An Insight into the Bicarbonate Effect in Photosystem II through the Prism of the JIP Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Choice of Samples
2.2. Chlorophyll a Fluorescence Measurements
2.3. The Maximum Quantum Yield of the PSII Photochemistry
2.4. The JIP Test
2.5. Calculations Related to the JIP Test
| Abbreviations | Formulas | Definitions of The JIP Test Parameters |
|---|---|---|
| Area | The area between the fluorescence curve and the line F = Fm | The total area over the O-J-I-P curve |
| F0 | F (50 µs) | The initial level of the chlorophyll a fluorescence (fluorescence at 50 µs) |
| Fm (FP) | The value of the maximum level of the fluorescence (FP for the JIP test) | |
| Fv | Fv = Fm − F0 (1) | The value of photo-induced changes of the Chl a fluorescence yield related to the photoreduction of the PSII primary quinone electron acceptor, QA (the variable chlorophyll fluorescence) |
| Fv/Fm | The maximum quantum yield of the PSII photochemistry in the dark-adapted samples | |
| F(t) | The value of photo-induced changes of the Chl a fluorescence yield at time t | |
| Fj | The value of photo-induced changes of the Chl a fluorescence yield at the step J (at 2–5 ms) | |
| τFj | The time of reaching of Fj, ms | |
| V(t) | V(t) = (F(t) − F0)/(Fm − F0) (2) | The relative variable fluorescence yield at time t (with values between zero and 1) in a double normalized O-J-I-P kinetics, i.e., at F0 = 0 and FP (Fm) = 1 |
| Vj | Vj = (Fj − F0)/(Fm − F0) (3) | The relative variable fluorescence yield at 2–5 ms in a double normalized O-J-I-P kinetics |
| 1-Fj | 1-Fj = 1 − (Fj − F0)/(Fm − F0) (4) | The probability of electrons to move into the electron transfer chain further than QA for a double normalized O-J-I-P kinetics |
| N | N = [Area/(Fm − F0)] × M0 × (1/Vj) (5) | The turn-over number of QA reduction events between time 0 to Fm for a double normalized O-J-I-P kinetics |
| M0 | M0 = [F(0.3 ms) − F0]/(Fm − F0) (6) | The initial slope of the O-J-I-P curve (slope of the O to J rise) for a double normalized O-J-I-P kinetics |
| F(0.3 ms) | The value of photo-induced changes of the Chl a fluorescence yield (the value of the fluorescence) at 0.3 ms | |
| Ra | Ra = [F(2.5 ms) − F(1 ms)]/Δt (7) | The rate of the fluorescence rises at the time range of 1–2.5 ms (which is marked as “the range a” in Figure 1A and Figure 2A), were Δt = 1.5 ms |
| F(2.5 ms) | The value of the fluorescence at 2.5 ms | |
| F(1 ms) | The value of the fluorescence at 1.0 ms | |
| Rb | Rb = [F(30 ms) − F(6 ms)]/Δt (8) | The rate of the fluorescence rises at the time range of 6–30 ms (which is marked as “the range b” in Figure 1A and Figure 2A), were Δt = 24 ms |
| F(30 ms) | The value of the fluorescence at 30 ms | |
| F(6 ms) | The value of the fluorescence at 6 ms | |
| Rc | Rc = [F(800 ms) − F(400 ms)]/Δt (9) | The rate of the fluorescence rises at the time range of 400–800 ms (which is marked as “the range c” in Figure 1A and Figure 2A), were Δt = 400 ms |
| F(800 ms) | The value of the fluorescence at 800 ms | |
| F(400 ms) | The value of the fluorescence at 400 ms |
2.6. Oxygen-Evolving Activity
2.7. Removal of Bicarbonate
2.8. Statistical Analysis
2.9. Chlorophyll Concentration
2.10. The Concentration of Photochemical Reaction Centers of PSII
2.11. Calculation of Kinetic Parameters (Vmax, Km, and kcat)
3. Results
3.1. The JIP Test in Thylakoids
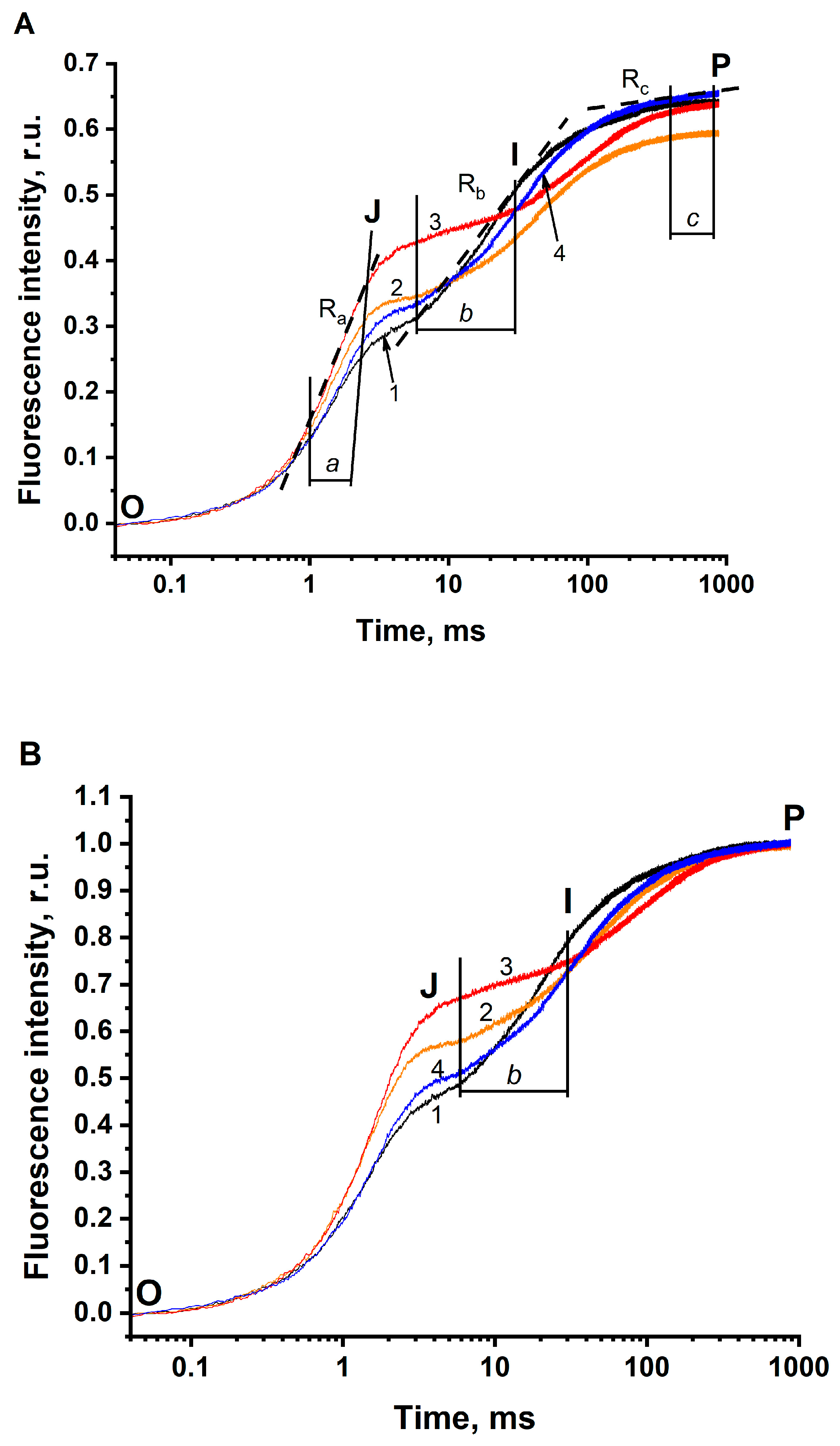
3.2. The JIP Test in BBYs (Photosystem II)
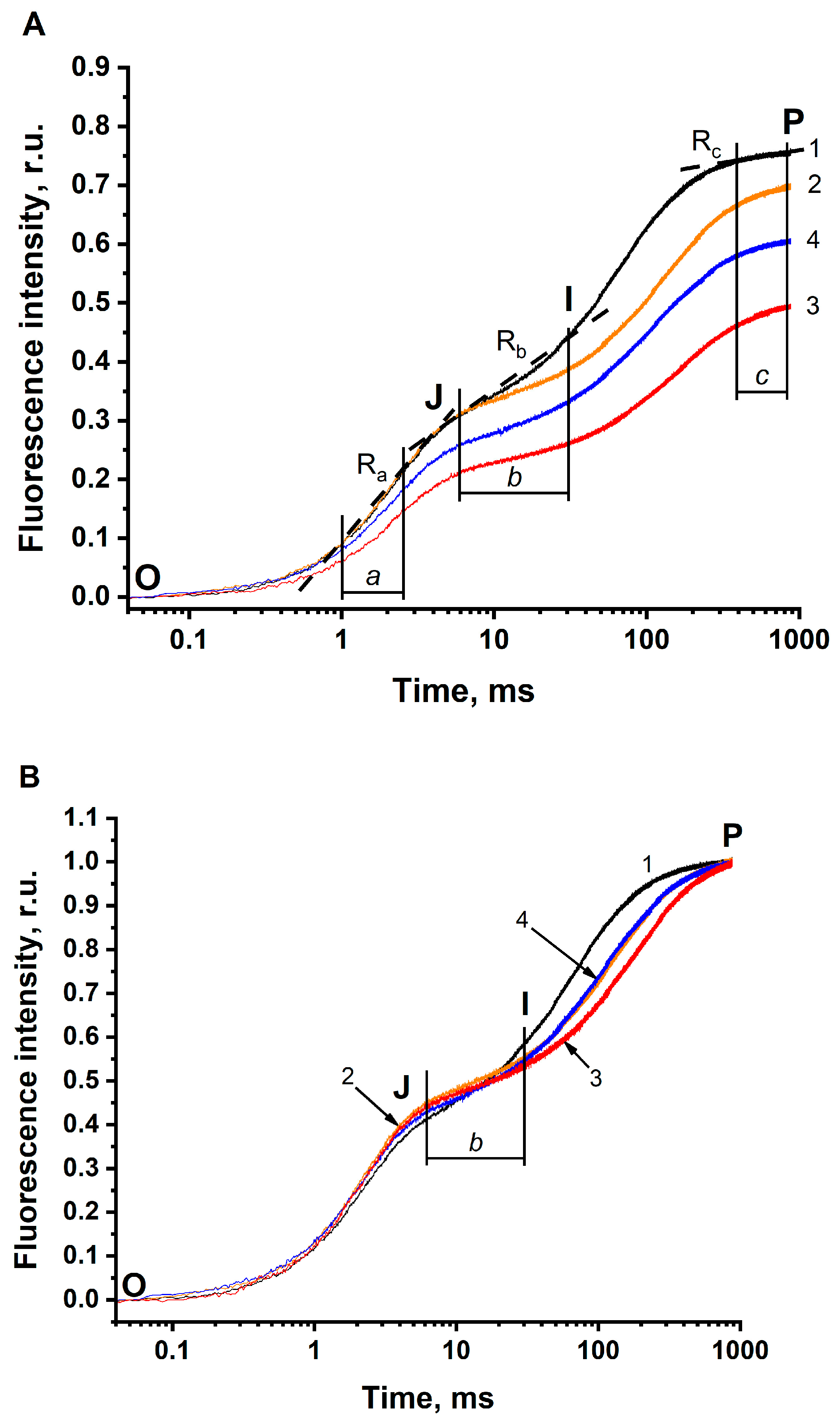
- τFj shifts to longer times. In BBYs, this shift was found to be less pronounced (the magnitude of the BC effect was 30%) than in the thylakoids (this magnitude equaled 66%). Remarkably, in BBY, τFj has longer times, as compared to that in thylakoids, even under optimal conditions (pH 6.5 in the buffer non-depleted of CO2/HCO3−).
- FP clearly decreases being more pronounced in BBY (the magnitude of the BC effect was equal to 34%), compared to that in the thylakoids (where the magnitude was only 9%, at least when comparing FP in solution 2) (see Table 3, column 5). This means that the BC effect on FP is seen more clearly in BBYs compared to that in the thylakoids.
- The Ra decreases. The magnitude of this decrease in BBY was similar to the decrease in 1-Ra in thylakoids.
- The Rb decreases, which was similar to that of thylakoids.
- The Rc increases and the 1-Rc decreases. The decrease in 1-Rc was found to be more pronounced in BBY (the magnitude of the BC effect was equal to 6%), compared to that in thylakoids (where the magnitude was only 2%).
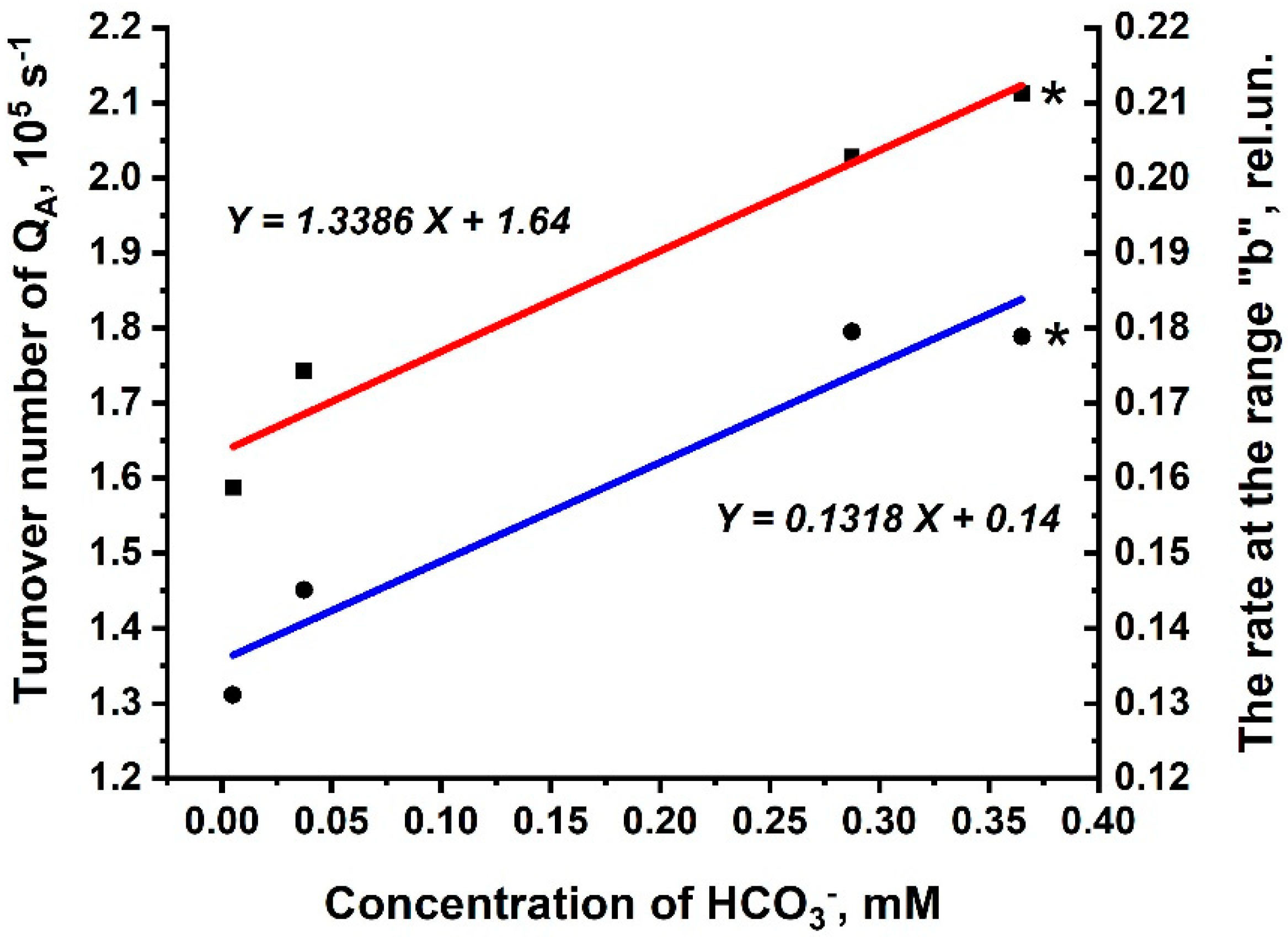
3.3. Kinetic Parameters of O-J-I-P Transients for the Rates Rb and 1-Rc in Thylakoid Membranes and in PSIIs (BBYs)
4. Discussion
4.1. HCO3− May Be Involved in a Single Turn-Over Event
4.1.1. The Shift of the τFj
4.1.2. The Increase in Fj or the Decrease in 1-Fj
4.2. HCO3− May Be Involved in Multiple Turn-Over Events
4.2.1. The Changes in J-I Rise May Be Associated with Events on the Acceptor Side of PSII Resulting from Removal of HCO3−
4.2.2. The Changes in I-P Rise May Be Associated with Events on the Donor Side of PSII Resulting from Removal of HCO3−
4.3. Perspectives of Using the Obtained Data for Research on Plants in a Field
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wydrzynski, T.J.; Satoh, K. (Eds.) Advances in Photosynthesis and Respiration. Photosystem II—The Light-Driven Water: Plastoquinone Oxidoreductase, 1st ed.; Springer: Dordrecht, The Netherlands, 2005; Volume 22, ISBN 978-1-4020-4249-2. [Google Scholar]
- Shevela, D.; Kern, J.F.; Govindjee, G.; Whitmarsh, J.; Messinger, J. Photosystem II. eLS 2021, 2, 1–16. [Google Scholar] [CrossRef]
- van Rensen, J.J.S.; Xu, C.; Govindjee. Role of Bicarbonate in Photosystem II, the Water-Plastoquinone Oxido-Reductase of Plant Photosynthesis. Physiol. Plant. 1999, 105, 585–592. [Google Scholar] [CrossRef]
- van Rensen, J.J.S.; Klimov, V.V. Bicarbonate Interactions. In Advances in Photosynthesis and Respiration. Photosystem II—The Light-driven Water: Plastoquinone Oxidoreductase; Wydrzynski, T.J., Satoh, K., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 22, pp. 329–345. [Google Scholar]
- Shevela, D.; Eaton-Rye, J.J.; Shen, J.-R.; Govindjee. Photosystem II and the Unique Role of Bicarbonate: A Historical Perspective. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Stemler, A.; Govindjee. Bicarbonate Ion as a Critical Factor in Photosynthetic Oxygen Evolution. Plant Physiol. 1973, 52, 119–123. [Google Scholar] [CrossRef]
- Wydrzynski, T.; Govindjee. A New Site of Bicarbonate Effect in Photosystem II of Photosynthesis: Evidence from Chlorophyll Fluorescence Transients in Spinach Chloroplasts. Biochim. Biophys. Acta Bioenerg. 1975, 387, 403–408. [Google Scholar] [CrossRef]
- Eaton-Rye, J.J.; Govindjee. Electron Transfer through the Quinone Acceptor Complex of Photosystem II after One or Two Actinic Flashes in Bicarbonate-Depleted Spinach Thylakoid Membranes. Biochim. Biophys. Acta Bioenerg. 1988, 935, 248–257. [Google Scholar] [CrossRef]
- Klimov, V.; Baranov, S. Bicarbonate Requirement for the Water-Oxidizing Complex of Photosystem II. Biochim. Biophys. Acta Bioenerg. 2001, 1503, 187–196. [Google Scholar] [CrossRef]
- Klimov, V.V.; Allakhverdiev, S.I.; Feyziev, Y.M.; Baranov, S.V. Bicarbonate Requirement for the Donor Side of Photosystem II. FEBS Lett. 1995, 363, 251–255. [Google Scholar] [CrossRef]
- Klimov, V.V.; Allakhverdiev, S.I.; Baranov, S.V.; Feyziev, Y.M. Effects of Bicarbonate and Formate on the Donor Side of Photosystem 2. Photosynth. Res. 1995, 46, 219–225. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Yruela, I.; Picorel, R.; Klimov, V.V. Bicarbonate Is an Essential Constituent of the Water-Oxidizing Complex of Photosystem II. Proc. Natl. Acad. Sci. USA 1997, 94, 5050–5054. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, K.N. Architecture of the Photosynthetic Oxygen-Evolving Center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal Structure of Oxygen-Evolving Photosystem II at a Resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native Structure of Photosystem II at 1.95 Å Resolution Viewed by Femtosecond X-Ray Pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, K.; Shevela, D.; Klimov, V.V.; Messinger, J. Quantification of Bound Bicarbonate in Photosystem II. Photosynthetica 2018, 56, 210–216. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Wang, J.; Liu, J.; Flesher, D.A.; Reiss, K.M.; Huang, H.-L.; Yang, K.R.; Armstrong, W.H.; Gunner, M.R.; Batista, V.S.; et al. High-Resolution Cryo-Electron Microscopy Structure of Photosystem II from the Mesophilic Cyanobacterium, Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2022, 119, e2116765118. [Google Scholar] [CrossRef]
- Brinkert, K.; De Causmaecker, S.; Krieger-Liszkay, A.; Fantuzzi, A.; Rutherford, A.W. Bicarbonate-Induced Redox Tuning in Photosystem II for Regulation and Protection. Proc. Natl. Acad. Sci. USA 2016, 113, 12144–12149. [Google Scholar] [CrossRef]
- Shutova, T.; Kenneweg, H.; Buchta, J.; Nikitina, J.; Terentyev, V.; Chernyshov, S.; Andersson, B.; Allakhverdiev, S.I.; Klimov, V.V.; Dau, H.; et al. The Photosystem II-Associated Cah3 in Chlamydomonas Enhances the O2 Evolution Rate by Proton Removal. EMBO J. 2008, 27, 782–791. [Google Scholar] [CrossRef]
- Koroidov, S.; Shevela, D.; Shutova, T.; Samuelsson, G.; Messinger, J. Mobile Hydrogen Carbonate Acts as Proton Acceptor in Photosynthetic Water Oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 6299–6304. [Google Scholar] [CrossRef]
- Shevela, D.; Do, H.-N.; Fantuzzi, A.; Rutherford, A.W.; Messinger, J. Bicarbonate-Mediated CO2 Formation on Both Sides of Photosystem II. Biochemistry 2020, 59, 2442–2449. [Google Scholar] [CrossRef]
- Stemler, A. Carbonic Anhydrase Associated with Thylakoids and Photosystem II Particles from Maize. Biochim. Biophys. Acta Bioenerg. 1986, 850, 97–107. [Google Scholar] [CrossRef]
- Pronina, N.A.; Allakhverdiev, S.I.; Kupriyanova, E.V.; Klyachko-Gurvich, G.L.; Klimov, V.V. Carbonic Anhydrase in Subchloroplast Particles of Pea Plants. Russ. J. Plant Physiol. 2002, 49, 303–310. [Google Scholar] [CrossRef]
- Moskvin, O.V.; Shutova, T.V.; Khristin, M.S.; Ignatova, L.K.; Villarejo, A.; Samuelsson, G.; Klimov, V.V.; Ivanov, B.N. Carbonic Anhydrase Activities in Pea Thylakoids. Photosynth. Res. 2004, 79, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.N.; Ignatova, L.K.; Ivanov, B.N. Multiple Sources of Carbonic Anhydrase Activity in Pea Thylakoids: Soluble and Membrane-Bound Forms. Photosynth. Res. 2007, 91, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, L.K.; Rudenko, N.N.; Mudrik, V.A.; Fedorchuk, T.P.; Ivanov, B.N. Carbonic Anhydrase Activity in Arabidopsis Thaliana Thylakoid Membrane and Fragments Enriched with PSI or PSII. Photosynth. Res. 2011, 110, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hillier, W.; McConnell, I.; Badger, M.R.; Boussac, A.; Klimov, V.V.; Dismukes, G.C.; Wydrzynski, T. Quantitative Assessment of Intrinsic Carbonic Anhydrase Activity and the Capacity for Bicarbonate Oxidation in Photosystem II. Biochemistry 2006, 45, 2094–2102. [Google Scholar] [CrossRef]
- Shitov, A.V.; Pobeguts, O.V.; Smolova, T.N.; Allakhverdiev, S.I.; Klimov, V.V. Manganese-Dependent Carboanhydrase Activity of Photosystem II Proteins. Biochemistry 2009, 74, 509–517. [Google Scholar] [CrossRef]
- Shitov, A.V.; Zharmukhamedov, S.K.; Shutova, T.V.; Allakhverdiev, S.I.; Samuelsson, G.; Klimov, V.V. A Carbonic Anhydrase Inhibitor Induces Bicarbonate-Reversible Suppression of Electron Transfer in Pea Photosystem 2 Membrane Fragments. J. Photochem. Photobiol. B Biol. 2011, 104, 366–371. [Google Scholar] [CrossRef]
- Shitov, A.V.; Terentyev, V.V.; Zharmukhamedov, S.K.; Rodionova, M.V.; Karacan, M.; Karacan, N.; Klimov, V.V.; Allakhverdiev, S.I. Is Carbonic Anhydrase Activity of Photosystem II Required for Its Maximum Electron Transport Rate? Biochim. Biophys. Acta Bioenerg. 2018, 1859, 292–299. [Google Scholar] [CrossRef]
- McConnell, I.L.; Badger, M.R.; Wydrzynski, T.; Hillier, W. A Quantitative Assessment of the Carbonic Anhydrase Activity in Photosystem II. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 639–647. [Google Scholar] [CrossRef]
- Bricker, T.M.; Frankel, L.K. Auxiliary Functions of the PsbO, PsbP and PsbQ Proteins of Higher Plant Photosystem II: A Critical Analysis. J. Photochem. Photobiol. B Biol. 2011, 104, 165–178. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Chapter 12; pp. 321–362. [Google Scholar]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Wungrampha, S.; Joshi, R.; Rathore, R.S.; Singla-Pareek, S.L.; Govindjee; Pareek, A. CO2 Uptake and Chlorophyll a Fluorescence of Suaeda Fruticosa Grown under Diurnal Rhythm and after Transfer to Continuous Dark. Photosynth. Res. 2019, 142, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A Non-Invasive Assay of the Plastoquinone Pool Redox State Based on the OJIP-Transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B.; Govindjee. Modeling Chlorophyll a Fluorescence Transient: Relation to Photosynthesis. Biochemistry 2014, 79, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.A.; Babcock, G.T.; Yocum, C.F. A Highly Resolved, Oxygen-Evolving Photosystem II Preparation from Spinach Thylakoid Membranes. FEBS Lett. 1981, 134, 231–234. [Google Scholar] [CrossRef]
- Ford, R.C.; Evans, M.C.W. Isolation of a Photosystem 2 Preparation from Higher Plants with Highly Enriched Oxygen Evolution Activity. FEBS Lett. 1983, 160, 159–164. [Google Scholar] [CrossRef]
- Schiller, H.; Dau, H. Preparation Protocols for High-Activity Photosystem II Membrane Particles of Green Algae and Higher Plants, PH Dependence of Oxygen Evolution and Comparison of the S2-State Multiline Signal by X-Band EPR Spectroscopy. J. Photochem. Photobiol. B Biol. 2000, 55, 138–144. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Kaminskaya, O.; Kern, J.; Shuvalov, V.A.; Renger, G. Extinction Coefficients of Cytochromes B559 and C550 of Thermosynechococcus Elongatus and Cyt B559/PS II Stoichiometry of Higher Plants. Biochim. Biophys. Acta Bioenerg. 2005, 1708, 333–341. [Google Scholar] [CrossRef]
- Terentyev, V.V.; Shukshina, A.K.; Ashikhmin, A.A.; Tikhonov, K.G.; Shitov, A.V. The Main Structural and Functional Characteristics of Photosystem-II-Enriched Membranes Isolated from Wild Type and Cia3 Mutant Chlamydomonas Reinhardtii. Life 2020, 10, 63. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and Dibromothymoquinone Treatments of Pea Leaves Reveal the Role of Photosystem I in the Chl a Fluorescence Rise OJIP. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Dark Recovery of the Chl a Fluorescence Transient (OJIP) after Light Adaptation: The QT-Component of Non-Photochemical Quenching Is Related to an Activated Photosystem I Acceptor Side. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Govindjee. Chlorophyll a Fluorescence Induction: A Personal Perspective of the Thermal Phase, the J-I-P Rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Puthur, J.T.; Nagy, V.; Garab, G. Experimental Evidence for Ascorbate-Dependent Electron Transport in Leaves with Inactive Oxygen-Evolving Complexes. Plant Physiol. 2009, 149, 1568–1578. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Kovács, L.; Holzwarth, A.R.; Garab, G. Evidence for a Fluorescence Yield Change Driven by a Light-Induced Conformational Change within Photosystem II during the Fast Chlorophyll a Fluorescence Rise. Biochim. Biophys. Acta 2011, 1807, 1032–1043. [Google Scholar] [CrossRef]
- Shinkarev, V.P.; Govindjee. Insight into the Relationship of Chlorophyll a Fluorescence Yield to the Concentration of Its Natural Quenchers in Oxygenic Photosynthesis. Proc. Natl. Acad. Sci. USA 1993, 90, 7466–7469. [Google Scholar] [CrossRef]
- Khanna, R.; Pfister, K.; Keresztes, Á.; van Rensen, J.J.S.; Govindjee. Evidence for a Close Spatial Location of the Binding Sites for CO2 and for Photosystem II Inhibitors. Biochim. Biophys. Acta Bioenerg. 1981, 634, 105–116. [Google Scholar] [CrossRef]
- van Rensen, J.J.S.; Vermaas, W.F.J. Action of Bicarbonate and Photosystem 2 Inhibiting Herbicides on Electron Transport in Pea Grana and in Thylakoids of a Blue-Green Alga. Physiol. Plant. 1981, 51, 106–110. [Google Scholar] [CrossRef]
- Eaton-Rye, J.J.; Govindjee. Electron Transfer through the Quinone Acceptor Complex of Photosystem II in Bicarbonate-Depleted Spinach Thylakoid Membranes as a Function of Actinic Flash Number and Frequency. Biochim. Biophys. Acta Bioenerg. 1988, 935, 237–247. [Google Scholar] [CrossRef]
- Khanna, R.; Govindjee; Wydrzynski, T. Site of Bicarbonate Effect in Hill Reaction. Evidence from the Use of Artificial Electron Acceptors and Donors. Biochim. Biophys. Acta Bioenerg. 1977, 462, 208–214. [Google Scholar] [CrossRef]
| Experimental Conditions | Characteristics of the O-J-I-P Fluorescence Transients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| τFj | Fj | 1-Fj | Fp | 1-Ra | Rb | 1-Rc | |||
| Norm. to F0 | Norm. to F0 | Norm. to F0 and Fp | Norm. to F0 and Fp | Norm. to F0 | Norm. to F0 | Norm. to F0 | Norm. to F0 and Fp | Norm. to F0 | |
| Solution 1 pH 6.5 not depleted of CO2/HCO3− (288 μM HCO3−) | 2.9 ms (100%) | 0.28 (100%) | 0.43 (100%) | 0.57 (100%) | 0.65 (100%) | 0.654 ± 0.011 (100%) | 0.299 ± 0.009 (100%) | 0.478 ± 0.023 (100%) | 0.988 ± 0.003 (100%) |
| Solution 2 pH 5.5 not depleted of CO2/HCO3− (38 μM HCO3−) | 3.8 ms (131%) | 0.34 (121%) | 0.57 (133%) | 0.43 (75%) | 0.59 (91%) | 0.570 ± 0.008 (87%) | 0.127 ± 0.002 (42%) | 0.210 ± 0.010 (44%) | 0.975 ± 0.001 (99%) |
| Solution 3 pH 5.5 depleted of CO2/HCO3− (5 μM HCO3−) | 4.8 ms (166%) | 0.42 (150%) | 0.66 (153%) | 0.34 (60%) | 0.64 (98%) | 0.464 ± 0.005 (71%) | 0.064 ± 0.003 (21%) | 0.100 ± 0.003 21%) | 0.964 ± 0.002 (98%) |
| Solution 4 pH 5.5 depleted of CO2/HCO3− + 3 mM of HCO3− (365 μM HCO3−) | 4.2 ms (145%) | 0.32 (114%) | 0.50 (116%) | 0.50 (88%) | 0.65 (100%) | 0.593 ± 0.004 (91%) | 0.190 ± 0.01 (64%) | 0.298 ± 0.009 (62%) | 0.972 ± 0.001 (98.4%) |
| Experimental Conditions | Characteristics of the O-J-I-P Fluorescence Transients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| τFj | Fj | 1-Fj | Fp | Ra | Rb | 1-Rc | |||
| Norm. To F0 | Norm. To F0 | Norm. to F0 and Fp | Norm. to F0 and Fp | Norm. To F0 | Norm. To F0 | Norm. To F0 | Norm. to F0 and Fp | Norm. To F0 | |
| Solution 1 pH 6.5 not depleted of CO2/HCO3− (288 μM HCO3−) | 3.7 ms (100%) | 0.27 (100%) | 0.35 (100%) | 0.65 (100%) | 0.76 (100%) | 0.316 ± 0.005 (100%) | 0.187 ± 0.012 (100%) | 0.249 ± 0.011 (100%) | 0.961 ± 0.004 (100%) |
| Solution 2 pH 5.5 not depleted of CO2/HCO3− (38 μM HCO3−) | 4.1 ms (111%) | 0.27 (100%) | 0.43 (123%) | 0.57 (88%) | 0.70 (92%) | 0.315 ± 0.007 (100%) | 0.101 ± 0.007 (54%) | 0.145 ± 0.009 (58%) | 0.914 ± 0.002 (95%) |
| Solution 3 pH 5.5 depleted of CO2/HCO3− (5 μM HCO3−) | 4.8 ms (130%) | 0.20 (74%) | 0.41 (117%) | 0.59 (91%) | 0.50 (66%) | 0.217 ± 0.006 (69%) | 0.065 ± 0.002 (35%) | 0.131 ± 0.004 (53%) | 0.903 ± 0.001 (94%) |
| Solution 4 pH 5.5 depleted of CO2/HCO3− + 3 mM of HCO3− (365 μM HCO3−) | 3.9 ms (105%) | 0.23 (85%) | 0.38 (109%) | 0.62 (95%) | 0.61 (80%) | 0.259 ± 0.004 (82%) | 0.102 ± 0.003 (55%) | 0.170 ± 0.007 (68%) | 0.929 ± 0.002 (97%) |
| Kinetic. Parameters | Thylakoids | BBY | ||
|---|---|---|---|---|
| For Rb | For 1-Rc | For Rb | For 1-Rc | |
| Km, mM | 11.4 × 10−3 | 7.4 × 10−5 | 3.9 × 10−3 | 2.0 × 10−4 |
| Vmax, rel.un. | 0.207 | 0.980 | 0.124 | 0.939 |
| kcat, s−1 | - | - | 2.0 × 106 | 1.6 × 107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shitov, A.V. An Insight into the Bicarbonate Effect in Photosystem II through the Prism of the JIP Test. Photochem 2022, 2, 779-797. https://doi.org/10.3390/photochem2030050
Shitov AV. An Insight into the Bicarbonate Effect in Photosystem II through the Prism of the JIP Test. Photochem. 2022; 2(3):779-797. https://doi.org/10.3390/photochem2030050
Chicago/Turabian StyleShitov, Alexandr V. 2022. "An Insight into the Bicarbonate Effect in Photosystem II through the Prism of the JIP Test" Photochem 2, no. 3: 779-797. https://doi.org/10.3390/photochem2030050
APA StyleShitov, A. V. (2022). An Insight into the Bicarbonate Effect in Photosystem II through the Prism of the JIP Test. Photochem, 2(3), 779-797. https://doi.org/10.3390/photochem2030050






