Phosphorescence of C5N− in Rare Gas Solids
Abstract
1. Introduction
2. Experimental
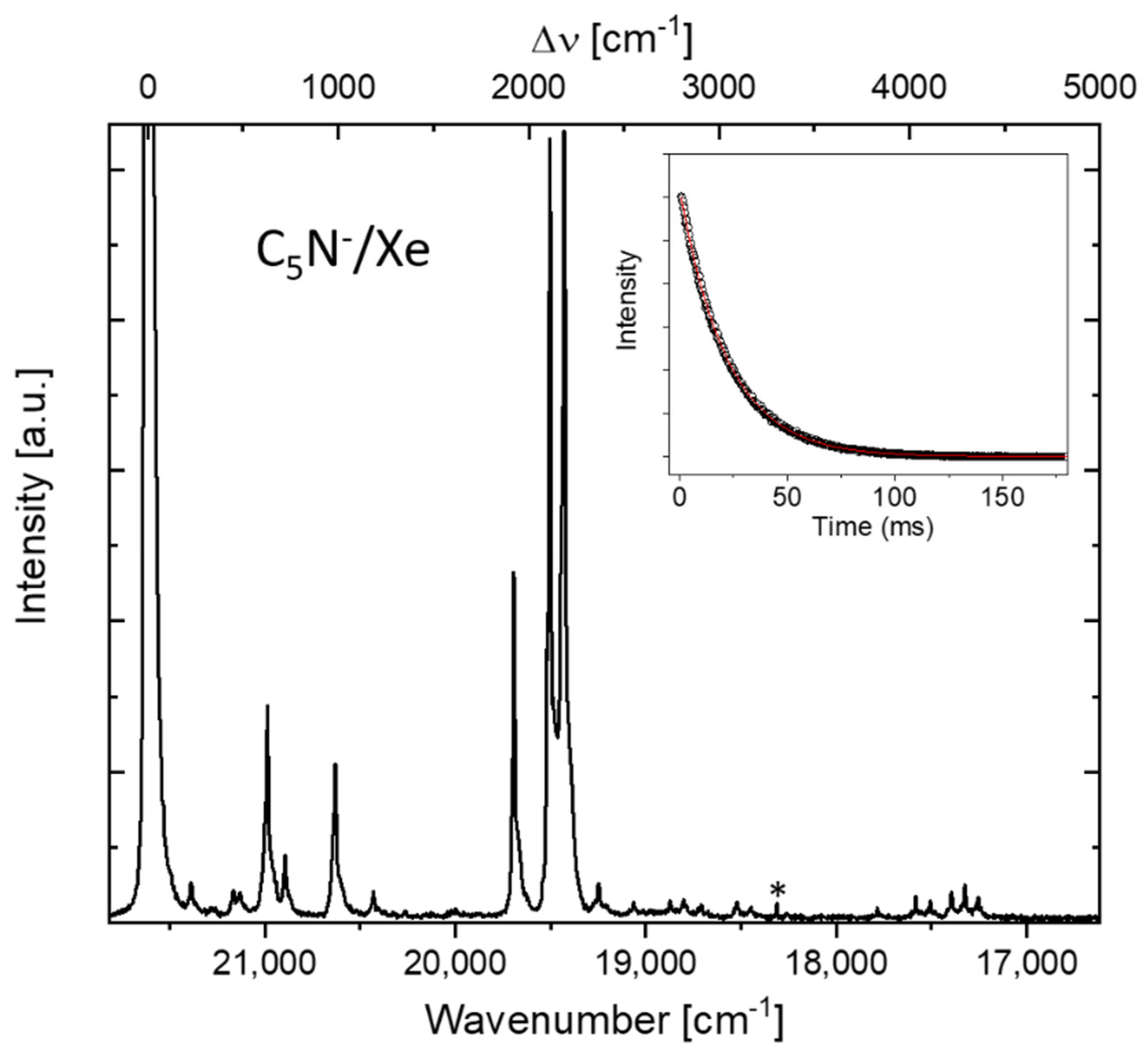
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, B.E. Detection of Interstellar Cyanoacetylene. Astrophys. J. Lett. 1971, 163, L35–L39. [Google Scholar] [CrossRef]
- Mauersberger, R.; Henkel, C.; Sage, L.J. Dense Gas in Nearby Galaxies. III-HC3N as an Extragalactic Density Probe. Astron. Astrophys. 1990, 236, 63–68. [Google Scholar]
- Kunde, V.G.; Aikin, A.C.; Hanel, R.A.; Jennings, D.E.; Maguire, W.C.; Samuelson, R.E. C4H2, HC3N and C2N2 in Titan’s Atmosphere. Nature 1981, 292, 686–688. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Lis, D.C.; Wink, J.E.; Despois, D.; Crovisier, J.; Bachiller, R.; Benford, D.J.; Biver, N.; Colom, P.; Davies, J.K.; et al. New Molecules Found in Comet C/1995 O1 (Hale-Bopp): Investigating the Link between Cometary and Interstellar Material. Astron. Astrophys. 2000, 353, 1101–1114. [Google Scholar]
- Snell, R.L.; Schloerb, F.P.; Young, J.S.; Hjalmarson, A.; Friberg, P. Observations of HC3N, HC5N, and HC7N in Molecular Clouds. Astrophys. J. 1981, 244, 45. [Google Scholar] [CrossRef]
- Broten, N.W.; Oka, T.; Avery, L.W.; MacLeod, J.M.; Kroto, H.W. The Detection of HC9N in Interstellar Space. Astrophys. J. 1978, 223, L105–L107. [Google Scholar] [CrossRef]
- Loomis, R.A.; Burkhardt, A.M.; Shingledecker, C.N.; Charnley, S.B.; Cordiner, M.A.; Herbst, E.; Kalenskii, S.; Lee, K.L.K.; Willis, E.R.; Xue, C.; et al. An Investigation of Spectral Line Stacking Techniques and Application to the Detection of HC11N. Nat. Astron. 2021, 5, 188–196. [Google Scholar] [CrossRef]
- Thaddeus, P.; Gottlieb, C.A.; Gupta, H.; Brünken, S.; McCarthy, M.C.; Agúndez, M.; Guélin, M.; Cernicharo, J. Laboratory and Astronomical Detection of the Negative Molecular Ion C3N−. Astrophys. J. 2008, 677, 1132–1139. [Google Scholar] [CrossRef]
- Cernicharo, J.; Guélin, M.; Agúndez, M.; McCarthy, M.C.; Thaddeus, P. Detection of C5N− and Vibrationally Excited C6H in IRC +10216. Astrophys. J. 2008, 688, L83–L86. [Google Scholar] [CrossRef]
- Vuitton, V.; Lavvas, P.; Yelle, R.V.; Galand, M.; Wellbrock, A.; Lewis, G.R.; Coates, A.J.; Wahlund, J.E. Negative Ion Chemistry in Titan’s Upper Atmosphere. Planet. Space Sci. 2009, 57, 1558–1572. [Google Scholar] [CrossRef]
- Kołos, R.; Gronowski, M.; Botschwina, P. Matrix Isolation IR Spectroscopic and Ab Initio Studies of C3N− and Related Species. J. Chem. Phys. 2008, 128, 154305. [Google Scholar] [CrossRef] [PubMed]
- Coupeaud, A.; Turowski, M.; Gronowski, M.; Piétri, N.; Couturier-Tamburelli, I.; Kołos, R.; Aycard, J.P. C5N− Anion and New Carbenic Isomers of Cyanodiacetylene: A Matrix Isolation IR Study. J. Chem. Phys. 2008, 128, 154303. [Google Scholar] [CrossRef] [PubMed]
- Stanca-Kaposta, E.C.; Schwaneberg, F.; Fagiani, M.R.; Wende, T.; Hagemann, F.; Wünschmann, A.; Wöste, L.; Asmis, K.R. Infrared Photodissociation Spectroscopy of C2n+1N− Anions with n = 1–5. Z. Phys. Chem. 2014, 228, 351–367. [Google Scholar] [CrossRef]
- Grutter, M.; Wyss, M.; Maier, J.P. Electronic Absorption Spectra of C2nH−, C2n−1N− (n = 4–7), and C2n−1N (n = 3–7) Chains in Neon Matrices. J. Chem. Phys. 1999, 110, 1492–1496. [Google Scholar] [CrossRef]
- Yen, T.A.; Garand, E.; Shreve, A.T.; Neumark, D.M. Anion Photoelectron Spectroscopy of C3N− and C5N−. J. Phys. Chem. A 2010, 114, 3215–3220. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, J.; Ekern, S.; Vala, M. Vibrational Spectroscopy of Small Matrix-Isolated Linear Carbon Cluster Anions. J. Phys. Chem. A 1997, 101, 1841–1847. [Google Scholar] [CrossRef]
- Botschwina, P.; Oswald, R. Carbon Chains of Type C2n+1N− (N = 2–6): A Theoretical Study of Potential Interstellar Anions. J. Chem. Phys. 2008, 129, 044305. [Google Scholar] [CrossRef]
- Skomorowski, W.; Gulania, S.; Krylov, A.I. Bound and Continuum-Embedded States of Cyanopolyyne Anions. Phys. Chem. Chem. Phys. 2018, 20, 4805–4817. [Google Scholar] [CrossRef]
- Turowski, M.; Gronowski, M.; Boyé-Péronne, S.; Douin, S.; Moneron, L.; Crépin, C.; Kołos, R. The C3N− Anion: First Detection of Its Electronic Luminescence in Rare Gas Solids. J. Chem. Phys. 2008, 128, 164304. [Google Scholar] [CrossRef] [PubMed]
- Trolez, Y.; Guillemin, J.-C. Synthesis and Characterization of 2,4-Pentadiynenitrile—A Key Compound in Space Science. Angew. Chem. Int. Ed. 2005, 44, 7224–7226. [Google Scholar] [CrossRef] [PubMed]
- Turowski, M.; Crépin, C.; Gronowski, M.; Guillemin, J.C.; Coupeaud, A.; Couturier-Tamburelli, I.; Pietri, N.; Kołos, R. Electronic Absorption and Phosphorescence of Cyanodiacetylene. J. Chem. Phys. 2010, 133, 074310. [Google Scholar] [CrossRef]
- Szczepaniak, U.; Kołos, R.; Gronowski, M.; Chevalier, M.; Guillemin, J.C.; Crépin, C. Synthesis and Electronic Phosphorescence of Dicyanooctatetrayne (NC10N) in Cryogenic Matrixes. J. Phys. Chem. A 2018, 122, 5580–5588. [Google Scholar] [CrossRef]
- Couturier-Tamburelli, I.; Piétri, N.; Crépin, C.; Turowski, M.; Guillemin, J.C.; Kołos, R. Synthesis and Spectroscopy of Cyanotriacetylene (HC7N) in Solid Argon. J. Chem. Phys. 2014, 140, 044329. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, U.; Kołos, R.; Gronowski, M.; Chevalier, M.; Guillemin, J.-C.; Turowski, M.; Custer, T.; Crépin, C. Cryogenic Photochemical Synthesis and Electronic Spectroscopy of Cyanotetracetylene. J. Phys. Chem. A 2017, 121, 7374–7384. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, U.; Ozaki, K.; Tanaka, K.; Ohnishi, Y.; Wada, Y.; Guillemin, J.C.; Crépin, C.; Kołos, R.; Morisawa, Y.; Suzuki, H.; et al. Phosphorescence Excitation Mapping and Vibrational Spectroscopy of HC9N and HC11N Cyanopolyynes in Organic Solvents. J. Mol. Struct. 2020, 1214, 128201. [Google Scholar] [CrossRef]
- Turowski, M.; Crépin, C.; Couturier-Tamburelli, I.; Pietri, N.; Kołos, R. Low-Temperature Phosphorescence of Dicyanoacetylene in Rare Gas Solids. Low Temp. Phys. 2012, 38, 723–726. [Google Scholar] [CrossRef][Green Version]
- Crépin, C.; Turowski, M.; Ceponkus, J.; Douin, S.; Boyé-Péronne, S.; Gronowski, M.; Kołos, R. UV-Induced Growth of Cyanopolyyne Chains in Cryogenic Solids. Phys. Chem. Chem. Phys. 2011, 13, 16780–16785. [Google Scholar] [CrossRef]
- Turowski, M.; Crépin, C.; Douin, S.; Kołos, R. Formation and Spectroscopy of Dicyanotriacetylene (NC8N) in Solid Kr. J. Phys. Chem. A 2015, 119, 2701–2708. [Google Scholar] [CrossRef]
- Szczepaniak, U. Spectroscopy and Photochemistry of Astrophysically-Relevant Molecules of the Cyanoactylene Family. Ph.D. Thesis, Institute of Physical Chemistry, Polish Academy of Sciences, Warsaw, Poland, Université Paris-Saclay, Université Paris-Sud-Paris XI, Orsay, France, 2017. Available online: https://tel.archives-ouvertes.fr/tel-01562041/ (accessed on 1 February 2022).
- Wright, M.R.; Frosch, R.P.; Robinson, G.W. Phosphorescence Lifetime of Benzene. an Intermolecular Heavy-Atom Effect, a Deuterium Effect, and a Temperature Effect. J. Chem. Phys. 1960, 33, 934–935. [Google Scholar] [CrossRef]
- Minaev, B.F. External Heavy-Atom Effects on Radiative Singlet-Triplet Transitions. J. Appl. Spectrosc. 1985, 43, 887–890. [Google Scholar] [CrossRef]
- Minaev, B. Theoretical Study of the External Heavy Atom Effect on Phosphorescence of Free-Base Porphin Molecule. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2004, 60, 3213–3224. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikov, G.; Minaev, B.; Ågren, H. Theory and Calculation of the Phosphorescence Phenomenon. Chem. Rev. 2017, 117, 6500–6537. [Google Scholar] [CrossRef] [PubMed]
- Turowski, M.; Gronowski, M.; Guillemin, J.C.; Kołos, R. Generation of H-Kr-C5N and H-Xe-C5N Molecules. J. Mol. Struct. 2012, 1025, 140–146. [Google Scholar] [CrossRef]
- Kunttu, H.M.; Seetula, J.A. Photogeneration of Ionic Species in Ar, Kr and Xe Matrices Doped with HCl, HBr and HI. Chem. Phys. 1994, 189, 273–292. [Google Scholar] [CrossRef]

| Ar | Kr | Xe | Involved Mode | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | IR | |||||||
| 21,720 | 21,721/21,697 | 21,614 | ||||||
| ~19,800 | 1920 | 1923.2 | 19,798/19,767 | 1923/1930 | 19,692 | 1922 | 1925.4 | ν1 |
| 19,610 | 2110 | 2111.3 | 19,610/19,577 | 2111/2120 | 19,505 | 2109 | 2119.1 | ν2 |
| 19,540 | 2180 | 2183.8 | 19,543/19,510 | 2178/2187 | 19,428 | 2186 | 2191.2 | ν3 |
| λ | Intensity 1 | Assignment | ||
|---|---|---|---|---|
| 462.7 | 21,614 | 0 | vvs | |
| 467.5 | 21,388 | 226 | vw | (or ) |
| 470.4 | 21,279 | 335 | vvw | |
| 472.5 | 21,164 | 450 | vw | (or ) |
| 473.3 | 21,130 | 484 | vw | |
| 476.5 | 20,985 | 629 | m | |
| 478.6 | 20,893 | 721 | w | |
| 484.8 | 20,629 | 985 | m | |
| 489.5 | 20,429 | 1184 | vw | |
| 507.8 | 19,692 | 1922 | s | |
| 512.7 | 19,505 | 2109 | vs | |
| 514.7 | 19,428 | 2185 | vs | |
| 519.6 | 19,246 | 2368 | vw | |
| 524.6 | 19,063 | 2551 | vvw | |
| 530.0 | 18,869 | 2744 | vvw | |
| 531.9 | 18,799 | 2815 | vvw | |
| 534.5 | 18,707 | 2906 | vvw | |
| 540.0 | 18,520 | 3094 | vvw | |
| 542.1 | 18,447 | 3167 | vvw | |
| 562.3 | 17,784 | 3830 | vvw | |
| 568.8 | 17,582 | 4032 | vw | |
| 571.3 | 17,505 | 4109 | vw | |
| 574.9 | 17,394 | 4220 | vw | |
| 577.2 | 17,324 | 4290 | vw | |
| 579.6 | 17,252 | 4361 | vw |
| Mode, Symmetry | Theory | Experiment | ||
|---|---|---|---|---|
| DFT 1 | CCSD(T) 2 | Ar Matrix 3 | Xe Matrix 4 | |
| ν1 σ | 2184 | 2202.6 | 2183.8 | 2186 |
| ν2 σ | 2116 | 2128.6 | 2111.3 | 2109 |
| ν3 σ | 1921 | 1927.2 | 1923.2 | 1922 |
| ν4 σ | 1166 | 1167.7 | 1184 | |
| ν5 σ | 612 | 614.4 | 629 | |
| ν6 π | 529 | 503.0 | ||
| ν7 π | 500 | 493.7 | 484 | |
| ν8 π | 242 | 227.4 | 226 | |
| ν9 π | 102 | 96.5 | 109 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepaniak, U.; Kołos, R.; Guillemin, J.-C.; Crépin, C. Phosphorescence of C5N− in Rare Gas Solids. Photochem 2022, 2, 263-271. https://doi.org/10.3390/photochem2020019
Szczepaniak U, Kołos R, Guillemin J-C, Crépin C. Phosphorescence of C5N− in Rare Gas Solids. Photochem. 2022; 2(2):263-271. https://doi.org/10.3390/photochem2020019
Chicago/Turabian StyleSzczepaniak, Urszula, Robert Kołos, Jean-Claude Guillemin, and Claudine Crépin. 2022. "Phosphorescence of C5N− in Rare Gas Solids" Photochem 2, no. 2: 263-271. https://doi.org/10.3390/photochem2020019
APA StyleSzczepaniak, U., Kołos, R., Guillemin, J.-C., & Crépin, C. (2022). Phosphorescence of C5N− in Rare Gas Solids. Photochem, 2(2), 263-271. https://doi.org/10.3390/photochem2020019







