: CID spectra of protonated benzoic acids. J. Mass Spectrom. 2017, 52, 230–238. [Google Scholar] [CrossRef]
- Frański, R.; Zalas, M.; Gierczyk, B.; Schroeder, G. Electro-oxidation of diclofenac in methanol as studied by high-performance liquid chromatography/electrospray ionization mass spectrometry: Letter to the Editor. Rapid Commun. Mass Spectrom. 2016, 30, 1662–1666. [Google Scholar] [CrossRef]
- Balta, B.; Aviyente, V.; Lifshitz, C. Elimination of water from the carboxyl group of GlyGlyH+. J. Am. Soc. Mass Spectrom. 2003, 14, 1192–1203. [Google Scholar] [CrossRef]
- Ma, Y.-C.; Kim, H.-Y. Determination of steroids by liquid chromatography/mass spectrometry. J. Am. Soc. Mass Spectrom. 1997, 8, 1010–1020. [Google Scholar] [CrossRef]
- D’Agostino, P.; Provost, L.; Hancock, J. Analysis of mustard hydrolysis products by packed capillary liquid chromatography–electrospray mass spectrometry. J. Chromatogr. A 1998, 808, 177–184. [Google Scholar] [CrossRef]
- da Silva, L.A.; Sandjo, L.P.; Misturini, A.; Caramori, G.F.; Biavatti, M.W. ESI-QTof-MS characterization of hirsutinolide and glaucolide sesquiterpene lactones: Fragmentation mechanisms and differentiation based on Na+ /H+ adducts interactions in complex mixture. J. Mass Spectrom. 2019, 54, 915–932. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physic; Taylor & Francis Group: Abingdon, UK, 2014. [Google Scholar]
- Hug, G.; Marciniak, B.; Bobrowski, K. Sensitized photo-oxidation of sulfur-containing amino acids and peptides in aqueous solution. J. Photochem. Photobiol. A Chem. 1996, 95, 81–88. [Google Scholar] [CrossRef]
- Marciniak, B.; Bobrowski, K. Photo- and Radiation-Induced One-Electron Oxidation of Methionine in Various Structural Environments Studied by Time-Resolved Techniques. Molecules 2022, 27, 1028. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, P.; Hug, G.L.; Bobrowski, K.; Pedzinski, T.; Kozubek, H.; Marciniak, B. Sensitized Photooxidation of S-Methylglutathione in Aqueous Solution: Intramolecular (S∴O) and (S∴N) Bonded Species. J. Phys. Chem. B 2013, 117, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski, K.; Hug, G.L.; Marciniak, B.; Kozubek, H. The 4-carboxybenzophenone-sensitized photooxidation of sulfur-containing amino acids in alkaline aqueous solutions. Secondary photoreactions kinetics. J. Phys. Chem. 1994, 98, 537–544. [Google Scholar] [CrossRef]
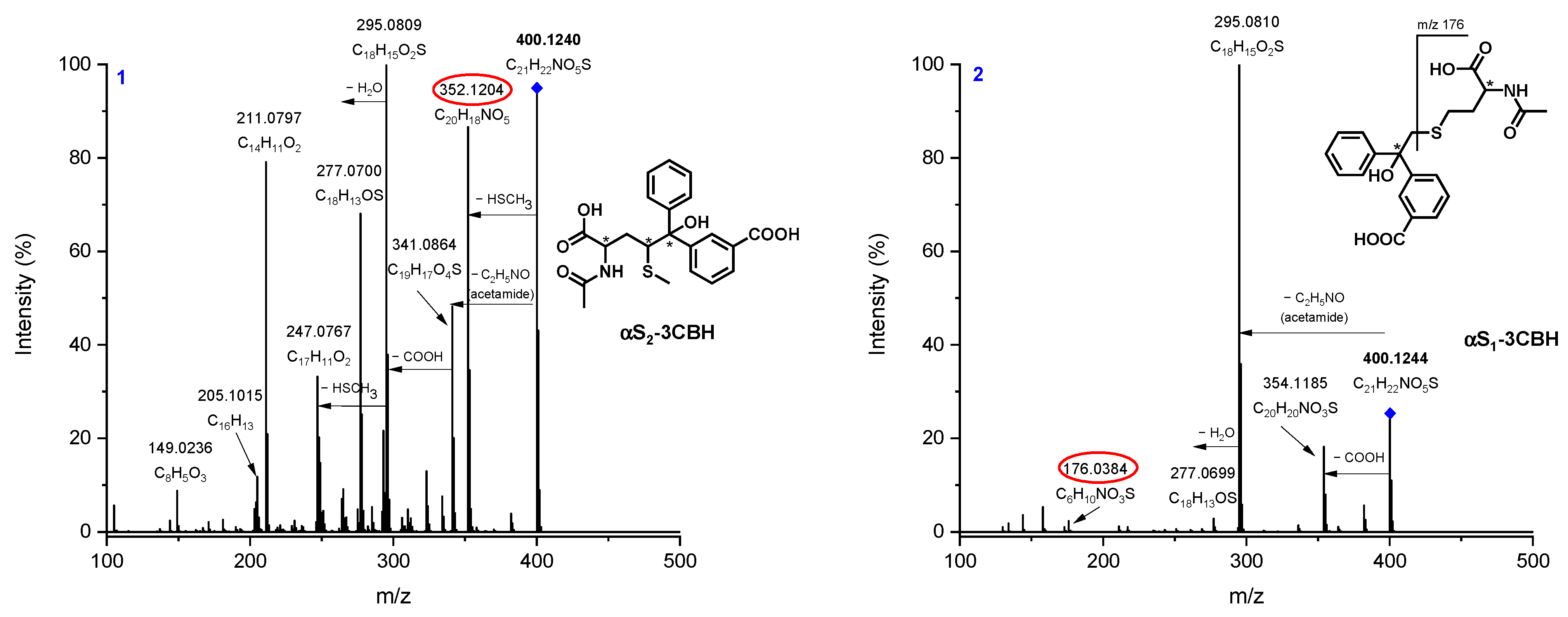
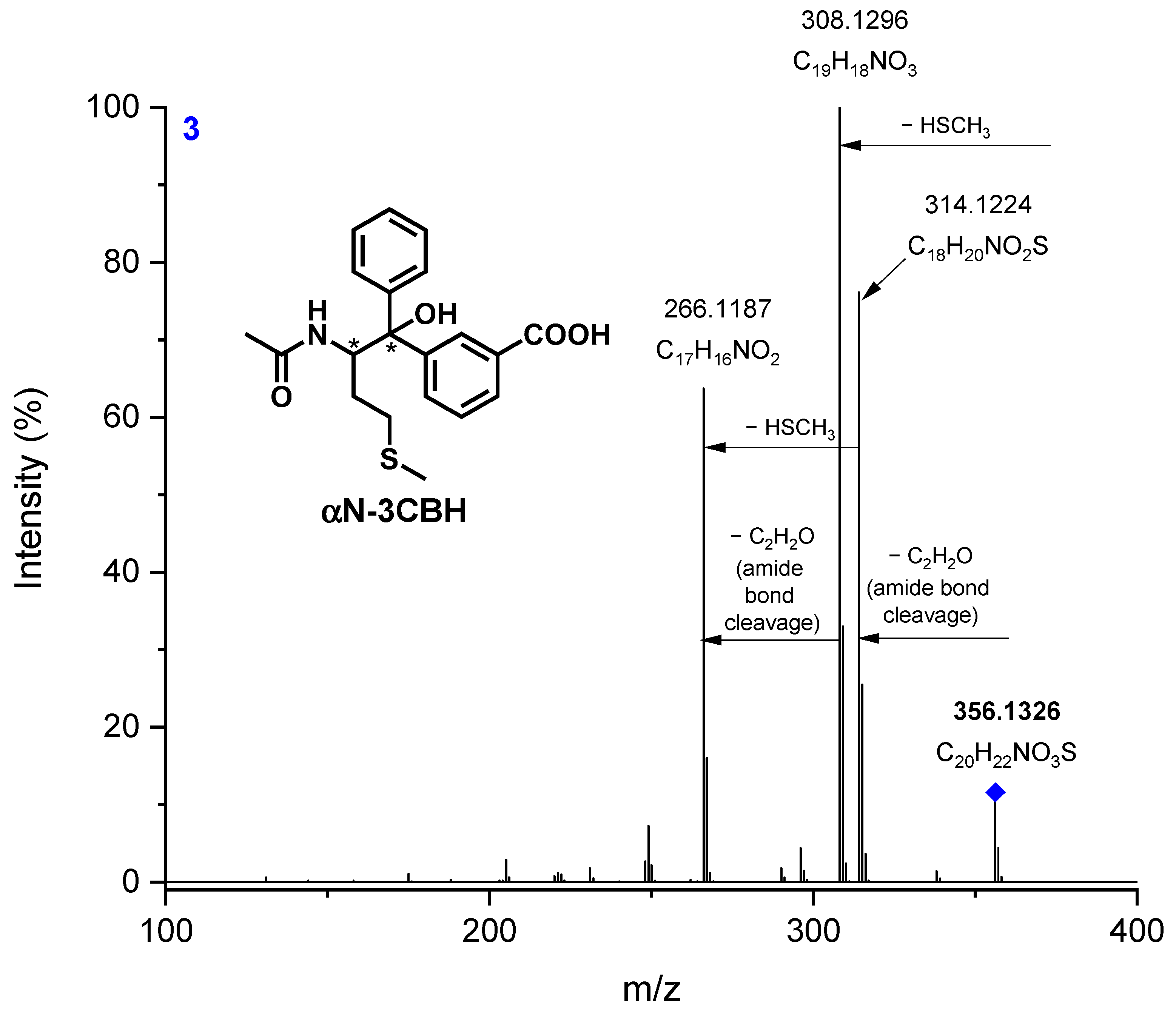

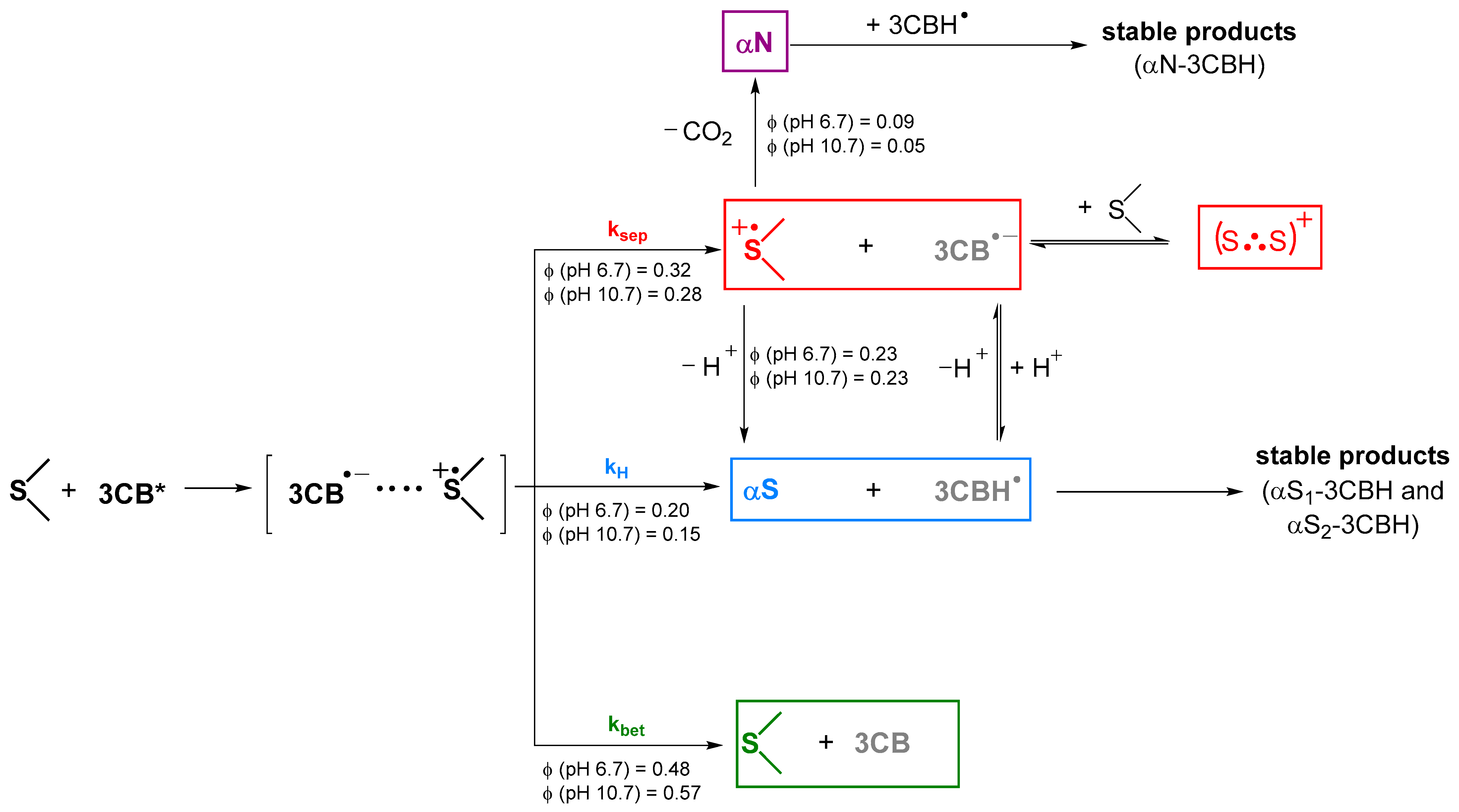
| Radical Species | Φ (pH = 6.7) | Φ (pH = 10.7) |
|---|---|---|
| 3CB●− | 0.32 | 0.28 |
| 3CBH● | 0.20 | 0.10 |
| Photoproduct | Accurate Mass (Measured) | Exact Mass (Calculated) | Mass Accuracy (ppm) | Molecular Composition |
|---|---|---|---|---|
| 1 (αS2-3CBH) | 400.1240 | 400.1219 | 5.33 | C21H22NO5S |
| 2 (αS1-3CBH) | 400.1244 | 400.1219 | 6.33 | C21H22NO5S |
| 3 (αN-3CBH) | 356.1326 | 356.1320 | 1.57 | C20H22NO3S |
| Reaction Pathway | Φ at pH = 6.4 | Φ at pH = 10.7 |
|---|---|---|
| Deprotonation (αS) | 0.23 | 0.23 |
| Decarboxylation (αN) | 0.09 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzyb, K.; Sehrawat, V.; Pedzinski, T. The Fate of Sulfur Radical Cation of N-Acetyl-Methionine: Deprotonation vs. Decarboxylation. Photochem 2023, 3, 98-108. https://doi.org/10.3390/photochem3010007
Grzyb K, Sehrawat V, Pedzinski T. The Fate of Sulfur Radical Cation of N-Acetyl-Methionine: Deprotonation vs. Decarboxylation. Photochem. 2023; 3(1):98-108. https://doi.org/10.3390/photochem3010007
Chicago/Turabian StyleGrzyb, Katarzyna, Vidhi Sehrawat, and Tomasz Pedzinski. 2023. "The Fate of Sulfur Radical Cation of N-Acetyl-Methionine: Deprotonation vs. Decarboxylation" Photochem 3, no. 1: 98-108. https://doi.org/10.3390/photochem3010007
APA StyleGrzyb, K., Sehrawat, V., & Pedzinski, T. (2023). The Fate of Sulfur Radical Cation of N-Acetyl-Methionine: Deprotonation vs. Decarboxylation. Photochem, 3(1), 98-108. https://doi.org/10.3390/photochem3010007