Photodynamic Effect of 5,10,15,20-Tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin towards the Human Pathogen Candida albicans under Different Culture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Strains and Cultures of C. albicans
2.4. PDI of C. albicans Planktonic Cells
2.5. PDI of C. albicans under Growing Conditions
2.6. PDI of C. albicans Pseudohyphae
2.7. Binding of TAPC to Pseudohyphae of C. albicans
2.8. PDI of C. albicans Cells in Albumin Suspensions
2.9. Controls and Statistical Analysis
3. Results
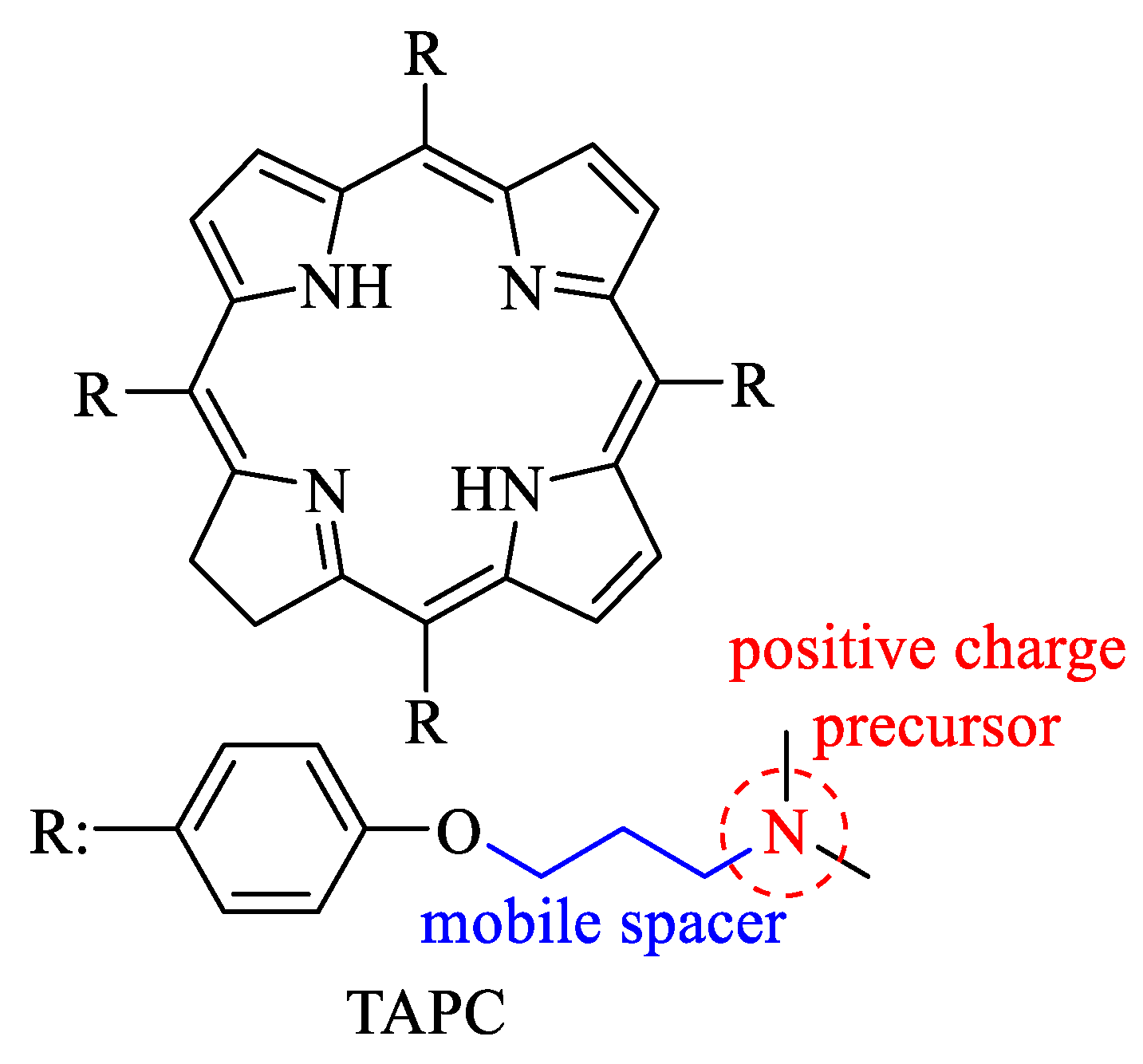
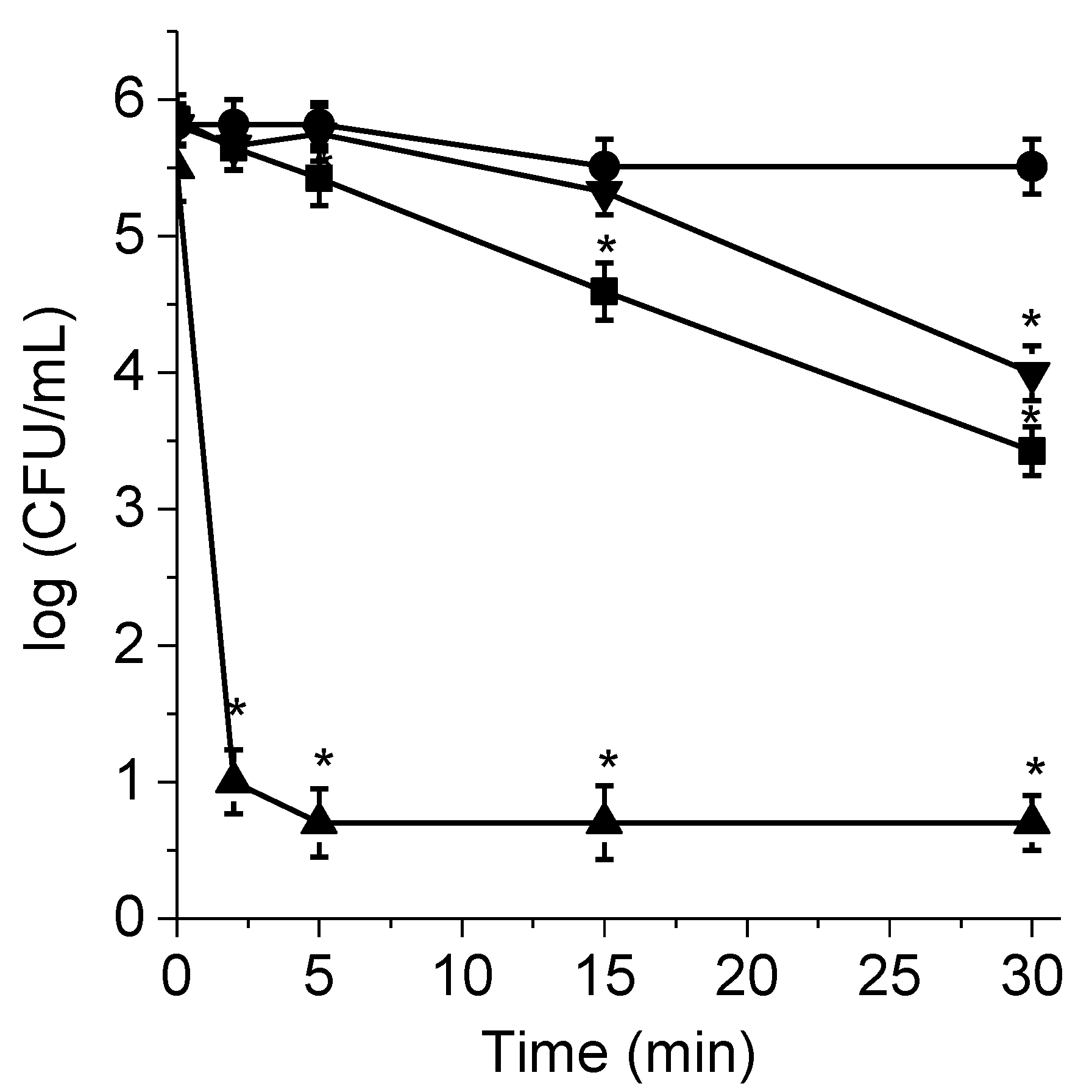
3.1. PDI of C. albicans Planktonic Cells and Photodynamic Action Mechanism
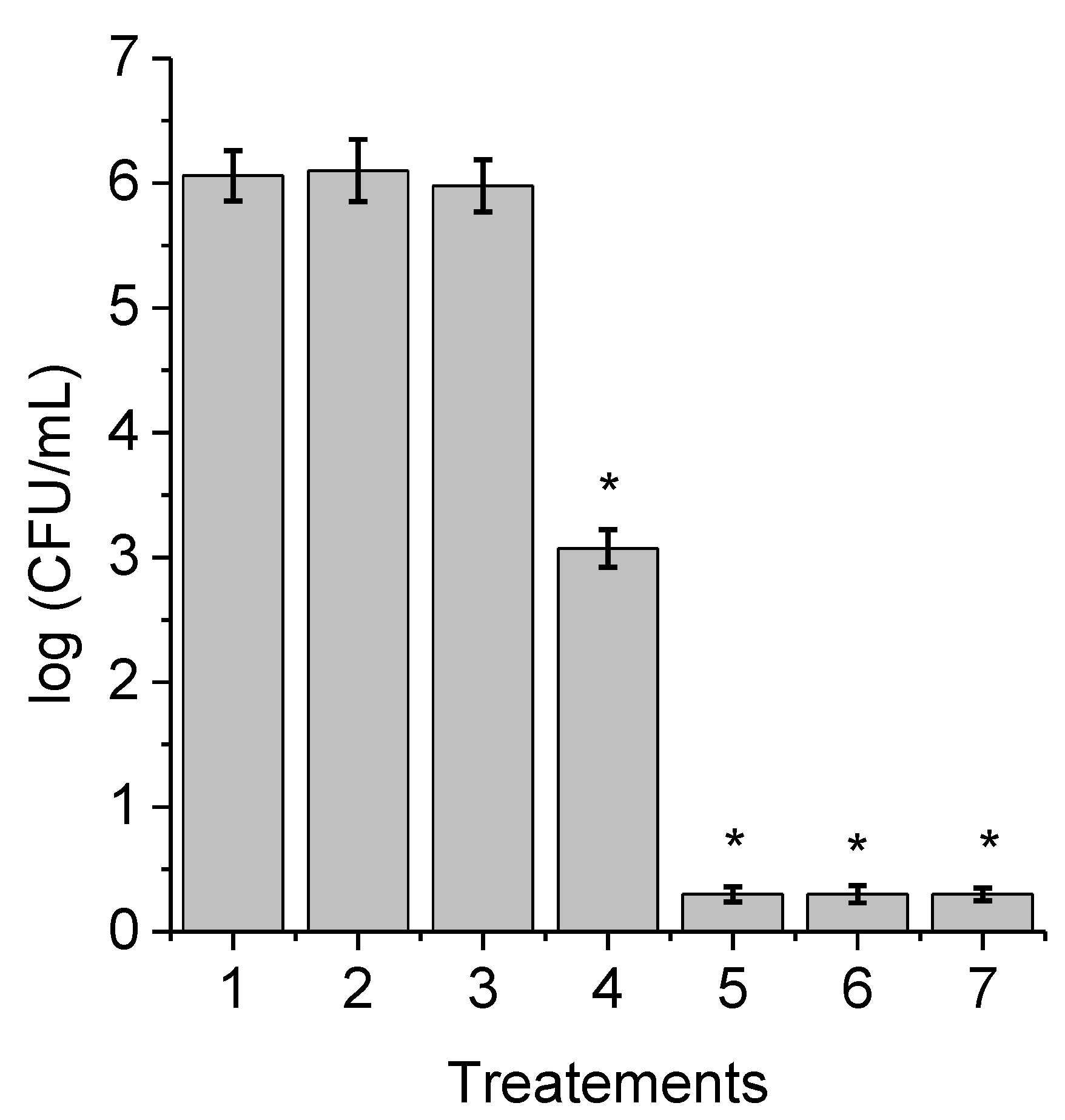
3.2. Photosensitized Effect of TAPC on the Growth of C. albicans Cells
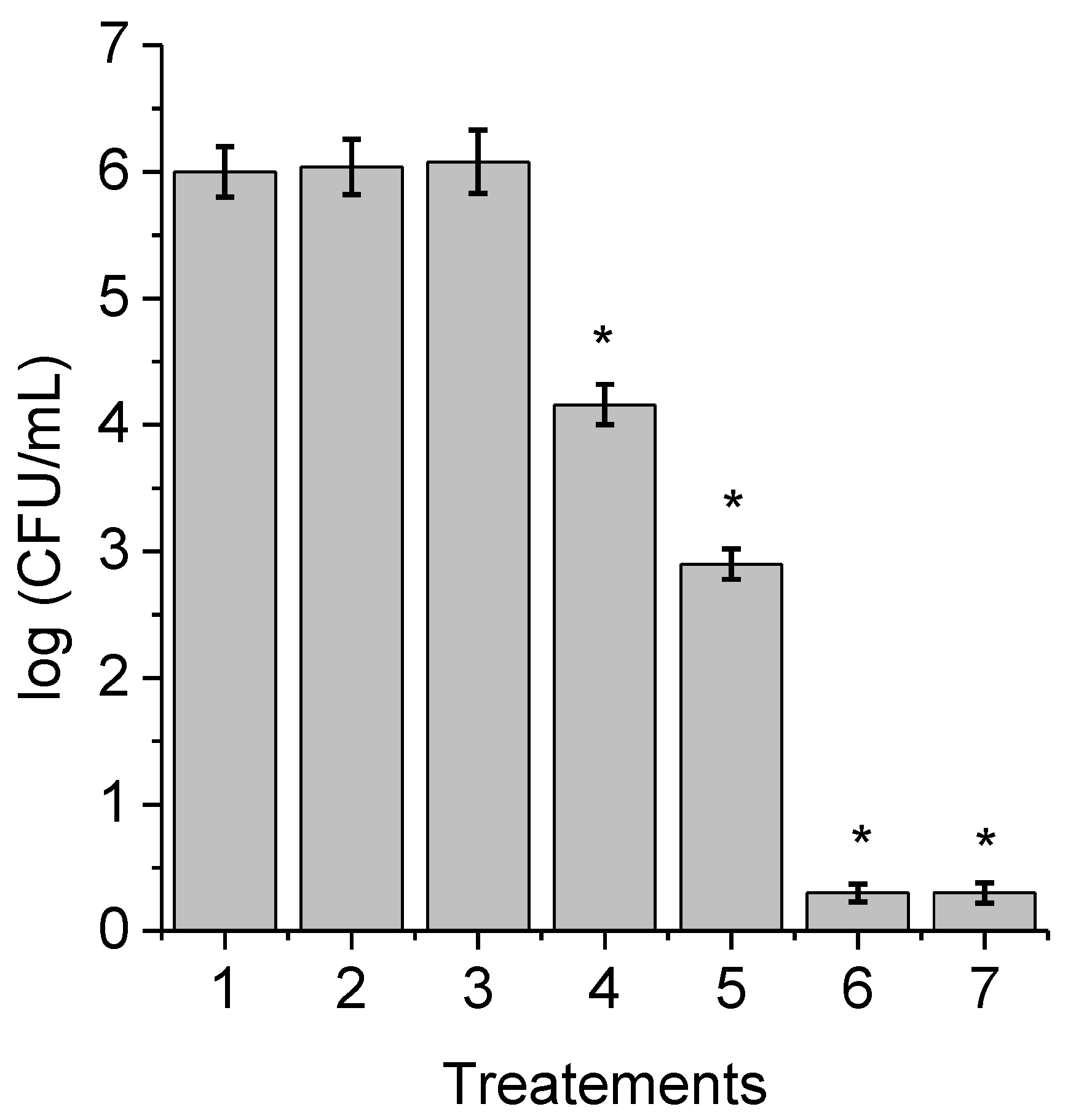
3.3. Photoinactivation of C. albicans Pseudohyphae
3.4. Binding of TAPC to C. albicans Pseudohyphae
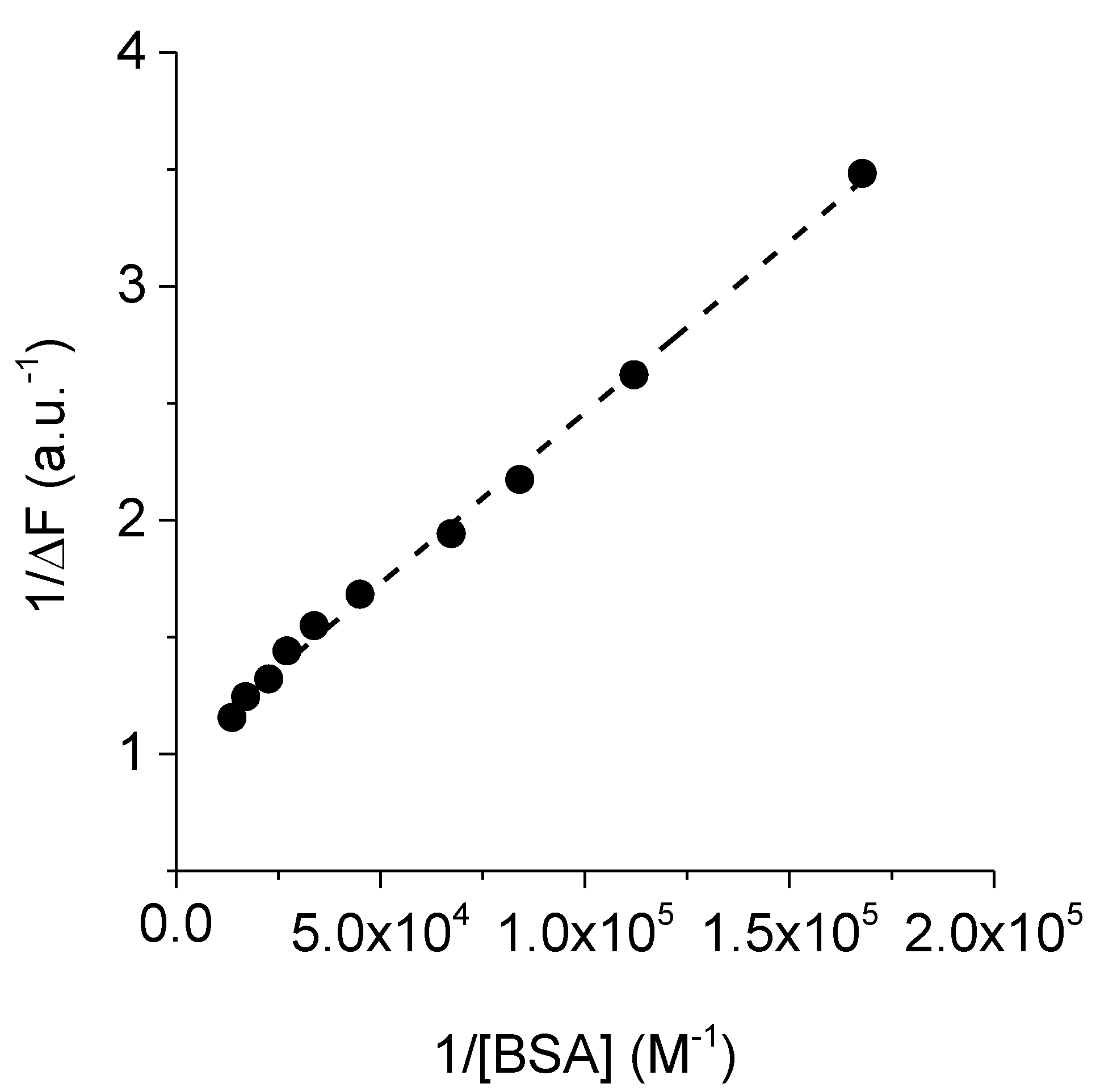
3.5. Interaction of TAPC with BSA
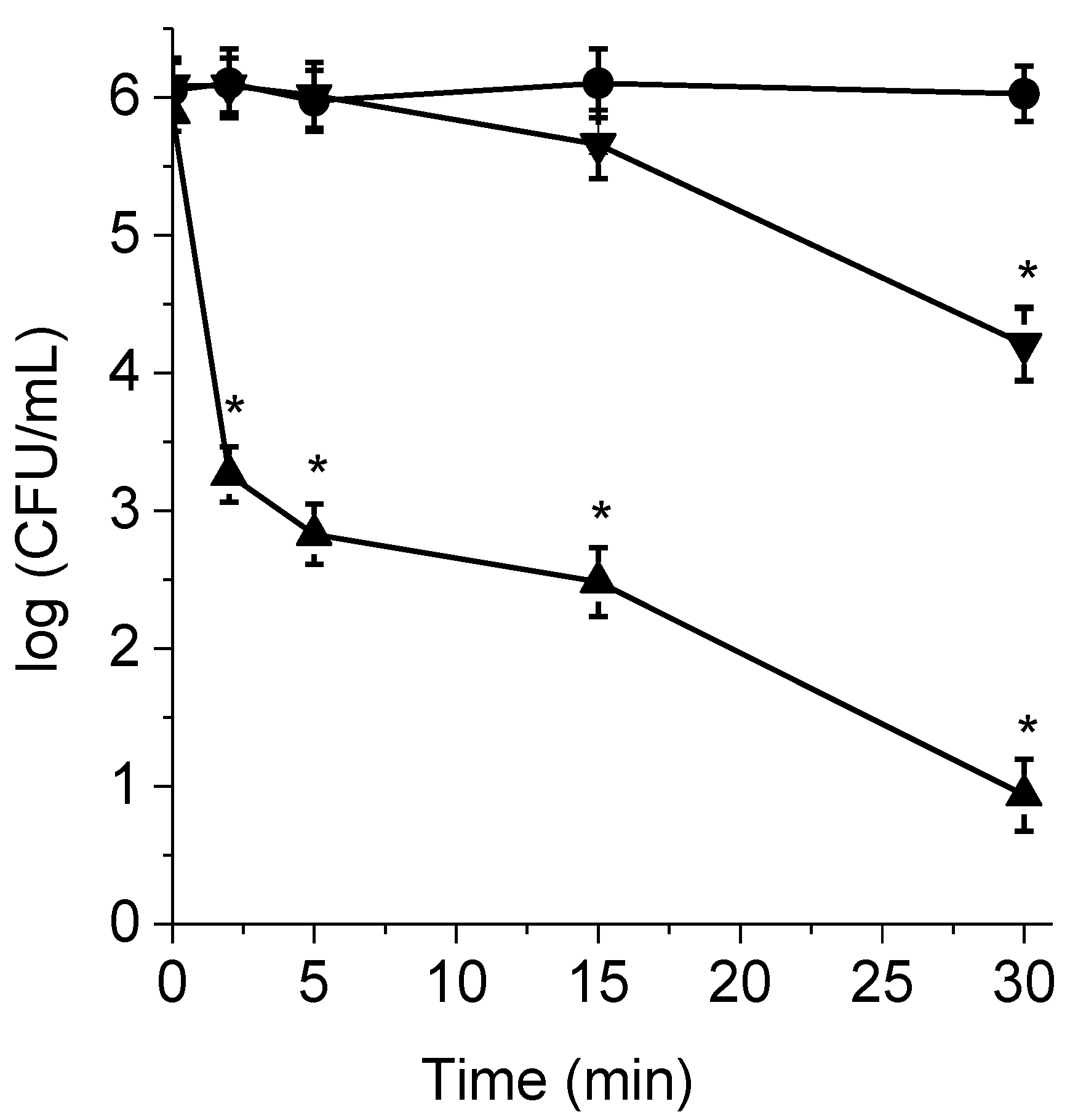
3.6. Inactivation of Planktonic Cells in Presence of BSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janbon, G.; Quintin, J.; Lanternier, F.; d’Enfert, C. Studying fungal pathogens of humans and fungal infections: Fungal diversity and diversity of approaches. Microbes Infect. 2019, 21, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef]
- Perfect, J.R.; Ghannoum, M. Emerging issues in antifungal resistance. Infect. Dis. Clin. N. Am. 2020, 34, 921–943. [Google Scholar] [CrossRef]
- Williams, T.J.; Harvey, S.; Armstrong-James, D. Immunotherapeutic approaches for fungal infections. Curr. Opin. Microbiol. 2020, 58, 130–137. [Google Scholar] [CrossRef]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal fungi in health and disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]
- Epelbaum, O.; Chasan, R. Candidemia in the intensive care unit. Clin. Chest Med. 2017, 38, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Capoor, M.R.; Subudhi, C.P.; Collier, A.; Bal, A.M. Antifungal stewardship with an emphasis on candidaemia. J. Glob. Antimicrob. Resist. 2019, 19, 262–268. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, M. Photodynamic antifungal chemotherapy. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Liu, Y.-C.; Sun, H.; Guo, D.-S. Type I photodynamic therapy by organic-inorganic hybrid materials: From strategies to applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Durantini, A.M.; Heredia, D.A.; Durantini, J.E.; Durantini, E.N. BODIPYs to the rescue: Potential applications in photodynamic inactivation. Eur. J. Med. Chem. 2018, 144, 651–661. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef] [Green Version]
- Martinez De Pinillos Bayona, A.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug. Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Ferreyra, D.D.; Reynoso, E.; Cordero, P.; Spesia, M.B.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Synthesis and properties of 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl] chlorin as potential broad-spectrum antimicrobial photosensitizers. J. Photochem. Photobiol. B Biol. 2016, 158, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, M.B.; Milanesio, M.E.; Fujita, H.; Lindsey, J.S.; Durantini, E.N. Bacteriochlorin-bis(spermine) conjugate affords an effective photodynamic action to eradicate microorganisms. J. Biophotonics 2020, 13, e201960061. [Google Scholar] [CrossRef]
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin-Schiff base conjugates bearing basic amino groups as antimicrobial phototherapeutic agents. Molecules 2021, 26, 5877. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.D.; Cordero, P.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Mechanistic aspects in the photodynamic inactivation of Candida albicans sensitized by a dimethylaminopropoxy porphyrin and its equivalent with cationic intrinsic charges. Photodiagn. Photodyn. Ther. 2020, 31, 101877. [Google Scholar] [CrossRef] [PubMed]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.D.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Photodynamic inactivation of Candida albicans by a tetracationictentacle porphyrin and its analogue without intrinsic charges inpresence of fluconazole. Photodiagn. Photodyn. Ther. 2016, 13, 334–340. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Cheung, B.P.K.; Yau, J.Y.Y.; Yeung, S.K.W.; Samaranayake, L.P. Human serum promotes candida albicans biofilm growth and virulence gene expression on silicone bio-material. PLoS ONE 2013, 8, e62902. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.F.F.; Pedersen, B.W.; Breitenbach, T.; Toftegaard, R.; Kuimova, M.K.; Arnaut, L.G.; Ogilby, P.R. Irradiation- and sensitizer-dependent changes in the lifetime of intracellular singlet oxygen produced in a photosensitized process. J. Phys. Chem. B 2012, 116, 445–461. [Google Scholar] [CrossRef]
- Cormick, M.P.; Quiroga, E.D.; Bertolotti, S.G.; Alvarez, M.G.; Durantini, E.N. Mechanistic insight of the photodynamic effect induced by tri- and tetra-cationic porphyrins on Candida albicans cells. Photochem. Photobiol. Sci. 2011, 10, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Bosl, C.; Szeimies, R.M.; Lehn, N.; Abels, C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob. Agents Chemother. 2005, 49, 1542–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gsponer, N.S.; Agazzi, M.L.; Spesia, M.B.; Durantini, E.N. Approaches to unravel pathways of reactive oxygen species in the photoinactivation of bacteria induced by a dicationic fulleropyrrolidini-um derivative. Methods 2016, 109, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Dalle, A.A.; Domergue, L.; Fourcade, F.; Assadi, A.A.; Djelal, H.; Lendormi, T.; Soutrel, I.; Taha, S.; Amrane, A. Efficiency of DMSO as hydroxyl radical probe in an electrochemical advanced oxidation process—Reactive oxygen species monitoring and impact of the current density. Electrochim. Acta 2017, 246, 1–8. [Google Scholar] [CrossRef]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: Highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef] [Green Version]
- An, W.; Jiao, Y.; Dong, C.; Yang, C.; Inoue, Y.; Shua, S. Spectroscopic and molecular modeling of the binding of meso-tetrakis(4-hydroxyphenyl)porphyrin to human serum albumin. Dyes Pigm. 2009, 81, 1–9. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Nakka, S.; Guruprasad, L.; Samanta, A. Interaction of bovine serum albumin with dipolar molecules: Fluorescence and molecular docking studies. J. Phys. Chem. B 2009, 113, 2143–2150. [Google Scholar] [CrossRef]
- Bose, B.; Dube, A. Interaction of chlorin p6 with bovine serum albumin and photodynamic oxidation of protein. J. Photochem. Photobiol. B Biol. 2006, 86, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Durmus, M.; Yaman, H.; Göl, C.; Ahsen, V.; Nyokong, T. Water-soluble quaternized mercaptopyridine-substituted zinc-phthalocyanines: Synthesis, photophysical, photochemical and bovine serum albumin binding properties. Dyes Pigm. 2011, 91, 153–163. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Zhou, B.; Liu, Y.-X.; Zhou, C.-X.; Ding, X.-L.; Liu, Y. Fluorescence study on the interaction of bovine serum albumin with p-aminoazobenzene. J. Fluoresc. 2008, 18, 109–118. [Google Scholar] [CrossRef]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and invasive candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Austermeier, S.; Kasper, L.; Westman, J.; Gresnigt, M.S. I want to break free-macrophage strategies to recognize and kill Candida albicans, and fungal counter-strategies to escape. Curr. Opin. Microbiol. 2020, 58, 15–23. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to antifungal drugs. Infect. Dis. Clin. N. Am. 2021, 35, 279–311. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Monteiro, M.C.; Mellado, E. Azole antifungal drugs: Mode of action and resistance. Encyclop. Mycol. 2021, 1, 427–437. [Google Scholar] [CrossRef]
- Rayer, A.V.; Sumon, K.Z.; Jaffari, L.; Henni, A. Dissociation constants (pKa) of tertiary and cyclic amines: Structural and temperature dependences. J. Chem. Eng. Data 2014, 59, 3805–3813. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Mechanistic insight into the photodynamic effect mediated by porphyrin-fullerene C60 dyads in solution and in Staphylococcus aureus cells. RSC Adv. 2018, 8, 22876–22886. [Google Scholar] [CrossRef] [Green Version]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Porphyrins containing basic aliphatic amino groups as potential broad spectrum antimicrobial agents. Photodiagn. Photodyn. Ther. 2018, 24, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. Susceptibility of Candida albicans to photodynamic action of 5,10,15,20-tetra(4-N-methylpyridyl)porphyrin in different media. FEMS Immunol. Med. Microbiol. 2010, 60, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wu, M.; Wang, Y.; Chen, Y.; Gao, J.; Ying, C. ERG11 couples oxidative stress adaptation, hyphal elongation and virulence in Candida albicans. FEMS Yeast Res. 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, N.S.; Gubarev, Y.A.; Yurina, E.S.; Syrbu, S.A. Features of interaction of tetraiodide meso-tetra(N-methyl-3-pyridyl) porphyrin with bovine serum albumin. J. Mol. Liq. 2018, 265, 664–667. [Google Scholar] [CrossRef]
- Kollara, J.; Machacek, M.; Jancarova, A.; Kubat, P.; Kucera, R.; Miletina, M.; Novakova, V.; Zimcika, P. Effect of bovine serum albumin on the photodynamic activity of sulfonated tetrapyrazino-porphyrazine. Dyes Pigm. 2019, 162, 358–366. [Google Scholar] [CrossRef]
- Bliss, J.M.; Bigelow, C.E.; Foster, T.H.; Haidaris, C.G. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob. Agents Chemother. 2004, 48, 2000–2006. [Google Scholar] [CrossRef] [Green Version]
- Chabrier-Roselló, Y.; Foster, T.H.; Pérez-Nazario, N.; Mitra, S.; Haidaris, C.G. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob. Agents Chemother. 2005, 49, 4288–4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baler, K.; Martin, O.A.; Carignano, M.A.; Ameer, G.A.; Vila, J.A.; Szleifer, I. Electrostatic unfolding and interactions of albumin driven by pH changes: A molecular dynamics study. J. Phys. Chem. B 2014, 118, 921–930. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Z.; Zhang, Y.; Li, R.; Xiao, Q.; Liu, Y.; Li, Z. Binding of cationic porphyrin to human serum albumin studied using comprehensive spectroscopic methods. J. Pharm. Sci. 2009, 98, 105–113. [Google Scholar] [CrossRef]
- Lambrechts, S.A.G.; Aalders, M.C.G.; Verbraak, F.D.; Lagerberg, J.W.M.; Dankert, J.B.; Schuitmaker, J.J. Effect of albumin on the photodynamic inactivation of microorganisms by a cationic porphyrin. J. Photochem. Photobiol. B Biol. 2005, 79, 51–57. [Google Scholar] [CrossRef]





| Medium | TAPC (nmol/106 Cells) | TAPC (%) |
|---|---|---|
| Cells-PBS a | 2.23 ± 0.26 | 43 |
| Supernatant-PBS b | 2.96 ± 0.41 | 57 |
| Cells-HS c | 0.45 ± 0.26 | 8 |
| Supernatant-HS d | 5.11 ± 0.74 | 92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero, P.V.; Ferreyra, D.D.; Pérez, M.E.; Alvarez, M.G.; Durantini, E.N. Photodynamic Effect of 5,10,15,20-Tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin towards the Human Pathogen Candida albicans under Different Culture Conditions. Photochem 2021, 1, 505-522. https://doi.org/10.3390/photochem1030033
Cordero PV, Ferreyra DD, Pérez ME, Alvarez MG, Durantini EN. Photodynamic Effect of 5,10,15,20-Tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin towards the Human Pathogen Candida albicans under Different Culture Conditions. Photochem. 2021; 1(3):505-522. https://doi.org/10.3390/photochem1030033
Chicago/Turabian StyleCordero, Paula V., Darío D. Ferreyra, María E. Pérez, María G. Alvarez, and Edgardo N. Durantini. 2021. "Photodynamic Effect of 5,10,15,20-Tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin towards the Human Pathogen Candida albicans under Different Culture Conditions" Photochem 1, no. 3: 505-522. https://doi.org/10.3390/photochem1030033
APA StyleCordero, P. V., Ferreyra, D. D., Pérez, M. E., Alvarez, M. G., & Durantini, E. N. (2021). Photodynamic Effect of 5,10,15,20-Tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin towards the Human Pathogen Candida albicans under Different Culture Conditions. Photochem, 1(3), 505-522. https://doi.org/10.3390/photochem1030033






