Progress in the Photoreforming of Carboxylic Acids for Hydrogen Production
Abstract
:1. Introduction
2. Details of the Photoreforming Process
3. Types of Organics (Oxygenates) in the Photoreforming Process for H2 Production
4. Importance of Carboxylic Acids
4.1. Formic Acid
4.2. Acetic Acid
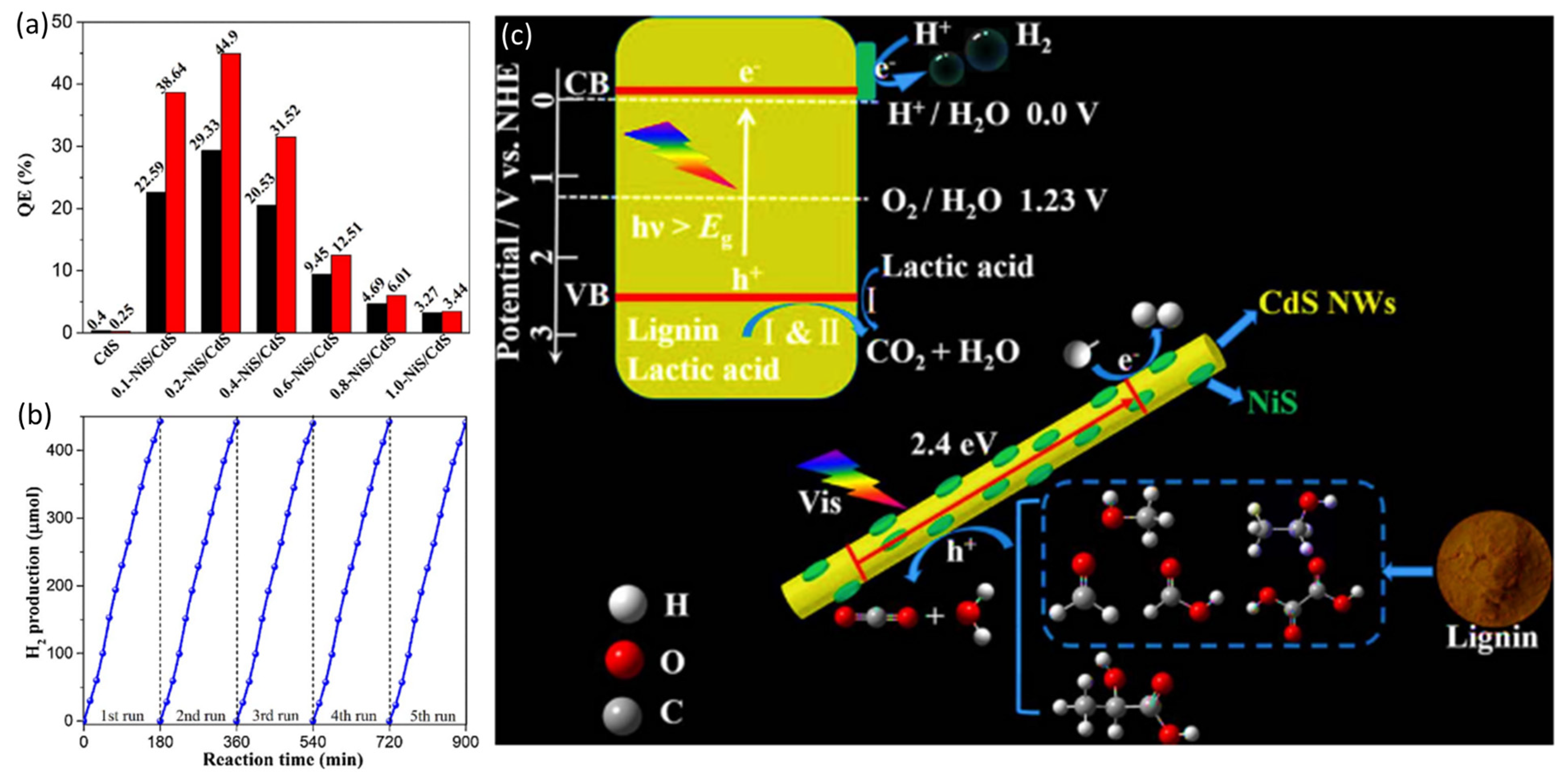
4.3. Lactic Acid
4.4. Other Carboxylic Acids
5. Future Aspects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balali, Y.; Stegen, S. Review of energy storage systems for vehicles based on technology, environmental impacts, and costs. Renew. Sustain. Energy Rev. 2021, 135, 110185. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.; Khan, H.U.R.; Zaman, K.; Hishan, S.S. Measuring the impact of global tropospheric ozone, carbon dioxide and sulfur dioxide concentrations on biodiversity loss. Environ. Res. 2018, 160, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Blanco, H.; Nijs, W.; Ruf, J.; Faaij, A. Potential for hydrogen and Power-to-Liquid in a low-carbon EU energy system using cost optimization. Appl. Energy 2018, 232, 617–639. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Xu, Z.; Jiaqiang, E.; Leng, E.; Zhang, F.; Liao, G. Process in supercritical water gasification of coal: A review of fundamentals, mechanisms, catalysts and element transformation. Energ. Convers. Manag. 2021, 237, 114122. [Google Scholar] [CrossRef]
- Chen, C.; Wood, P. A turbulence closure model for dilute gas-particle flows. Can. J. Chem. Eng. 1985, 63, 349–360. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Sunarso, J.; Li, C.; Pham, G.H.; Phan, C.; Liu, S. Microwave-assisted catalytic methane reforming: A review. Appl. Catal. A. Gen. 2020, 599, 117620. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Ghazvini, M.; Alhuyi Nazari, M.; Ahmadi, M.A.; Pourfayaz, F.; Lorenzini, G.; Ming, T. Renewable energy harvesting with the application of nanotechnology: A review. Int. J. Energy Res. 2019, 43, 1387–1410. [Google Scholar] [CrossRef]
- Luo, P. perspectives in photo-and electrochemical-oxidation of biomass for sustainable chemicals and hydrogen production. Adv. Energy Mater 2021, 11, 2101180. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Huang, D.; Lai, C.; Cheng, M.; Wang, W.; Xiong, W. Recent progress on metal-organic frameworks based-and derived-photocatalysts for water splitting. Chem. Eng. J. 2020, 383, 123196. [Google Scholar] [CrossRef]
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sanchez-Sanchez, M.C.; Alvarez-Galvan, M.C.; Valle, F.; Fierro, J.L. Hydrogen production from renewable sources: Biomass and photocatalytic opportunities. Energy Environ. Science. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Aramendia, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navio, J.A. Influence of the strong metal support interaction effect (SMSI) of Pt/TiO2 and Pd/TiO2 systems in the photocatalytic biohydrogen production from glucose solution. Catal. Commun. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Bowker, M. Sustainable hydrogen production by the application of ambient temperature photocatalysis. Green Chem. 2011, 13, 2235–2246. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhera, S.K.; Rajan, A.; Rugma, T.; Bernaurdshaw, N. A review on particulate photocatalytic hydrogen production system: Progress made in achieving high energy conversion efficiency and key challenges ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar] [CrossRef]
- Arkhipova, N.; Kuznetsova, A. Photocatalytic activity and physicochemical characteristics of modified potassium polytitanates in the reaction of decomposition of aqueous-alcoholic solutions. Russ. J. Appl. Chem. 2017, 90, 186–192. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today. 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Christoforidis, K.; Fornasiero, P. Photocatalytic hydrogen production: A rift into the future energy supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef] [Green Version]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Quispe, C.A.; Coronado, C.J.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Edition 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Toe, C.Y.; Tsounis, C.; Zhang, J.; Masood, H.; Gunawan, D.; Scott, J.; Amal, R. Advancing photoreforming of organics: Highlights on photocatalyst and system designs for selective oxidation reactions. Energy Environ. Sci. 2021, 14, 1140–1175. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.-P.; Yu, J.-S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef] [Green Version]
- McKone, J.R.; Pieterick, A.P.; Gray, H.B.; Lewis, N.S. Hydrogen evolution from Pt/Ru-coated p-type WSe2 photocathodes. J. Am. Chem. Soc. 2013, 135, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Walte, M.; Warren, E.; McKone, J.; Boettcher, S.; Qixi, M.; Santori, E.; Lewis, N. Solar water splitting cell. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I. Hydrogen production by photoreforming of renewable substrates. Int. Sch. Res. Notices 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Sagar, R.U.R.; Zhang, B.; Huang, W.; Mahmood, A.; Mahmood, N.; Khan, K.; Zhang, H. Recent progress, challenges, and prospects in two-dimensional photocatalyst materials and environmental remediation. Micro Nano Lett. 2020, 12, 1–77. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Sakata, T.; Hashimoto, K.; Kawai, T. Catalytic properties of ruthenium oxide on n-type semiconductors under illumination. J. Phys. Chem. A 1984, 88, 5214–5221. [Google Scholar] [CrossRef]
- Pajares, A.; Wang, Y.; Kronenberg, M.; de la Piscina, P.R.; Homs, N. Photocatalytic H2 production from ethanol aqueous solution using TiO2 with tungsten carbide nanoparticles as co-catalyst. Int. J. Hydrogen Energy 2020, 45, 20558–20567. [Google Scholar] [CrossRef]
- Alshehri, A.; Narasimharao, K. PtOx-TiO2 anatase nanomaterials for photocatalytic reformation of methanol to hydrogen: Effect of TiO2 morphology. J. Mater. Res. Technol. 2020, 9, 14907–14921. [Google Scholar] [CrossRef]
- Hippargi, G.; Anjankar, S.; Krupadam, R.J.; Rayalu, S.S. Simultaneous wastewater treatment and generation of blended fuel methane and hydrogen using Au-Pt/TiO2 photo-reforming catalytic material. Fuel 2021, 291, 120113. [Google Scholar] [CrossRef]
- Huang, C.-W.; Nguyen, B.-S.; Wu, J.C.-S.; Nguyen, V.-H. A current perspective for photocatalysis towards the hydrogen production from biomass-derived organic substances and water. Int. J. Hydrogen Energy 2020, 45, 18144–18159. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic hydrogen production: Role of sacrificial reagents on the activity of oxide, carbon, and sulfide catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Guzman, F.; Chuang, S.S.; Yang, C. Role of methanol sacrificing reagent in the photocatalytic evolution of hydrogen. Ind. Eng. Chem. Res. 2013, 52, 61–65. [Google Scholar] [CrossRef]
- Ismael, M. Latest progress on the key operating parameters affecting the photocatalytic activity of TiO2-based photocatalysts for hydrogen fuel production: A comprehensive review. Fuel 2021, 303, 121207. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Liu, S.; Duan, X.; Hu, Z. Synthesis and characterization of Cu2O/TiO2 photocatalysts for H2 evolution from aqueous solution with different scavengers. Appl. Surf. Sci. 2015, 324, 736–744. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Forni, L.; Selli, E. Photocatalytic hydrogen production by liquid-andgas-phasereforming of CH3OHoverflame-made TiO2 andAu1 TiO2. Catal. Today 2009, 144, 69–74. [Google Scholar] [CrossRef]
- Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; Andreozzi, R. Near UV-Irradiation of CuOx-Impregnated TiO2 Providing Active Species for H2 Production Through Methanol Photoreforming. ChemCatChem 2019, 11, 4314–4326. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Machado, V.A. Photodegradation of Organic Pollutants in Seawater and Hydrogen Production via Methanol Photoreforming with Hydrated Niobium Pentoxide Catalysts. Sustain. Chem. 2022, 3, 172–191. [Google Scholar] [CrossRef]
- Saha, A.; Sinhamahapatra, A.; Kang, T.-H.; Ghosh, S.C.; Yu, J.-S.; Panda, A.B. Hydrogenated MoS2 QD-TiO2 heterojunction mediated efficient solar hydrogen production. Nanoscale 2017, 9, 17029–17036. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Lee, H.-Y.; Shen, S.; Mao, S.S.; Yu, J.-S. H-doped TiO2-x prepared with MgH2 for highly efficient solar-driven hydrogen production. Appl. Catal. B 2018, 237, 613–621. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Hachisuka, K.; Katsumata, H.; Suzuki, T.; Funasaka, K.; Kaneco, S. Enhanced photocatalytic hydrogen production from aqueous methanol solution using ZnO with simultaneous photodeposition of Cu. Int. J. Hydrogen Energy 2013, 38, 11840–11846. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.-P.; Kang, J.; Han, B.; Yu, J.-S. Oxygen-deficient zirconia (ZrO2−x): A new material for solar light absorption. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Phan, T.-D.; Baber, A.E.; Rodriguez, J.A.; Senanayake, S.D. Au and Pt nanoparticle supported catalysts tailored for H2 production: From models to powder catalysts. Appl. Catal. A Gen. 2016, 518, 18–47. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, M.J.; Oliveira, J.W.; Sombrio, C.I.; Baptista, D.L.; Teixeira, S.R.; Carabineiro, S.A.; Silva, C.G.; Faria, J.L. Photocatalytic performance of Au/ZnO nanocatalysts for hydrogen production from ethanol. Appl. Catal. A Gen. 2016, 518, 198–205. [Google Scholar] [CrossRef]
- Montini, T.; Gombac, V.; Sordelli, L.; Delgado, J.J.; Chen, X.; Adami, G.; Fornasiero, P. Nanostructured Cu/TiO2 Photocatalysts for H2 Production from Ethanol and Glycerol Aqueous Solutions. ChemCatChem 2011, 3, 574–577. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, L.; Yun, R.; Pu, M.; Zhang, B.; Xiang, X. Increasing the activity and selectivity of TiO2-supported Au catalysts for renewable hydrogen generation from ethanol photoreforming by engineering Ti3+ defects. ACS Sustain. Chem. Eng. 2019, 7, 13856–13864. [Google Scholar] [CrossRef]
- Gunawan, D.; Toe, C.Y.; Kumar, P.; Scott, J.; Amal, R. Synergistic Cyanamide Functionalization and Charge-Induced Activation of Nickel/Carbon Nitride for Enhanced Selective Photoreforming of Ethanol. ACS Appl. Mater. Interfaces 2021, 13, 49916–49926. [Google Scholar] [CrossRef]
- Tran, N.H.; Kannangara, G.K. Conversion of glycerol to hydrogen rich gas. Chem. Soc. Rev. 2013, 42, 9454–9479. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, C.; Zhao, H.; Peng, S.; Tian, Z.; Shu, R.; Chen, Y. Synergistic Effect of Photo-thermal Catalytic Glycerol Reforming Hydrogen Production over 2D Au/TiO2 Nanoflakes. Chem. Eng. J. 2022, 137063. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Murcia, J.; Lara, A.; Hernández, J.; Rojas, H.; Navío, J.; Hidalgo, M. Photocatalytic production of hydrogen and methane from glycerol reforming over Pt/TiO2–Nb2O5. Int. J. Hydrogen Energy 2021, 46, 38678–38691. [Google Scholar] [CrossRef]
- Tahir, M.; Siraj, M.; Tahir, B.; Umer, M.; Alias, H.; Othman, N. Au-NPs embedded Z–scheme WO3/TiO2 nanocomposite for plasmon-assisted photocatalytic glycerol-water reforming towards enhanced H2 evolution. Appl. Surf. Sci. 2020, 503, 144344. [Google Scholar] [CrossRef]
- St. John, M.R.; Furgala, A.J.; Sammells, A.F. Hydrogen generation by photocatalytic oxidation of glucose by platinized n-titania powder. J. Phys. Chem. A 1983, 87, 801–805. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrogen Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Ciambelli, P. Production of hydrogen from glucose by LaFeO3 based photocatalytic process during water treatment. Int. J. Hydrogen Energy 2016, 41, 959–966. [Google Scholar] [CrossRef]
- Nguyen, V.-C.; Nimbalkar, D.B.; Nam, L.D.; Lee, Y.-L.; Teng, H. Photocatalytic cellulose reforming for H2 and formate production by using graphene oxide-dot catalysts. ACS Catal. 2021, 11, 4955–4967. [Google Scholar] [CrossRef]
- Mondal, A.; Biswas, S.; Kumar, A.; Yu, J.S.; Sinhamahapatra, A. Sub 10 nm CoO nanoparticle-decorated graphitic carbon nitride for solar hydrogen generation via efficient charge separation. Nanoscale Adv. 2020, 2, 4473–4481. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic hydrogen production from water by the decomposition of poly-vinylchloride, protein, algae, dead insects, and excrement. Chem. Lett. 1981, 10, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Razzaq, A.; Sinhamahapatra, A.; Kang, T.-H.; Grimes, C.A.; Yu, J.-S.; In, S.-I. Efficient solar light photoreduction of CO2 to hydrocarbon fuels via magnesiothermally reduced TiO2 photocatalyst. Appl. Catal. B 2017, 215, 28–35. [Google Scholar] [CrossRef]
- Greenbaum, E.; Tevault, C.V.; Ma, C. New Photosynthesis: Direct photoconversion of biomass to molecular oxygen and volatile hydrocarbons. Energy Fuels 1995, 9, 163–167. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Speltini, A.; Gualco, F.; Maraschi, F.; Sturini, M.; Dondi, D.; Malavasi, L.; Profumo, A. Photocatalytic hydrogen evolution assisted by aqueous (waste) biomass under simulated solar light: Oxidized g-C3N4 vs. P25 titanium dioxide. Int. J. Hydrogen Energy 2019, 44, 4072–4078. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, L.; Xi, Q.; Lei, Y.; Wang, F. Edge functionalization of terminal amino group in carbon nitride by in-situ C–N coupling for photoreforming of biomass into H2. Chem. Eng. J. 2020, 383, 123792. [Google Scholar] [CrossRef]
- Guo, P.; Xiong, Z.; Yuan, S.; Xie, K.; Wang, H.; Gao, Y. The synergistic effect of Co/CoO hybrid structure combined with biomass materials promotes photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 420, 130372. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial hydrogen production over TiO2-based photocatalysts: Polyols, carboxylic acids, and saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- Tan, H.; Kong, P.; Liu, M.; Gu, X.; Zheng, Z. Enhanced photocatalytic hydrogen production from aqueous-phase methanol reforming over cyano-carboxylic bifunctionally-modified carbon nitride. ChemComm. 2019, 55, 12503–12506. [Google Scholar] [CrossRef]
- Liu, X.; Duan, X.; Wei, W.; Wang, S.; Ni, B.-J. Photocatalytic conversion of lignocellulosic biomass to valuable products. Green Chem. 2019, 21, 4266–4289. [Google Scholar] [CrossRef]
- AlSalka, Y.; Al-Madanat, O.; Curti, M.; Hakki, A.; Bahnemann, D.W. Photocatalytic H2 evolution from oxalic acid: Effect of cocatalysts and carbon dioxide radical anion on the surface charge transfer mechanisms. ACS Appl. Energy Mater. 2020, 3, 6678–6691. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Li, S. Photocatalytic hydrogen generation and decomposition of oxalic acid over platinized TiO2. Appl. Catal. A Gen. 2001, 214, 179–185. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Li, S. Photocatalytic production of hydrogen in single component and mixture systems of electron donors and monitoring adsorption of donors by in situ infrared spectroscopy. Chemosphere 2003, 52, 843–850. [Google Scholar] [CrossRef]
- Zieliñska, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Photocatalytic hydrogen generation over alkaline-earth titanates in the presence of electron donors. Int. J. Hydrogen Energy 2008, 33, 1797–1802. [Google Scholar] [CrossRef]
- Reuss, G.; Disteldorf, W.; Grundler, O.; Hilt, A. Ullmann’s Encycl; Society of Chemical Industry: Chichester, UK, 1985. [Google Scholar]
- Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Cover Picture: Selective Catalytic Synthesis Using the Combination of Carbon Dioxide and Hydrogen: Catalytic Chess at the Interface of Energy and Chemistry (Angew. Chem. Int. Ed. 26/2016). Angew. Chem. Int. Ed. 2016, 55, 7267. [Google Scholar] [CrossRef]
- Tao, C.; Guopeng, W.; Zhaochi, F.; Gengshen, H.; Weiguang, S.; Pinliang, Y.; Can, L. In situ FT-IR study of photocatalytic decomposition of formic acid to hydrogen on Pt/TiO2 catalyst. Chin. J. Catal. 2008, 29, 105–107. [Google Scholar]
- Lanese, V.; Spasiano, D.; Marotta, R.; Di Somma, I.; Lisi, L.; Cimino, S.; Andreozzi, R. Hydrogen production by photoreforming of formic acid in aqueous copper/TiO2 suspensions under UV-simulated solar radiation at room temperature. Int. J. Hydrogen Energy 2013, 38, 9644–9654. [Google Scholar]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic hydrogen generation using metal-decorated TiO2: Sacrificial donors vs. true water splitting. ACS Energy Lett. 2018, 3, 542–545. [Google Scholar]
- Nasir, J.A.; Hafeez, M.; Arshad, M.; Ali, N.Z.; Teixeira, I.F.; McPherson, I.; Khan, M.A. Photocatalytic dehydrogenation of formic acid on CdS nanorods through Ni and Co redox mediation under mild conditions. ChemSusChem 2018, 11, 2587–2592. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- Song, R.; Luo, B.; Liu, M.; Geng, J.; Jing, D.; Liu, H. Synergetic coupling of photo and thermal energy for efficient hydrogen production by formic acid reforming. AIChE J. 2017, 63, 2916–2925. [Google Scholar] [CrossRef]
- Willner, I.; Goren, Z. Photodecomposition of formic acid by cadmium sulphide semiconductor particles. J. Chem. Soc. Chem. Commun. 1986, 2, 172–173. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Peng, S.; Xie, D.; Lu, G.; Li, S. Enhancement of photocatalytic activity of cadmium sulfide for hydrogen evolution by photoetching. Int. J. Hydrogen Energy. 2008, 33, 2007–2013. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Peng, S.; Lu, G.; Li, S. Synthesis of CdS nanorods by an ethylenediamine assisted hydrothermal method for photocatalytic hydrogen evolution. J. Phys. Chem. A C 2009, 113, 9352–9358. [Google Scholar] [CrossRef]
- Matsumura, M.; Hiramoto, M.; Iehara, T.; Tsubomura, H. Photocatalytic and photoelectrochemical reactions of aqueous solutions of formic acid, formaldehyde, and methanol on platinized cadmium sulfide powder and at a cadmium sulfide electrode. J. Phys. Chem. A 1984, 88, 248–250. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, S.-W.; Liao, Y.; Xue, C. Selective photocatalytic decomposition of formic acid over AuPd nanoparticle-decorated TiO2 nanofibers toward high-yield hydrogen production. Appl. Catal. B 2015, 162, 204–209. [Google Scholar] [CrossRef]
- Puangpetch, T.; Chavadej, S.; Sreethawong, T. Hydrogen production over Au-loaded mesoporous-assembled SrTiO3 nanocrystal photocatalyst: Effects of molecular structure and chemical properties of hole scavengers. Energy Convers. Manag. 2011, 52, 2256–2261. [Google Scholar] [CrossRef]
- Zhu, R.; Tian, F.; Yang, R.; He, J.; Zhong, J.; Chen, B. Z scheme system ZnIn2S4/RGO/BiVO4 for hydrogen generation from water splitting and simultaneous degradation of organic pollutants under visible light. Renew. Energy 2019, 139, 22–27. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Su, W.; Zhou, G.; Zong, X.; Lei, Z.; Li, C. H2 production with ultra-low CO selectivity via photocatalytic reforming of methanol on Au/TiO2 catalyst. Int. J. Hydrogen Energy 2008, 33, 1243–1251. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, L. Photocatalytic degradation of formic acid with simultaneous production of hydrogen over Pt and Ru-loaded CdS/Al-HMS photocatalysts. Desalination 2009, 249, 1017–1021. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, L.; Li, S. Synthesis of Al-substituted mesoporous silica coupled with CdS nanoparticles for photocatalytic generation of hydrogen. Int. J. Hydrogen Energy 2010, 35, 438–444. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Wakerley, D.W.; Orchard, K.L.; Reisner, E. Photocatalytic formic acid conversion on CdS nanocrystals with controllable selectivity for H2 or CO. Angew. Chem. Int. Ed. 2015, 54, 9627–9631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, H.; Lo, S.; Chen, M.; Chen, H. Hydrogen production from formic acid solution by modified TiO2 and titanate nanotubes in a two-step system under visible light irradiation. Water Sci. Technol. 2014, 69, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Lo, S.-L.; Lai, Y.-C.; Liou, Y.-H. Titanate nanotubes coupled with Pt nanoparticles for the inhibition of CdS photocorrosion during visible-light-driven hydrogen production from formic acid. Mater. Res. Express. 2018, 5, 095507. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Peng, S.; Li, Z.; Lu, G. Phosphate-assisted hydrothermal synthesis of hexagonal CdS for efficient photocatalytic hydrogen evolution. CrystEngComm 2012, 14, 6974–6982. [Google Scholar]
- Cai, Y.; Li, X.; Zhang, Y.; Wei, X.; Wang, K.; Chen, J. Highly Efficient Dehydrogenation of Formic Acid over a Palladium-Nanoparticle-Based Mott-Schottky Photocatalyst. Angew. Chem. 2013, 125, 12038–12041. [Google Scholar] [CrossRef]
- Kakuta, S.; Abe, T. A novel example of molecular hydrogen generation from formic acid at visible-light-responsive photocatalyst. ACS Appl. Mater. Interfaces 2009, 1, 2707–2710. [Google Scholar] [CrossRef] [PubMed]
- Loges, B.; Boddien, A.; Gärtner, F.; Junge, H.; Beller, M. Catalytic generation of hydrogen from formic acid and its derivatives: Useful hydrogen storage materials. Top. Catal. 2010, 53, 902–914. [Google Scholar]
- Park, J.Y.; Lee, I.H. Decomposition of acetic acid by advanced oxidation processes. Korean J. Chem Eng. 2009, 26, 387–391. [Google Scholar]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic decomposition of saturated carboxylic acids on titanium dioxide powder. Decarboxylative Route Alkanes. J. Am. Chem. Soc. 1978, 100, 5985–5992. [Google Scholar]
- Yoneyama, H.; Takao, Y.; Tamura, H.; Bard, A.J. Factors influencing product distribution in photocatalytic decomposition of aqueous acetic acid on platinized titania. J. Phys. Chem. A 1983, 87, 1417–1422. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Wei, L.-F.; Zhang, Z.-H.; Jiang, Q.-J.; Wei, Y.-J.; Xie, B.; Wei, M.-B. Research on photocatalytic H2 production from acetic acid solution by Pt/TiO2 nanoparticles under UV irradiation. Int. J. Hydrogen Energy 2009, 34, 9033–9041. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Wei, Y.-J.; Wei, L.-F.; Xie, B.; Wei, M.-B. Photocatalytic H2 production from acetic acid solution over CuO/SnO2 nanocomposites under UV irradiation. Int. J. Hydrogen Energy 2010, 35, 11709–11718. [Google Scholar] [CrossRef]
- Imizcoz, M.; Puga, A.V. Optimising hydrogen production via solar acetic acid photoreforming on Cu/TiO2. Catal. Sci. Technol. 2019, 9, 1098–1102. [Google Scholar] [CrossRef] [Green Version]
- Asal, S.; Saif, M.; Hafez, H.; Mozia, S.; Heciak, A.; Moszyński, D.; Abdel-Mottaleb, M. Photocatalytic generation of useful hydrocarbons and hydrogen from acetic acid in the presence of lanthanide modified TiO2. Int. J. Hydrogen Energy 2011, 36, 6529–6537. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, S.; Liu, Z.; Wang, C.; Mao, Z. Studies on the enhanced photocatalytic hydrogen evolution over Pt/PEG-modified TiO2 photocatalysts. Int. J. Hydrogen Energy 2008, 33, 1112–1117. [Google Scholar] [CrossRef]
- Sreethawong, T.; Puangpetch, T.; Chavadej, S.; Yoshikawa, S. Quantifying influence of operational parameters on photocatalytic H2 evolution over Pt-loaded nanocrystalline mesoporous TiO2 prepared by single-step sol–gel process with surfactant template. J. Power Sources 2007, 165, 861–869. [Google Scholar] [CrossRef]
- Hamid, S.; Ivanova, I.; Jeon, T.H.; Dillert, R.; Choi, W.; Bahnemann, D.W. Photocatalytic conversion of acetate into molecular hydrogen and hydrocarbons over Pt/TiO2: pH dependent formation of Kolbe and Hofer-Moest products. J. Catal. 2017, 349, 128–135. [Google Scholar] [CrossRef]
- Sato, S. Photo-Kolbe reaction at gas-solid interfaces. J. Phys. Chem. A 1983, 87, 3531–3537. [Google Scholar] [CrossRef]
- Sakata, T.; Kawai, T.; Hashimoto, K. Heterogeneous photocatalytic reactions of organic acids and water. New React. Paths Besides Photo-Kolbe React. J. Phys. Chem. A 1984, 88, 2344–2350. [Google Scholar] [CrossRef]
- Heciak, A.; Morawski, A.W.; Grzmil, B.; Mozia, S. Cu-modified TiO2 photocatalysts for decomposition of acetic acid with simultaneous formation of C1–C3 hydrocarbons and hydrogen. Appl. Catal. B 2013, 140, 108–114. [Google Scholar] [CrossRef]
- Mozia, S.; Heciak, A.; Morawski, A.W. Preparation of Fe-modified photocatalysts and their application for generation of useful hydrocarbons during photocatalytic decomposition of acetic acid. J. Photochem. Photobiol. 2010, 216, 275–282. [Google Scholar] [CrossRef]
- Mozia, S.; Heciak, A.; Morawski, A.W. Photocatalytic acetic acid decomposition leading to the production of hydrocarbons and hydrogen on Fe-modified TiO2. Catal. Today 2011, 161, 189–195. [Google Scholar] [CrossRef]
- Sclafani, A.; Palmisano, L.; Schiavello, M.; Augugliaro, V.; Coluccia, S.; Marchese, L. The photodecomposition of ethanoic acid adsorbed over semiconductor and insulator oxides. I. Pure Oxides. New J. Chem. (1987) 1988, 12, 129–135. [Google Scholar]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Singhvi, M.; Khire, J.; Gokhale, D. Strain improvement of Lactobacillus lactis for D-lactic acid production. Biotechnol. Lett. 2010, 32, 517–520. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, Z.; Zhong, Z.; Xu, R. Highly efficient and noble metal-free NiS/CdS photocatalysts for H 2 evolution from lactic acid sacrificial solution under visible light. ChemComm 2010, 46, 7631–7633. [Google Scholar] [CrossRef]
- Harada, H.; Sakata, T.; Ueda, T. Effect of semiconductor on photocatalytic decomposition of lactic acid. J. Am. Chem. Soc. 1985, 107, 1773–1774. [Google Scholar] [CrossRef]
- Harada, H.; Sakata, T.; Ueda, T. Semiconductor effect on the selective photocatalytic reaction of. alpha.-hydroxycarboxylic acids. J. Phys. Chem. A 1989, 93, 1542–1548. [Google Scholar]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef]
- Zong, X.; Han, J.; Ma, G.; Yan, H.; Wu, G.; Li, C. Photocatalytic H2 evolution on CdS loaded with WS2 as cocatalyst under visible light irradiation. J. Phys. Chem. A C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Hu, Z.; Jimmy, C.Y. Pt 3 Co-loaded CdS and TiO2 for photocatalytic hydrogen evolution from water. J. Mater. Chem. 2013, 1, 12221–12228. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Naghadeh, S.B.; Zhang, J.Z.; Fang, P. Visible light driven hydrogen evolution by photocatalytic reforming of lignin and lactic acid using one-dimensional NiS/CdS nanostructures. Appl. Catal. B 2018, 227, 229–239. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.; Wu, G.; Ma, G.; Wen, F.; Wang, L.; Li, C. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef]
- Chen, G.; Li, D.; Li, F.; Fan, Y.; Zhao, H.; Luo, Y.; Yu, R.; Meng, Q. Ball-milling combined calcination synthesis of MoS2/CdS photocatalysts for high photocatalytic H2 evolution activity under visible light irradiation. Appl. Catal. A Gen. 2012, 443, 138–144. [Google Scholar] [CrossRef]
- Lang, D.; Shen, T.; Xiang, Q. Roles of MoS2 and graphene as cocatalysts in the enhanced visible-light photocatalytic H2 production activity of multiarmed CdS nanorods. ChemCatChem 2015, 7, 943–951. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Peng, S. Tunable photodeposition of MoS2 onto a composite of reduced graphene oxide and CdS for synergic photocatalytic hydrogen generation. J. Phys. Chem. A C 2014, 118, 19842–19848. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Shaislamov, U.; Yang, B.L. CdS-sensitized single-crystalline TiO2 nanorods and polycrystalline nanotubes for solar hydrogen generation. J. Mater. Res. 2013, 28, 418–423. [Google Scholar] [CrossRef]
- Hou, Y.; Laursen, A.B.; Zhang, J.; Zhang, G.; Zhu, Y.; Wang, X.; Dahl, S.; Chorkendorff, I. Layered nanojunctions for hydrogen-evolution catalysis. Angew. Chem. 2013, 125, 3709–3713. [Google Scholar] [CrossRef]
- Pham, M.-H.; Dinh, C.-T.; Vuong, G.-T.; Ta, N.-D.; Do, T.-O. Visible light induced hydrogen generation using a hollow photocatalyst with two cocatalysts separated on two surface sides. Phys. Chem. Chem. Phys. 2014, 16, 5937–5941. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Xu, L.; Mou, F.; Guan, J. Single crystalline tantalum oxychloride microcubes: Controllable synthesis, formation mechanism and enhanced photocatalytic hydrogen production activity. ChemComm 2015, 51, 12455–12458. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Y.; Wang, C.-J.; Lv, X.-J.; Fu, W.-F. Spectacular photocatalytic hydrogen evolution using metal-phosphide/CdS hybrid catalysts under sunlight irradiation. ChemComm 2015, 51, 8708–8711. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Chen, Y.; Kang, L.; Lin, Z.; Fu, W.-F. Enhanced photocatalytic H2-evolution by immobilizing CdS nanocrystals on ultrathin Co0.85 Se/RGO–PEI nanosheets. J. Mater. Chem. A 2015, 3, 18711–18717. [Google Scholar] [CrossRef]
- Pan, Y.X.; Zhuang, H.; Hong, J.; Fang, Z.; Liu, H.; Liu, B.; Huang, Y.; Xu, R. Cadmium Sulfide Quantum Dots Supported on Gallium and Indium Oxide for Visible-Light-Driven Hydrogen Evolution from Water. ChemSusChem 2014, 7, 2537–2544. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Yang, L.; Shao, L.; Xu, L. A highly efficient and noble metal-free photocatalytic system using NixB/CdS as photocatalyst for visible light H2 production from aqueous solution. Catal. Commun. 2015, 67, 45–48. [Google Scholar] [CrossRef]
- Hu, J.; Liu, A.; Jin, H.; Ma, D.; Yin, D.; Ling, P.; Wang, S.; Lin, Z.; Wang, J. A versatile strategy for shish-kebab-like multi-heterostructured chalcogenides and enhanced photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 11004–11010. [Google Scholar] [CrossRef]
- Shen, L.; Luo, M.; Liu, Y.; Liang, R.; Jing, F.; Wu, L. Noble-metal-free MoS2 co-catalyst decorated UiO-66/CdS hybrids for efficient photocatalytic H2 production. Appl. Catal. B Environ. 2015, 166, 445–453. [Google Scholar] [CrossRef]
- Liu, K.; Litke, A.; Su, Y.; Van Campenhout, B.G.; Pidko, E.A.; Hensen, E.J. Photocatalytic decarboxylation of lactic acid by Pt/TiO2. ChemComm 2016, 52, 11634–11637. [Google Scholar] [CrossRef]
- Shiragami, T.; Tomo, T.; Tsumagari, H.; Yuki, R.; Yamashita, T.; Yasuda, M. Pentose acting as a sacrificial multielectron source in photocatalytic hydrogen evolution from water by Pt-doped TiO2. Chem. Lett. 2012, 41, 29–31. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Li, S. Photocatalytic transformation of rhodamine B and its effect on hydrogen evolution over Pt/TiO2 in the presence of electron donors. J. Photochem. Photobiol. 2002, 152, 219–228. [Google Scholar] [CrossRef]
- Wei, L.F.; Zheng, X.J.; Zhang, Z.H.; Wei, Y.J.; Xie, B.; Wei, M.B.; Sun, X.L. A systematic study of photocatalytic H2 production from propionic acid solution over Pt/TiO2 photocatalyst. Int. J. Energy Res. 2012, 36, 75–86. [Google Scholar] [CrossRef]
- Scandura, G.; Rodríguez, J.; Palmisano, G. Hydrogen and propane production from butyric acid photoreforming over Pt-TiO2. Front. Chem. 2019, 7, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]

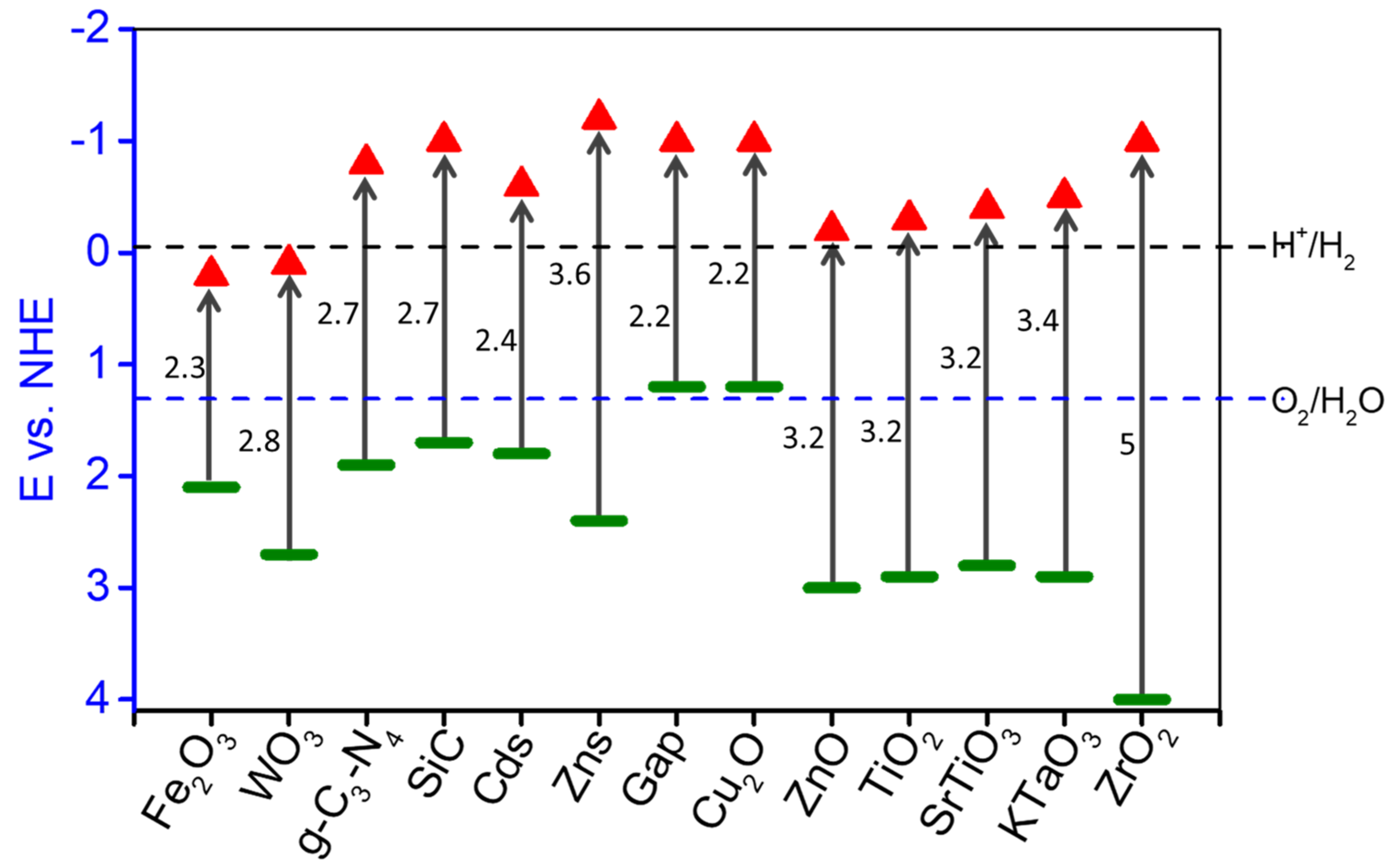
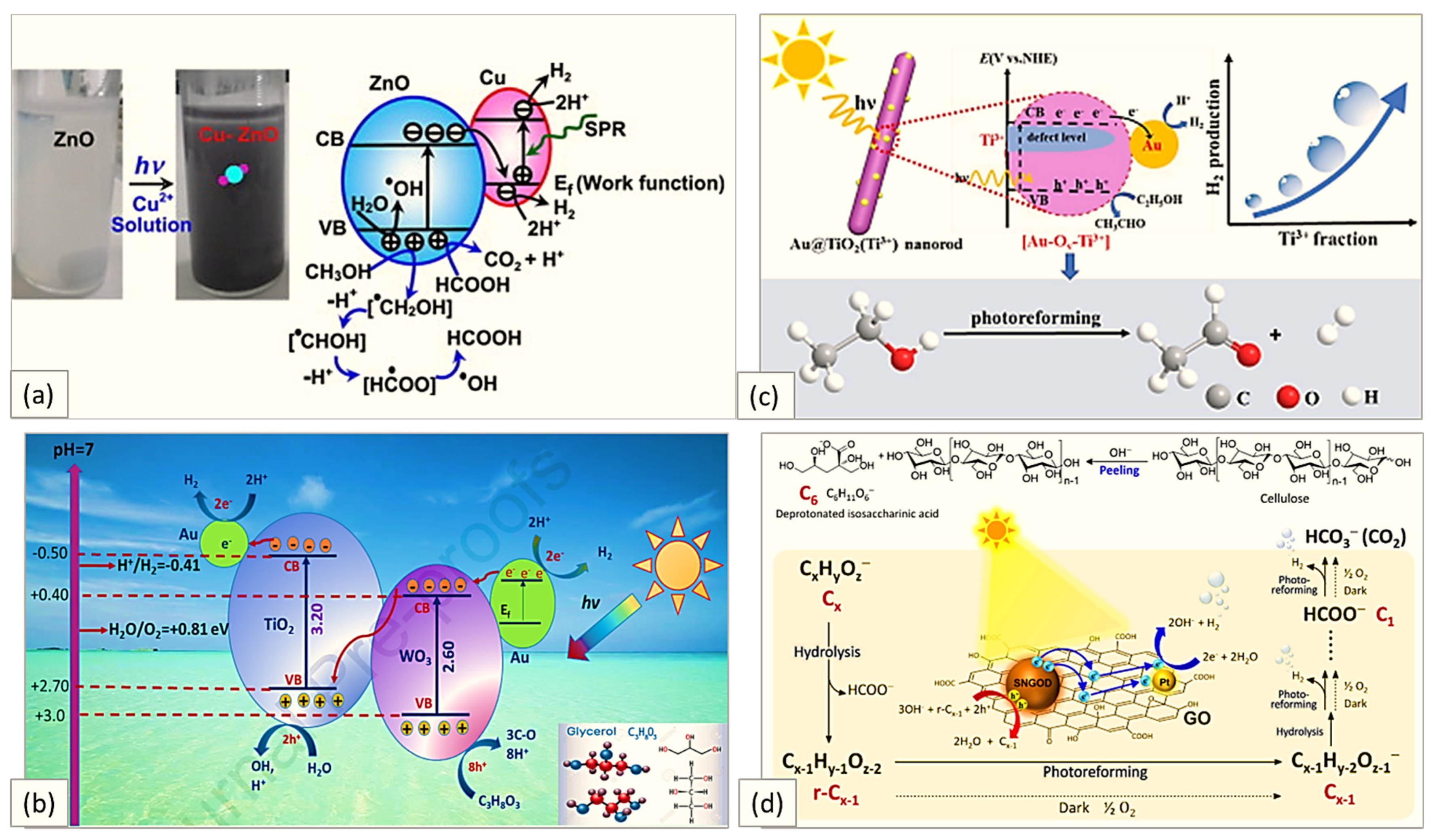
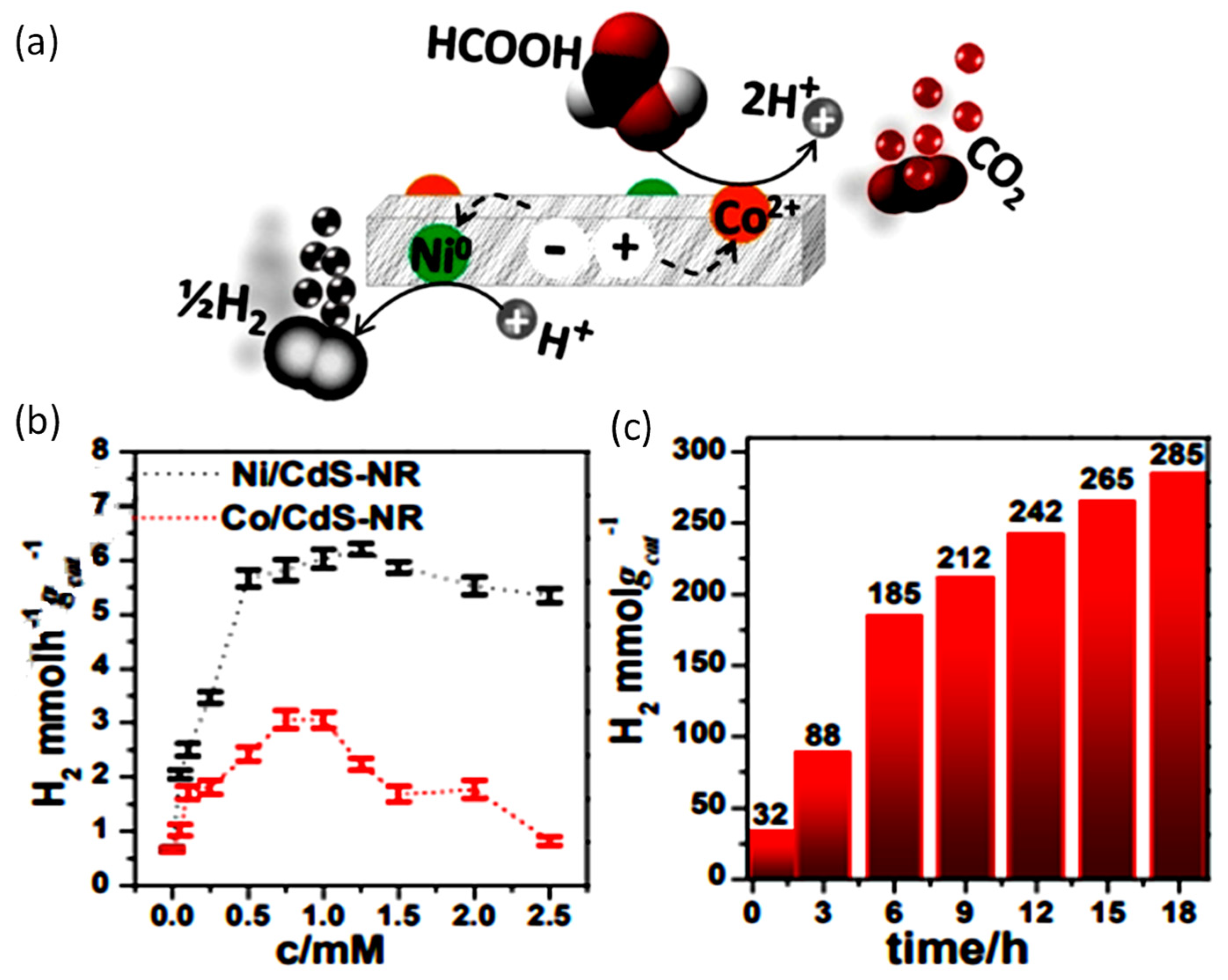
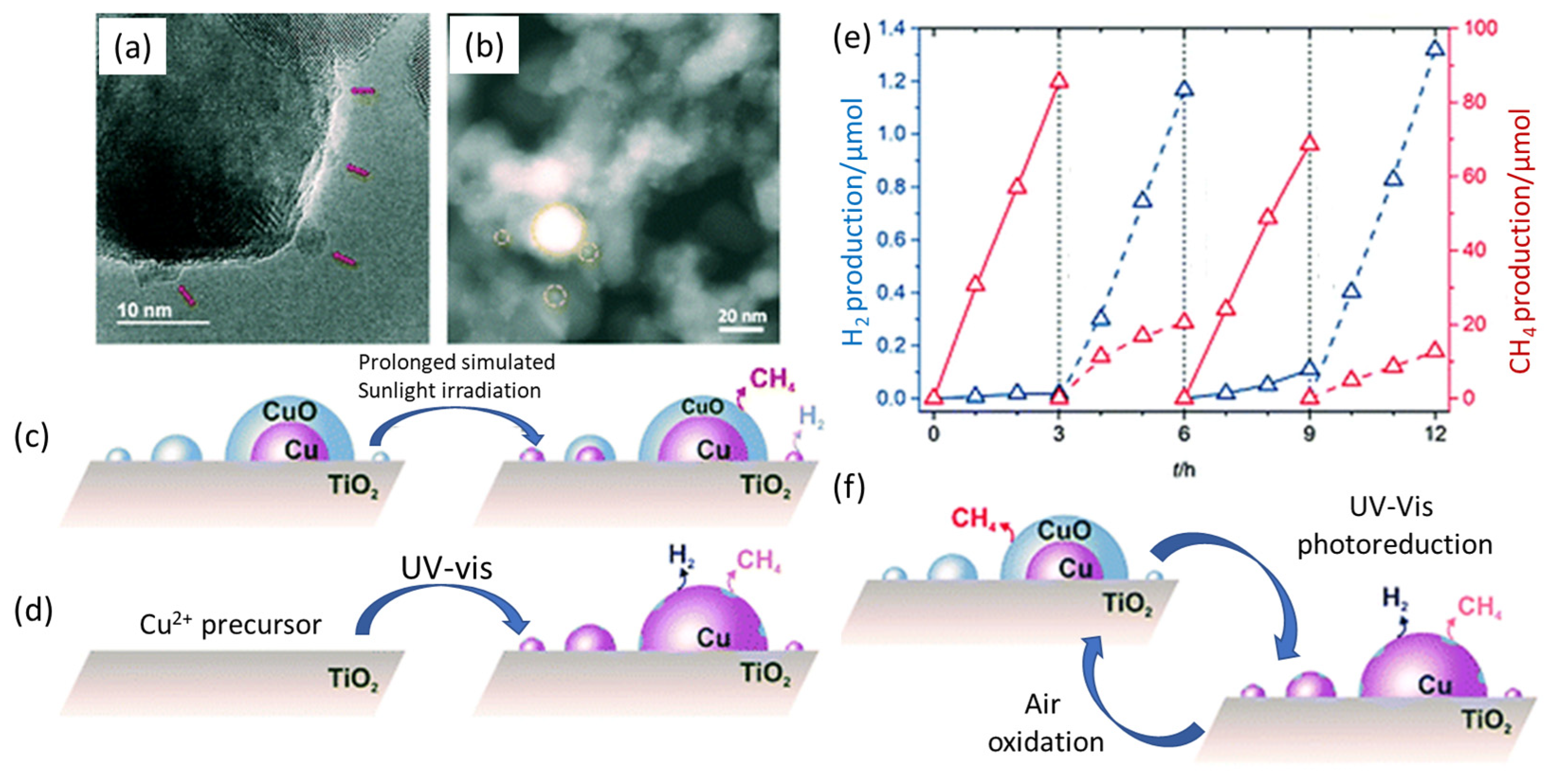
| Concentration | Light Source, Wavelength (nm) | Photocatalyst | Co-Catalyst | Time Course (h) | Rate of Production (μmolg−1 h−1) | Reference | ||
|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | CO | ||||||
| aq. 1.3 mM | solar | 0.5%-Pt/TiO2 | Pt | 20 | 1275 | - | - | [17] |
| aq. 0.01 M | Hg | 0.5%-Pt/TiO2 | Pt | 5 | 1150 | - | - | [72] |
| H2O/HCOOH (10:1), v | Hg, 350–450 | FP-0.5%-Pt/TiO2 | Pt | - | 5400 | 4100 | 80 | [80] |
| 10 vol%, (35 °C) | UV light, 200–800 | 1%-Pt/TiO2 | Pt (photo) | 8 | 61.5 | - | - | [81] |
| 10 vol%, (90 °C) | UV light | 1%-Pt/TiO2 | Pt (thermal) | 8 | 119.3 | - | - | [81] |
| 10 vol%, (photo + 90 °C) | UV light | 1%-Pt/TiO2 | Pt (photothermal) | 8 | 499.8 | - | - | [81] |
| 0.5 M HCO2−/H2O | UV light | CdS | - | 12 | 420 (μL) | - | [82] | |
| 0.5 M DCO2−/H2O | UV light | CdS | - | 12 | 110 (μL) | - | [82] | |
| aq, 5 mL of 88% HCOOH | Hg, >300 | 1.5%-Pt/CdS (photoetching) | Pt | 10 | 1128 | - | - | [83] |
| aq, 5 mL of 88 wt% HCOOH | Hg | CdS | - | 10 | 79 | - | - | [83] |
| aq, 5 mL of 88 wt% HCOOH | Hg, 420 | 0.05%-Pt/CdS | Pt | 10 | 4460 | - | - | [84] |
| aq, 5 mL of 88 wt% HCOOH | Hg | CdS | - | 10 | 219 | - | - | [84] |
| aq, 2 M | Hg, 400 | Pt/CdS | Pt | 20 | 385 | 385 | 77 | [85] |
| aq, 20 mL | Xe, >420 | Co-Ni/CdS-NR | Co, Ni | 18 | 32,600 | - | - | [79] |
| aq, 20 mL | Xe | Co/CdS-NR | Co | 12 | 14,200 | - | - | [79] |
| aq, 20 mL | Xe | Ni/CdS-NR | Ni | 12 | 22,800 | - | - | [79] |
| aq, 20 mL | Xe | CdS-NR | - | 12 | 13,400 | - | - | [79] |
| aq, 2.7 M | Solar, 420–780 | 0.75%-Au, 0.25%-Pd/TiO2 | Au, Pd | 10 | 17,700 | - | - | [86] |
| aq, 200 mL of 2.5 vol% CHOOH | Hg | 1%-Au-loaded mesoporous assembled SrTiO3 | Au | 5 | 647 | - | - | [87] |
| aq, 150 mL H2O + HCOOH | Xe | CdS-ZnS | Cd:Zn (0.8:0.2) | 4 | 1263 | - | - | [88] |
| aq, 150 mL H2O + HCOOH | Xe | 5%-Ru/CdS-ZnS | Ru, Cd:Zn (0.8:0.2) | 4 | 5800 | - | - | [88] |
| aq, 200 mL, 0.05 M | Xe, <355 | Au/TiO2 | Au | 4 | 452 | - | - | [89] |
| aq, (4:1 in volume) H2O:HCOOH | Xe, <420 | 0.34%-Pt/2.5%-CdS/Al-HMS | Pt | 6 | 1705 | - | - | [90] |
| aq, (9:1 in volume) H2O:HCOOH | Xe, <420 | 0.99%Ru/21%-CdS/Al-HMS | Ru | 6 | 2753 | - | - | [91] |
| aq, 1 M | Hg, 399 | SrTiO3: TiO2 | SrTiO3 | 5 | 280 | - | - | [73] |
| aq, 2.5 M | UV light, >420 | QD-MPA | - | 168 | 52,100 | - | [92] | |
| aq, 2.5 M | UV light | QD-MPA/CoCl2 | CoCl2 | 168 | 116,000 | - | [92] | |
| aq, 20 vol% HCOOH | UV light | 0.01%-Pt/CdS/TNT | Pt | 3 | 42,600 | - | - | [93] |
| aq, 10 vol% HCOOH | UV light, 254 | 1%-Pt(P)/CdS/TNT | Pt | 3 | 3300 | - | - | [94] |
| 5 mL HCOOH in 100 mL H2O + 180 °C | UV light, ≥420 | 0.0.25%-Pt/CdS-QD | Pt | 30 | 12,200 | - | - | [95] |
| aq, 10 mL, 1 M HCOOH | UV light, ≥420 | Pd/C3N4 | Pd | 6 | 53,400 | - | - | [96] |
| aq, 1 M HCOOH | UV light | Cu/TiO2 (anatase) | Cu | 5 | 5000 | - | - | [77] |
| aq, 2.5 M HCOOH, pH 5 | Halogen, 420 | 0.5%-Pt/Cu2O | Pt | 40 | 155 | 158 | - | [97] |
| aq, 2.5 M HCOOH, pH 5 | Halogen | Cu2O | - | 40 | 65 | 64 | - | [97] |
| 1 mL DMF, 5 mL 5HCO2H ·2NEt3 | Xe | [Fe3 (CO)12] + PPh3,2,2′:6′,2″-terpyridine | 3 | 2700 | - | trace | [98] | |
| Concentration | Light Source, Wavelength (nm) | Photocatalyst | Co-Catalyst | Time Course (h) | Rate of Production (μmolg−1 h−1) | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | CH4 | C2H6 | ||||||
| aq, 1 M CH3COOH | UV light | Ln3+(0.02%-Eu)/TiO2 | Ln3+ (Eu3+) | 27 | 9 | 88 | 108 | 4 | [106] |
| aq, 1 M CH3COOH | UV light | Ln3+(0.05%-Sm)/TiO2 | Ln3+ (Sm3+) | 27 | 3 | 131 | 124 | 3 | [106] |
| aq, 0.87 mM CH3COOH | Xe | 0.5%-Pt/P25TiO2 | Pt | 20 | 278 | - | - | - | [17] |
| aq, H2O/CH3COOH (5:1) | Hg | 0.5%Pt/PEG-TiO2 | Pt | 6 | 1000 | - | - | - | [107] |
| aq, H2O/CH3COOH (10:1) | Hg | 0.6%-Pt/TiO2 (mesoporous) | Pt | 5 | 390 | - | - | - | [108] |
| aq, 50 mL (0.5 M) CH3COOH and H2O, pH 2 | Xe | 1%-Pt/TiO2 | Pt | 15 | 22 (μmol/h) | 65 (μmol/h) | 35 (μmol/h) | 2 (μmol/h) | [109] |
| aq, 450 mL CH3COOH (4.35 g/L), pH 1.0 | Hg | 1%Pt-TiO2 | Pt | 4 | 28,478 | - | - | - | [103] |
| l, 15 mL solution CH3COOH/Na HAC | Xe-Hg | 1–5%-Pt/TiO2(anatase) | Pt | - | 1600 | - | - | [101] | |
| l, H2O/CH3COOH (1:9) | Xe-Hg | 1–5%-Pt/TiO2(anatase) | Pt | - | 4060 | - | - | [101] | |
| l, H2O/CH3COOH (1:1) | Xe-Hg | 1–5%-Pt/TiO2(anatase) | Pt | - | 1563 | 1322 | 120 | [101] | |
| aq, AcOH/Na [AcO] (4:0.6 M), pH 3.9 | Hg-conc. | 3%-Pt/TiO2 (anatase) | Pt | 24 | 65 | 43 | 4 | [102] | |
| aq, AcOH/Na [AcO] (4:0.6 M), pH 3.9 | Hg-conc. | 3%-Pt/TiO2 (rutile) | Pt | 9 | 11 | 10 | 1 | [102] | |
| v, CH3COOH (11 torr) | Hg | 2%-Pt/TiO2(anatase) | Pt | 3 | 46 | 132 | 54 | 43 | [110] |
| v, H2O/CH3COOH (24:11 torr) | Hg | 2%-Pt/TiO2(anatase) | Pt | 3 | 180 | 453 | 104 | 180 | [110] |
| l, CH3COOH (1 mL) | Hg | 2%-Pt/TiO2(anatase) | Pt | 3 | 46 | 290 | 163 | 12 | [110] |
| l, H2O/CH3COOH (6:1), pH 2.1 | Hg, 366 | 7%-Pt/TiO2 (rutile) | Pt | 77 | - | 397 | - | [111] | |
| l, H2O/CH3COOH (6:1), pH 8.8 | Hg | 7%-Pt/TiO2 (rutile) | Pt | 367 | - | 2 | - | [111] | |
| aq, Na (AcO) (1.7% w/v), pH 7.4 | Hg | 7%-Pt/TiO2 (anatase) | Pt | 165 | 27 | 0.24 | - | [111] | |
| aq, 1 M CH3COOH, pH 2.6 | UV light | 10%-Cu/TiO2 | Cu | 5 | 144 | 640 | 590 | 66 | [112] |
| aq, 1 M CH3COOH | UV light, 366 | P25TiO2 | 27 | 2 | 141 | 50 | 4 | [113] | |
| aq, 1 M CH3COOH | UV light, 366 | 10%-Fe/TiO2 | Fe | 27 | 7 | 102 | 93 | 4 | [113] |
| aq, 1 M CH3COOH | Hg | 20-%Fe/TiO2 | Fe | 5 | 7 | 257 | 260 | 15 | [114] |
| v, CH3COOH (665 Pa) | Hg-Xe, >420 | TiO2 | - | - | 0.370 | 0.018 | - | [115] | |
| v, CH3COOH (665 Pa) | Hg-Xe | ZnO2 | - | - | 0.098 | 0.034 | - | [115] | |
| v, CH3COOH (665 Pa) | Hg-Xe | MgO | - | - | 0.923 | 0.179 | - | [115] | |
| v, CH3COOH (665 Pa) | Hg-Xe | SiO2 | - | - | 0.336 | 0.168 | - | [115] | |
| v, CH3COOH (665 Pa) | Hg-Xe | WO3 | - | - | 0.026 | 0.03 | - | [115] | |
| v, CH3COOH (665 Pa) | Hg-Xe | γ-Al2O3 | - | - | 0.336 | 0.086 | - | [115] | |
| Concentration | Light Source, Wavelength (nm) | Photocatalyst | Co-Catalyst | Time Course (h) | Rate of Production (μmolg−1 h−1) | Reference | |
|---|---|---|---|---|---|---|---|
| H2 | CO2 | ||||||
| l, CH3CH(OH)COOH/H2O (1:10) pH 2 | Xe, 360–520 | 5%-Pt/TiO2 (rutile) | Pt | 4 | 1008 | 1192 | [120] |
| l, CH3CH(OH)COOH/H2O (1:10) pH 2 | Xe | 5%-Pt/CdS | Pt | 4 | 1000 | 13 | [120] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe, 420 | 1%-Pt/CdS | Pt | 8170 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt3Co)/CdS | Pt, Co | 15,860 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt2.3-Co)/CdS | Pt, Co | 13,010 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(PtCo)/CdS | Pt, Co | 7050 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-Co/CdS | Co | 1070 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt3Au)/CdS | Pt, Au | 14,900 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt3Ni)/CdS | Pt, Ni | 12,810 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt3Cu)/CdS | Pt, Cu | 3890 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-Pt/P25TiO2 | Pt | 690 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt3Co)/P25TiO2 | Pt, Co | 1040 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt3Au)/P25TiO2 | Pt, Au | 890 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-(Pt3Ni)/P25TiO2 | Pt, Ni | 820 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe, 420 | 1%-(Pt3Cu)/P25TiO2 | Pt, Cu | 460 | - | [123] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 0.2%-MoS2/CdS | MoS2 | 5400 | - | [125] | |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 0.2%-Pt/CdS | Pt | 5 | 4400 | - | [125] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 0.2%-Ru/CdS | Ru | 5 | 3650 | - | [125] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 0.2%-Rh/CdS | Rh | 5 | 2500 | - | [125] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 0.2%-Pd/CdS | Pd | 5 | 1800 | - | [125] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 0.2%-Au/CdS | Au | 5 | 400 | - | [125] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe, 420 | CdS | - | 5 | 150 | - | [125] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe, 420 | 1%-WS2/CdS | WS2 | 5 | 4000 | - | [122] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-Pt/CdS | Pt | 5 | 3550 | - | [122] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-Ru/CdS | Ru | 5 | 2930 | - | [122] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-Rh/CdS | Rh | 5 | 2070 | - | [122] |
| l, CH3CH(OH)COOH/H2O (1:9) | Xe | 1%-Au/CdS | Au | 5 | 455 | - | [122] |
| aq, H2O/CH3CH(OH)COOH (10:1) | Xe | 0.9%-MoS2/CdS | MoS2 | 5 | 13,151 | - | [126] |
| aq, H2O/CH3CH(OH)COOH (10:1) | Xe, 420 | 0.2%-Pt/CdS | Pt | 5 | 4880 | - | [126] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 2.5%-MoS2-RGO/CdS | MoS2-RGO | 621.3 | - | [127] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | 2.5%-MoS2/CdS | MoS2 | 551.3 | [127] | ||
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | 0.25%-Pt/CdS | Pt | 450 | - | [127] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 1.5%-RGO/CdS/1.5%-MoS2 | RGO, MoS2 | 5 | 1980 | - | [128] |
| aq, H2O/CH3CH(OH)COOH (4:1) | Xe, 420 | 2%-(MoS2/RGO)/CdS | MoS2, RGO | 5 | 9000 | - | [129] |
| aq, H2O/CH3CH(OH)COOH (7:3) | Xe, 420 | 1.2%-NiS/CdS | NiS | 7267 | - | [118] | |
| aq, H2O/CH3CH(OH)COOH (7:3) | Xe, 420 | CdS | - | 210 | [118] | ||
| aq, H2O/CH3CH(OH)COOH (7:3) | Xe | 1%-Pt/CdS | Pt | 1333 | - | [118] | |
| aq, H2O/CH3CH(OH)COOH (7:3) | Xe | 1.2%-CoS/CdS | CoS | 1000 | - | [118] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | TiO2-1.2%-Pt/CdS | Pt, TiO2 NPs | 14,750 | - | [130] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | P25TiO2-2%Pt/Cds | Pt, TiO2 NPs | 13,750 | - | [130] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 2%-Pt/P25TiO2 | 3875 | - | [130] | ||
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 0.5%-Pt/0.5%-RGO/CdS | Pt, RGO | 19,000 | - | [121] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | 0.5%-Pt/1%-RGO/CdS | Pt, RGO | 56,000 | - | [121] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | 0.5%-Pt/2.5%-RGO/CdS | Pt, RGO | 27,500 | - | [121] | |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 0.2%-MoS2/g-C3N4(mesoporous) | MoS2 | 4 | 1375 | - | [131] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | 2%-Pt/g-C3N4(mesoporous) | Pt | 4 | 1000 | - | [131] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | 0.5%-WS2/g-C3N4(mesoporous) | WS2 | 4 | 340 | - | [131] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | Fe2O3-TiO2-PtOx | Fe2O3, PtOx | 5 | 1100 | - | [132] |
| aq, H2O/CH3CH(OH)COOH (7:3) | Xe, 280 | 5%-Pt/TaO2.18Cl0.64 | Pt | 8 | 1500 | - | [133] |
| aq, H2O/CH3CH(OH)COOH (19:1), pH 3 | LED, 420-780 | CoP/CdS | CoP | 10 | 251,500 | - | [134] |
| aq, H2O/CH3CH(OH)COOH (19:1), pH 3 | LED | Ni2P/CdS | Ni2P | 10 | 143,600 | - | [134] |
| aq, H2O/CH3CH(OH)COOH (19:1), pH 3 | LED | Cu3P/CdS | Cu3P | 10 | 77,600 | - | [134] |
| aq, H2O/CH3CH(OH)COOH (19:1), pH 3 | LED | Pt/CdS | Pt | 10 | 77,300 | - | [134] |
| aq, H2O/CH3CH(OH)COOH (20:3) | LED, 420 | Co0.85Se/RGO–PEI nanosheets/CdS | Co0.85Se/RGO–PEI nanosheets | 10 | 17,600 | - | [135] |
| aq, H2O/CH3CH(OH)COOH (20:3) | LED | 0.1%-Pt/CdS | Pt | 10 | 18,600 | - | [135] |
| aq, H2O/CH3CH(OH)COOH (4:1) | Xe, 420 | 9%-CdS/1%-Pt/In2O3 | Pt | >10 | 9384 | - | [136] |
| aq, H2O/CH3CH(OH)COOH (4:1) | Xe | 9%-CdS/1%-Pt/Ga2O3 | Pt | >10 | 9053 | - | [136] |
| aq, H2O/CH3CH(OH)COOH (4:1) | Xe, 420 | 9%-CdS/1%-Pt/P25TiO2 | Pt | >10 | 5482 | - | [136] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 0.8%-NiBx/CdS | NiBx | 10 | 4800 | - | [137] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 9%-NiS-37%-CdS/Te | NiS, Te | 12 | 317 | - | [138] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe, 420 | 11%-Pt/11%-Pd/31%-CdS/Te | Pt, Pd, Te | 12 | 236 | - | [138] |
| aq, H2O/CH3CH(OH)COOH (9:1) | Xe | 1.5%-MoS2/UiO-66//CdS | MoS2 | 4 | 32,500 | - | [139] |
| aq, CH3CH(OH)COOH (0.1 M) | Hg-Xe, 420 | TiO2 | 3 | 0 | 693 | [140] | |
| aq, CH3CH(OH)COOH (0.1 M) | Hg-Xe | 1%-Pt/TiO2 | Pt | 3 | 13,890 | 13,500 | [140] |
| aq, CH3CH(OH)COOH (0.1 M) | Hg-Xe | 1%-Au/TiO2 | Au | 3 | 6667 | 6600 | [140] |
| aq, CH3CH(OH)COOH (0.1 M) | Hg-Xe | 1%-Pd/TiO2 | Pd | 3 | 8300 | 8700 | [140] |
| aq, CH3CH(OH)COOH (0.1 M) | Hg-Xe | 1%-Ru/TiO2 | Ru | 3 | 11,500 | 11,120 | [140] |
| Acid Concentration | Light Source, Wavelength (nm) | Photocatalyst | Co-Catalyst | Time Course (h) | Rate of Production (μmolg−1 h−1) | Reference | ||
|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | C2H6 | ||||||
| aq, 0.01 M HOOCCOOH (Oxalic acid), pH 2 | Hg | 0.5%-Pt/P25TiO2 | Pt | 5 | 2850 | - | - | [72] |
| aq, 0.049 M HOOCCOOH (Oxalic acid) | Hg | 0.5%-Pt/P25TiO2 | Pt | 5 | 1160 | - | - | [71] |
| aq, 0.001 M HOOCCOOH (Oxalic acid) | Hg | 0.3%-Pt/P25TiO2 | Pt | 5.5 | 3750 | - | - | [142] |
| aq, 1.3 g/L HOCH2(CHOH)4COOH (Gluconic acid) | Hg | 2%-Pt/TiO2 | Pt | 30 | 675 | 296 | - | [141] |
| aq, 0.51 g/L H2O/HOCH2COOH (Glycolic acid) | Hg | 2%-Pt/TiO2 | Pt | 46 | 284 | 109 | - | [141] |
| H2O/HOCH2COOH (Glycolic acid) (1:10 v/v) | Xe, 360–520 | 5%-Pt/TiO2(rutile) | Pt | 5 | 105 | 76 | - | [120] |
| H2O/HOCH2COOH (Glycolic acid) (1:10) | Xe | 5%-Pt/CdS | Pt | 5 | 392 | 3 | - | [120] |
| aq, C18H36O2 or CH3(CH2)16COOH (Stearic acid) (1.7%w/v) | Xe | 5%-Pt/TiO2 | Pt | 24 | 29 | 0 | - | [60] |
| aq, H2O/CH3CH2COOH (Propionic acid) (6:1) | Hg, 320 | 7%-Pt/TiO2(rutile) | Pt | 51 | - | 490 | [111] | |
| aq, H2O/CH3CH2CH2COOH (L-Propionic acid) (6:1) | Hg, 320 | 7%-Pt/TiO2(rutile) | Pt | 111 | - | 332 | [111] | |
| aq, H2O/CH3(CH2)3COOH (6:1) | Hg | 7%-Pt/TiO2(rutile) | Pt | 174 | - | 339 | [111] | |
| l, 6.66 g/L CH3CH2COOH (Butyric acid) | Hg, 420 | 1%-Pt/TiO2 | Pt | >16 | 6800 (μmol h−1) | - | - | [143] |
| aq, 5 mM CH3CH2CH2COOH (Valeric acid) (400 °C) | LED, 360–370 | Pt/TiO2-NTs | Pt | 8 | 387.5 | 362.5 | - | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samage, A.; Gupta, P.; Halakarni, M.A.; Nataraj, S.K.; Sinhamahapatra, A. Progress in the Photoreforming of Carboxylic Acids for Hydrogen Production. Photochem 2022, 2, 580-608. https://doi.org/10.3390/photochem2030040
Samage A, Gupta P, Halakarni MA, Nataraj SK, Sinhamahapatra A. Progress in the Photoreforming of Carboxylic Acids for Hydrogen Production. Photochem. 2022; 2(3):580-608. https://doi.org/10.3390/photochem2030040
Chicago/Turabian StyleSamage, Anita, Pooja Gupta, Mahaveer A. Halakarni, Sanna Kotrappanavar Nataraj, and Apurba Sinhamahapatra. 2022. "Progress in the Photoreforming of Carboxylic Acids for Hydrogen Production" Photochem 2, no. 3: 580-608. https://doi.org/10.3390/photochem2030040
APA StyleSamage, A., Gupta, P., Halakarni, M. A., Nataraj, S. K., & Sinhamahapatra, A. (2022). Progress in the Photoreforming of Carboxylic Acids for Hydrogen Production. Photochem, 2(3), 580-608. https://doi.org/10.3390/photochem2030040





